Mitochondrial Dysfunction in Neurodegenerative Diseases: Mechanisms and Corresponding Therapeutic Strategies
Abstract
1. Introduction
2. Basic Structure and Homeostasis of Mitochondria
2.1. Basic Structure and Functional Areas
2.2. Core Dimensions of Mitochondrial Homeostasis
2.2.1. Energy Metabolism
2.2.2. Mitochondrial Dynamics
2.2.3. Mitophagy
2.2.4. Regulation of Cytokines
3. Neurodegenerative Diseases and Mitochondria
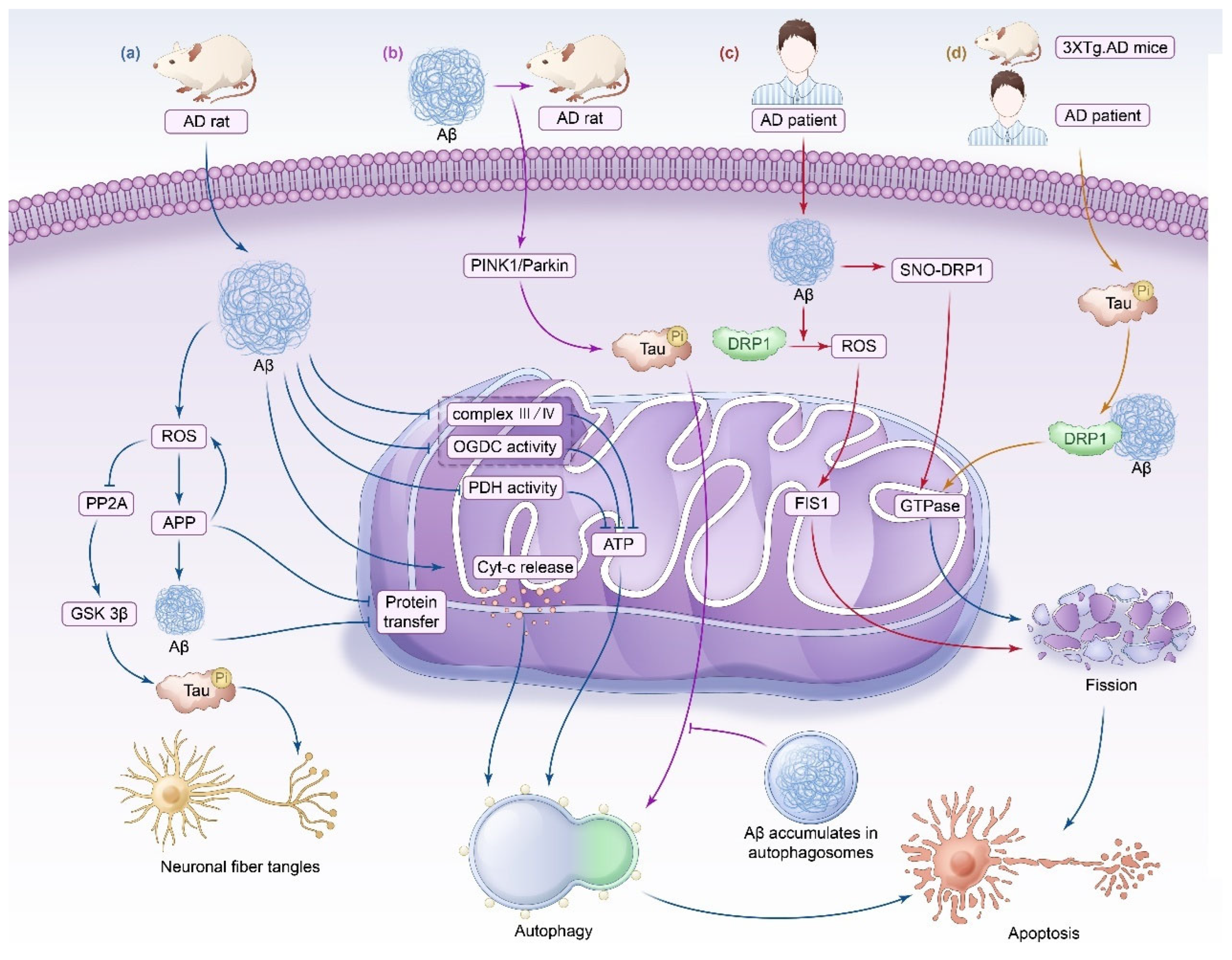
3.1. Alzheimer’s Disease
3.1.1. Impaired Mitochondrial Energy Metabolism
3.1.2. Imbalance in Mitochondrial Dynamics
3.1.3. Impaired Mitophagy
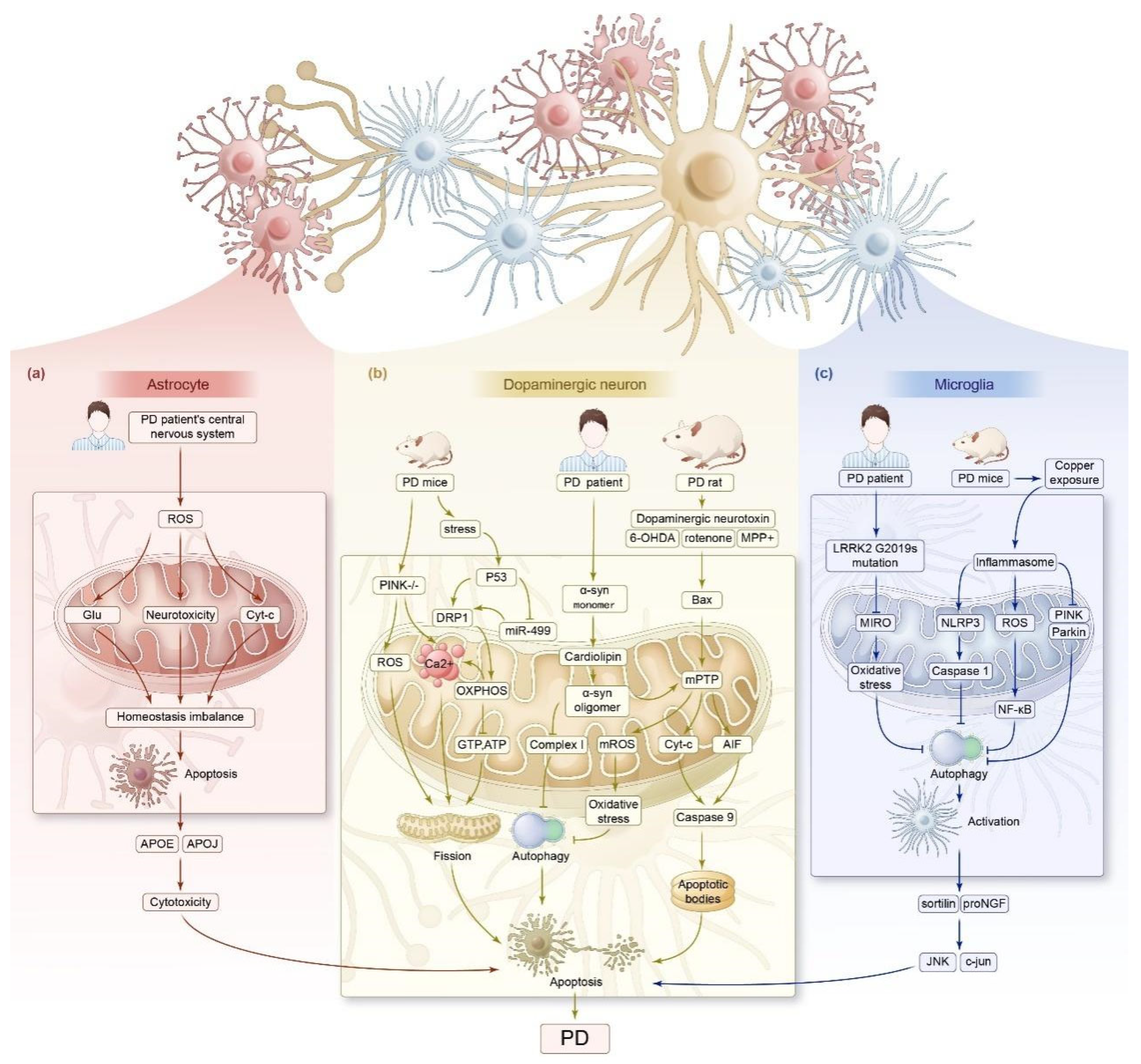
3.2. Parkinson’s Disease
3.2.1. Impaired Mitochondrial Energy Metabolism
3.2.2. Imbalance in Mitochondrial Dynamics
3.2.3. Impaired Mitophagy
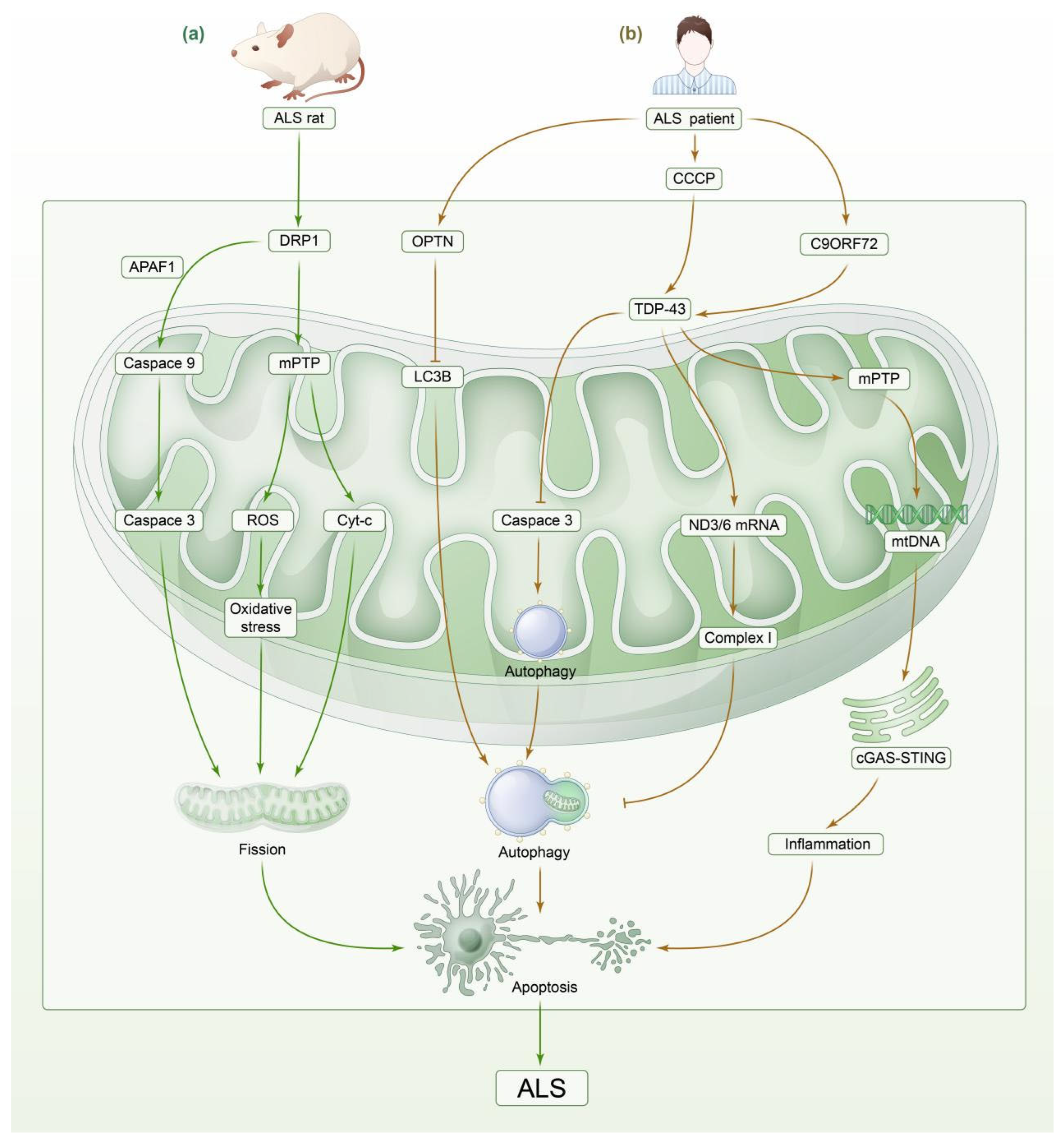
3.3. Amyotrophic Lateral Sclerosis
3.3.1. Impaired Mitochondrial Energy Metabolism
3.3.2. Imbalance in Mitochondrial Dynamics
3.3.3. Impaired Mitophagy
4. Mitochondrial-Targeted Therapies for Neurodegenerative Diseases
5. Summary and Outlook
| Neurological Disease | Model/Resources | Molecular Mechanism | Outcome | Reference(s) |
|---|---|---|---|---|
| Alzheimer’s disease | AD patients | Mitochondrial complex I gene expression is downregulated, and the expression of complexes III and IV is upregulated. | Reduced energy generation, increased production of ROS. | [37] |
| AD mice | Elevated ROS, mitochondrial membrane depolarization, mitochondrial swelling, Cyt-c release, and increased ATP/ADP ratio. | Caspase-3 activation, neuronal apoptosis in rat brain. | [46] | |
| AD mice | Aβ decreases mitochondrial complexes III and IV, cytochrome oxidase, α-ketoglutarate dehydrogenase, and pyruvate dehydrogenase activities. | Decreasing energy within neurons, leading to neuronal apoptosis. | [45] | |
| AD mice | Elevated ROS levels promote APP production and increase Aβ synthesis, while APP and Aβ block protein transport in mitochondria. | The ETC is disrupted, causing neuronal injury. | [161] | |
| AD mice | Aβ mediates lysosomal membrane degradation. | Neuronal apoptosis. | [41] | |
| AD patients | ROS inhibit PP2A, promote GSK 3β activation, and cause Tau hyperphosphorylation. | NFTs. | [49] | |
| AD patients | Aβ interacts with DRP1 and activates DRP1 and FIS1, resulting in mitochondrial fission and impaired mitochondrial transport. | Neuronal energy metabolism is affected, leading to neuronal apoptosis. | [47] | |
| AD patients | Aβ induces DRP1 S-nitrosylation and accelerates mitochondrial fission. | Synapse loss and neuronal apoptosis. | [47] | |
| AD patients | Tau and interaction with Aβ lead to MMP dissipation, ROS overproduction, enhanced oxidative stress, mtDNA loss, impaired mitochondrial transport, and increased mitophagy. | AD. | [53] | |
| AD rats | A lack of PINK1 leads to the hyperphosphorylation of Tau and impairs mitophagy mediated by the PINK1/Parkin pathway. | Neuronal synaptic damage. | [55] | |
| Parkinson’s disease | PD mice | Microglia BV2 promotes ROS generation, activates the NF-κB pathway, decreases mitochondrial membrane potential, downregulates Parkin and PINK1, and upregulates NLRP3/caspase-1/GSDMD axis proteins. | Inhibited mitophagy, leading to focal death of dopaminergic neurons. | [95] |
| PD patients | Excessive levels of ROS lead to the dysregulation of mitochondrial homeostasis and Cyt-c release and mediate their own apoptosis and the loss of neuroprotection. | PD. | [71] | |
| SH-SY5Y neuroblastoma cells | Stimuli such as dopaminergic neurotoxins induce the overexpression of DRP1 and promote sustained cleavage of mitochondrial membranes. | Death of dopaminergic neurons. | [74,75,76,77] | |
| PD mice | Bax translocates to the OMM, resulting in a continuous opening of the MPTP, which leads to a gradual decrease in the mitochondrial membrane potential. | Release of AIF, which leads to neuronal apoptosis. | [81] | |
| PD mice | Hypertonicity of the MIM and opening of the mPTP lead to the swelling of the mitochondrial matrix and reduction in OMM folds, which are prone to rupture and release intermembrane pro-apoptotic proteins. | Apoptosis. | [78] | |
| PD mice | Sustained opening of the mPTP leads to excessive release of Cyt-c, which binds to Apaf-1 in the presence of dATP, contributing to the formation of apoptotic vesicles and the activation of caspase-9. | Mitochondria-dependent apoptosis in cardiomyocytes. | [162,163] | |
| PD mice | P53 translocates to the OMM and aggregates and interacts with pro-apoptotic proteins such as Bax and PUMA | Apoptosis. | [164] | |
| SH-SY5Y cell line | PINK1 deficiency results in DRP1-dependent mitochondrial swelling, cristae reduction, and mitochondrial fission. | Neuronal mitochondrial homeostasis and function are affected. | [165] | |
| PD patients IPSC | Co-localization and rapid oligomerization of the A53T α-syn monomer with cardiolipin of the OMM promotes opening of the mPTP, facilitates mROS production, accelerates mitochondrial oxidative stress, inhibits mitophagy, and inhibits complex I synthesis. | Mitochondrial depolarization, neuronal apoptosis, and cytotoxicity. | [66] | |
| PD patients | LRRK2 G2019S mutation delays mitophagy, impairs cellular respiration and metabolism, and generates increased oxidative stress through the inhibition of Miro clearance. The deletion or mutation of LRRK2 results in impaired mitochondrial Ca2+ buffering capacity. | Impairment of mitochondrial function, leading to neuronal death. | [98,99] | |
| Amyotrophic lateral sclerosis | ALS mice | Elevated ROS promote SOD1 aggregation in neurons, triggering endoplasmic reticulum stress, mitochondrial dysfunction, and disruption of axonal transport, which in turn lead to neuronal loss and fission of the mitochondrial network. This further promotes free radical production. | A vicious cycle is formed, leading to neuronal apoptosis and necrosis and inducing ND. | [111,112,113,114] |
| ALS mice | Damage to microglia mitochondria along with increased ROS production leads to a decrease in the number of mitochondria and the release of large amounts of inflammatory factors with elevated levels of COX-2 and PGE2. | Damage to nerve fibers and neuronal apoptosis, inducing ALS. | [166,167,168] | |
| ALS mice | The overexpression of DRP1 leads to binding to the mitochondrial membrane, resulting in mitochondrial membrane depolarization, increased ROS and oxidative stress, and decreased ATP production. DRP1 binds to APAF1, which recruits and activates the Caspase-9 precursor and upregulates the apoptosis execution protein caspase-3. | Mitochondrial fission, neuronal apoptosis. | [120,122,169,170] | |
