Short-Chain Fatty Acids and Their Metabolic Interactions in Heart Failure
Abstract
1. Introduction
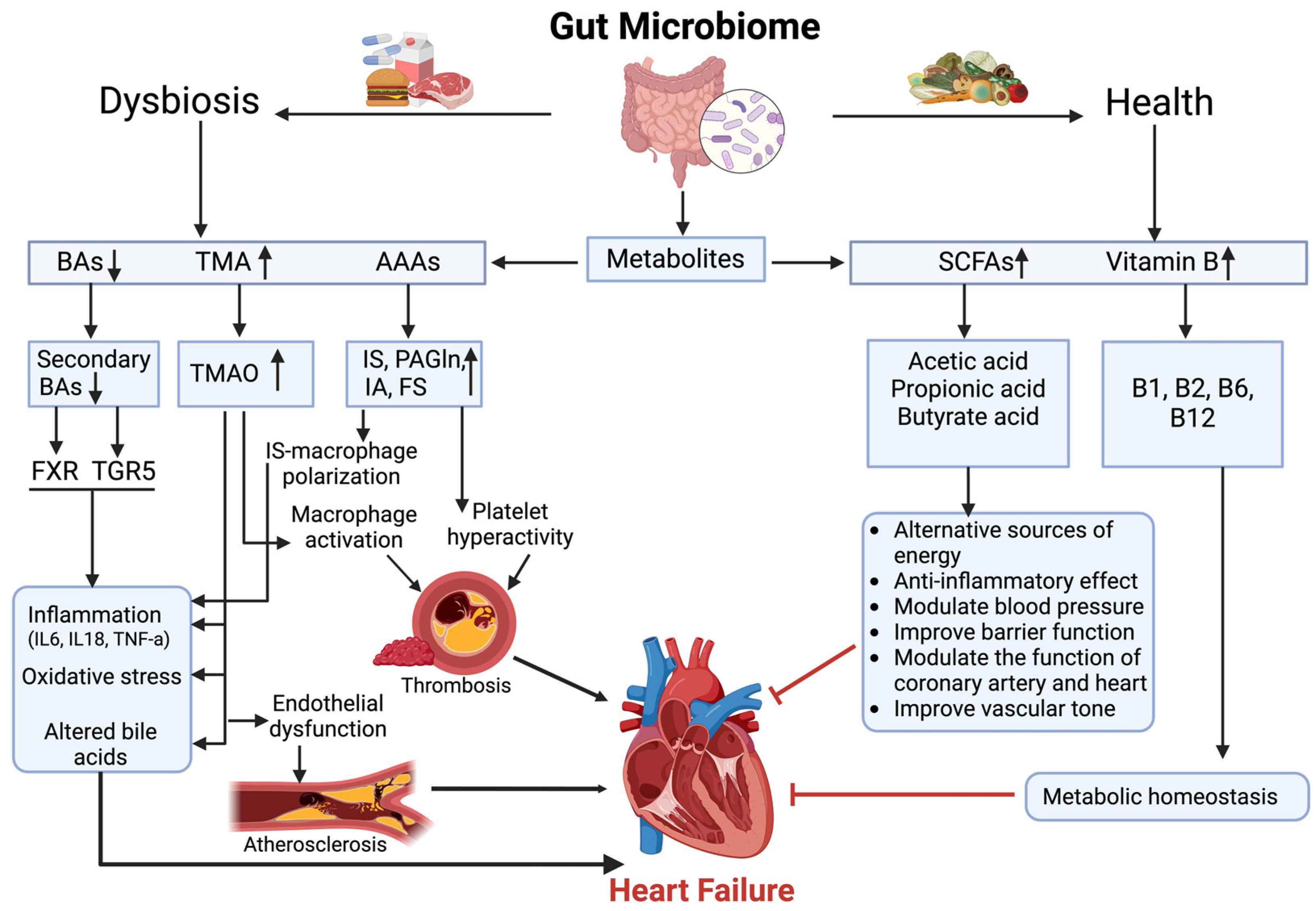
2. Gut Microbial Metabolites in Heart Failure
2.1. Production and Role of SCFAs
2.2. SCFAs and Gut–Heart Axis
2.3. SCFAs and Related Metabolic Pathways in Heart Failure
2.3.1. SCFAs and TMAO Interaction in Heart Failure
2.3.2. SCFAs and Aromatic Amino Acid Metabolism in Heart Failure
2.3.3. SCFAs and B-Vitamin Synthesis in Heart Failure
2.3.4. SCFA and BA Interactions in Heart Failure
3. Short-Chain Fatty Acids and Their Association with Biomarkers of HF
3.1. Relationship of SCFAs and HF
3.2. Left Ventricular Ejection Fraction and Its Association with SCFAs in HF
3.3. NT-proBNP and Its Association with SCFAs in HF
3.4. Glomerular Filtration Rate and Its Association with SCFAs in HF
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shahim, B.; Kapelios, C.J.; Savarese, G.; Lund, L.H. Global Public Health Burden of Heart Failure: An Updated Review. Card. Fail. Rev. 2023, 9, e11. [Google Scholar] [CrossRef]
- Roger, V.L. Epidemiology of Heart Failure. Circ. Res. 2021, 128, 1421–1434. [Google Scholar] [CrossRef] [PubMed]
- Norhammar, A.; Bodegard, J.; Vanderheyden, M.; Tangri, N.; Karasik, A.; Maggioni, A.P.; Sveen, K.A.; Taveira-Gomes, T.; Botana, M.; Hunziker, L.; et al. Prevalence, outcomes and costs of a contemporary, multinational population with heart failure. Heart 2023, 109, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Metra, M.; Tomasoni, D.; Adamo, M.; Bayes-Genis, A.; Filippatos, G.; Abdelhamid, M.; Adamopoulos, S.; Anker, S.D.; Antohi, L.; Böhm, M.; et al. Worsening of chronic heart failure: Definition, epidemiology, management and prevention. A clinical consensus statement by the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2023, 25, 776–791. [Google Scholar] [CrossRef]
- Groenewegen, A.; Rutten, F.H.; Mosterd, A.; Hoes, A.W. Epidemiology of Heart Failure; John Wiley and Sons Ltd.: Hoboken, NJ, USA, 2020. [Google Scholar] [CrossRef]
- Bozkurt, B.; Coats, A.; Coats, A.; Tsutsui, H.; Tsutsui, H. Universal definition and classification of heart failure: A report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure: Endorsed by the Canadian Heart Failure Society, Heart Failure Association of India, Cardiac Society of Australia and New Zealand, and Chinese Heart Failure Association. Eur. J. Heart Fail. 2021, 23, 352–380. [Google Scholar] [CrossRef]
- Castiglione, V.; Aimo, A.; Vergaro, G.; Saccaro, L.; Passino, C.; Emdin, M. Biomarkers for the diagnosis and management of heart failure. Heart Fail. Rev. 2022, 27, 625–643. [Google Scholar] [CrossRef] [PubMed]
- Sata, Y.; Marques, F.Z.; Kaye, D.M. The Emerging Role of Gut Dysbiosis in Cardio-metabolic Risk Factors for Heart Failure. Curr. Hypertens. Rep. 2020, 22, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mamic, P.; Chaikijurajai, T.; Tang, W.H.W. Gut microbiome—A potential mediator of pathogenesis in heart failure and its comorbidities: State-of-the-art review. J. Mol. Cell. Cardiol. 2021, 152, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Wu, Q.; Yao, Q.; Jiang, K.; Yu, J.; Tang, Q. Short-chain fatty acid metabolism and multiple effects on cardiovascular diseases. Ageing Res. Rev. 2022, 81, 101706. [Google Scholar] [CrossRef] [PubMed]
- Overby, H.B.; Ferguson, J.F. Gut Microbiota-Derived Short-Chain Fatty Acids Facilitate Microbiota:Host Cross talk and Modulate Obesity and Hypertension. Curr. Hypertens. Rep. 2021, 23, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Spehlmann, M.E.; Rangrez, A.Y.; Dhotre, D.P.; Schmiedel, N.; Chavan, N.; Bang, C.; Müller, O.J.; Shouche, Y.S.; Franke, A.; Frank, D.; et al. Heart Failure Severity Closely Correlates with Intestinal Dysbiosis and Subsequent Metabolomic Alterations. Biomedicines 2022, 10, 809. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.F.; Caparrós-Martin, J.A.; Gray, N.; Lodge, S.; Wist, J.; Lee, S.; O’gara, F.; Shah, A.; Ward, N.C.; Dwivedi, G. Insights into the associations between the gut microbiome, its metabolites, and heart failure. Am. J. Physiol. Circ. Physiol. 2023, 325, H1325–H1336. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Hu, Y.; Chen, X.; Luo, Y.; Chen, J.; Wang, H. Effects of Gut Microbiota and Metabolites on Heart Failure and Its Risk Factors: A Two-Sample Mendelian Randomization Study. Front. Nutr. 2022, 9, 899746. [Google Scholar] [CrossRef]
- Wang, M.; Wichienchot, S.; He, X.; Fu, X.; Huang, Q.; Zhang, B. In vitro colonic fermentation of dietary fibers: Fermentation rate, short-chain fatty acid production and changes in microbiota. Trends Food Sci. Technol. 2019, 88, 1–9. [Google Scholar] [CrossRef]
