Abstract
Background/Objectives: Catheter ablation is an effective treatment for atrial fibrillation (AF) but is associated with femoral vascular complications. While anticoagulation therapy has been linked to these complications, specific risk factors remain unclear. This study assessed the incidence and predictors of vascular complications after catheter ablation using systematic duplex ultrasound (DUS) as well as their outcomes. Methods: A single-center observational study was conducted with 404 consecutive AF patients who underwent catheter ablation between March 2023 and February 2024. DUS was performed systematically post-procedure in all patients to identify complications; these were primarily treated with DUS-guided manual compression. Results: Vascular complications were observed in 6.4% of patients, higher than reported in previous studies. Hematomas (3.5%) and arteriovenous fistulas (AVFs, 2.0%) were the most common such complications. Multivariate analysis identified repeat ablation (odds ratio [OR] 3.09, 95% confidence interval [CI] 1.10–8.64, p = 0.03) and months of experience <6 months (OR 3.42, 95% CI 1.36–8.63, p = 0.01) as significant predictors of complications. DUS-guided compression managed most complications successfully, with three pseudoaneurysms resolved through compression and one through embolization. However, AVFs were relatively resistant to conservative management, often necessitating prolonged observation or intervention. Conclusions: Systematic DUS following catheter ablation revealed a higher-than-expected incidence of vascular complications. Repeat ablation and months of experience <6 months are potential predictors of femoral vascular complications. DUS-guided compression is effective for most complications, although AVFs present greater treatment challenges.
1. Introduction
The catheter ablation is a widely utilized intervention for the treatment of cardiac arrhythmias, including atrial fibrillation (AF). However, this procedure is not always safe [1,2,3]. Complication rates associated with catheter ablation vary depending on the type of procedure [4], patient characteristics, and operator experience [5]. Among these complications, vascular complications are particularly noteworthy due to their frequency and potential severity.
The femoral vein is a common access site for catheter ablation, and the use of ultrasound (US) guidance for femoral venipuncture has been proposed to reduce both major and minor complications associated with the procedure [6]. A meta-analysis indicated that US-guided femoral venipuncture can reduce the incidence of complications such as hematomas, arteriovenous fistulas (AVFs), and pseudoaneurysms [7]. Despite these findings, a randomized controlled trial reported that US-guided femoral venipuncture improved inter-procedural outcomes such as puncture time, number of puncture attempts, inadvertent arterial punctures, and unsuccessful cannulations, but did not significantly decrease the overall rate of major complications [8].
Vascular complications remain the most prevalent type of complication following catheter ablation for AF, with an incidence rate of approximately 1.31% [9]. However, the real incidence of vascular complications, including those that may be clinically silent or “hidden”, is not well understood. Traditional clinical assessments may underestimate the true frequency of these complications, highlighting the need for more sensitive and comprehensive diagnostic tools.
Predictors of vascular complications following catheter ablation are not as well characterized as those for other major complications. Factors such as patient demographics [10], comorbid conditions, procedural specifics, and operator technique may all influence the likelihood of vascular complications. Understanding these predictors is crucial for risk stratification, patient counseling, and preventative strategies.
Given the importance of early detection and management of vascular complications, our study aimed to investigate the incidence of these complications using DUS following catheter ablation. Additionally, we sought to identify predictors of vascular complications to better inform clinical practice and improve patient outcomes. This study addresses a significant gap in the current literature by providing a comprehensive analysis of postoperative vascular complications and their predictors, contributing to the optimization of catheter ablation procedures for AF.
2. Materials and Methods
This single-center observational study consisted of 404 consecutive patients with AF who underwent catheter ablation at Samsung Medical Center between March 2023 and February 2024. Detailed patient data and clinical outcomes were obtained from medical records. The hospital’s institutional review board approved the study protocol (IRB No. SMC-2024-01-023-001), which complied with the Declaration of Helsinki.
2.1. Procedural Protocols
As recommended in the consensus, anticoagulation therapy in AF ablation was uninterrupted during the periprocedural period [11,12]. The procedure was performed under general anesthesia or sedation with midazolam and propofol. Femoral access was obtained using the modified Seldinger technique, and a 6.5-Fr/7.5-Fr/8.5-Fr-sized sheath for ablation catheter insertion was cannulated. The months of experience were defined as months of venipuncture experience for AF ablation and categorized into less than 6 months and 6 months or more. The operators who attempted vascular puncture were first-year fellows in cardiac electrophysiology and none had prior venipuncture experience for AF catheter ablation. Our institution’s fellowship begins in March, and a new fellow starts performing venipuncture the following year. We defined months of experience < 6 months from early March to the end of August, and months of experience ≥ 6 months from early September to the end of February of the following year. The latest consensus emphasizes the necessity of US-guided venipuncture to reduce vascular complications during catheter ablation of AF, yet 24% among the writing group members still perform venipuncture without US guidance [12]. In most cases, the first attempt used a technique where the femoral artery pulse was palpated, followed by puncturing medially without the aid of US. Venipuncture was performed with the basic principle of puncturing from the lateral to the medial side, without crossing the height of the inguinal crease and without invading the outer boundary where the femoral artery pulse is palpable. There were no additional rules that restricted the operators beyond this.
A transseptal puncture was performed using a Brockenbrough needle (Abbott, St. Paul, MN, USA) and an 8.5-Fr-long sheath (SL-0, St. Jude Medical, Inc., St. Paul, MN, USA). Intracardiac echocardiography was performed during transseptal puncture and ablation procedures for monitoring complications. For reducing complications including cardiac tamponade, intravenous heparin was administered immediately following transseptal puncture during ablation procedures and adjusted to achieve and maintain an activated clotting time (ACT) of at least 300 s [13]. The latest consensus supports initial heparin bolus administration before transseptal puncture to reduce thrombus [12]. Pulmonary vein isolation was performed with either a 28-mm second-generation cryoballoon (Arctic Front Advance, Medtronic, Minneapolis, MN, USA or Polar X, Boston Scientific, St. Paul, MN, USA) via a 15-Fr steerable sheath (FlexCath, Medtronic, Minneapolis, MN, USA or POLARSHEATH, Boston Scientific, St. Paul, MN, USA) or an irrigated-tip radiofrequency catheter (SmartTouch Surround Flow, Biosense Webster, Irvine, CA, USA or FlexAbility, Abbott, St. Paul, MN, USA) via an 8.5-Fr-long sheath (SL-0) guided by a three-dimensional (3D) mapping system (CARTO3, Biosense Webster, Irvine, CA, USA or ENSITE, Abbott, St. Paul, MN, USA). Additional substrate modification was performed according to the operator’s preference.
2.2. Postoperative Management
Hemostasis of the vascular access site was achieved using a figure-eight suture after protamine administration. Simple manual compression was used only in cases of failure, and venous vascular closure systems were not utilized. Post-procedure DUS evaluations for all patients that underwent catheter ablation for AF were performed by the vascular surgeons on the day of the procedure or the following day. If symptoms indicating complications, such as pain, swelling, or bleeding, were observed in the patient, DUS was conducted on the same day, otherwise, it was performed the next day. The femoral vascular complications after the procedure were reported to include three types: hematoma of Bleeding Academic Research Consortium (BARC) scale 2 or higher, AVF, and pseudoaneurysm [14]. In cases where complications occurred, most were treated with manual compression hemostasis guided by DUS [15]. For lesions that could not be controlled by compression hemostasis, methods such as embolization were utilized. In instances where embolization was unsuccessful or deemed inappropriate, surgical intervention was considered [16].
2.3. Statistical Analysis
Continuous variables are presented as mean ± standard deviation and were compared using Student’s t-test for normally distributed data. For categorical variables, frequencies and percentages were calculated, and comparisons between groups were performed using the chi-square test or Fisher’s exact test as appropriate. Variables with a p-value of less than 0.3 in the univariate analysis were selected for inclusion in a multivariable logistic regression analysis to identify independent predictors of femoral vascular complications. Multivariable logistic regression was performed using a backward elimination method, and results are expressed as odds ratios (ORs) with corresponding 95% confidence intervals (CIs). Statistical significance was defined as a two-sided p-value less than 0.05. All statistical analyses were performed using SPSS software for Windows, version 20.0.0 (IBM Corp., Armonk, NY, USA).