| SOD1 G93A transgenic mouse model | The dephosphorylation of DRP1 by PP1 induces excessive mitochondrial fission. PP1 also regulates the activity of some subunits of mitochondrial complex I through dephosphorylation. | Neurodegeneration and ALS. | [123,124] | |
| ALS patients | Decreased PFN1 expression leads to downregulation of PTEN levels, resulting in abnormal mitophagy. | The development of ALS. | [128,129] | |
| E478G ubiquitin-binding-deficient OPTN mutant | OPTN mutation leads to the inhibition of mitophagy degradation. | ALS | [130,131] | |
| ALS patients | TDP-43 inhibits the production of OXPHOS complex I by mediating ND3/6 mRNA-specific transcription, leading to mitochondrial dysfunction, mPTP activation, mediated mtDNA release, and induced the activation of the cGAS-STING signaling pathway. | Cellular inflammation in the nervous system due to impaired mitophagy, and neuronal apoptosis caused by severe neuroinflammation. | [132,133] | |
| ALS mice | Binding of NO to superoxide anion leads to NFTs. Abnormally activated microglia also produce large amounts of TNF-a, which induces upregulation of MHCI expression on the cell surface of neurons. | Abnormal neuronal shape and transit dysfunction resulting in motor neuron apoptosis. | [22] | |
| ALS mice | Large amounts of TNF-a promote neuronal apoptosis, directly oxidize lipids and proteins in neuroinflammation, and lead to the release of toxic substances from microglia, exacerbating the neurotoxic effects of microglia. | Promoted motor neuron apoptosis and ALS. | [24] |
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Seifan, A.; Ganzer, C.A.; Ryon, K.; Lin, M.; Mahmudur, R.; Adolfo, H.; Shih, C.; Jacobs, A.R.; Greenwald, M.; Isaacson, R.S. Detecting Non-cognitive Features of Prodromal Neurodegenerative Diseases. Curr. Aging Sci. 2019, 11, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Brody, J.A.; Stanhope, J.M.; Kurland, L.T. Patterns of Amyotrophic Lateral Sclerosis and Parkinsonism-Dementia on Guam. Contemp. Neurol. Ser. 1975, 12, 45–70. [Google Scholar] [PubMed]
- de Haan, P.; Klein, H.C.; ’t Hart, B.A. Autoimmune Aspects of Neurodegenerative and Psychiatric Diseases: A Template for Innovative Therapy. Front. Psychiatry 2017, 8, 46. [Google Scholar] [CrossRef] [PubMed]
- Messner, D.A.; Rabins, P.; Downing, A.C.; Irizarry, M.; Foster, N.L.; Al Naber, J.; Dabbous, O.; Fillit, H.; Gabler, S.; Krakauer, R.; et al. Designing Trials of Disease Modifying Agents for Early and Preclinical Alzheimer’s Disease Intervention: What Evidence is Meaningful to Patients, Providers, and Payers? J. Prev. Alzheimers Dis. 2019, 6, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Jethwa, K.D. Antipsychotics for the management of Parkinson’s disease psychosis. Int. J. Geriatr. Psychiatry 2017, 32, 464–465. [Google Scholar] [CrossRef] [PubMed]
- Rummel, N.G.; Butterfield, D.A. Altered Metabolism in Alzheimer Disease Brain: Role of Oxidative Stress. Antioxid. Redox Signal. 2022, 36, 1289–1305. [Google Scholar] [CrossRef]
- Bereiter-Hahn, J.; Vöth, M. Dynamics of mitochondria in living cells: Shape changes, dislocations, fusion, and fission of mitochondria. Microsc. Res. Tech. 1994, 27, 198–219. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, J.; Ordureau, A.; Harper, J.W.; Holzbaur, E.L.F. Brain-derived autophagosome profiling reveals the engulfment of nucleoid-enriched mitochondrial fragments by basal autophagy in neurons. Neuron 2022, 110, 967–976.e968. [Google Scholar] [CrossRef]
- Chua, J.P.; De Calbiac, H.; Kabashi, E.; Barmada, S.J. Autophagy and ALS: Mechanistic insights and therapeutic implications. Autophagy 2022, 18, 254–282. [Google Scholar] [CrossRef]
- Toyama, E.Q.; Herzig, S.; Courchet, J.; Lewis, T.L., Jr.; Losón, O.C.; Hellberg, K.; Young, N.P.; Chen, H.; Polleux, F.; Chan, D.C.; et al. AMP-activated protein kinase mediates mitochondrial fission in response to energy stress. Science 2016, 351, 275–281. [Google Scholar] [CrossRef]
- Yun, H.R.; Jo, Y.H.; Kim, J.; Shin, Y.; Kim, S.S.; Choi, T.G. Roles of Autophagy in Oxidative Stress. Int. J. Mol. Sci. 2020, 21, 3289. [Google Scholar] [CrossRef] [PubMed]
- Klecker, T.; Westermann, B. Pathways shaping the mitochondrial inner membrane. Open Biol. 2021, 11, 210238. [Google Scholar] [CrossRef] [PubMed]
- Holmström, K.M.; Finkel, T. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat. Rev. Mol. Cell Biol. 2014, 15, 411–421. [Google Scholar] [CrossRef]
- Cobley, J.N.; Fiorello, M.L.; Bailey, D.M. 13 reasons why the brain is susceptible to oxidative stress. Redox Biol. 2018, 15, 490–503. [Google Scholar] [CrossRef] [PubMed]
- Salim, S. Oxidative Stress and the Central Nervous System. J. Pharmacol. Exp. Ther. 2017, 360, 201–205. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Ru, X.; Wen, T. NRF2, a Transcription Factor for Stress Response and Beyond. Int. J. Mol. Sci. 2020, 21, 4777. [Google Scholar] [CrossRef]
- Wang, J.; Mao, J.; Wang, R.; Li, S.; Wu, B.; Yuan, Y. Kaempferol Protects Against Cerebral Ischemia Reperfusion Injury Through Intervening Oxidative and Inflammatory Stress Induced Apoptosis. Front. Pharmacol. 2020, 11, 424. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Long, X.; Tang, J.; Li, X.; Zhang, X.; Luo, C.; Zhou, Y.; Zhang, P. The Attenuation of Traumatic Brain Injury via Inhibition of Oxidative Stress and Apoptosis by Tanshinone IIA. Oxid. Med. Cell Longev. 2020, 2020, 4170156. [Google Scholar] [CrossRef]
- Archer, S.L. Mitochondrial Dynamics—Mitochondrial Fission and Fusion in Human Diseases. N. Engl. J. Med. 2013, 369, 2236–2251. [Google Scholar] [CrossRef]
- Chen, H.; Detmer, S.A.; Ewald, A.J.; Griffin, E.E.; Fraser, S.E.; Chan, D.C. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J. Cell Biol. 2003, 160, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.L.; Meng, S.; Chen, Y.; Feng, J.X.; Gu, D.D.; Yu, B.; Li, Y.J.; Yang, J.Y.; Liao, S.; Chan, D.C.; et al. MFN1 structures reveal nucleotide-triggered dimerization critical for mitochondrial fusion. Nature 2017, 542, 372–376. [Google Scholar] [CrossRef]
- Szabadkai, G.; Simoni, A.M.; Chami, M.; Wieckowski, M.R.; Youle, R.J.; Rizzuto, R. Drp-1-dependent division of the mitochondrial network blocks intraorganellar Ca2+ waves and protects against Ca2+-mediated apoptosis. Mol. Cell 2004, 16, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Manczak, M.; Reddy, P.H. Abnormal interaction between the mitochondrial fission protein Drp1 and hyperphosphorylated tau in Alzheimer’s disease neurons: Implications for mitochondrial dysfunction and neuronal damage. Hum. Mol. Genet. 2012, 21, 2538–2547. [Google Scholar] [CrossRef]
- Ashrafian, H.; Docherty, L.; Leo, V.; Towlson, C.; Neilan, M.; Steeples, V.; Lygate, C.A.; Hough, T.; Townsend, S.; Williams, D.; et al. A mutation in the mitochondrial fission gene Dnm1l leads to cardiomyopathy. PLoS Genet. 2010, 6, e1001000. [Google Scholar] [CrossRef] [PubMed]