- Prasad, K.N.; Bondy, S.C. Dietary fibers and their fermented short-chain fatty acids in prevention of human diseases. Bioact. Carbohydr. Diet. Fibre 2019, 17, 100170. [Google Scholar] [CrossRef]
- Ma, J.; Piao, X.; Mahfuz, S.; Long, S.; Wang, J. The interaction among gut microbes, the intestinal barrier and short chain fatty acids. Anim. Nutr. 2022, 9, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Si, X.; Shang, W.; Zhou, Z.; Strappe, P.; Wang, B.; Bird, A.; Blanchard, C. Gut Microbiome-Induced Shift of Acetate to Butyrate Positively Manages Dysbiosis in High Fat Diet. Mol. Nutr. Food Res. 2017, 62, 1700670. [Google Scholar] [CrossRef]
- He, J.; Zhang, P.; Shen, L.; Niu, L.; Tan, Y.; Chen, L.; Zhao, Y.; Bai, L.; Hao, X.; Li, X.; et al. Short-chain fatty acids and their association with signalling pathways in inflammation, glucose and lipid metabolism. Int. J. Mol. Sci. 2020, 21, 6356. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Yu, B.; Sun, J.; Liu, Z.; Chen, H.; Ge, L.; Chen, D. Short-chain fatty acids can improve lipid and glucose metabolism independently of the pig gut microbiota. J. Anim. Sci. Biotechnol. 2021, 12, 1–14. [Google Scholar] [CrossRef]
- Vinolo, M.A.R.; Rodrigues, H.G.; Nachbar, R.T.; Curi, R. Regulation of inflammation by short chain fatty acids. Nutrients 2011, 3, 858–887. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-F.; Shao, J.-H.; Liao, Y.-T.; Wang, L.-N.; Jia, Y.; Dong, P.-J.; Liu, Z.-Z.; He, D.-D.; Li, C.; Zhang, X. Regulation of short-chain fatty acids in the immune system. Front. Immunol. 2023, 14, 1186892. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Zhao, J.; Tao, S.; Zhou, X.; Pi, Y.; Gerrits, W.J.; Johnston, L.J.; Zhang, S.; Yang, H.; Liu, L.; et al. Effect of dietary fiber fermentation on short-chain fatty acid production and microbial composition in vitro. J. Sci. Food Agric. 2020, 100, 4282–4291. [Google Scholar] [CrossRef] [PubMed]
- Ashaolu, T.J.; Ashaolu, J.O.; Adeyeye, S.A. Fermentation of prebiotics by human colonic microbiota in vitro and short-chain fatty acids production: A critical review. J. Appl. Microbiol. 2021, 130, 677–687. [Google Scholar] [CrossRef]
- Carley, A.N.; Maurya, S.K.; Fasano, M.; Wang, Y.; Selzman, C.H.; Drakos, S.G.; Lewandowski, E.D. Short-Chain Fatty Acids Outpace Ketone Oxidation in the Failing Heart. Circulation 2021, 143, 1797–1808. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Lv, Y.-W.; Long, J.; Chen, J.-M.; He, J.-M.; Ruan, X.-Z.; Zhu, H.-B. Butyrate Improves the Metabolic Disorder and Gut Microbiome Dysbiosis in Mice Induced by a High-Fat Diet. Front. Pharmacol. 2019, 10, 1040. [Google Scholar] [CrossRef]
- Magnusson, M.K.; Isaksson, S.; Öhman, L. The Anti-inflammatory Immune Regulation Induced by Butyrate Is Impaired in Inflamed Intestinal Mucosa from Patients with Ulcerative Colitis. Inflammation 2020, 43, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Kummen, M.; Mayerhofer, C.C.K.; Vestad, B.; Broch, K.; Awoyemi, A.; Storm-Larsen, C.; Ueland, T.; Yndestad, A.; Hov, J.R.; Trøseid, M. Gut Microbiota Signature in Heart Failure Defined From Profiling of 2 Independent Cohorts. J. Am. Coll. Cardiol. 2018, 71, 1184–1186. [Google Scholar] [CrossRef]
- Okamoto, T.; Morino, K.; Ugi, S.; Nakagawa, F.; Lemecha, M.; Ida, S.; Ohashi, N.; Sato, D.; Fujita, Y.; Maegawa, H. Microbiome potentiates endurance exercise through intestinal acetate production. Am. J. Physiol. Metab. 2019, 316, E956–E966. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Xu, P.; Jiang, Q.; Xu, Q.; Zheng, Y.; Yan, J.; Ji, H.; Ning, J.; Zhang, X.; Li, C.; et al. Depletion of acetate-producing bacteria from the gut microbiota facilitates cognitive impairment through the gut-brain neural mechanism in diabetic mice. Microbiome 2021, 9, 1–19. [Google Scholar] [CrossRef]
- Rastelli, M.; Knauf, C.; Cani, P.D. Gut Microbes and Health: A Focus on the Mechanisms Linking Microbes, Obesity, and Related Disorders. Obesity 2018, 26, 792–800. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, H.-Y.; Hu, X.-M.; Zhang, Y.; Zhang, S.-Y. Current understanding of gut microbiota alterations and related therapeutic intervention strategies in heart failure. Chin. Med J. 2019, 132, 1843–1855. [Google Scholar] [CrossRef] [PubMed]
- Yukino-Iwashita, M.; Nagatomo, Y.; Kawai, A.; Taruoka, A.; Yumita, Y.; Kagami, K.; Yasuda, R.; Toya, T.; Ikegami, Y.; Masaki, N.; et al. Short-Chain Fatty Acids in Gut–Heart Axis: Their Role in the Pathology of Heart Failure. J. Pers. Med. 2022, 12, 1805. [Google Scholar] [CrossRef]