3. Results
3.1. Baseline Characteristics
In total, 222 (55.0%) and 182 (45.0%) patients underwent cryoablation and radiofrequency catheter ablation for AF, respectively. Baseline characteristics of the 404 patients are shown in Table 1. All patients underwent DUS after catheter ablation for AF before discharge. Among these patients, 378 (93.6%) experienced no complications after catheter ablation. The average age of patients included in the study was 56.8 ± 9.6 years, and there were more male patients (315 men, 78%) than female patients. A total of 395 patients, accounting for 98% of the entire group, was on anticoagulants. Average left atrial size was 43.7 ± 7.0 mm, indicating a relatively enlarged left atrium. Baseline characteristics were not significantly different between the two groups, except for diabetes. Diabetes was not only significantly more prevalent in the group without vascular complications, but it was also found exclusively in that group (p < 0.04).

Table 1.
Baseline characteristics.
3.2. Procedural Characteristics
Table 2 summarizes the procedural characteristics of the study population, categorized by the presence or absence of femoral vascular complications. A history of previous ablation was significantly associated with a higher rate of femoral vascular complications (23.1% vs. 10.1%, p = 0.039). However, no significant differences were found between groups regarding the number of venous sheaths used (mean 4.4 ± 0.5 for both groups, p = 0.520) or the use of cryoballoon ablation (57.7% vs. 54.8%, p = 0.771). Procedure duration was slightly shorter in patients with femoral vascular complications than those without, but this difference was not significant (92.5 ± 23.5 min vs. 102.7 ± 35.9 min, p = 0.140).

Table 2.
Procedural characteristics.
Additionally, ACT values including minimum (322.5 ± 26.7 s vs. 314.6 ± 29.1 s, respectively, p = 0.734), maximum (359.8 ± 31.7 s vs. 360.0 ± 33.2 s, respectively, p = 0.763), and mean ACT (335.1 ± 27.7 s vs. 331.0 ± 23.0 s, respectively, p = 0.325), showed no significant differences between the groups. The heparin dose administered was similar, with no significant difference observed between patients with and without complications (10,661.5 ± 442.0 IU vs. 10,491.2 ± 146.3 IU, respectively, p = 0.361).
3.3. Incidence of Femoral Vascular Complications
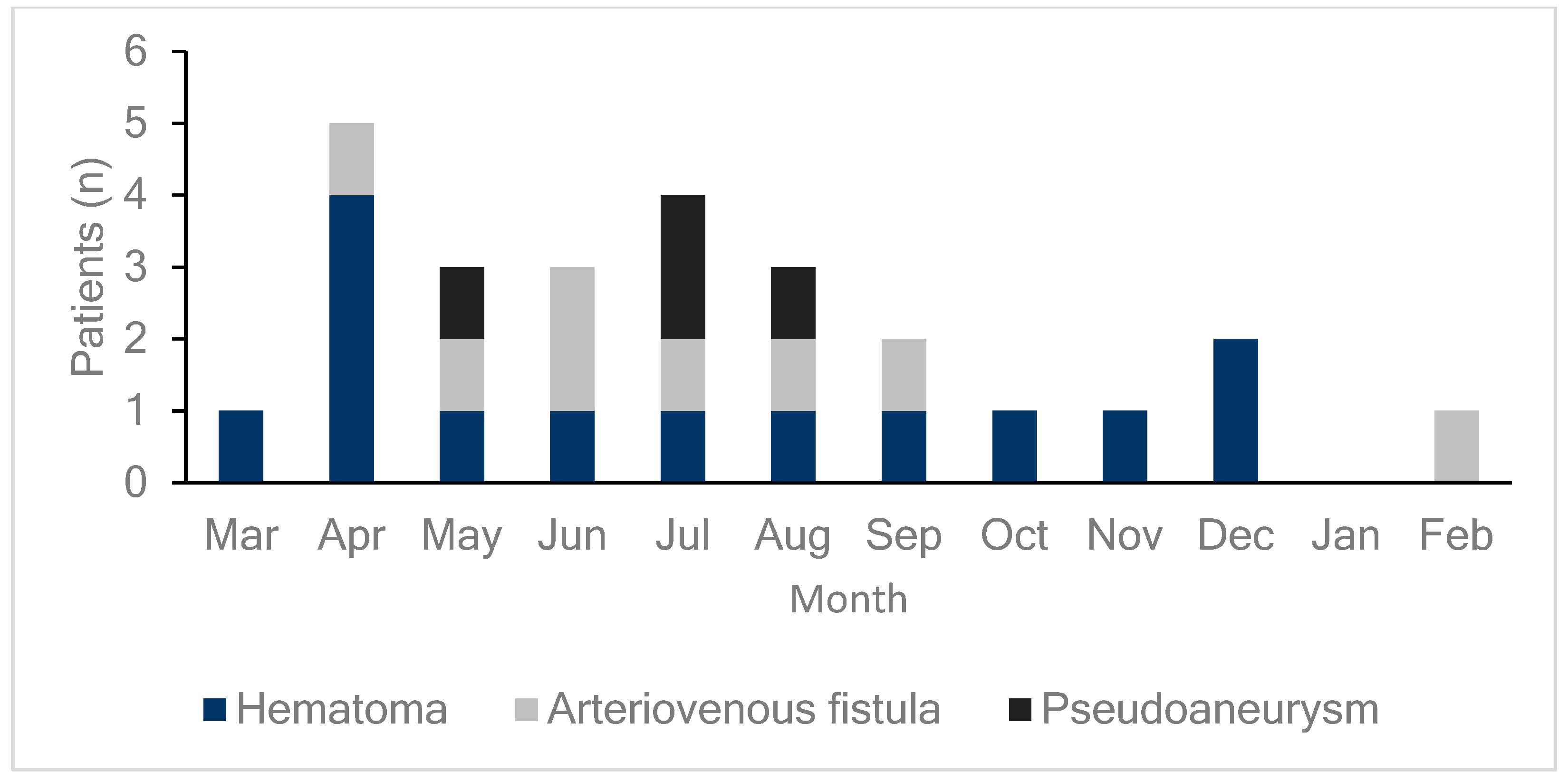
DUS was performed on all patients either on the day of the procedure or the day after the procedure to diagnose the occurrence of femoral vascular complications. As shown in Figure 1, 26 patients (6.4%) exhibited vascular complications at the femoral access site, including hematomas, AVFs, and pseudoaneurysms in 14 (3.5%), eight (2.0%), and four (1.0%) patients, respectively. DUS, performed on all patients regardless of the presence of symptoms, detected early complications in 24 of 26 patients (92%) with confirmed complications. This study compared patients who underwent radiofrequency ablation (RFCA) and cryoballoon ablation and found no differences in vascular complication rates or outcomes between the two groups. Additionally, hematoma was more common in the cryoballoon ablation group (2.2% vs. 4.5%), while pseudoaneurysm occurred more frequently in the RFCA group (0% vs. 2.2%, Supplementary Figure S1). However, these differences were not statistically significant (Table 2). As illustrated in Figure 2, the monthly incidence of femoral vascular complications varied throughout the year. Complications were most frequently observed in the early months after the new practitioner began performing venipuncture procedures, with a notable peak in April (five cases), followed by a gradual decrease over the subsequent months.