- Lazarou, M.; Sliter, D.A.; Kane, L.A.; Sarraf, S.A.; Wang, C.; Burman, J.L.; Sideris, D.P.; Fogel, A.I.; Youle, R.J. The ubiquitin kinase PINK1 recruits autophagy receptors to induce mitophagy. Nature 2015, 524, 309–314. [Google Scholar] [CrossRef]
- Onishi, M.; Yamano, K.; Sato, M.; Matsuda, N.; Okamoto, K. Molecular mechanisms and physiological functions of mitophagy. EMBO J. 2021, 40, e104705. [Google Scholar] [CrossRef] [PubMed]
- Stavoe, A.K.H.; Holzbaur, E.L.F. Autophagy in Neurons. Annu. Rev. Cell Dev. Biol. 2019, 35, 477–500. [Google Scholar] [CrossRef]
- Zhang, J.M.; An, J. Cytokines, inflammation, and pain. Int. Anesth. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef]
- Tur, J.; Pereira-Lopes, S.; Vico, T.; Marín, E.A.; Muñoz, J.P.; Hernández-Alvarez, M.; Cardona, P.J.; Zorzano, A.; Lloberas, J.; Celada, A. Mitofusin 2 in Macrophages Links Mitochondrial ROS Production, Cytokine Release, Phagocytosis, Autophagy, and Bactericidal Activity. Cell Rep. 2020, 32, 108079. [Google Scholar] [CrossRef] [PubMed]
- Tresse, E.; Riera-Ponsati, L.; Jaberi, E.; Sew, W.Q.G.; Ruscher, K.; Issazadeh-Navikas, S. IFN-β rescues neurodegeneration by regulating mitochondrial fission via STAT5, PGAM5, and Drp1. EMBO J. 2021, 40, e106868. [Google Scholar] [CrossRef]
- Wei, R.M.; Zhang, Y.M.; Zhang, K.X.; Liu, G.X.; Li, X.Y.; Zhang, J.Y.; Lun, W.Z.; Liu, X.C.; Chen, G.H. An enriched environment ameliorates maternal sleep deprivation-induced cognitive impairment in aged mice by improving mitochondrial function via the Sirt1/PGC-1α pathway. Aging 2024, 16, 1128–1144. [Google Scholar] [CrossRef]
- Alzheimer’s Association Report. Alzheimer’s disease facts and figures. Alzheimers Dement. 2024, 20, 3708–3821. [Google Scholar] [CrossRef] [PubMed]
- Breijyeh, Z.; Karaman, R. Comprehensive Review on Alzheimer’s Disease: Causes and Treatment. Molecules 2020, 25, 5789. [Google Scholar] [CrossRef] [PubMed]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [PubMed]
- Karch, C.M.; Goate, A.M. Alzheimer’s disease risk genes and mechanisms of disease pathogenesis. Biol. Psychiatry 2015, 77, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.H.; Oliver, D.M. Amyloid Beta and Phosphorylated Tau-Induced Defective Autophagy and Mitophagy in Alzheimer’s Disease. Cells 2019, 8, 488. [Google Scholar] [CrossRef] [PubMed]
- Manczak, M.; Park, B.S.; Jung, Y.; Reddy, P.H. Differential expression of oxidative phosphorylation genes in patients with Alzheimer’s disease: Implications for early mitochondrial dysfunction and oxidative damage. Neuromolecular Med. 2004, 5, 147–162. [Google Scholar] [CrossRef] [PubMed]
- Rak, M.; Bénit, P.; Chrétien, D.; Bouchereau, J.; Schiff, M.; El-Khoury, R.; Tzagoloff, A.; Rustin, P. Mitochondrial cytochrome c oxidase deficiency. Clin. Sci. 2016, 130, 393–407. [Google Scholar] [CrossRef] [PubMed]
- Tabner, B.J.; El-Agnaf, O.M.; German, M.J.; Fullwood, N.J.; Allsop, D. Protein aggregation, metals and oxidative stress in neurodegenerative diseases. Biochem. Soc. Trans. 2005, 33, 1082–1086. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Drake, J.; Pocernich, C.; Castegna, A. Evidence of oxidative damage in Alzheimer’s disease brain: Central role for amyloid beta-peptide. Trends Mol. Med. 2001, 7, 548–554. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.D.; Wang, Y.; Wu, J.C.; Lin, F.; Han, R.; Han, F.; Fukunaga, K.; Qin, Z.H. Down-regulation of Bcl-2 enhances autophagy activation and cell death induced by mitochondrial dysfunction in rat striatum. J. Neurosci. Res. 2009, 87, 3600–3610. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Song, H.; Ding, J.; Wang, D.J.; Zhu, S.P.; Liu, C.; Jin, X.; Chen, J.W. The Mechanism of TNF-α-Mediated Accumulation of Phosphorylated Tau Protein and Its Modulation by Propofol in Primary Mouse Hippocampal Neurons: Role of Mitophagy, NLRP3, and p62/Keap1/Nrf2 Pathway. Oxid. Med. Cell Longev. 2022, 2022, 8661200. [Google Scholar] [CrossRef] [PubMed]
- Nunomura, A.; Castellani, R.J.; Zhu, X.; Moreira, P.I.; Perry, G.; Smith, M.A. Involvement of oxidative stress in Alzheimer disease. J. Neuropathol. Exp. Neurol. 2006, 65, 631–641. [Google Scholar] [CrossRef]
- Subbarao, K.V.; Richardson, J.S.; Ang, L.C. Autopsy samples of Alzheimer’s cortex show increased peroxidation in vitro. J. Neurochem. 1990, 55, 342–345. [Google Scholar] [CrossRef] [PubMed]
- Casley, C.S.; Canevari, L.; Land, J.M.; Clark, J.B.; Sharpe, M.A. Beta-amyloid inhibits integrated mitochondrial respiration and key enzyme activities. J. Neurochem. 2002, 80, 91–100. [Google Scholar] [CrossRef]
- Faizi, M.; Seydi, E.; Abarghuyi, S.; Salimi, A.; Nasoohi, S.; Pourahmad, J. A Search for Mitochondrial Damage in Alzheimer’s Disease Using Isolated Rat Brain Mitochondria. Iran. J. Pharm. Res. 2016, 15, 185–195. [Google Scholar] [PubMed]
- Cho, D.H.; Nakamura, T.; Fang, J.; Cieplak, P.; Godzik, A.; Gu, Z.; Lipton, S.A. S-nitrosylation of Drp1 mediates beta-amyloid-related mitochondrial fission and neuronal injury. Science 2009, 324, 102–105. [Google Scholar] [CrossRef]
- Wang, X.; Su, B.; Lee, H.G.; Li, X.; Perry, G.; Smith, M.A.; Zhu, X. Impaired balance of mitochondrial fission and fusion in Alzheimer’s disease. J. Neurosci. 2009, 29, 9090–9103. [Google Scholar] [CrossRef]
- Toral-Rios, D.; Pichardo-Rojas, P.S.; Alonso-Vanegas, M.; Campos-Pena, V. GSK3beta and Tau Protein in Alzheimer’s Disease and Epilepsy. Front. Cell Neurosci. 2020, 14, 19. [Google Scholar] [CrossRef] [PubMed]
- Manczak, M.; Kandimalla, R.; Fry, D.; Sesaki, H.; Reddy, P.H. Protective effects of reduced dynamin-related protein 1 against amyloid beta-induced mitochondrial dysfunction and synaptic damage in Alzheimer’s disease. Hum. Mol. Genet. 2016, 25, 5148–5166. [Google Scholar] [CrossRef]
- DuBoff, B.; Götz, J.; Feany, M.B. Tau promotes neurodegeneration via DRP1 mislocalization in vivo. Neuron 2012, 75, 618–632. [Google Scholar] [CrossRef]
- Kandimalla, R.; Manczak, M.; Fry, D.; Suneetha, Y.; Sesaki, H.; Reddy, P.H. Reduced dynamin-related protein 1 protects against phosphorylated Tau-induced mitochondrial dysfunction and synaptic damage in Alzheimer’s disease. Hum. Mol. Genet. 2016, 25, 4881–4897. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, K.; Yamamoto, S.; Hattori, S.; Nishimura, N.; Matsumoto, H.; Miyakawa, T.; Nakada, K. Neuronal degeneration and cognitive impairment can be prevented via the normalization of mitochondrial dynamics. Pharmacol. Res. 2021, 163, 105246. [Google Scholar] [CrossRef]
- Fang, E.F.; Hou, Y.; Palikaras, K.; Adriaanse, B.A.; Kerr, J.S.; Yang, B.; Lautrup, S.; Hasan-Olive, M.M.; Caponio, D.; Dan, X.; et al. Mitophagy inhibits amyloid-beta and tau pathology and reverses cognitive deficits in models of Alzheimer’s disease. Nat. Neurosci. 2019, 22, 401–412. [Google Scholar] [CrossRef]