- O’Riordan, K.J.; Collins, M.K.; Moloney, G.M.; Knox, E.G.; Aburto, M.R.; Fülling, C.; Morley, S.J.; Clarke, G.; Schellekens, H.; Cryan, J.F. Short chain fatty acids: Microbial metabolites for gut-brain axis signalling. Mol. Cell. Endocrinol. 2022, 546, 111572. [Google Scholar] [CrossRef]
- Balikçi, C.; Gökçay, G.; Erdoğan, S.; Erdoğan, H.; Ural, K. GUT-HEART AXIS. Vet. Farmakoloji Toksikoloji Dern. Bul. 2023, 14, 49–58. [Google Scholar] [CrossRef]
- Wang, A.; Li, Z.; Sun, Z.; Zhang, D.; Ma, X. Gut-derived short-chain fatty acids bridge cardiac and systemic metabolism and immunity in heart failure. J. Nutr. Biochem. 2023, 120, 109370. [Google Scholar] [CrossRef]
- Palm, C.L.; Nijholt, K.T.; Bakker, B.M.; Westenbrink, B.D. Short-Chain Fatty Acids in the Metabolism of Heart Failure—Rethinking the Fat Stigma. Front. Cardiovasc. Med. 2022, 9, 915102. [Google Scholar] [CrossRef] [PubMed]
- Simadibrata, D.M.; Auliani, S.; Widyastuti, P.A.; Wijaya, A.D.; Amin, H.Z.; Muliawan, H.S.; Siswanto, B.B.; Simadibrata, M. The Gut Microbiota Profile in Heart Failure Patients: A Systematic Review. J. Gastrointest. Liver Dis. 2023, 32, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Nagatomo, Y.; Tang, W.H.W. Intersections Between Microbiome and Heart Failure: Revisiting the Gut Hypothesis. J. Card. Fail. 2015, 21, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Lupu, V.V.; Raileanu, A.A.; Mihai, C.M.; Morariu, I.D.; Lupu, A.; Starcea, I.M.; Frasinariu, O.E.; Mocanu, A.; Dragan, F.; Fotea, S. The Implication of the Gut Microbiome in Heart Failure. Cells 2023, 12, 1158. [Google Scholar] [CrossRef] [PubMed]
- Shanmugham, M.; Bellanger, S.; Leo, C.H. Gut-Derived Metabolite, Trimethylamine-N-oxide (TMAO) in Cardio-Metabolic Diseases: Detection, Mechanism, and Potential Therapeutics. Pharmaceuticals 2023, 16, 504. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, Y.; Wang, P.; Huang, Y.; Wang, F. Short-Chain Fatty Acids Manifest Stimulative and Protective Effects on Intestinal Barrier Function Through the Inhibition of NLRP3 Inflammasome and Autophagy. Cell. Physiol. Biochem. 2018, 49, 190–205. [Google Scholar] [CrossRef] [PubMed]
- Elamin, E.E.; Masclee, A.A.; Dekker, J.; Pieters, H.J.; Jonkers, D.M. Short-chain fatty acids activate AMP-activated protein kinase and ameliorate ethanol-induced intestinal barrier dysfunction in Caco-2 cell monolayers. J. Nutr. 2013, 143, 1872–1881. [Google Scholar] [CrossRef]
- Vu, V.; Kim, Y.M.; Cho, M. Effects of SCFAs and TMAO on non-alcoholic fatty liver disease indicating the therapeutic benefits of plant-based diet, and supplemental prebiotics, probiotics and synbiotics. Appl. Biol. Chem. 2023, 66, 1–15. [Google Scholar] [CrossRef]
- Liu, P.; Wang, Y.; Yang, G.; Zhang, Q.; Meng, L.; Xin, Y.; Jiang, X. The role of short-chain fatty acids in intestinal barrier function, inflammation, oxidative stress, and colonic carcinogenesis. Pharmacol. Res. 2021, 165, 105420. [Google Scholar] [CrossRef]
- Cai, Y.Y.; Huang, F.Q.; Lao, X.; Lu, Y.; Gao, X.; Alolga, R.N.; Yin, K.; Zhou, X.; Wang, Y.; Liu, B.; et al. Integrated metagenomics identifies a crucial role for trimethylamine-producing Lachnoclostridium in promoting atherosclerosis. NPJ Biofilms Microbiomes 2022, 8, 11. [Google Scholar] [CrossRef] [PubMed]
- Romano, K.A.; Vivas, E.I.; Amador-Noguez, D.; Rey, F.E. Intestinal microbiota composition modulates choline bioavailability from diet and accumulation of the proatherogenic metabolite trimethylamine-N-oxide. mBio 2015, 6, e02481-14. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Gao, R.; Zhang, Y.; Pan, D.; Zhu, Y.; Zhang, X.; Yang, R.; Jiang, R.; Xu, Y.; Qin, H. Dysbiosis signatures of gut microbiota in coronary artery disease. Physiol. Genom. 2018, 50, 893–903. [Google Scholar] [CrossRef]
- Farmer, N.; Gutierrez-Huerta, C.A.; Turner, B.S.; Mitchell, V.M.; Collins, B.S.; Baumer, Y.; Wallen, G.R.; Powell-Wiley, T.M. Neighborhood environment associates with trimethylamine-n-oxide (Tmao) as a cardiovascular risk marker. Int. J. Environ. Res. Public Health 2021, 18, 4296. [Google Scholar] [CrossRef]
- Subramaniam, S.; Fletcher, C. Trimethylamine N-oxide: Breathe new life. Br. J. Pharmacol. 2018, 175, 1344–1353. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; He, J.-Q. Pathogenic Mechanisms of Trimethylamine N-Oxide-induced Atherosclerosis and Cardiomyopathy. Curr. Vasc. Pharmacol. 2021, 20, 29–36. [Google Scholar] [CrossRef]
- Mutengo, K.H.; Masenga, S.K.; Mweemba, A.; Mutale, W.; Kirabo, A. Gut microbiota dependant trimethylamine N-oxide and hypertension. Front. Physiol. 2023, 14, 1075641. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Y.; Ke, B.; Du, J. TMAO: How gut microbiota contributes to heart failure. Transl. Res. 2021, 228, 109–125. [Google Scholar] [CrossRef]