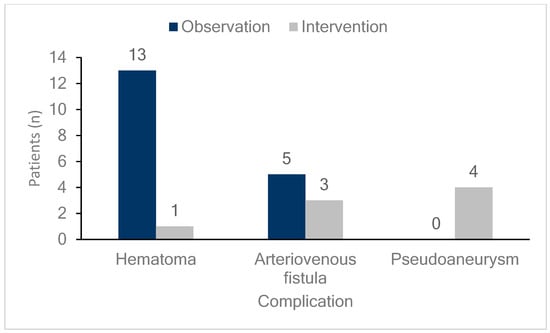
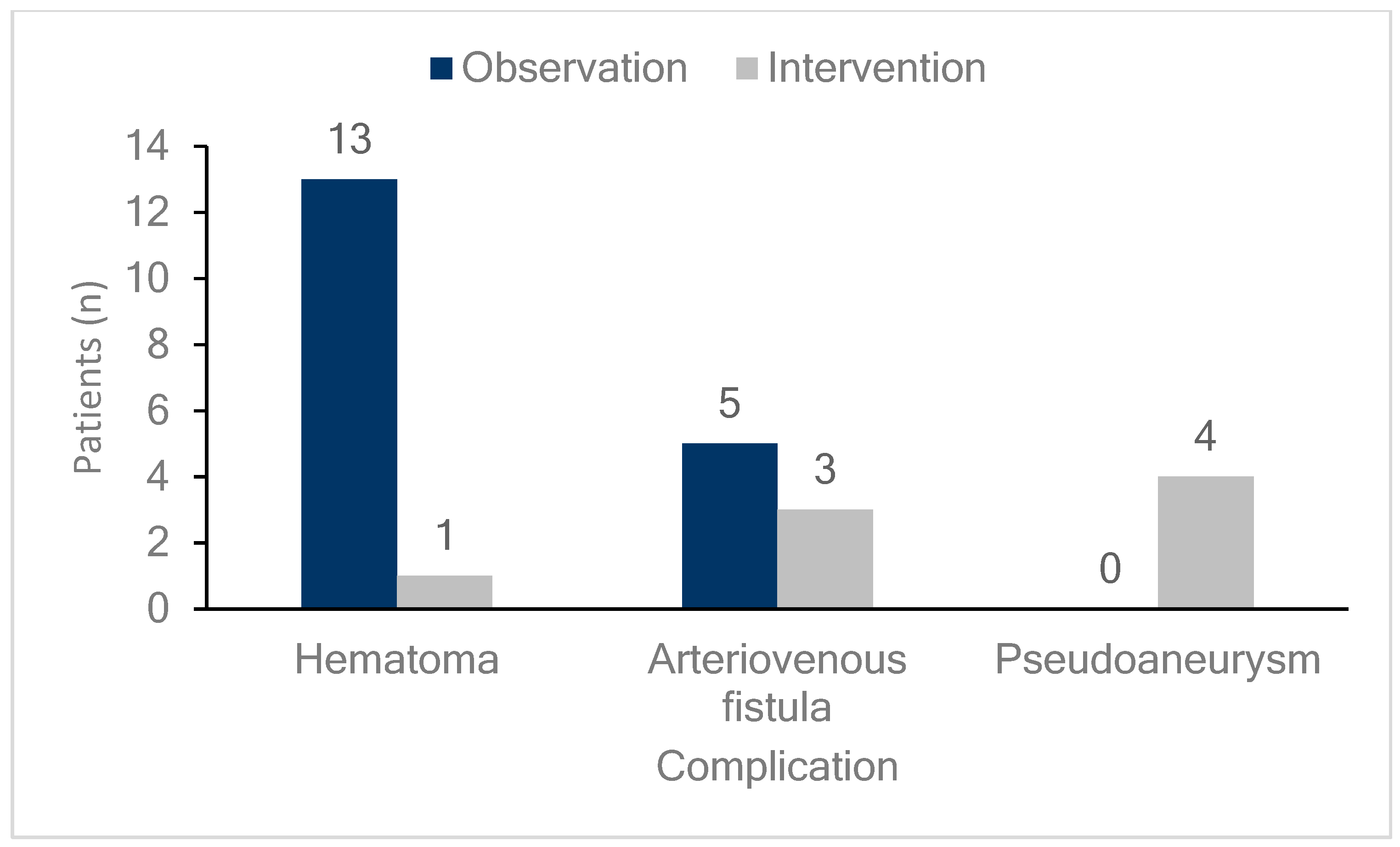
Figure 1.
Incidence and management of femoral vascular complications. The bar graph shows the number and percentage of patients experiencing various femoral vascular complications (hematomas, arteriovenous fistulas, and pseudoaneurysms) along with their management strategies. Dark blue bars represent patients managed through observation, while light gray bars indicate those managed with intervention. Hematomas occurred in 14 patients, with 13 (92.9%) managed by observation and one (7.1%) by intervention. Arteriovenous fistulas were observed in eight patients, with five (62.5%) managed by observation and three (37.5%) by intervention. Pseudoaneurysm occurred in four patients, all of whom (100%) were managed with intervention.
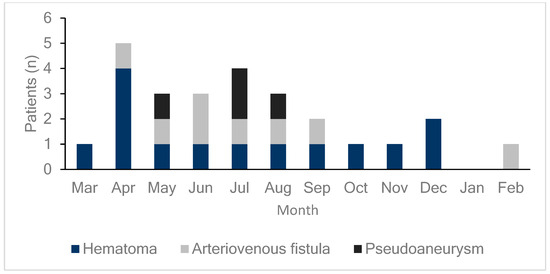
Figure 2.
Monthly incidence of femoral vascular complications. This bar graph displays the distribution of femoral vascular complications (hematomas, arteriovenous fistulas, and pseudoaneurysms) by month. Each color represents a specific type of complication: dark blue for hematomas, light gray for arteriovenous fistulas, and black for pseudoaneurysms. Complications peaked in April, with a total of five cases (four hematomas and one arteriovenous fistula), which may reflect the initial learning curve of a new practitioner that started in March. As the new practitioner gains experience over the year, complication rates may decrease, until a new, inexperienced practitioner takes over each March, potentially leading to similar early peaks annually.
3.4. Management of Femoral Vascular Complications
Management of each complication is detailed in Figure 1. Among hematomas, 93% (13 cases) were observed without any specific intervention. One case of hematoma, which was not detected by DUS but identified through CT angiography, was managed with simple compression without DUS guidance. For AVFs, 38% (3 cases) required additional intervention, with two patients undergoing compression under DUS guidance and one patient receiving embolization. All patients with pseudoaneurysms required intervention; three underwent compression under DUS guidance, and one underwent embolization.