- Wang, X.J.; Qi, L.; Cheng, Y.F.; Ji, X.F.; Chi, T.Y.; Liu, P.; Zou, L.B. PINK1 overexpression prevents forskolin-induced tau hyperphosphorylation and oxidative stress in a rat model of Alzheimer’s disease. Acta Pharmacol. Sin. 2022, 43, 1916–1927. [Google Scholar] [CrossRef] [PubMed]
- Rowland, A.M.; Richmond, J.E.; Olsen, J.G.; Hall, D.H.; Bamber, B.A. Presynaptic terminals independently regulate synaptic clustering and autophagy of GABAA receptors in Caenorhabditis elegans. J. Neurosci. 2006, 26, 1711–1720. [Google Scholar] [CrossRef] [PubMed]
- Li, J.H.; Li, M.Y.; Ge, Y.Y.; Chen, J.Y.; Ma, J.M.; Wang, C.C.; Sun, M.M.; Wang, L.; Yao, S.L.; Yao, C.Y. β-amyloid protein induces mitophagy-dependent ferroptosis through the CD36/PINK/PARKIN pathway leading to blood-brain barrier destruction in Alzheimer’s disease. Cell Biosci. 2022, 12, 69. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.S.; Shen, L.L.; Bu, X.L.; Zhang, W.W.; Chen, S.H.; Huang, Z.L.; Xiong, J.X.; Gao, C.Y.; Dong, Z.; He, Y.N.; et al. Peritoneal dialysis reduces amyloid-beta plasma levels in humans and attenuates Alzheimer-associated phenotypes in an APP/PS1 mouse model. Acta Neuropathol. 2017, 134, 207–220. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Del Tredici, K.; Rüb, U.; de Vos, R.A.; Jansen Steur, E.N.; Braak, E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging 2003, 24, 197–211. [Google Scholar] [CrossRef]
- Zulai, L.C.; Albuquerque, A.M.; Papcke, C.; Louzada, F.M.; Scheeren, E.M. Postural impairments in Parkinson’s disease are not associated with changes in circadian rhythms changes. Chronobiol. Int. 2020, 37, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Kiernan, M.C.; Vucic, S.; Cheah, B.C.; Turner, M.R.; Eisen, A.; Hardiman, O.; Burrell, J.R.; Zoing, M.C. Amyotrophic lateral sclerosis. Lancet 2011, 377, 942–955. [Google Scholar] [CrossRef]
- Zaman, V.; Shields, D.C.; Shams, R.; Drasites, K.P.; Matzelle, D.; Haque, A.; Banik, N.L. Cellular and molecular pathophysiology in the progression of Parkinson’s disease. Metab. Brain Dis. 2021, 36, 815–827. [Google Scholar] [CrossRef] [PubMed]
- González-Rodríguez, P.; Zampese, E.; Stout, K.A.; Guzman, J.N.; Ilijic, E.; Yang, B.; Tkatch, T.; Stavarache, M.A.; Wokosin, D.L.; Gao, L.; et al. Disruption of mitochondrial complex I induces progressive parkinsonism. Nature 2021, 599, 650–656. [Google Scholar] [CrossRef]
- Subramaniam, S.R.; Chesselet, M.F. Mitochondrial dysfunction and oxidative stress in Parkinson’s disease. Prog. Neurobiol. 2013, 106–107, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Shao, M.; Guo, B.; Li, C.; Lu, Y.; Yang, X.; Li, S.; Li, H.; Zhu, Q.; Zhong, H.; et al. Tetramethylpyrazine Analogue T-006 Promotes the Clearance of Alpha-synuclein by Enhancing Proteasome Activity in Parkinson’s Disease Models. Neurotherapeutics 2019, 16, 1225–1236. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.L.; Chappard, A.; Singh, B.P.; Maclachlan, C.; Rodrigues, M.; Fedotova, E.I.; Berezhnov, A.V.; De, S.; Peddie, C.J.; Athauda, D.; et al. Author Correction: Pathological structural conversion of α-synuclein at the mitochondria induces neuronal toxicity. Nat. Neurosci. 2022, 25, 1582. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xie, Z.; Turkson, S.; Zhuang, X. A53T human alpha-synuclein overexpression in transgenic mice induces pervasive mitochondria macroautophagy defects preceding dopamine neuron degeneration. J. Neurosci. 2015, 35, 890–905. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Sesaki, H.; Qi, X. Drp1 stabilizes p53 on the mitochondria to trigger necrosis under oxidative stress conditions in vitro and in vivo. Biochem. J. 2014, 461, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Booth, H.D.E.; Hirst, W.D.; Wade-Martins, R. The Role of Astrocyte Dysfunction in Parkinson’s Disease Pathogenesis. Trends Neurosci. 2017, 40, 358–370. [Google Scholar] [CrossRef]
- Kuter, K.; Olech, L.; Glowacka, U. Prolonged Dysfunction of Astrocytes and Activation of Microglia Accelerate Degeneration of Dopaminergic Neurons in the Rat Substantia Nigra and Block Compensation of Early Motor Dysfunction Induced by 6-OHDA. Mol. Neurobiol. 2018, 55, 3049–3066. [Google Scholar] [CrossRef] [PubMed]
- Gollihue, J.L.; Norris, C.M. Astrocyte mitochondria: Central players and potential therapeutic targets for neurodegenerative diseases and injury. Ageing Res. Rev. 2020, 59, 101039. [Google Scholar] [CrossRef] [PubMed]
- Kirkley, K.S.; Popichak, K.A.; Hammond, S.L.; Davies, C.; Hunt, L.; Tjalkens, R.B. Genetic suppression of IKK2/NF-κB in astrocytes inhibits neuroinflammation and reduces neuronal loss in the MPTP-Probenecid model of Parkinson’s disease. Neurobiol. Dis. 2019, 127, 193–209. [Google Scholar] [CrossRef] [PubMed]
- Valero, T. Mitochondrial biogenesis: Pharmacological approaches. Curr. Pharm. Des. 2014, 20, 5507–5509. [Google Scholar] [CrossRef]
- Lin, C.Y.; Chen, W.J.; Fu, R.H.; Tsai, C.W. Upregulation of OPA1 by carnosic acid is mediated through induction of IKKγ ubiquitination by parkin and protects against neurotoxicity. Food Chem. Toxicol. 2020, 136, 110942. [Google Scholar] [CrossRef] [PubMed]
- Dagda, R.K.; Zhu, J.; Kulich, S.M.; Chu, C.T. Mitochondrially localized ERK2 regulates mitophagy and autophagic cell stress: Implications for Parkinson’s disease. Autophagy 2008, 4, 770–782. [Google Scholar] [CrossRef] [PubMed]
- Quintero-Espinosa, D.A.; Velez-Pardo, C.; Jimenez-Del-Rio, M. High Yield of Functional Dopamine-like Neurons Obtained in NeuroForsk 2.0 Medium to Study Acute and Chronic Rotenone Effects on Oxidative Stress, Autophagy, and Apoptosis. Int. J. Mol. Sci. 2023, 24, 15744. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.-K.; Agarwal, S.; Kuo, C.-H.; Kung, Y.-L.; Day, C.H.; Lin, P.-Y.; Lin, S.-Z.; Hsieh, D.J.-Y.; Huang, C.-Y.; Chiang, C.-Y. Artemisia Leaf Extract protects against neuron toxicity by TRPML1 activation and promoting autophagy/mitophagy clearance in both in vitro and in vivo models of MPP+/MPTP-induced Parkinson’s disease. Phytomedicine 2022, 104, 154250. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Yao, Y.; Guo, M.; Li, J.; Yang, P.; Xu, H.; Lin, D. Sevoflurane increases intracellular calcium to induce mitochondrial injury and neuroapoptosis. Toxicol. Lett. 2021, 336, 11–20. [Google Scholar] [CrossRef]
- Li, D.W.; Qi, X.D.; Zhang, C.H.; Sun, W.P. Annexin A2 degradation contributes to dopaminergic cell apoptosis via regulating p53 in neurodegenerative conditions. Neuroreport 2021, 32, 1263–1268. [Google Scholar] [CrossRef]
- Zingales, V.; Fernandez-Franzon, M.; Ruiz, M.J. Sterigmatocystin-induced DNA damage triggers cell-cycle arrest via MAPK in human neuroblastoma cells. Toxicol. Mech. Methods 2021, 31, 479–488. [Google Scholar] [CrossRef]