- Chaar, D.; Dumont, B.; Vulesevic, B.; Neagoe, P.; Rakel, A.; Sirois, M.G.; White, M. Neutrophils pro-inflammatory and anti-inflammatory cytokine release in patients with heart failure and reduced ejection fraction. ESC Heart Fail. 2021, 8, 3855–3864. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Yamashita, T.; Watanabe, H.; Kami, K.; Yoshida, N.; Tabata, T.; Emoto, T.; Sasaki, N.; Mizoguchi, T.; Irino, Y.; et al. Gut microbiome and plasma microbiome-related metabolites in patients with decompensated and compensated heart failure. Circ. J. 2019, 83, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Swer, N.M.; Venkidesh, B.S.; Murali, T.S.; Mumbrekar, K.D. Gut microbiota-derived metabolites and their importance in neurological disorders. Mol. Biol. Rep. 2023, 50, 1663–1675. [Google Scholar] [CrossRef] [PubMed]
- Oliphant, K.; Allen-Vercoe, E. Macronutrient metabolism by the human gut microbiome: Major fermentation by-products and their impact on host health. Microbiome 2019, 7, 91. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Xu, K.; Liu, H.; Liu, G.; Bai, M.; Peng, C.; Li, T.; Yin, Y. Impact of the gut microbiota on intestinal immunity mediated by tryptophan metabolism. Front. Cell. Infect. Microbiol. 2018, 8, 13. [Google Scholar] [CrossRef]
- Kaur, H.; Bose, C.; Mande, S.S. Tryptophan Metabolism by Gut Microbiome and Gut-Brain-Axis: An in silico Analysis. Front. Neurosci. 2019, 13, 1365. [Google Scholar] [CrossRef]
- Roager, H.M.; Licht, T.R. Microbial tryptophan catabolites in health and disease. Nat. Commun. 2018, 9, 3294. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.M.; Cao, S. Harnessing and delivering microbial metabolites as therapeutics via advanced pharmaceutical approaches. Pharmacol. Ther. 2024, 256, 108605. [Google Scholar] [CrossRef]
- Gasaly, N.; de Vos, P.; Hermoso, M.A. Impact of Bacterial Metabolites on Gut Barrier Function and Host Immunity: A Focus on Bacterial Metabolism and Its Relevance for Intestinal Inflammation. Front. Immunol. 2021, 12, 658354. [Google Scholar] [CrossRef]
- Galligan, J.J. Beneficial actions of microbiota-derived tryptophan metabolites. Neurogastroenterol. Motil. 2018, 30, 13283. [Google Scholar] [CrossRef]
- Cienkowski, K.; Cienkowska, A.; Kupczynska, K.; Bielecka-Dabrowa, A. The Role of Gut Microbiota and Its Metabolites in Patients with Heart Failure. Biomedicines 2024, 12, 894. [Google Scholar] [CrossRef] [PubMed]
- Bartolomaeus, H.; Balogh, A.; Yakoub, M.; Homann, S.; Markó, L.; Höges, S.; Tsvetkov, D.; Krannich, A.; Wundersitz, S.; Avery, E.G.; et al. Short-Chain Fatty Acid Propionate Protects from Hypertensive Cardiovascular Damage. Circulation 2019, 139, 1407–1421. [Google Scholar] [CrossRef]
- Shah, S.; Fillier, T.; Pham, T.H.; Thomas, R.; Cheema, S.K. Intraperitoneal administration of short-chain fatty acids improves lipid metabolism of long–evans rats in a sex-specific manner. Nutrients 2021, 13, 892. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-C.; Koay, Y.C.; Pan, C.; Zhou, Z.; Tang, W.; Wilcox, J.; Li, X.S.; Zagouras, A.; Marques, F.; Allayee, H.; et al. Indole-3-Propionic Acid Protects Against Heart Failure with Preserved Ejection Fraction. Circ. Res. 2024, 134, 371–389. [Google Scholar] [CrossRef]
- Imazu, M.; Fukuda, H.; Kanzaki, H.; Amaki, M.; Hasegawa, T.; Takahama, H.; Hitsumoto, T.; Tsukamoto, O.; Morita, T.; Ito, S.; et al. Plasma indoxyl sulfate levels predict cardiovascular events in patients with mild chronic heart failure. Sci. Rep. 2020, 10, 16528. [Google Scholar] [CrossRef]
- Cao, X.-S.; Chen, J.; Zou, J.-Z.; Zhong, Y.-H.; Teng, J.; Ji, J.; Chen, Z.-W.; Liu, Z.-H.; Shen, B.; Nie, Y.-X.; et al. Association of indoxyl sulfate with heart failure among patients on hemodialysis. Clin. J. Am. Soc. Nephrol. 2015, 10, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Lekawanvijit, S.; Adrahtas, A.; Kelly, D.J.; Kompa, A.R.; Wang, B.H.; Krum, H. Does indoxyl sulfate, a uraemic toxin, have direct effects on cardiac fibroblasts and myocytes? Eur. Heart J. 2010, 31, 1771–1779. [Google Scholar] [CrossRef]
- Romano, K.A.; Nemet, I.; Saha, P.P.; Haghikia, A.; Li, X.S.; Mohan, M.L.; Lovano, B.; Castel, L.; Witkowski, M.; Buffa, J.A.; et al. Gut Microbiota-Generated Phenylacetylglutamine and Heart Failure. Circ. Heart Fail. 2023, 16, e009972. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.W.; Nemet, I.; Li, X.S.; Wu, Y.; Haghikia, A.; Witkowski, M.; Koeth, R.A.; Demuth, I.; König, M.; Steinhagen-Thiessen, E.; et al. Prognostic value of gut microbe-generated metabolite phenylacetylglutamine in patients with heart failure. Eur. J. Heart Fail. 2024, 26, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Soto-Martin, E.C.; Warnke, I.; Farquharson, F.M.; Christodoulou, M.; Horgan, G.; Derrien, M.; Faurie, J.-M.; Flint, H.J.; Duncan, S.H.; Louis, P. Vitamin biosynthesis by human gut butyrate-producing bacteria and cross-feeding in synthetic microbial communities. mBio 2020, 11, e00886-20. [Google Scholar] [CrossRef]