3.5. Follow-Up of Femoral Vascular Complications
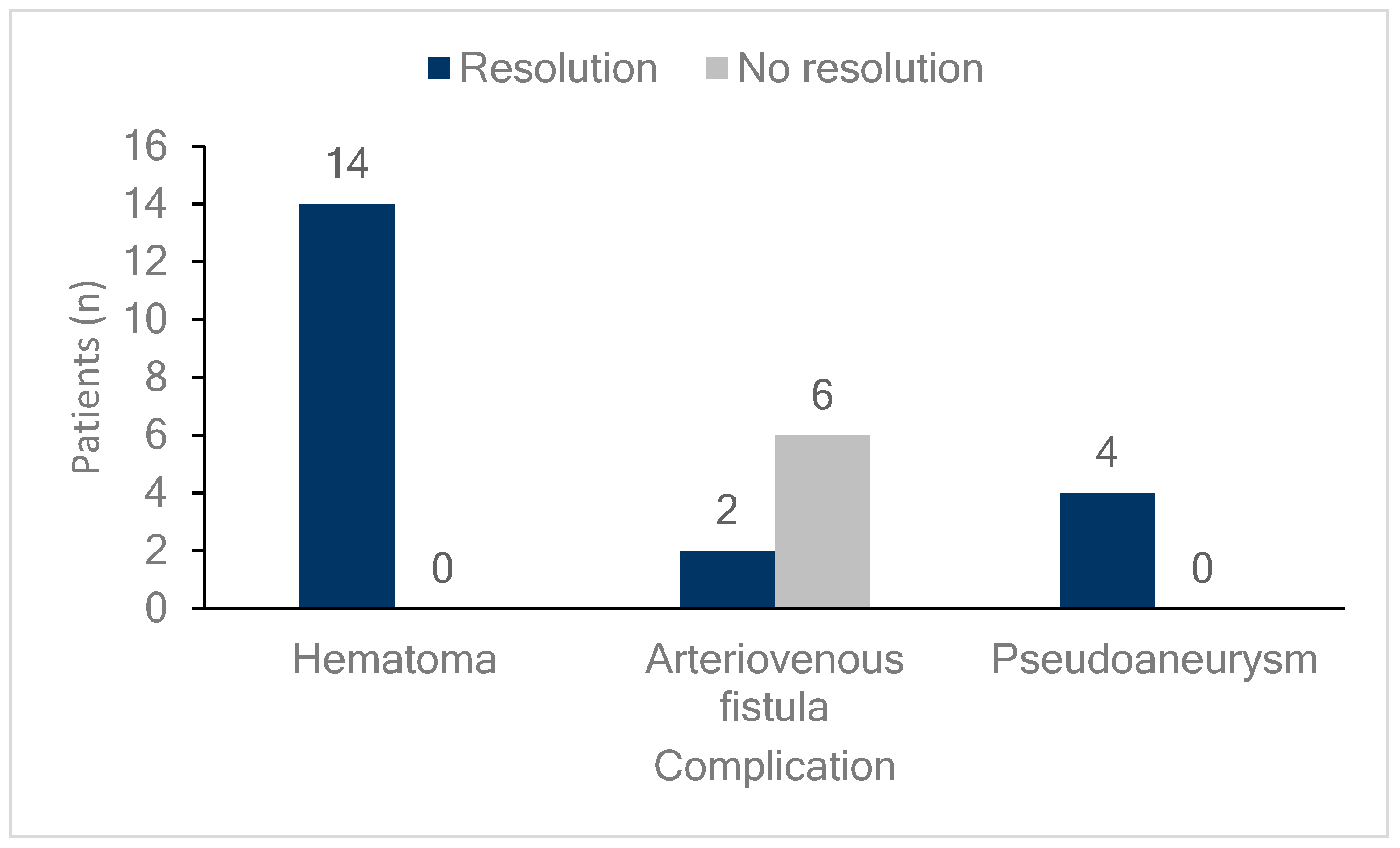
Figure 3 shows the outcomes of the femoral vascular complications. All patients who developed hematomas or pseudoaneurysms, whether they were observed or received additional interventions, experienced complete resolution of the lesions. Meanwhile, 75% (six cases) of patients who developed AVFs did not achieve resolution, and this group included all three patients who received additional interventions. However, none of the patients in any group required surgical intervention.
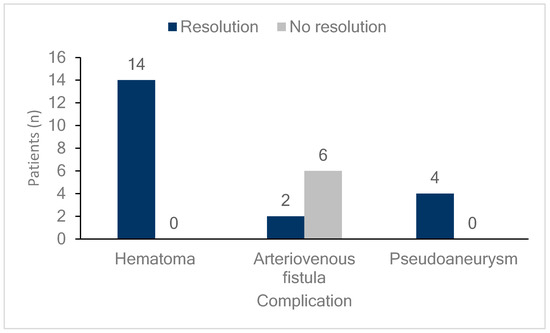
Figure 3.
Follow-up of femoral vascular complications. The bar graph illustrates the resolution status of patients with different femoral vascular complications (hematomas, arteriovenous fistulas, and pseudoaneurysms). Dark blue bars indicate patients whose complications resolved, while light gray bars represent those without resolution. Among patients with hematomas, all 14 (100%) showed resolution. For arteriovenous fistulas, two patients (25%) achieved resolution, while six (75%) did not. In cases of pseudoaneurysm, all four patients (100%) experienced resolution.
3.6. Predictors of Vascular Complications
Univariate and multivariate analyses were conducted to identify predictors of femoral vascular complications (Table 3). In the multivariate analysis, repeat ablation remained a significant predictor, with an increased odds of complications (OR 3.09; 95% CI 1.10–8.64; p = 0.032). Additionally, months of experience < 6 months showed a significant association with higher complication rates (OR 3.42; 95% CI 1.36–8.63; p = 0.009), suggesting a substantial effect of the learning curve on complication risk.

Table 3.
Predictors of vascular complications.
4. Discussion
To our knowledge, this study is the first to report the results of systematic conventional venipuncture and post-procedural DUS to identify and manage complications following catheter ablation for AF, including both RFCA and cryoballoon ablation techniques. The main findings of this study are: (1) post-procedural DUS detected a higher incidence of complications than reported in previous studies; (2) repeat ablation and months of experience < 6 months were identified as risk factors for vascular complications; and (3) most vascular complications were managed successfully with DUS-guided compression, though AVFs were relatively challenging to treat.
Ströker E. et al. presented outcomes from one of the three groups in their study, which underwent conventional puncture followed by US evaluation for complications after catheter ablation [17]. Moreover, our findings align with those of prior study, demonstrating that systematic DUS evaluation can detect more complications. However, our study differs from previous research in several important aspects and provides new clinical implications. First, our study is the first to include both cryoballoon ablation and RFCA as catheter ablation techniques in a systematic DUS study. From a population perspective, our study evaluated not only cryoballoon ablation but also RFCA performed with three-dimensional mapping. Importantly, we found no significant differences in vascular complication outcomes between the two techniques (p = 0.772). Second, unlike previous research, which did not identify risk factors for vascular complications beyond the absence of US-guided venipuncture, our study identified two additional factors associated with vascular complications: (i) operator-related factors, specifically months of experience <6 months, and (ii) patient-related factors, repeat ablation. Finally, in the previous study, only 265 out of 1435 patients underwent venipuncture without US guidance and were subsequently evaluated by US, with 800 patients not receiving pre- or post-procedure US. In contrast, our study assessed 404 patients who underwent venipuncture without US guidance, with systematic post-procedural US evaluation, highlighting a notable difference in population size.
In our study, vascular complications were observed in 6.4% of patients, a higher incidence than the previously reported rate of approximately 1.31% based on traditional clinical assessment. In another study on cryoballoon ablation for AF, where conventional venipuncture was performed followed by systematic DUS, a major US event rate of 3.8% and a minor US event rate of 7.5% were found, indicating a higher incidence of complications than previously reported [17]. This discrepancy highlights the limitations of conventional methods in detecting “hidden” or subclinical vascular issues. DUS, with its ability to visualize both vascular anatomy and blood flow, was instrumental in identifying complications such as AVFs, pseudoaneurysms, and hematomas that might not have been detected through clinical examination alone. These results reinforce the value of routine postoperative DUS evaluation for early identification and management of vascular complications, which can improve patient outcomes. With the studies of same-day discharge in AF catheter ablation, cardiac implantable electronic device and various procedures requiring venipuncture [18,19,20,21], DUS can help facilitate same-day discharge after the procedures.
An early study comparing conventional venipuncture and US-guided venipuncture in AF ablation showed that US-guided venipuncture was associated with a lower rate of BARC 2+ bleeding (10.4% vs. 19.9%) than conventional venipuncture [22]. A recent study compared vascular complication rates within 30 days between conventional venipuncture and US-guided venipuncture, and reported fewer pseudoaneurysms in the US-guided group. However, there were no statistically significant differences in AV fistula or hematoma incidence requiring treatment between the groups [23]. This study also did not include systematic post-procedural imaging, so the full extent of potential complications could not be captured. The complication rate observed in the conventional venipuncture group in our study was not higher than the rates reported in the US-guided venipuncture groups in these previous studies.
A previous study demonstrated that practitioners who underwent mastery learning with a simulator had a higher success rate in central venous catheterization in the intensive care unit (95% vs. 81%) and a lower incidence of arterial puncture (1% vs. 14%) than those who did not have this training [24]. A relatively recent study found that, while practitioners with one year of training and experience showed an overall reduced complication rate in central vein catheterization compared to their first year with limited experience, a significant reduction was observed only in infection rates [25]. Both of these studies focused on venipuncture for central vein catheterization; no prior studies have compared the learning curve and complication rates specifically for femoral venous access. The most recent prospective multicenter cohort study reported that less experienced practitioners (<100 catheterizations) had a higher incidence of major mechanical complications during US-guided central vein catheterization, including femoral venous access, than more experienced practitioners [26]. This study demonstrated that, even in hospitals where procedures are performed under US guidance, complication rates are higher for practitioners with limited experience.