- Filichia, E.; Hoffer, B.; Qi, X.; Luo, Y. Inhibition of Drp1 mitochondrial translocation provides neural protection in dopaminergic system in a Parkinson’s disease model induced by MPTP. Sci. Rep. 2016, 6, 32656. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.X.; Jiao, J.Q.; Li, Q.; Long, B.; Wang, K.; Liu, J.P.; Li, Y.R.; Li, P.F. miR-499 regulates mitochondrial dynamics by targeting calcineurin and dynamin-related protein-1. Nat. Med. 2011, 17, 71–78. [Google Scholar] [CrossRef]
- Konstantakou, E.G.; Voutsinas, G.E.; Velentzas, A.D.; Basogianni, A.S.; Paronis, E.; Balafas, E.; Kostomitsopoulos, N.; Syrigos, K.N.; Anastasiadou, E.; Stravopodis, D.J. 3-BrPA eliminates human bladder cancer cells with highly oncogenic signatures via engagement of specific death programs and perturbation of multiple signaling and metabolic determinants. Mol. Cancer 2015, 14, 135. [Google Scholar] [CrossRef] [PubMed]
- Rios, L.; Pokhrel, S.; Li, S.J.; Heo, G.; Haileselassie, B.; Mochly-Rosen, D. Targeting an allosteric site in dynamin-related protein 1 to inhibit Fis1-mediated mitochondrial dysfunction. Nat. Commun. 2023, 14, 4356. [Google Scholar] [CrossRef] [PubMed]
- Joshi, A.U.; Ebert, A.E.; Haileselassie, B.; Mochly-Rosen, D. Drp1/Fis1-mediated mitochondrial fragmentation leads to lysosomal dysfunction in cardiac models of Huntington’s disease. J. Mol. Cell Cardiol. 2019, 127, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Krzystek, T.J.; Banerjee, R.; Thurston, L.; Huang, J.; Swinter, K.; Rahman, S.N.; Falzone, T.L.; Gunawardena, S. Differential mitochondrial roles for alpha-synuclein in DRP1-dependent fission and PINK1/Parkin-mediated oxidation. Cell Death Dis. 2021, 12, 796. [Google Scholar] [CrossRef]
- Smith, M.A.; Zhu, X.; Tabaton, M.; Liu, G.; McKeel, D.W., Jr.; Cohen, M.L.; Wang, X.; Siedlak, S.L.; Dwyer, B.E.; Hayashi, T.; et al. Increased iron and free radical generation in preclinical Alzheimer disease and mild cognitive impairment. J. Alzheimers Dis. 2010, 19, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Narendra, D.P.; Jin, S.M.; Tanaka, A.; Suen, D.F.; Gautier, C.A.; Shen, J.; Cookson, M.R.; Youle, R.J. PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol. 2010, 8, e1000298. [Google Scholar] [CrossRef]
- Soto, I.; McManus, R.; Navarrete, W.; Kasanga, E.A.; Doshier, K.; Nejtek, V.A.; Salvatore, M.F. Aging accelerates locomotor decline in PINK1 knockout rats in association with decreased nigral, but not striatal, dopamine and tyrosine hydroxylase expression. Exp. Neurol. 2024, 376, 114771. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.Z.; Qiao, J.; Knox, T.; Iriah, S.; Kulkarni, P.; Madularu, D.; Morrison, T.; Waszczak, B.; Hartner, J.C.; Ferris, C.F. In search of early neuroradiological biomarkers for Parkinson’s Disease: Alterations in resting state functional connectivity and gray matter microarchitecture in PINK1−/− rats. Brain Res. 2019, 1706, 58–67. [Google Scholar] [CrossRef]
- Pullara, F.; Forsmann, M.C.; General, I.J.; Ayoob, J.C.; Furbee, E.; Castro, S.L.; Hu, X.; Greenamyre, J.T.; Di Maio, R. NADPH oxidase 2 activity disrupts Calmodulin/CaMKIIα complex via redox modifications of CaMKIIα-contained Cys30 and Cys289: Implications in Parkinson’s disease. Redox Biol. 2024, 75, 103254. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Ye, Q.; Xu, Y.; He, B.; Wu, L.; Zhu, G.; Xie, J.; Yao, L.; Xiao, Z. Mitochondrial calcium uptake 3 mitigates cerebral amyloid angiopathy-related neuronal death and glial inflammation by reducing mitochondrial dysfunction. Int. Immunopharmacol. 2023, 117, 109614. [Google Scholar] [CrossRef]
- Gegg, M.E.; Cooper, J.M.; Schapira, A.H.; Taanman, J.W. Silencing of PINK1 expression affects mitochondrial DNA and oxidative phosphorylation in dopaminergic cells. PLoS ONE 2009, 4, e4756. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Zhang, L. Resveratrol Suppresses Aβ-Induced Microglial Activation Through the TXNIP/TRX/NLRP3 Signaling Pathway. DNA Cell Biol. 2019, 38, 874–879. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Zhang, Y.; Lu, L.; Zhang, H.; Zhao, C.; Pu, Y.; Yin, L. Copper induces microglia-mediated neuroinflammation through ROS/NF-κB pathway and mitophagy disorder. Food Chem. Toxicol. 2022, 168, 113369. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.L.; Xie, X.H.; Ding, J.H.; Du, R.H.; Hu, G. Astragaloside IV inhibits astrocyte senescence: Implication in Parkinson’s disease. J. Neuroinflammation 2020, 17, 105. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, C.; Yu, M.; Cui, J.; Liu, L.; Xu, Z. ULK1 and JNK are involved in mitophagy incurred by LRRK2 G2019S expression. Protein Cell 2013, 4, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Conradson, D.M.; Bharat, V.; Kim, M.J.; Hsieh, C.H.; Minhas, P.S.; Papakyrikos, A.M.; Durairaj, A.S.; Ludlam, A.; Andreasson, K.I.; et al. A mitochondrial membrane-bridging machinery mediates signal transduction of intramitochondrial oxidation. Nat. Metab. 2021, 3, 1242–1258. [Google Scholar] [CrossRef] [PubMed]
- Ludtmann, M.H.R.; Kostic, M.; Horne, A.; Gandhi, S.; Sekler, I.; Abramov, A.Y. LRRK2 deficiency induced mitochondrial Ca2+ efflux inhibition can be rescued by Na+/Ca2+/Li+ exchanger upregulation. Cell Death Dis. 2019, 10, 265. [Google Scholar] [CrossRef]
- D’Amico, E.; Grosso, G.; Nieves, J.W.; Zanghi, A.; Factor-Litvak, P.; Mitsumoto, H. Metabolic Abnormalities, Dietary Risk Factors and Nutritional Management in Amyotrophic Lateral Sclerosis. Nutrients 2021, 13, 2273. [Google Scholar] [CrossRef]
- Hardiman, O.; Al-Chalabi, A.; Chio, A.; Corr, E.M.; Logroscino, G.; Robberecht, W.; Shaw, P.J.; Simmons, Z.; van den Berg, L.H. Amyotrophic lateral sclerosis. Nat. Rev. Dis. Primers 2017, 3, 17071. [Google Scholar] [CrossRef] [PubMed]
- Cozzolino, M.; Pesaresi, M.G.; Gerbino, V.; Grosskreutz, J.; Carrì, M.T. Amyotrophic Lateral Sclerosis: New Insights into Underlying Molecular Mechanisms and Opportunities for Therapeutic Intervention. Antioxid. Redox Signal. 2012, 17, 1277–1330. [Google Scholar] [CrossRef]
- Pasinelli, P.; Brown, R.H. Molecular biology of amyotrophic lateral sclerosis: Insights from genetics. Nat. Rev. Neurosci. 2006, 7, 710–723. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.J. Mitochondrial pathobiology in ALS. J. Bioenerg. Biomembr. 2011, 43, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Barber, S.C.; Shaw, P.J. Oxidative stress in ALS: Key role in motor neuron injury and therapeutic target. Free Radic. Biol. Med. 2010, 48, 629–641. [Google Scholar] [CrossRef] [PubMed]