- Das, P.; Babaei, P.; Nielsen, J. Metagenomic analysis of microbe-mediated vitamin metabolism in the human gut microbiome. BMC Genom. 2019, 20, 208. [Google Scholar] [CrossRef]
- LeBlanc, J.G.; Milani, C.; de Giori, G.S.; Sesma, F.; van Sinderen, D.; Ventura, M. Bacteria as vitamin suppliers to their host: A gut microbiota perspective. Curr. Opin. Biotechnol. 2013, 24, 160–168. [Google Scholar] [CrossRef]
- Eshak, E.S.; Arafa, A.E. Thiamine deficiency and cardiovascular disorders. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Keith, M.; Quach, S.; Ahmed, M.; Azizi-Namini, P.; Al-Hesayen, A.; Azevedo, E.; James, R.; Leong-Poi, H.; Ong, G.; Desjardins, S.; et al. Thiamin supplementation does not improve left ventricular ejection fraction in ambulatory heart failure patients: A randomized controlled trial. Am. J. Clin. Nutr. 2019, 110, 1287–1295. [Google Scholar] [CrossRef] [PubMed]
- Balmain, B.N.; Jay, O.; Morris, N.R.; Stewart, G.M.; Shiino, K.; McFarland, A.J.; Jayasinghe, R.; Chan, J.; Sabapathy, S. Folic acid supplementation improves vascular endothelial function, yet not skin blood flow during exercise in the heat, in patients with heart failure. Am. J. Physiol. Integr. Comp. Physiol. 2018, 315, R810–R819. [Google Scholar] [CrossRef] [PubMed]
- Piquereau, J.; Boitard, S.E.; Ventura-Clapier, R.; Mericskay, M. Metabolic therapy of heart failure: Is there a future for B vitamins? Int. J. Mol. Sci. 2021, 23, 30. [Google Scholar] [CrossRef]
- Wan, Z.; Zheng, J.; Zhu, Z.; Sang, L.; Zhu, J.; Luo, S.; Zhao, Y.; Wang, R.; Zhang, Y.; Hao, K.; et al. Intermediate role of gut microbiota in vitamin B nutrition and its influences on human health. Front. Nutr. 2022, 9, 1031502. [Google Scholar] [CrossRef]
- Xu, Q.; Cao, Y.; Zhong, X.; Qin, X.; Feng, J.; Peng, H.; Su, Y.; Ma, Z.; Zhou, S. Riboflavin protects against heart failure via SCAD-dependent DJ-1–Keap1–Nrf2 signalling pathway. Br. J. Pharmacol. 2023, 180, 3024–3044. [Google Scholar] [CrossRef]
- Miao, Y.; Guo, Y.; Chen, Y.; Lin, Y.; Lu, Y.; Guo, Q. The effect of B-vitamins on the prevention and treatment of cardiovascular diseases: A systematic review and meta-analysis. Nutr. Rev. 2023, 82, 1386–1401. [Google Scholar] [CrossRef]
- Oniszczuk, A.; Oniszczuk, T.; Gancarz, M.; Szymańska, J. Role of gut microbiota, probiotics and prebiotics in the cardiovascular diseases. Molecules 2021, 26, 1172. [Google Scholar] [CrossRef]
- Lu, X.; Liu, J.; Zhou, B.; Wang, S.; Liu, Z.; Mei, F.; Luo, J.; Cui, Y. Microbial metabolites and heart failure: Friends or enemies? Front. Microbiol. 2022, 13, 956516. [Google Scholar] [CrossRef]
- Yntema, T.; Koonen, D.P.Y.; Kuipers, F. Emerging Roles of Gut Microbial Modulation of Bile Acid Composition in the Etiology of Cardiovascular Diseases. Nutrients 2023, 15, 1850. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Ma, W.-Q.; Fu, M.-J.; Li, J.; Hu, C.-H.; Chen, Y.; Zhou, M.-M.; Gao, Z.-J.; He, Y.-L. Overview of bile acid signaling in the cardiovascular system. World J. Clin. Cases 2021, 9, 308–320. [Google Scholar] [CrossRef] [PubMed]
- Di Ciaula, A.; Garruti, G.; Baccetto, R.L.; Molina-Molina, E.; Bonfrate, L.; Wang, D.Q.-H.; Portincasa, P. Bile acid physiology. Ann. Hepatol. 2017, 16, S4–S14. [Google Scholar] [CrossRef]
- Ishimwe, J.A.; Dola, T.; Ertuglu, L.A.; Kirabo, A. Bile acids and salt-sensitive hypertension: A role of the gut-liver axis. Am. J. Physiol. Circ. Physiol. 2022, 322, H636–H646. [Google Scholar] [CrossRef] [PubMed]
- Mayerhofer, C.C.; Ueland, T.; Broch, K.; Vincent, R.P.; Cross, G.F.; Dahl, C.P.; Aukrust, P.; Gullestad, L.; Hov, J.R.; Trøseid, M. Increased Secondary/Primary Bile Acid Ratio in Chronic Heart Failure. J. Card. Fail. 2017, 23, 666–671. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhou, J.; Wu, W.; Zhu, Y.; Liu, X. The Role of Bile Acids in Cardiovascular Diseases: From Mechanisms to Clinical Implications. Aging Dis. 2023, 14, 261–282. [Google Scholar] [CrossRef]
- Vasavan, T.; Ferraro, E.; Ibrahim, E.; Dixon, P.; Gorelik, J.; Williamson, C. Heart and bile acids—Clinical consequences of altered bile acid metabolism. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2018, 1864, 1345–1355. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Wei, J.; Yuan, H.; Li, Y.; Guo, Z. The role of the gut microbiota and bile acids in heart failure: A review. Medicine 2023, 102, e35795. [Google Scholar] [CrossRef] [PubMed]