Our data indicate that procedures performed during the months of experience <6 months were associated with a higher complication risk (Table 3). This heightened risk may be attributed to the practitioner’s limited experience, as the incidence of complications tended to decrease as the practitioner became more skilled over time. Figure 2 supports this finding, showing a peak in complications in the early months following the transition of individual practitioners, particularly in April, when the number of complications was notably high. This recurring pattern suggests that the institutional practice of rotating practitioners annually may inadvertently contribute to increased complication rates each spring. Addressing this learning curve effect through targeted training or extended mentorship could potentially mitigate early complications and enhance patient safety. Further studies to explore structured intervention strategies that reduce the impact of practitioner transitions on complication rates are warranted. Patients who had undergone prior catheter ablation were approximately three times more likely to experience vascular complications, possibly due to cumulative trauma to the femoral vessels or scar tissue formation from prior procedures.
A previous study described a causal relationship between anticoagulation therapy and procedure-related complications [27]. In our study, the association between the total administered dose of anticoagulants and procedure-related complications was not significant. Rather than contradicting previous findings, our study findings suggest that the method of administering anticoagulants and the dosing rate per unit time may be more important than the total amount of anticoagulants used. And recent study has shown an association between age and major complications following venipuncture [28]. However, this study did not reveal such finding, which may be attributed to the lower mean age and narrower standard deviation compared to recent study.
Two patients had complications that could not be diagnosed on DUS; however, due to their symptoms, computed tomography (CT) angiography was performed, which identified a hematoma and a pseudoaneurysm, both requiring interventions. Similar to previous study highlighting the utility of the imaging modality [29], these findings suggest that CT angiography can be beneficial in cases where significant symptoms are present but no abnormalities are detected on DUS, particularly in situations such as deep-seated hemorrhage [30,31].
Figure 4 and Figure 5 illustrate two representative cases of femoral vascular complications: an AVF and a pseudoaneurysm, respectively, highlighting differences in progression and treatment outcomes. Figure 4 depicts a case where an AVF, despite early detection, continued to progress over a five-month period, indicating that conservative management alone may be insufficient for complete resolution in such cases. Differing from previous study [32], this suggests that AVFs may require surgical intervention for effective management due to their potential for prolonged persistence and progression. In contrast, Figure 5 presents a pseudoaneurysm case managed initially with DUS-guided compression. Although the initial compression was unsuccessful, follow-up DUS one month later demonstrated spontaneous thrombotic occlusion and improvement of the lesion. Similar to previous study [33], this finding suggests that pseudoaneurysms may respond positively to conservative management with DUS-guided compression, even if immediate success is not achieved. This trend is consistent with observations from Figure 1 and Figure 2, where all pseudoaneurysm cases demonstrated gradual improvement over time following DUS-guided hemostasis or embolization, regardless of short-term treatment success. Conversely, AVF cases exhibited persistent progression, even when detected early. These results underscore the importance of early detection for both complications, while indicating distinct management approaches: pseudoaneurysms can often be managed conservatively with DUS-guided compression, whereas AVFs may require surgical intervention due to the higher likelihood of treatment resistance and progression, even with early detection. This distinction suggests that tailored management strategies are necessary for femoral vascular complications. For AVF cases, early consideration of surgical intervention may result in better long-term outcomes. These differences emphasize the need for individualized treatment strategies when managing femoral vascular complications.
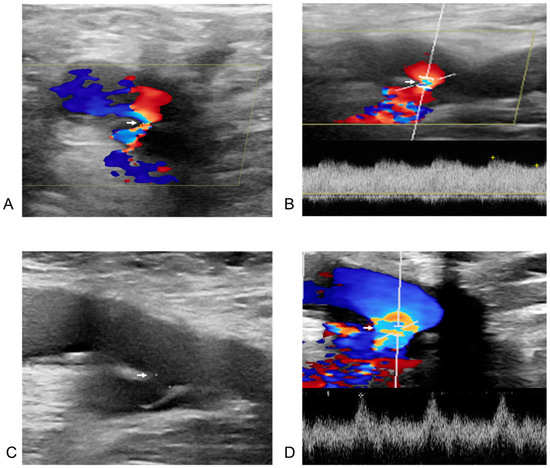
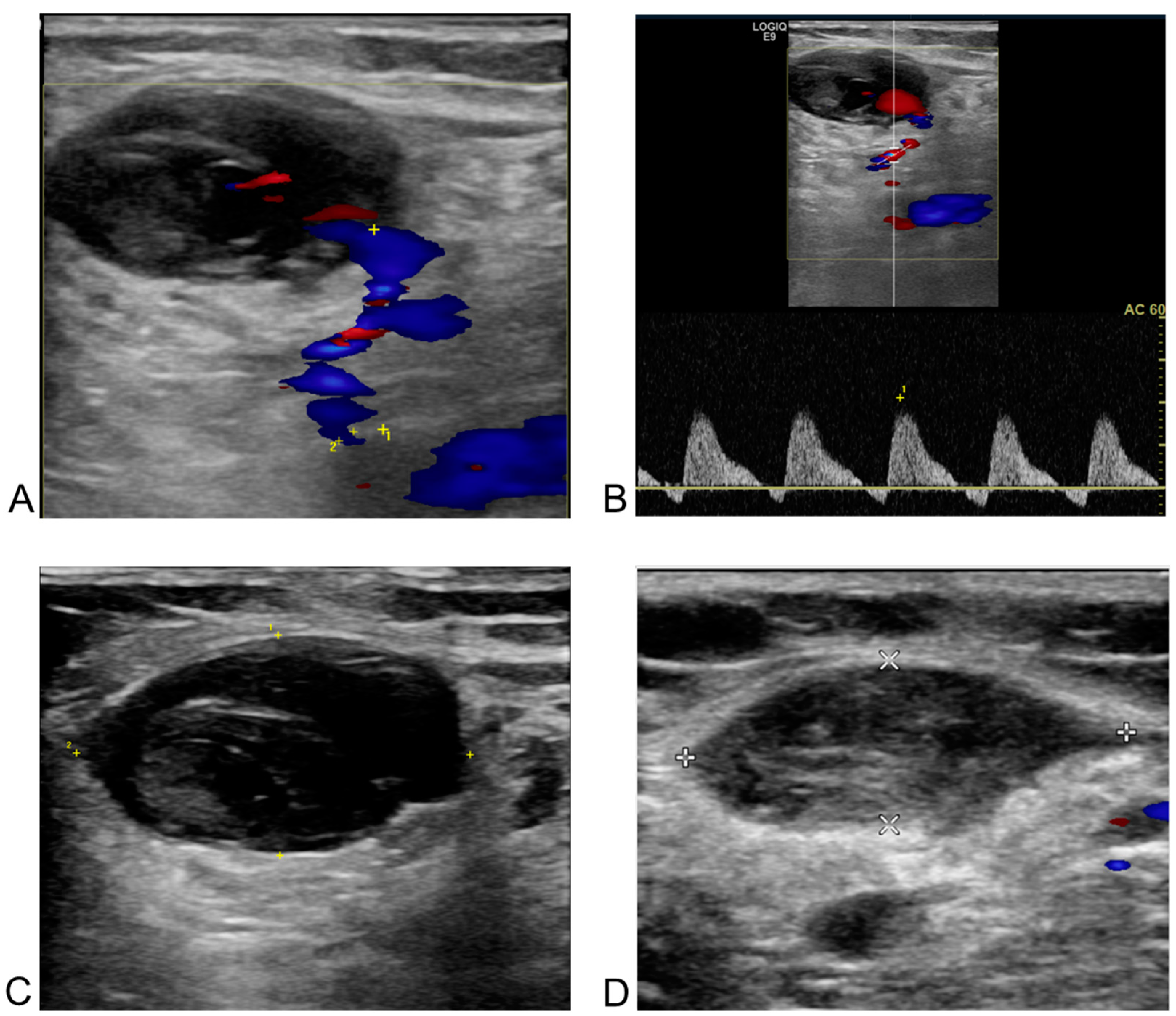
Figure 4.