- Delic, V.; Kurien, C.; Cruz, J.; Zivkovic, S.; Barretta, J.; Thomson, A.; Hennessey, D.; Joseph, J.; Ehrhart, J.; Willing, A.E.; et al. Discrete mitochondrial aberrations in the spinal cord of sporadic ALS patients. J. Neurosci. Res. 2018, 96, 1353–1366. [Google Scholar] [CrossRef]
- Beckers, J.; Tharkeshwar, A.K.; Van Damme, P. C9orf72 ALS-FTD: Recent evidence for dysregulation of the autophagy-lysosome pathway at multiple levels. Autophagy 2021, 17, 3306–3322. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.R.; Gregory, J.M.; Dando, O.; Carter, R.N.; Burr, K.; Nanda, J.; Story, D.; McDade, K.; Smith, C.; Morton, N.M.; et al. Mitochondrial bioenergetic deficits in C9orf72 amyotrophic lateral sclerosis motor neurons cause dysfunctional axonal homeostasis. Acta Neuropathol. 2021, 141, 257–279. [Google Scholar] [CrossRef]
- Mitsumoto, H.; Santella, R.M.; Liu, X.; Bogdanov, M.; Zipprich, J.; Wu, H.C.; Mahata, J.; Kilty, M.; Bednarz, K.; Bell, D.; et al. Oxidative stress biomarkers in sporadic ALS. Amyotroph. Lateral Scler. 2008, 9, 177–183. [Google Scholar] [CrossRef]
- Cioffi, F.; Adam, R.H.I.; Broersen, K. Molecular Mechanisms and Genetics of Oxidative Stress in Alzheimer’s Disease. J. Alzheimers Dis. 2019, 72, 981–1017. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.F.; Shaw, P.J.; De Vos, K.J. The role of mitochondria in amyotrophic lateral sclerosis. Neurosci. Lett. 2019, 710, 132933. [Google Scholar] [CrossRef] [PubMed]
- Stein, J.; Walkenfort, B.; Cihankaya, H.; Hasenberg, M.; Bader, V.; Winklhofer, K.F.; Roderer, P.; Matschke, J.; Theiss, C.; Matschke, V. Increased ROS-Dependent Fission of Mitochondria Causes Abnormal Morphology of the Cell Powerhouses in a Murine Model of Amyotrophic Lateral Sclerosis. Oxid. Med. Cell Longev. 2021, 2021, 6924251. [Google Scholar] [CrossRef] [PubMed]
- Malacarne, C.; Giagnorio, E.; Chirizzi, C.; Cattaneo, M.; Saraceno, F.; Cavalcante, P.; Bonanno, S.; Mantegazza, R.; Moreno-Manzano, V.; Lauria, G.; et al. FM19G11-loaded nanoparticles modulate energetic status and production of reactive oxygen species in myoblasts from ALS mice. Biomed. Pharmacother. 2024, 173, 116380. [Google Scholar] [CrossRef]
- Rakhit, R.; Cunningham, P.; Furtos-Matei, A.; Dahan, S.; Qi, X.F.; Crow, J.P.; Cashman, N.R.; Kondejewski, L.H.; Chakrabartty, A. Oxidation-induced misfolding and aggregation of superoxide dismutase and its implications for amyotrophic lateral sclerosis. J. Biol. Chem. 2002, 277, 47551–47556. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Wang, X.; Chen, L.; Lenahan, C.; Fu, Z.; Fang, Y.; Yu, W. Crosstalk Between the Oxidative Stress and Glia Cells After Stroke: From Mechanism to Therapies. Front. Immunol. 2022, 13, 852416. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xia, X.; Wang, Y.; Zheng, J.C. Mitochondrial dysfunction in microglia: A novel perspective for pathogenesis of Alzheimer’s disease. J. Neuroinflammation 2022, 19, 248. [Google Scholar] [CrossRef]
- Lopez, D.E.; Ballaz, S.J. The Role of Brain Cyclooxygenase-2 (Cox-2) Beyond Neuroinflammation: Neuronal Homeostasis in Memory and Anxiety. Mol. Neurobiol. 2020, 57, 5167–5176. [Google Scholar] [CrossRef]
- Joshi, A.U.; Saw, N.L.; Vogel, H.; Cunnigham, A.D.; Shamloo, M.; Mochly-Rosen, D. Inhibition of Drp1/Fis1 interaction slows progression of amyotrophic lateral sclerosis. EMBO Mol. Med. 2018, 10, e8166. [Google Scholar] [CrossRef]
- Liu, W.; Yamashita, T.; Tian, F.; Morimoto, N.; Ikeda, Y.; Deguchi, K.; Abe, K. Mitochondrial fusion and fission proteins expression dynamically change in a murine model of amyotrophic lateral sclerosis. Curr. Neurovasc Res. 2013, 10, 222–230. [Google Scholar] [CrossRef]
- Srinivasan, S.; Stevens, M.; Wiley, J.W. Diabetic peripheral neuropathy: Evidence for apoptosis and associated mitochondrial dysfunction. Diabetes 2000, 49, 1932–1938. [Google Scholar] [CrossRef]
- Feng, W.; Wang, J.; Yan, X.; Zhang, Q.; Chai, L.; Wang, Q.; Shi, W.; Chen, Y.; Liu, J.; Qu, Z.; et al. ERK/Drp1-dependent mitochondrial fission contributes to HMGB1-induced autophagy in pulmonary arterial hypertension. Cell Prolif. 2021, 54, e13048. [Google Scholar] [CrossRef] [PubMed]
- Hengartner, M.O. The biochemistry of apoptosis. Nature 2000, 407, 770–776. [Google Scholar] [CrossRef]
- Choi, S.Y.; Lee, J.H.; Chung, A.Y.; Jo, Y.; Shin, J.H.; Park, H.C.; Kim, H.; Lopez-Gonzalez, R.; Ryu, J.R.; Sun, W. Prevention of mitochondrial impairment by inhibition of protein phosphatase 1 activity in amyotrophic lateral sclerosis. Cell Death Dis. 2020, 11, 888. [Google Scholar] [CrossRef] [PubMed]
- Petruzzella, V.; Papa, S. Mutations in human nuclear genes encoding for subunits of mitochondrial respiratory complex I: The NDUFS4 gene. Gene 2002, 286, 149–154. [Google Scholar] [CrossRef]
- Arico, S.; Petiot, A.; Bauvy, C.; Dubbelhuis, P.F.; Meijer, A.J.; Codogno, P.; Ogier-Denis, E. The tumor suppressor PTEN positively regulates macroautophagy by inhibiting the phosphatidylinositol 3-kinase/protein kinase B pathway. J. Biol. Chem. 2001, 276, 35243–35246. [Google Scholar] [CrossRef] [PubMed]
- Teyssou, E.; Chartier, L.; Roussel, D.; Perera, N.D.; Nemazanyy, I.; Langui, D.; Albert, M.; Larmonier, T.; Saker, S.; Salachas, F.; et al. The Amyotrophic Lateral Sclerosis M114T PFN1 Mutation Deregulates Alternative Autophagy Pathways and Mitochondrial Homeostasis. Int. J. Mol. Sci. 2022, 23, 5694. [Google Scholar] [CrossRef]
- Padman, B.S.; Nguyen, T.N.; Uoselis, L.; Skulsuppaisarn, M.; Nguyen, L.K.; Lazarou, M. LC3/GABARAPs drive ubiquitin-independent recruitment of Optineurin and NDP52 to amplify mitophagy. Nat. Commun. 2019, 10, 408. [Google Scholar] [CrossRef]
- Yang, L.; Cheng, Y.; Jia, X.; Liu, X.; Li, X.; Zhang, K.; Shen, D.; Liu, M.; Guan, Y.; Liu, Q.; et al. Four novel optineurin mutations in patients with sporadic amyotrophic lateral sclerosis in Mainland China. Neurobiol. Aging 2021, 97, e141–e149. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, H.; Morino, H.; Ito, H.; Izumi, Y.; Kato, H.; Watanabe, Y.; Kinoshita, Y.; Kamada, M.; Nodera, H.; Suzuki, H.; et al. Mutations of optineurin in amyotrophic lateral sclerosis. Nature 2010, 465, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Wong, Y.C.; Holzbaur, E.L.F. Optineurin is an autophagy receptor for damaged mitochondria in parkin-mediated mitophagy that is disrupted by an ALS-linked mutation. Proc. Natl. Acad. Sci. USA 2014, 111, E4439–E4448. [Google Scholar] [CrossRef] [PubMed]
- Rogov, V.V.; Suzuki, H.; Fiskin, E.; Wild, P.; Kniss, A.; Rozenknop, A.; Kato, R.; Kawasaki, M.; McEwan, D.G.; Lohr, F.; et al. Structural basis for phosphorylation-triggered autophagic clearance of Salmonella. Biochem. J. 2013, 454, 459–466. [Google Scholar] [CrossRef]