- Emoto, T.; Yamashita, T.; Sasaki, N.; Hirota, Y.; Hayashi, T.; So, A.; Kasahara, K.; Yodoi, K.; Matsumoto, T.; Mizoguchi, T.; et al. Analysis of gut microbiota in coronary artery disease patients: A possible link between gut microbiota and coronary artery disease. J. Atheroscler. Thromb. 2016, 23, 908–921. [Google Scholar] [CrossRef]
- Tang, W.H.W.; Hazen, S.L. The Gut Microbiome and Its Role in Cardiovascular Diseases. Circulation 2017, 135, 1008–1010. [Google Scholar] [CrossRef]
- Matsiras, D.; Bezati, S.; Ventoulis, I.; Verras, C.; Parissis, J.; Polyzogopoulou, E. Gut Failure: A Review of the Pathophysiology and Therapeutic Potentials in the Gut–Heart Axis. J. Clin. Med. 2023, 12, 2567. [Google Scholar] [CrossRef] [PubMed]
- Luqman, A.; Hassan, A.; Ullah, M.; Naseem, S.; Ullah, M.; Zhang, L.; Din, A.U.; Ullah, K.; Ahmad, W.; Wang, G. Role of the intestinal microbiome and its therapeutic intervention in cardiovascular disorder. Front. Immunol. 2024, 15, 1321395. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Du, D.; Fu, T.; Han, Y.; Li, P.; Ju, H. Alterations of the Gut Microbiota in Patients with Severe Chronic Heart Failure. Front. Microbiol. 2022, 12, 813289. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Jiang, S.; Qian, D.; Duan, J. Modulation of microbially derived short-chain fatty acids on intestinal homeostasis, metabolism, and neuropsychiatric disorder. Appl. Microbiol. Biotechnol. 2020, 104, 589–601. [Google Scholar] [CrossRef]
- Han, X.; Ma, Y.; Ding, S.; Fang, J.; Liu, G. Regulation of dietary fiber on intestinal microorganisms and its effects on animal health. Anim. Nutr. 2023, 14, 356–369. [Google Scholar] [CrossRef] [PubMed]
- Portincasa, P.; Bonfrate, L.; Vacca, M.; De Angelis, M.; Farella, I.; Lanza, E.; Khalil, M.; Wang, D.Q.-H.; Sperandio, M.; Di Ciaula, A. Gut Microbiota and Short Chain Fatty Acids: Implications in Glucose Homeostasis. Int. J. Mol. Sci. 2022, 23, 1105. [Google Scholar] [CrossRef]
- Peng, J.; Gong, H.; Lyu, X.; Liu, Y.; Li, S.; Tan, S.; Dong, L.; Zhang, X. Characteristics of the fecal microbiome and metabolome in older patients with heart failure and sarcopenia. Front. Cell. Infect. Microbiol. 2023, 13, 1127041. [Google Scholar] [CrossRef] [PubMed]
- Marques, F.Z.; Nelson, E.; Chu, P.-Y.; Horlock, D.; Fiedler, A.; Ziemann, M.; Tan, J.K.; Kuruppu, S.; Rajapakse, N.W.; El-Osta, A.; et al. High-fiber diet and acetate supplementation change the gut microbiota and prevent the development of hypertension and heart failure in hypertensive mice. Circulation 2017, 135, 964–977. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-C.; Liu, Y.-W.; Chang, K.-C.; Wu, Y.-W.; Chen, Y.-M.; Chao, Y.-K.; You, M.-Y.; Lundy, D.J.; Lin, C.-J.; Hsieh, M.L.; et al. Gut butyrate-producers confer post-infarction cardiac protection. Nat. Commun. 2023, 14, 7249. [Google Scholar] [CrossRef]
- Bartolomaeus, H.; Avery, E.G.; Bartolomaeus, T.U.P.; Kozhakhmetov, S.; Zhumadilov, Z.; Müller, D.N.; Wilck, N.; Kushugulova, A.; Forslund, S.K. Blood pressure changes correlate with short-chain fatty acid production potential shifts under a synbiotic intervention. Cardiovasc. Res. 2020, 116, 1252–1253. [Google Scholar] [CrossRef]
- Modin, D.; Sengeløv, M.; Jørgensen, P.G.; Olsen, F.J.; Bruun, N.E.; Fritz-Hansen, T.; Andersen, D.M.; Jensen, J.S.; Biering-Sørensen, T. Prognostic Value of Left Atrial Functional Measures in Heart Failure with Reduced Ejection Fraction. J. Card. Fail. 2019, 25, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Mullens, W.; Mullens, W.; Mullens, W.; Auricchio, A.; Auricchio, A.; Auricchio, A.; Martens, P.; Martens, P.; Martens, P.; Witte, K.; et al. Optimized implementation of cardiac resynchronization therapy: A call for action for referral and optimization of care. Europace 2021, 23, 1324–1342. [Google Scholar] [CrossRef] [PubMed]
- Borlaug, B.A. Evaluation and management of heart failure with preserved ejection fraction. Nat. Rev. Cardiol. 2020, 17, 559–573. [Google Scholar] [CrossRef]
- Alhakak, A.S.; Teerlink, J.R.; Lindenfeld, J.; Böhm, M.; Rosano, G.M.; Biering-Sørensen, T. The significance of left ventricular ejection time in heart failure with reduced ejection fraction. Eur. J. Heart Fail. 2021, 23, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Cai, B.; Sun, Y.; Deng, H.; Wang, H.; Qiao, Z. Alteration of the gut microbiota and metabolite phenylacetylglutamine in patients with severe chronic heart failure. Front. Cardiovasc. Med. 2023, 9, 1076806. [Google Scholar] [CrossRef]