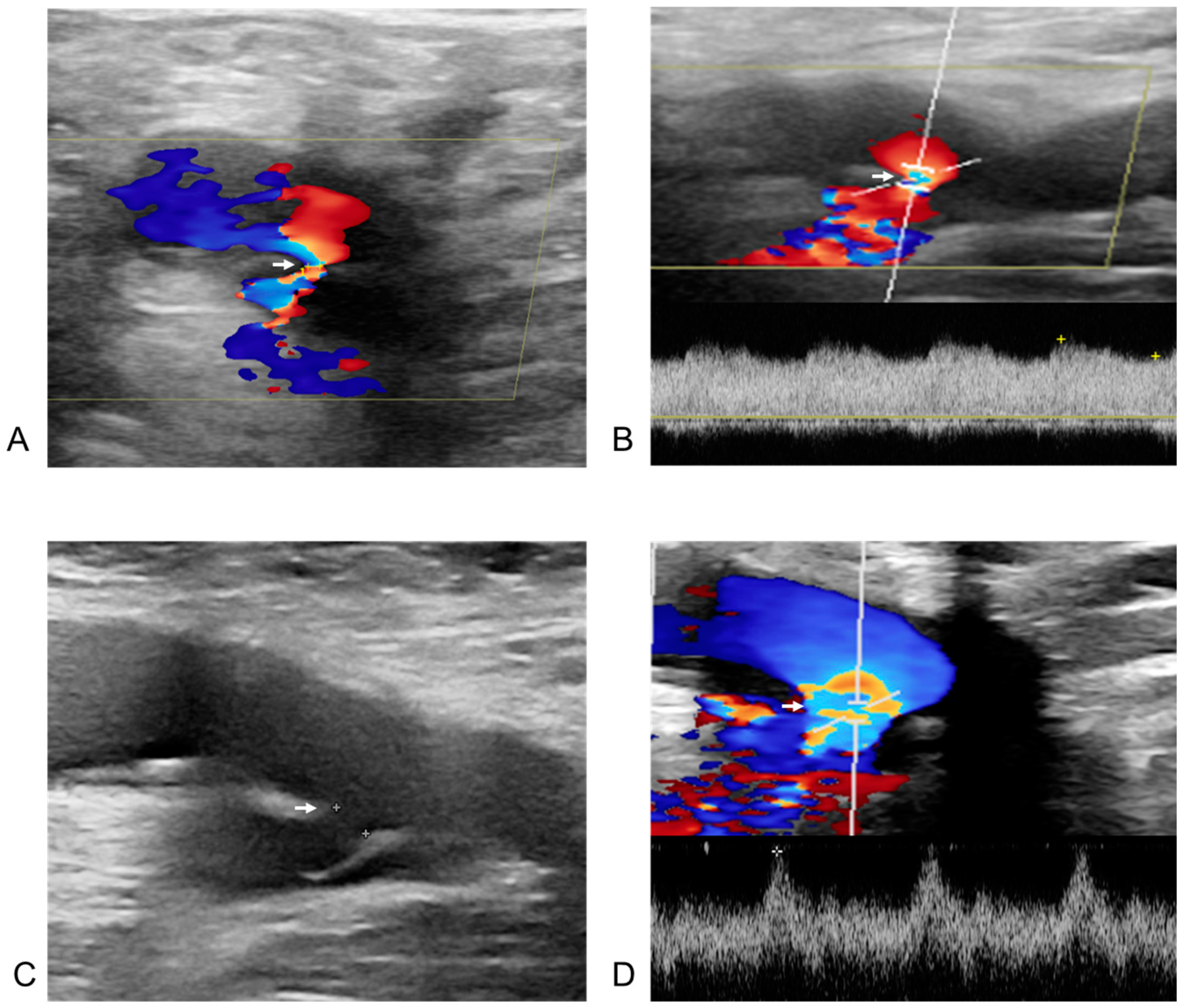
Post-procedural and follow-up duplex ultrasonography (DUS) of an arteriovenous fistula from the right deep femoral artery to the right common femoral vein in a 60-year-old male patient after radiofrequency ablation for atrial fibrillation. The white arrow indicates the neck of the arteriovenous fistula in all images. (A) DUS obtained one day after the procedure. The right deep femoral artery is shown above the arrow, and the right common femoral vein below it. Doppler flow acceleration (the colored area indicated by the white arrow) was observed as blood flowed from the right deep femoral artery through the neck into the right common femoral vein. (B) Spectral Doppler image from one day post-procedure. The diameter of the neck (the colored area indicated by the white arrow) was measured as 1.0 mm, with a flow velocity of 333.2 cm/s. (C) Two-dimensional image at six months after the procedure. The size of the fistula neck (the white arrow) increased from 1.0 mm to 2.6 mm (diameter measured by white spot to spot). (D) Spectral Doppler image at six months post-procedure. A more arterialized waveform (the colored area indicated by the white arrow) was observed within the right common femoral vein.
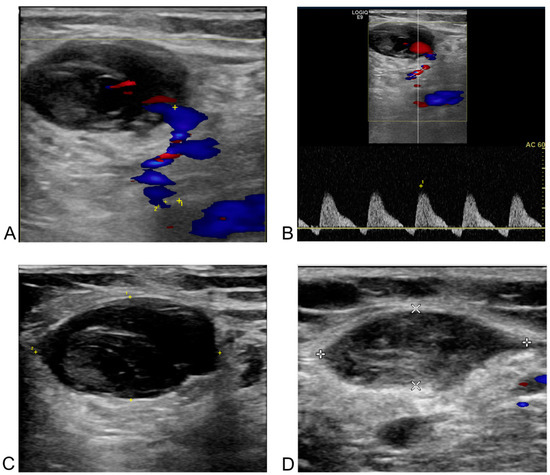
Figure 5.
Post-procedural and follow-up duplex ultrasonography (DUS) of a pseudoaneurysm at the left common femoral artery in a 66-year-old female patient after redo-radiofrequency ablation for atrial fibrillation. (A) DUS one day after the procedure. The blue area in the bottom right corresponds to the body of the pseudoaneurysm, and the unnumbered cross corresponds to the major axis of the neck. A neck with a 1.4 mm orifice diameter (the distance between the yellow cross labeled 1 and the cross labeled 2) and a length of 16.9 mm (the distance between the two unnumbered yellow crosses) was observed between the left common femoral artery and the pseudoaneurysm on the Doppler image. (B) Spectral Doppler image one day after the procedure. “To-and-from” flow was observed with waveforms above and below the baseline in the pseudoaneurysm neck between the arterial vessel and the sac. (C) Two-dimensional image taken a day after the procedure. A low echogenicity pseudoaneurysm and its neck are present on the left of the cross-section of the left common femoral artery, though this was challenging to confirm without Doppler imaging. (D) Two-dimensional image one month after the procedure. Thrombotic occlusion of the pseudoaneurysm, previously observed in the branch of the common femoral artery in the left inguinal area, was confirmed. The line connecting the two white crosses represents the major axis, and the line connecting the two white Xs represents the minor axis of left common femoral artery.
Limitations
There are several limitations to our study that warrant discussion. First, as a single-center study with limited populations, the findings may not be generalizable to other institutions with different patient populations or procedural techniques. Second, although the latest consensus recommends US-guided venipuncture, our study included patients who underwent conventional venipuncture due to the resource limitations and needs of our institution. However, 24–33% among the writing group members still perform conventional venipuncture, according to the most recent two consensus statements [12,13]. Third, while we identified significant predictors of vascular complications, our analyses were limited to factors available in the medical records. Other potential predictors, such as genetic predispositions or specific operator techniques, were not examined in detail. Fourth, the relatively short follow-up period may have led to an underestimation of late-onset complications, including those that might manifest weeks after the procedure. Finally, post-procedure management was conducted using the figure-of-eight suture which has been shown to have benefits in several studies as the primary hemostasis method [34,35], despite the proven utility of venous vascular closure systems [36,37,38]. In the future, it will be necessary to investigate vascular complication outcomes in studies utilizing venous vascular closure systems for hemostasis.