- Shao, W.; Todd, T.W.; Wu, Y.; Jones, C.Y.; Tong, J.; Jansen-West, K.; Daughrity, L.M.; Park, J.; Koike, Y.; Kurti, A.; et al. Two FTD-ALS genes converge on the endosomal pathway to induce TDP-43 pathology and degeneration. Science 2022, 378, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, L.; Lu, J.; Siedlak, S.L.; Fujioka, H.; Liang, J.; Jiang, S.; Ma, X.; Jiang, Z.; da Rocha, E.L.; et al. The inhibition of TDP-43 mitochondrial localization blocks its neuronal toxicity. Nat. Med. 2016, 22, 869–878. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Shao, J.; Wang, Y.; Wu, K.; Huang, M. OPA1, a molecular regulator of dilated cardiomyopathy. J. Cell Mol. Med. 2023, 27, 3017–3025. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.; Bharathi, V.; Sivalingam, V.; Girdhar, A.; Patel, B.K. Molecular Mechanisms of TDP-43 Misfolding and Pathology in Amyotrophic Lateral Sclerosis. Front. Mol. Neurosci. 2019, 12, 25. [Google Scholar] [CrossRef] [PubMed]
- Vahsen, B.F.; Nalluru, S.; Morgan, G.R.; Farrimond, L.; Carroll, E.; Xu, Y.; Cramb, K.M.L.; Amein, B.; Scaber, J.; Katsikoudi, A.; et al. C9orf72-ALS human iPSC microglia are pro-inflammatory and toxic to co-cultured motor neurons via MMP9. Nat. Commun. 2023, 14, 5898. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.W.; Chen, M.T.; Lin, L.T.; Huang, P.I.; Lo, W.L.; Yang, Y.P.; Lu, K.H.; Chen, Y.W.; Chiou, S.H.; Wu, C.W. TDP-43/HDAC6 axis promoted tumor progression and regulated nutrient deprivation-induced autophagy in glioblastoma. Oncotarget 2017, 8, 56612–56625. [Google Scholar] [CrossRef] [PubMed]
- Buratti, E. Targeting TDP-43 proteinopathy with drugs and drug-like small molecules. Br. J. Pharmacol. 2021, 178, 1298–1315. [Google Scholar] [CrossRef]
- Geng, J.; Liu, W.; Gao, J.; Jiang, C.; Fan, T.; Sun, Y.; Qin, Z.H.; Xu, Q.; Guo, W.; Gao, J. Andrographolide alleviates Parkinsonism in MPTP-PD mice via targeting mitochondrial fission mediated by dynamin-related protein 1. Br. J. Pharmacol. 2019, 176, 4574–4591. [Google Scholar] [CrossRef] [PubMed]
- Bido, S.; Soria, F.N.; Fan, R.Z.; Bezard, E.; Tieu, K. Mitochondrial division inhibitor-1 is neuroprotective in the A53T-α-synuclein rat model of Parkinson’s disease. Sci. Rep. 2017, 7, 7495. [Google Scholar] [CrossRef]
- Lian, W.W.; Zhou, W.; Zhang, B.Y.; Jia, H.; Xu, L.J.; Liu, A.L.; Du, G.H. DL0410 ameliorates cognitive disorder in SAMP8 mice by promoting mitochondrial dynamics and the NMDAR-CREB-BDNF pathway. Acta Pharmacol. Sin. 2021, 42, 1055–1068. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Yang, X.; Li, J.; Shu, Z.; Dai, J.; Liu, X.; Li, B.; Jia, S.; Kou, X.; Yang, Y.; et al. Spermidine coupled with exercise rescues skeletal muscle atrophy from D-gal-induced aging rats through enhanced autophagy and reduced apoptosis via AMPK-FOXO3a signal pathway. Oncotarget 2017, 8, 17475–17490. [Google Scholar] [CrossRef]
- Kong, W.; Liu, Y.; Ai, P.; Bi, Y.; Wei, C.; Guo, X.; Cai, Z.; Gao, G.; Hu, P.; Zheng, J.; et al. Genetically modified E. coli secreting melanin (E. melanin) activates the astrocytic PSAP-GPR37L1 pathway and mitigates the pathogenesis of Parkinson’s disease. J. Nanobiotechnology 2024, 22, 690. [Google Scholar] [CrossRef]
- Kaur, G.; Arora, J.; Sodhi, A.S.; Bhatia, S.; Batra, N. Nanotechnology and CRISPR/Cas-Mediated Gene Therapy Strategies: Potential Role for Treating Genetic Disorders. Mol. Biotechnol. 2024, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Lei, Z.G.; Chu, K.; Lam, O.J.H.; Chiang, C.Y.; Zhang, Z.J. N, N-Dimethyltryptamine, a natural hallucinogen, ameliorates Alzheimer’s disease by restoring neuronal Sigma-1 receptor-mediated endoplasmic reticulum-mitochondria crosstalk. Alzheimers Res. Ther. 2024, 16, 95. [Google Scholar] [CrossRef]
- Jhurry, N.D.; Chakrabarti, M.; McCormick, S.P.; Holmes-Hampton, G.P.; Lindahl, P.A. Biophysical investigation of the ironome of human jurkat cells and mitochondria. Biochemistry 2012, 51, 5276–5284. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.H.; Nguyen, C.D.; Lee, G.; Na, C.S. Insamgobonhwan Protects Neuronal Cells from Lipid ROS and Improves Deficient Cognitive Function. Antioxidants 2022, 11, 295. [Google Scholar] [CrossRef] [PubMed]
- Fei, Y.; Ding, Y. The role of ferroptosis in neurodegenerative diseases. Front. Cell Neurosci. 2024, 18, 1475934. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Song, X.; Long, D. Ferroptosis regulation through Nrf2 and implications for neurodegenerative diseases. Arch. Toxicol. 2024, 98, 579–615. [Google Scholar] [CrossRef] [PubMed]
- Netea, M.G.; Nold-Petry, C.A.; Nold, M.F.; Joosten, L.A.; Opitz, B.; van der Meer, J.H.; van de Veerdonk, F.L.; Ferwerda, G.; Heinhuis, B.; Devesa, I.; et al. Differential requirement for the activation of the inflammasome for processing and release of IL-1beta in monocytes and macrophages. Blood 2009, 113, 2324–2335. [Google Scholar] [CrossRef] [PubMed]
- Bauernfeind, F.G.; Horvath, G.; Stutz, A.; Alnemri, E.S.; MacDonald, K.; Speert, D.; Fernandes-Alnemri, T.; Wu, J.; Monks, B.G.; Fitzgerald, K.A.; et al. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J. Immunol. 2009, 183, 787–791. [Google Scholar] [CrossRef]
- He, W.T.; Wan, H.; Hu, L.; Chen, P.; Wang, X.; Huang, Z.; Yang, Z.H.; Zhong, C.Q.; Han, J. Gasdermin D is an executor of pyroptosis and required for interleukin-1β secretion. Cell Res. 2015, 25, 1285–1298. [Google Scholar] [CrossRef] [PubMed]
- Billingham, L.K.; Stoolman, J.S.; Vasan, K.; Rodriguez, A.E.; Poor, T.A.; Szibor, M.; Jacobs, H.T.; Reczek, C.R.; Rashidi, A.; Zhang, P.; et al. Mitochondrial electron transport chain is necessary for NLRP3 inflammasome activation. Nat. Immunol. 2022, 23, 692–704. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.Q.; Zheng, R.; Liu, Y.; Ruan, Y.; Lin, Z.H.; Xue, N.J.; Chen, Y.; Zhang, B.R.; Pu, J.L. Parkin regulates microglial NLRP3 and represses neurodegeneration in Parkinson’s disease. Aging Cell 2023, 22, e13834. [Google Scholar] [CrossRef] [PubMed]
- Andhale, R.; Shrivastava, D. Huntington’s Disease: A Clinical Review. Cureus 2022, 14, e28484. [Google Scholar] [CrossRef]
- Walker, F.O. Huntington’s disease. Lancet 2007, 369, 218–228. [Google Scholar] [CrossRef]
- Túnez, I.; Sánchez-López, F.; Agüera, E.; Fernández-Bolaños, R.; Sánchez, F.M.; Tasset-Cuevas, I. Important role of oxidative stress biomarkers in Huntington’s disease. J. Med. Chem. 2011, 54, 5602–5606. [Google Scholar] [CrossRef] [PubMed]
- Shirendeb, U.; Reddy, A.P.; Manczak, M.; Calkins, M.J.; Mao, P.; Tagle, D.A.; Reddy, P.H. Abnormal mitochondrial dynamics, mitochondrial loss and mutant huntingtin oligomers in Huntington’s disease: Implications for selective neuronal damage. Hum. Mol. Genet. 2011, 20, 1438–1455. [Google Scholar] [CrossRef] [PubMed]
- Niatsetskaya, Z.; Sosunov, S.; Stepanova, A.; Goldman, J.; Galkin, A.; Neginskaya, M.; Pavlov, E.; Ten, V. Cyclophilin D-dependent oligodendrocyte mitochondrial ion leak contributes to neonatal white matter injury. J. Clin. Investig. 2020, 130, 5536–5550. [Google Scholar] [CrossRef]