- Modrego, J.; Ortega-Hernández, A.; Goirigolzarri, J.; Restrepo-Córdoba, M.A.; Bäuerl, C.; Cortés-Macías, E.; Sánchez-González, S.; Esteban-Fernández, A.; Pérez-Villacastín, J.; Collado, M.C.; et al. Gut Microbiota and Derived Short-Chain Fatty Acids Are Linked to Evolution of Heart Failure Patients. Int. J. Mol. Sci. 2023, 24, 13892. [Google Scholar] [CrossRef] [PubMed]
- Kirschner, S.K.; Deutz, N.E.P.; Rijnaarts, I.; Smit, T.J.; Larsen, D.J.; Engelen, M.P.K.J. Impaired intestinal function is associated with lower muscle and cognitive health and well-being in patients with congestive heart failure. J. Parenter. Enter. Nutr. 2022, 46, 660–670. [Google Scholar] [CrossRef]
- Zhao, P.; Zhao, S.; Tian, J.; Liu, X. Significance of Gut Microbiota and Short-Chain Fatty Acids in Heart Failure. Nutrients 2022, 14, 3758. [Google Scholar] [CrossRef]
- Chen, M.; Peng, L.; Zhang, C.; Liu, Q.; Long, T.; Xie, Q. Gut microbiota might mediate the benefits of high-fiber/acetate diet to cardiac hypertrophy mice. J. Physiol. Biochem. 2023, 79, 745–756. [Google Scholar] [CrossRef] [PubMed]
- Panagia, M.; He, H.; Baka, T.; Pimentel, D.R.; Croteau, D.; Bachschmid, M.M.; Balschi, J.A.; Colucci, W.S.; Luptak, I. Increasing mitochondrial ATP synthesis with butyrate normalizes ADP and contractile function in metabolic heart disease. NMR Biomed. 2020, 33, e4258. [Google Scholar] [CrossRef] [PubMed]
- Challa, A.A.; Lewandowski, E.D. Short-Chain Carbon Sources: Exploiting Pleiotropic Effects for Heart Failure Therapy. JACC Basic Transl. Sci. 2022, 7, 730–742. [Google Scholar] [CrossRef] [PubMed]
- Werhahn, S.M.; Becker, C.; Mende, M.; Haarmann, H.; Nolte, K.; Laufs, U.; Zeynalova, S.; Löffler, M.; Dagres, N.; Husser, D.; et al. NT-proBNP as a marker for atrial fibrillation and heart failure in four observational outpatient trials. ESC Heart Fail. 2022, 9, 100–109. [Google Scholar] [CrossRef]
- Nendl, A.; Raju, S.C.; Broch, K.; Mayerhofer, C.C.K.; Holm, K.; Halvorsen, B.; Lappegård, K.T.; Moscavitch, S.; Hov, J.R.; Seljeflot, I.; et al. Intestinal fatty acid binding protein is associated with cardiac function and gut dysbiosis in chronic heart failure. Front. Cardiovasc. Med. 2023, 10, 1160030. [Google Scholar] [CrossRef]
- Han, Y.; Gong, Z.; Sun, G.; Xu, J.; Qi, C.; Sun, W.; Jiang, H.; Cao, P.; Ju, H. Dysbiosis of Gut Microbiota in Patients with Acute Myocardial Infarction. Front. Microbiol. 2021, 12, 680101. [Google Scholar] [CrossRef]
- Yang, C.; Li, X.; Hu, M.; Li, T.; Jiang, L.; Zhang, Y. Gut Microbiota as Predictive Biomarker for Chronic Heart Failure in Patients with Different Nutritional Risk. J. Cardiovasc. Transl. Res. 2024, 17, 1240–1257. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; An, Y.; Wang, H.; Zhang, N.; Deng, S. The clinical significance of changes in cTnT, CRP and NT-proBNP levels in patients with heart failure. Am. J. Transl. Res. 2021, 13, 2947. [Google Scholar] [PubMed]
- Matin, S.S.; Shidfar, F.; Naderi, N.; Amin, A.; Hosseini-Baharanchi, F.S.; Dehnad, A. The Effect of Synbiotic Consumption on Serum NTproBNP, hsCRP and Blood Pressure in Patients with Chronic Heart Failure: A Randomized, Triple-Blind, Controlled Trial. Front. Nutr. 2022, 8, 822498. [Google Scholar] [CrossRef]
- Vindhyal, M.R.; Khayyat, S.; Shaaban, A.; A Duran, B.; Kallail, K.J. Decreased Renal Function is Associated with Heart Failure Readmissions. Cureus 2018, 10, e3122. [Google Scholar] [CrossRef] [PubMed]
- House, A.A.; Wanner, C.; Sarnak, M.J.; Piña, I.L.; McIntyre, C.W.; Komenda, P.; Kasiske, B.L.; Deswal, A.; Defilippi, C.R.; Cleland, J.G.F.; et al. Heart failure in chronic kidney disease: Conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2019, 95, 1304–1317. [Google Scholar] [CrossRef] [PubMed]
- Gregg, L.P.; Hedayati, S.S. Management of Traditional Cardiovascular Risk Factors in CKD: What Are the Data? Am. J. Kidney Dis. 2018, 72, 728–744. [Google Scholar] [CrossRef]
- Altamura, S.; Pietropaoli, D.; Lombardi, F.; Del Pinto, R.; Ferri, C. An Overview of Chronic Kidney Disease Pathophysiology: The Impact of Gut Dysbiosis and Oral Disease. Biomedicines 2023, 11, 3033. [Google Scholar] [CrossRef] [PubMed]
- A Bryniarski, M.; Hamarneh, F.; Yacoub, R. The role of chronic kidney disease-associated dysbiosis in cardiovascular disease. Exp. Biol. Med. 2019, 244, 514–525. [Google Scholar] [CrossRef]
- Onal, E.M.; Afsar, B.; Covic, A.; Vaziri, N.D.; Kanbay, M. Gut microbiota and inflammation in chronic kidney disease and their roles in the development of cardiovascular disease. Hypertens. Res. 2019, 42, 123–140. [Google Scholar] [CrossRef]
- Zhong, C.; Dai, Z.; Chai, L.; Wu, L.; Li, J.; Guo, W.; Zhang, J.; Zhang, Q.; Xue, C.; Lin, H.; et al. The change of gut microbiota-derived short-chain fatty acids in diabetic kidney disease. J. Clin. Lab. Anal. 2021, 35, e24062. [Google Scholar] [CrossRef]