5. Conclusions
The incidence of vascular complications following catheter ablation for AF is higher than traditionally reported, particularly when sensitive imaging modalities like DUS are used for detection. Repeat ablation and months of experience <6 months were identified as significant predictors of these complications. Most vascular complications were managed successfully with DUS-guided compression, though AVF was relatively challenging to treat.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biomedicines13020314/s1, Figure S1: Comparison of vascular complications between patients who underwent radiofrequency ablation and cryoballoon ablation. CBA, cryoballoon ablation; RFCA, radiofrequency ablation.
Author Contributions
Conceptualization, K.-M.P.; methodology, K.-M.P., H.J.L. and S.H.L.; validation, H.J.L., J.K. and J.Y.K.; formal analysis, S.H.L., H.J.L. and J.K.; investigation, H.J.L., S.H.L., S.P., M.J.K. and J.Y.K.; data curation, S.H.L., H.J.L., S.P. and M.J.K.; writing—original draft preparation, H.J.L., S.H.L. and K.-M.P.; writing—review and editing, K.-M.P., Y.K.O. and S.-J.P.; visualization, S.H.L. and H.J.L.; supervision, K.-M.P., Y.K.O. and S.-J.P.; project administration, K.-M.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of Samsung Medical Center (IRB No. SMC-2024-01-023-001 and 23 January 2024).
Informed Consent Statement
Patient consent was waived due to the retrospective nature of this study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Deshmukh, A.; Patel, N.J.; Pant, S.; Shah, N.; Chothani, A.; Mehta, K.; Grover, P.; Singh, V.; Vallurupalli, S.; Savani, G.T.; et al. In-hospital complications associated with catheter ablation of atrial fibrillation in the United States between 2000 and 2010: Analysis of 93,801 procedures. Circulation 2013, 128, 2104–2112. [Google Scholar] [CrossRef] [PubMed]
- Steinbeck, G.; Sinner, M.F.; Lutz, M.; Müller-Nurasyid, M.; Kääb, S.; Reinecke, H. Incidence of complications related to catheter ablation of atrial fibrillation and atrial flutter: A nationwide in-hospital analysis of administrative data for Germany in 2014. Eur. Heart J. 2018, 39, 4020–4029. [Google Scholar] [CrossRef]
- Freeman, J.V.; Tabada, G.H.; Reynolds, K.; Sung, S.H.; Liu, T.I.; Gupta, N.; Go, A.S. Contemporary Procedural Complications, Hospitalizations, and Emergency Visits After Catheter Ablation for Atrial Fibrillation. Am. J. Cardiol. 2018, 121, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Eckardt, L.; Doldi, F.; Anwar, O.; Gessler, N.; Scherschel, K.; Kahle, A.K.; von Falkenhausen, A.S.; Thaler, R.; Wolfes, J.; Metzner, A.; et al. Major in-hospital complications after catheter ablation of cardiac arrhythmias: Individual case analysis of 43,031 procedures. Europace 2023, 26, euad361. [Google Scholar] [CrossRef] [PubMed]
- Bohnen, M.; Stevenson, W.G.; Tedrow, U.B.; Michaud, G.F.; John, R.M.; Epstein, L.M.; Albert, C.M.; Koplan, B.A. Incidence and predictors of major complications from contemporary catheter ablation to treat cardiac arrhythmias. Heart Rhythm 2011, 8, 1661–1666. [Google Scholar] [CrossRef]
- Sharma, P.S.; Padala, S.K.; Gunda, S.; Koneru, J.N.; Ellenbogen, K.A. Vascular Complications During Catheter Ablation of Cardiac Arrhythmias: A Comparison Between Vascular Ultrasound Guided Access and Conventional Vascular Access. J. Cardiovasc. Electrophysiol. 2016, 27, 1160–1166. [Google Scholar] [CrossRef]
- Kupó, P.; Pap, R.; Sághy, L.; Tényi, D.; Bálint, A.; Debreceni, D.; Basu-Ray, I.; Komócsi, A. Ultrasound guidance for femoral venous access in electrophysiology procedures-systematic review and meta-analysis. J. Interv. Card. Electrophysiol. 2020, 59, 407–414. [Google Scholar] [CrossRef]
- Yamagata, K.; Wichterle, D.; Roubícek, T.; Jarkovský, P.; Sato, Y.; Kogure, T.; Peichl, P.; Konecný, P.; Jansová, H.; Kucera, P.; et al. Ultrasound-guided versus conventional femoral venipuncture for catheter ablation of atrial fibrillation: A multicentre randomized efficacy and safety trial (ULTRA-FAST trial). Europace 2018, 20, 1107–1114. [Google Scholar] [CrossRef]
- Benali, K.; Khairy, P.; Hammache, N.; Petzl, A.; Da Costa, A.; Verma, A.; Andrade, J.G.; Macle, L. Procedure-Related Complications of Catheter Ablation for Atrial Fibrillation. J. Am. Coll. Cardiol. 2023, 81, 2089–2099. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, Y.; Miyamoto, K.; Nakai, M.; Sumita, Y.; Ueda, N.; Nakajima, K.; Kamakura, T.; Wada, M.; Yamagata, K.; Ishibashi, K.; et al. Complications Associated with Catheter Ablation in Patients with Atrial Fibrillation: A Report from the JROAD-DPC Study. J. Am. Heart Assoc. 2021, 10, e019701. [Google Scholar] [CrossRef] [PubMed]
- Joglar, J.A.; Chung, M.K.; Armbruster, A.L.; Benjamin, E.J.; Chyou, J.Y.; Cronin, E.M.; Deswal, A.; Eckhardt, L.L.; Goldberger, Z.D.; Gopinathannair, R.; et al. 2023 ACC/AHA/ACCP/HRS Guideline for the Diagnosis and Management of Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2024, 149, e1–e156. [Google Scholar] [CrossRef]
- Tzeis, S.; Gerstenfeld, E.P.; Kalman, J.; Saad, E.B.; Shamloo, A.S.; Andrade, J.G.; Barbhaiya, C.R.; Baykaner, T.; Boveda, S.; Calkins, H.; et al. 2024 European Heart Rhythm Association/Heart Rhythm Society/Asia Pacific Heart Rhythm Society/Latin American Heart Rhythm Society expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm 2024, 21, e31–e149. [Google Scholar] [CrossRef] [PubMed]
- Calkins, H.; Hindricks, G.; Cappato, R.; Kim, Y.H.; Saad, E.B.; Aguinaga, L.; Akar, J.G.; Badhwar, V.; Brugada, J.; Camm, J.; et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm 2017, 14, e275–e444. [Google Scholar] [CrossRef] [PubMed]
- Mehran, R.; Rao, S.V.; Bhatt, D.L.; Gibson, C.M.; Caixeta, A.; Eikelboom, J.; Kaul, S.; Wiviott, S.D.; Menon, V.; Nikolsky, E.; et al. Standardized bleeding definitions for cardiovascular clinical trials: A consensus report from the Bleeding Academic Research Consortium. Circulation 2011, 123, 2736–2747. [Google Scholar] [CrossRef]
- Ohlow, M.A.; Secknus, M.A.; von Korn, H.; Neumeister, A.; Wagner, A.; Yu, J.; Lauer, B. Incidence and outcome of femoral vascular complications among 18,165 patients undergoing cardiac catheterisation. Int. J. Cardiol. 2009, 135, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Waigand, J.; Uhlich, F.; Gross, C.M.; Thalhammer, C.; Dietz, R. Percutaneous treatment of pseudoaneurysms and arteriovenous fistulas after invasive vascular procedures. Catheter. Cardiovasc. Interv. 1999, 47, 157–164. [Google Scholar] [CrossRef]