- Lee, M.Y.; Sumpter, R., Jr.; Zou, Z.; Sirasanagandla, S.; Wei, Y.; Mishra, P.; Rosewich, H.; Crane, D.I.; Levine, B. Peroxisomal protein PEX13 functions in selective autophagy. EMBO Rep. 2017, 18, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.H.; Beal, M.F. Amyloid beta, mitochondrial dysfunction and synaptic damage: Implications for cognitive decline in aging and Alzheimer’s disease. Trends Mol. Med. 2008, 14, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Li, H.; Chang, L.; Wei, C.; Yin, Y.; Bei, H.; Wang, Z.; Liang, J.; Wu, Y. Qiliqiangxin Attenuates Oxidative Stress-Induced Mitochondrion-Dependent Apoptosis in Cardiomyocytes via PI3K/AKT/GSK3β Signaling Pathway. Biol. Pharm. Bull. 2019, 42, 1310–1321. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Jia, P.; Huang, Z.; Liu, S.; Miao, J.; Guo, Y.; Wu, N.; Jia, D. Lycopene protects against myocardial ischemia-reperfusion injury by inhibiting mitochondrial permeability transition pore opening. Drug Des. Devel Ther. 2019, 13, 2331–2342. [Google Scholar] [CrossRef] [PubMed]
- Shin, E.J.; Nam, Y.; Lee, J.W.; Nguyen, P.T.; Yoo, J.E.; Tran, T.V.; Jeong, J.H.; Jang, C.G.; Oh, Y.J.; Youdim, M.B.H.; et al. N-Methyl, N-propynyl-2-phenylethylamine (MPPE), a Selegiline Analog, Attenuates MPTP-induced Dopaminergic Toxicity with Guaranteed Behavioral Safety: Involvement of Inhibitions of Mitochondrial Oxidative Burdens and p53 Gene-elicited Pro-apoptotic Change. Mol. Neurobiol. 2016, 53, 6251–6269. [Google Scholar] [CrossRef] [PubMed]
- Dagda, R.K.; Cherra, S.J., 3rd; Kulich, S.M.; Tandon, A.; Park, D.; Chu, C.T. Loss of PINK1 function promotes mitophagy through effects on oxidative stress and mitochondrial fission. J. Biol. Chem. 2009, 284, 13843–13855. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.H.; Davidson, S.; Harapas, C.R.; Hilton, J.B.; Mlodzianoski, M.J.; Laohamonthonkul, P.; Louis, C.; Low, R.R.J.; Moecking, J.; De Nardo, D.; et al. TDP-43 Triggers Mitochondrial DNA Release via mPTP to Activate cGAS/STING in ALS. Cell 2020, 183, 636–649. [Google Scholar] [CrossRef]
- Bond, S.T.; Moody, S.C.; Liu, Y.Y.; Civelek, M.; Villanueva, C.J.; Gregorevic, P.; Kingwell, B.A.; Hevener, A.L.; Lusis, A.J.; Henstridge, D.C.; et al. The E3 ligase MARCH5 is a PPARγ target gene that regulates mitochondria and metabolism in adipocytes. Am. J. Physiol.-Endocrinol. Metab. 2019, 316, E293–E304. [Google Scholar] [CrossRef]
- Zhou, Y.Q.; Mei, W.; Tian, X.B.; Tian, Y.K.; Liu, D.Q.; Ye, D.W. The therapeutic potential of Nrf2 inducers in chronic pain: Evidence from preclinical studies. Pharmacol. Ther. 2021, 225, 107846. [Google Scholar] [CrossRef]
- Schmeichel, A.M.; Schmelzer, J.D.; Low, P.A. Oxidative injury and apoptosis of dorsal root ganglion neurons in chronic experimental diabetic neuropathy. Diabetes 2003, 52, 165–171. [Google Scholar] [CrossRef]
- Pacher, P.; Liaudet, L.; Soriano, F.G.; Mabley, J.G.; Szabo, E.; Szabo, C. The role of poly(ADP-ribose) polymerase activation in the development of myocardial and endothelial dysfunction in diabetes. Diabetes 2002, 51, 514–521. [Google Scholar] [CrossRef] [PubMed]
| Neurological Disease | Treatment | Model/Resources | Outcome | Reference |
|---|---|---|---|---|
| AD | Water extract of Centella asiatica (CAW) | 5xFAD mouse model | The accumulation of Aβ plaques in mice cortex decreased; cAW targeted the activation of NRF2, and the cognitive changes in mice were accompanied by an increase in NRF2 antioxidant response genes in the frontal cortex. | [145] |
| Quercetin | AD patients | The inhibition of MAPK pathway activation and inhibition of tau phosphorylation, thereby improving memory, cognitive function, synaptic plasticity and neuronal metabolism. | [146] | |
| Andrographolide (AGA) | Apoe4 mouse model | Targeting of the SIRT3-FOXO3a signaling pathway, activation of mitophagy, and inhibition of the production of NLRP3 inflammasome, inhibiting neuroinflammation and alleviating cognitive impairment in mice. | [147] | |
| PD | Geniposide | Rotenone-induced PD mouse model | The inhibition of rotenone-induced oxidative damage of Nrf2 signaling neurons and inhibition of mTOR-involved anti-apoptotic pathway activation, thereby improving motor dysfunction in mice, restoring neurotransmitter levels, and reducing dopaminergic neurodegeneration. | [148] |
| Corydine (Cory) | MPTP-induced PD mouse model | Reduced phosphorylation of glycogen synthase kinase 3β (GSK-3β) at Tyr216 and enhanced mitophagy; reduced MPTP-induced cell damage; and upregulated LC3-II/LC3-I to improve motor coordination in PD mice. | [149] | |
| ALS | Sodium butyrate (NaB) | R97-116 peptide-induced autoimmune myasthenia gravis (EAMG) mice | Stimulation of anti-inflammatory cells and inhibited activation of NF-κB and TNF-α secretion, thereby inhibiting pro-inflammatory cytokines. Reversal of Th17/Treg cell imbalance, reduction in the number of Tfh and B cells, and alleviation of MG symptoms in mice. | [150] |
| Antioxidant genistein | ALS SOD1-G93A transgenic mouse model | Inhibition of the production of pro-inflammatory cytokines and glial proliferation in the spinal cord, mitophagy, and alleviation of ALS-related symptoms. | [151] | |
| Oxymatrine (OMT) | Transgenic SOD1-G93A mice | Neuroprotective effects via reduction in the activation of microglia and astrocytes, downregulating pro-inflammatory mediators and upregulating anti-inflammatory factors. | [152] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, K.; Jia, H.; Hou, X.; Zhu, Z.; Lu, Y.; Feng, Y.; Feng, J.; Xia, Y.; Tan, R.; Cui, F.; et al. Mitochondrial Dysfunction in Neurodegenerative Diseases: Mechanisms and Corresponding Therapeutic Strategies. Biomedicines 2025, 13, 327. https://doi.org/10.3390/biomedicines13020327
Meng K, Jia H, Hou X, Zhu Z, Lu Y, Feng Y, Feng J, Xia Y, Tan R, Cui F, et al. Mitochondrial Dysfunction in Neurodegenerative Diseases: Mechanisms and Corresponding Therapeutic Strategies. Biomedicines. 2025; 13(2):327. https://doi.org/10.3390/biomedicines13020327
Chicago/Turabian StyleMeng, Kai, Haocheng Jia, Xiaoqing Hou, Ziming Zhu, Yuguang Lu, Yingying Feng, Jingwen Feng, Yong Xia, Rubin Tan, Fen Cui, and et al. 2025. "Mitochondrial Dysfunction in Neurodegenerative Diseases: Mechanisms and Corresponding Therapeutic Strategies" Biomedicines 13, no. 2: 327. https://doi.org/10.3390/biomedicines13020327
APA StyleMeng, K., Jia, H., Hou, X., Zhu, Z., Lu, Y., Feng, Y., Feng, J., Xia, Y., Tan, R., Cui, F., & Yuan, J. (2025). Mitochondrial Dysfunction in Neurodegenerative Diseases: Mechanisms and Corresponding Therapeutic Strategies. Biomedicines, 13(2), 327. https://doi.org/10.3390/biomedicines13020327