- Wang, S.; Lv, D.; Jiang, S.; Jiang, J.; Liang, M.; Hou, F.; Chen, Y. Quantitative reduction in short-chain fatty acids, especially butyrate, contributes to the progression of chronic kidney disease. Clin. Sci. 2019, 133, 1857–1870. [Google Scholar] [CrossRef]
- Lee, D.-H.; Kim, M.-T.; Han, J.-H. GPR41 and GPR43: From development to metabolic regulation. Biomed. Pharmacother. 2024, 175, 116735. [Google Scholar] [CrossRef]
- Xu, J.; Moore, B.N.; Pluznick, J.L. Short-Chain Fatty Acid Receptors and Blood Pressure Regulation: Council on Hypertension Mid-Career Award for Research Excellence 2021. Hypertension 2022, 79, 2127–2137. [Google Scholar] [CrossRef]
- Li, Y.J.; Chen, X.; Kwan, T.K.; Loh, Y.W.; Singer, J.; Liu, Y.; Ma, J.; Tan, J.; Macia, L.; Mackay, C.R.; et al. Dietary fiber protects against diabetic nephropathy through short-chain fatty acid–mediated activation of G protein–coupled receptors GPR43 and GPR109A. J. Am. Soc. Nephrol. 2020, 31, 1267–1281. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, X.; Kwan, T.; Loh, Y.; Singer, J.; Tan, J.; Macia, L.; Chadban, S.; Wu, H. SAT-160 dietary fibre and bacterial scfa modulate renal inflammation in diabetic nephropathy through activation of g-protein coupled receptors gpr43 and gpr109a. Kidney Int. Rep. 2020, 5, S68–S69. [Google Scholar] [CrossRef][Green Version]
- Cai, K.; Ma, Y.; Cai, F.; Huang, X.; Xiao, L.; Zhong, C.; Ren, P.; Luo, Q.; Chen, J.; Han, F. Changes of gut microbiota in diabetic nephropathy and its effect on the progression of kidney injury. Endocrine 2022, 76, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Magliocca, G.; Mone, P.; Di Iorio, B.R.; Heidland, A.; Marzocco, S. Short-Chain Fatty Acids in Chronic Kidney Disease: Focus on Inflammation and Oxidative Stress Regulation. Int. J. Mol. Sci. 2022, 23, 5354. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Wang, L.; Bhat, O.M.; Lohner, H.; Li, P.-L. Differential effects of short chain fatty acids on endothelial Nlrp3 inflammasome activation and neointima formation: Antioxidant action of butyrate. Redox Biol. 2018, 16, 21–31. [Google Scholar] [CrossRef]
- Hobby, G.P.; Karaduta, O.; Dusio, G.F.; Singh, M.; Zybailov, B.L.; Arthur, J.M. Chronic kidney disease and the gut microbiome. Am. J. Physiol. Physiol. 2019, 316, F1211–F1217. [Google Scholar] [CrossRef]
- Gryp, T.; Huys, G.R.; Joossens, M.; Van Biesen, W.; Glorieux, G.; Vaneechoutte, M. Isolation and Quantification of Uremic Toxin Precursor-Generating Gut Bacteria in Chronic Kidney Disease Patients. Int. J. Mol. Sci. 2020, 21, 1986. [Google Scholar] [CrossRef] [PubMed]
- Mair, R.D.; Sirich, T.L.; Plummer, N.S.; Meyer, T.W. Characteristics of Colon-Derived Uremic Solutes. Clin. J. Am. Soc. Nephrol. 2018, 13, 1398–1404. [Google Scholar] [CrossRef]
- Cheng, T.-H.; Ma, M.-C.; Liao, M.-T.; Zheng, C.-M.; Lu, K.-C.; Liao, C.-H.; Hou, Y.-C.; Liu, W.-C.; Lu, C.-L. Indoxyl Sulfate, a Tubular Toxin, Contributes to the Development of Chronic Kidney Disease. Toxins 2020, 12, 684. [Google Scholar] [CrossRef] [PubMed]
- Steenbeke, M.; Valkenburg, S.; Gryp, T.; Van Biesen, W.; Delanghe, J.R.; Speeckaert, M.M.; Glorieux, G. Gut Microbiota and Their Derived Metabolites, a Search for Potential Targets to Limit Accumulation of Protein-Bound Uremic Toxins in Chronic Kidney Disease. Toxins 2021, 13, 809. [Google Scholar] [CrossRef] [PubMed]
- Jadoon, A.; Mathew, A.V.; Byun, J.; Gadegbeku, C.A.; Gipson, D.S.; Afshinnia, F.; Pennathur, S. Gut microbial product predicts cardiovascular risk in chronic kidney disease patients. Am. J. Nephrol. 2018, 48, 269–277. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chulenbayeva, L.; Issilbayeva, A.; Sailybayeva, A.; Bekbossynova, M.; Kozhakhmetov, S.; Kushugulova, A. Short-Chain Fatty Acids and Their Metabolic Interactions in Heart Failure. Biomedicines 2025, 13, 343. https://doi.org/10.3390/biomedicines13020343
Chulenbayeva L, Issilbayeva A, Sailybayeva A, Bekbossynova M, Kozhakhmetov S, Kushugulova A. Short-Chain Fatty Acids and Their Metabolic Interactions in Heart Failure. Biomedicines. 2025; 13(2):343. https://doi.org/10.3390/biomedicines13020343
Chicago/Turabian StyleChulenbayeva, Laura, Argul Issilbayeva, Aliya Sailybayeva, Makhabbat Bekbossynova, Samat Kozhakhmetov, and Almagul Kushugulova. 2025. "Short-Chain Fatty Acids and Their Metabolic Interactions in Heart Failure" Biomedicines 13, no. 2: 343. https://doi.org/10.3390/biomedicines13020343
APA StyleChulenbayeva, L., Issilbayeva, A., Sailybayeva, A., Bekbossynova, M., Kozhakhmetov, S., & Kushugulova, A. (2025). Short-Chain Fatty Acids and Their Metabolic Interactions in Heart Failure. Biomedicines, 13(2), 343. https://doi.org/10.3390/biomedicines13020343