- Ströker, E.; de Asmundis, C.; Kupics, K.; Takarada, K.; Mugnai, G.; De Cocker, J.; Stockman, D.; Sieira, J.; Schwagten, B.; Brugada, P.; et al. Value of ultrasound for access guidance and detection of subclinical vascular complications in the setting of atrial fibrillation cryoballoon ablation. Europace 2019, 21, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.E.; Boppana, L.K.T.; Abdullah, A.S.; Chuprun, D.; Shah, A.; Rao, M.; Bhatt, D.L.; Depta, J.P. Safety and Feasibility of Same-Day Discharge After Left Atrial Appendage Closure with the WATCHMAN Device. Circ. Cardiovasc. Interv. 2021, 14, e009669. [Google Scholar] [CrossRef] [PubMed]
- Deyell, M.W.; Hoskin, K.; Forman, J.; Laksman, Z.W.; Hawkins, N.M.; Bennett, M.T.; Yeung-Lai-Wah, J.A.; Chakrabarti, S.; Krahn, A.D.; Andrade, J.G. Same-day discharge for atrial fibrillation ablation: Outcomes and impact of ablation modality. Europace 2023, 25, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Eldadah, Z.A.; Al-Ahmad, A.; Bunch, T.J.; Delurgio, D.B.; Doshi, R.N.; Hook, B.G.; Hranitzky, P.M.; Joyner, C.A.; Mittal, S.; Porterfield, C.; et al. Same-day discharge following catheter ablation and venous closure with VASCADE MVP: A postmarket registry. J. Cardiovasc. Electrophysiol. 2023, 34, 348–355. [Google Scholar] [CrossRef]
- Gianni, C.; Elchouemi, M.; Helmy, R.; Spinetta, L.; La Fazia, V.M.; Pierucci, N.; Asfour, I.; Della Rocca, D.G.; Mohanty, S.; Bassiouny, M.A.; et al. Safety and feasibility of same-day discharge following uncomplicated transvenous lead extraction. J. Cardiovasc. Electrophysiol. 2024, 35, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Wynn, G.J.; Haq, I.; Hung, J.; Bonnett, L.J.; Lewis, G.; Webber, M.; Waktare, J.E.; Modi, S.; Snowdon, R.L.; Hall, M.C.; et al. Improving safety in catheter ablation for atrial fibrillation: A prospective study of the use of ultrasound to guide vascular access. J. Cardiovasc. Electrophysiol. 2014, 25, 680–685. [Google Scholar] [CrossRef]
- Krimphoff, A.; Urbanek, L.; Bordignon, S.; Schaack, D.; Tohoku, S.; Chen, S.; Chun, K.R.J.; Schmidt, B. The impact of ultrasound-guided vascular access for catheter ablation of left atrial arrhythmias in a high-volume centre. J. Interv. Card. Electrophysiol. 2024, 67, 1247–1255. [Google Scholar] [CrossRef]
- Barsuk, J.H.; McGaghie, W.C.; Cohen, E.R.; O’Leary, K.J.; Wayne, D.B. Simulation-based mastery learning reduces complications during central venous catheter insertion in a medical intensive care unit. Crit. Care Med. 2009, 37, 2697–2701. [Google Scholar]
- Hanauer, L.P.T.; Comerlato, P.H.; Papke, A.; Butzke, M.; Daga, A.; Hoffmeister, M.C.; Boniatti, M.M.; John, J.F.; Schaan, B.D.; Rados, D.V. Reducing central vein catheterization complications with a focused educational program: A retrospective cohort study. Sci. Rep. 2020, 10, 17530. [Google Scholar] [CrossRef] [PubMed]
- Adrian, M.; Borgquist, O.; Kröger, T.; Linné, E.; Bentzer, P.; Spångfors, M.; Åkeson, J.; Holmström, A.; Linnér, R.; Kander, T. Mechanical complications after central venous catheterisation in the ultrasound-guided era: A prospective multicentre cohort study. Br. J. Anaesth. 2022, 129, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Prudente, L.A.; Moorman, J.R.; Lake, D.; Xiao, Y.; Greebaum, H.; Mangrum, J.M.; DiMarco, J.P.; Ferguson, J.D. Femoral vascular complications following catheter ablation of atrial fibrillation. J. Interv. Card. Electrophysiol. 2009, 26, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Teumer, Y.; Eckart, D.; Katov, L.; Graf, M.; Bothner, C.; Rottbauer, W.; Weinmann-Emhardt, K. Ultrasound-Guided Venous Puncture Reduces Groin Complications in Electrophysiological Procedures. Biomedicines 2024, 12, 2375. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, E.; Miyazaki, S.; Mukai, M.; Aoyama, D.; Nodera, M.; Hasegawa, K.; Kaseno, K.; Miyahara, K.; Matsui, A.; Shiomi, Y.; et al. Femoral vascular complications after catheter ablation in the current era: The utility of computed tomography imaging. J. Cardiovasc. Electrophysiol. 2020, 31, 1385–1393. [Google Scholar] [CrossRef]
- Tonolini, M.; Ierardi, A.M.; Carrafiello, G.; Laganà, D. Multidetector CT of iatrogenic and self-inflicted vascular lesions and infections at the groin. Insights Imaging 2018, 9, 631–642. [Google Scholar] [CrossRef]
- Kolluri, R.; Fowler, B.; Nandish, S. Vascular access complications: Diagnosis and management. Curr. Treat. Options Cardiovasc. Med. 2013, 15, 173–187. [Google Scholar] [CrossRef] [PubMed]
- Kelm, M.; Perings, S.M.; Jax, T.; Lauer, T.; Schoebel, F.C.; Heintzen, M.P.; Perings, C.; Strauer, B.E. Incidence and clinical outcome of iatrogenic femoral arteriovenous fistulas: Implications for risk stratification and treatment. J. Am. Coll. Cardiol. 2002, 40, 291–297. [Google Scholar] [CrossRef]
- Schaub, F.; Theiss, W.; Busch, R.; Heinz, M.; Paschalidis, M.; Schömig, A. Management of 219 consecutive cases of postcatheterization pseudoaneurysm. J. Am. Coll. Cardiol. 1997, 30, 670–675. [Google Scholar] [CrossRef] [PubMed]
- Aytemir, K.; Canpolat, U.; Yorgun, H.; Evranos, B.; Kaya, E.B.; Şahiner, M.L.; Özer, N. Usefulness of ‘figure-of-eight’ suture to achieve haemostasis after removal of 15-French calibre femoral venous sheath in patients undergoing cryoablation. Europace 2016, 18, 1545–1550. [Google Scholar] [CrossRef] [PubMed]
- Mujer, M.T.; Al-Abcha, A.; Flores, J.; Saleh, Y.; Robinson, P. A comparison of figure-of-8-suture versus manual compression for venous access closure after cardiac procedures: An updated meta-analysis. Pacing Clin. Electrophysiol. 2020, 43, 856–865. [Google Scholar] [CrossRef]
- Natale, A.; Mohanty, S.; Liu, P.Y.; Mittal, S.; Al-Ahmad, A.; De Lurgio, D.B.; Horton, R.; Spear, W.; Bailey, S.; Bunch, J.; et al. Venous Vascular Closure System Versus Manual Compression Following Multiple Access Electrophysiology Procedures: The AMBULATE Trial. JACC Clin. Electrophysiol. 2020, 6, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Tilz, R.R.; Feher, M.; Vogler, J.; Bode, K.; Duta, A.I.; Ortolan, A.; Lopez, L.D.; Küchler, M.; Mamaev, R.; Lyan, E.; et al. Venous vascular closure system vs. figure-of-eight suture following atrial fibrillation ablation: The STYLE-AF Study. Europace 2024, 26. [Google Scholar] [CrossRef] [PubMed]
- Tabaja, C.; Hight, N.; Younis, A.; Jadam, S.; Demian, J.; Hussein, A.; Sroubek, J.; Saliba, W.; Kanj, M.; Bhargava, M.; et al. Vascular access complications after catheter ablation of ventricular arrhythmias: Impact of vascular closure devices. Heart Rhythm 2024. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).