Moringa oleifera Leaf Extract Improves Cognitive Function in Rat Offspring Born to Protein-Deficient Mothers
Abstract
1. Introduction
2. Material and Methods
2.1. Laboratory Animals
2.2. Experimental Design
2.3. Amino Acids Composition in EEMO, Normal Diet, and Low Protein Diet
2.4. Anthropometric and Nutritional Determination
2.5. Cognitive Measurement
2.6. Hb and Albumin Measurement
2.7. Blood Urea Nitrogen (BUN), Ghrelin, and Leptin Measurement
2.8. Histological Analysis
2.9. Statistical Analysis
3. Results
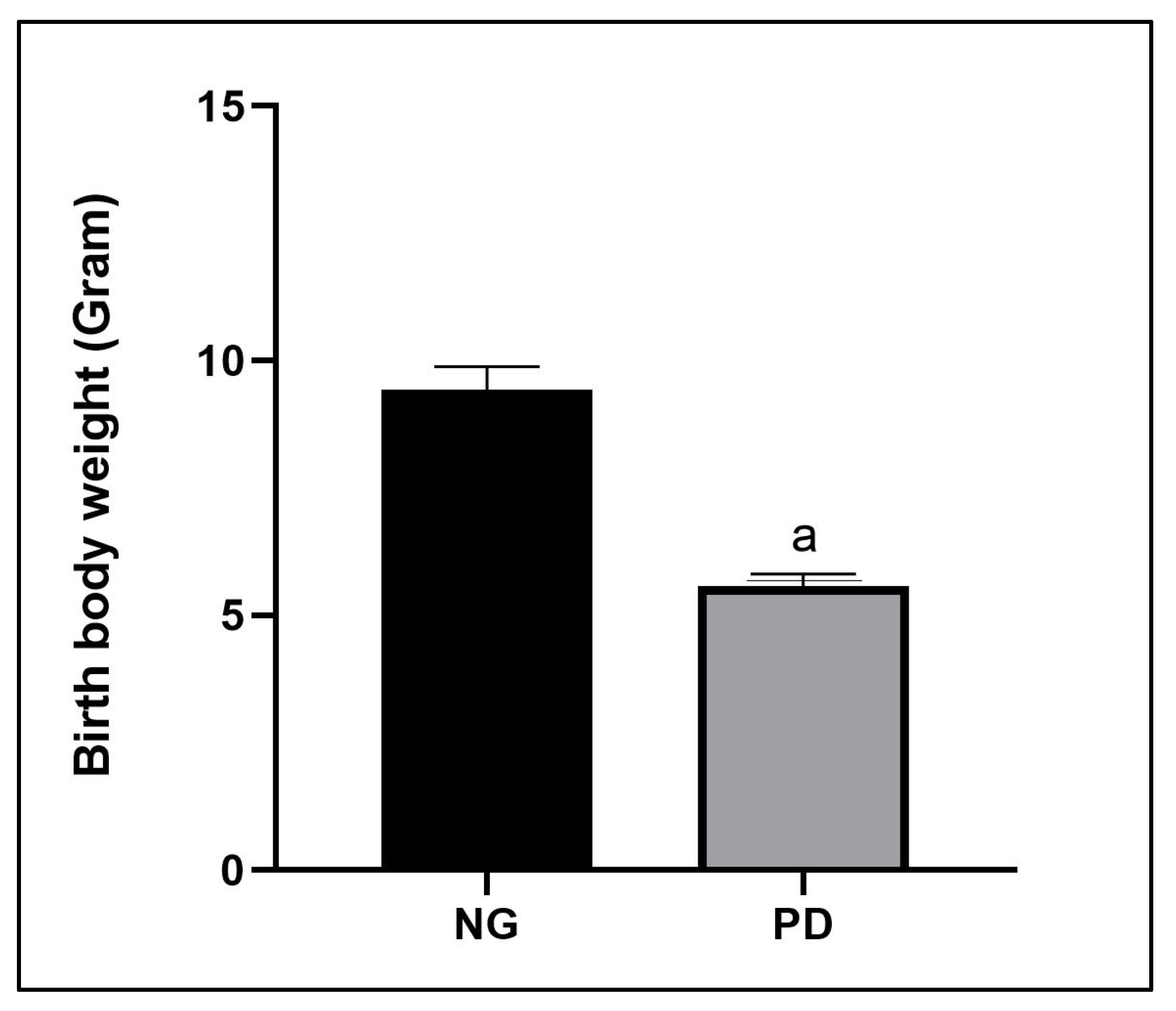
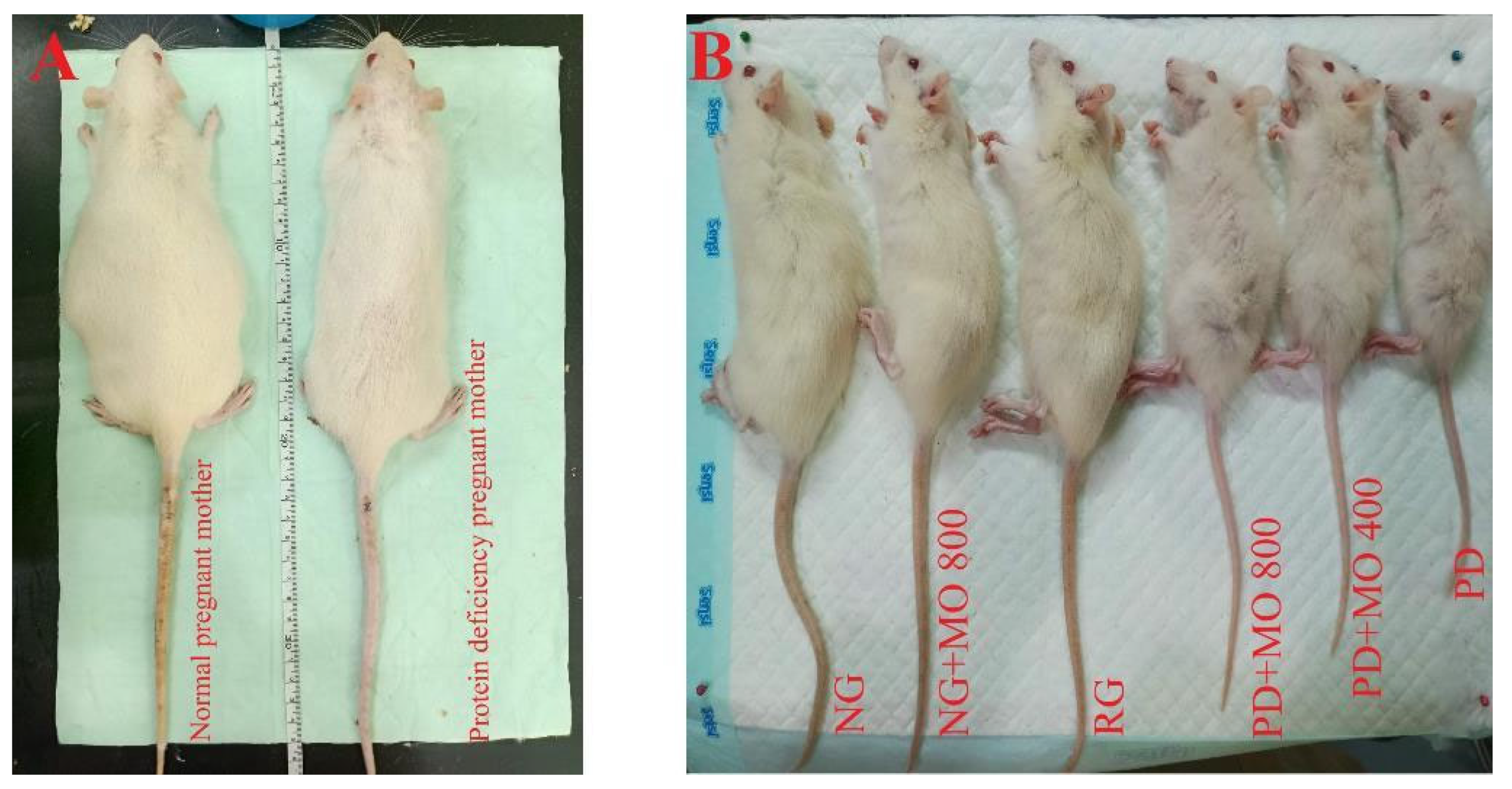
3.1. Characteristics of Offspring Born Form Protein Deficient Mothers
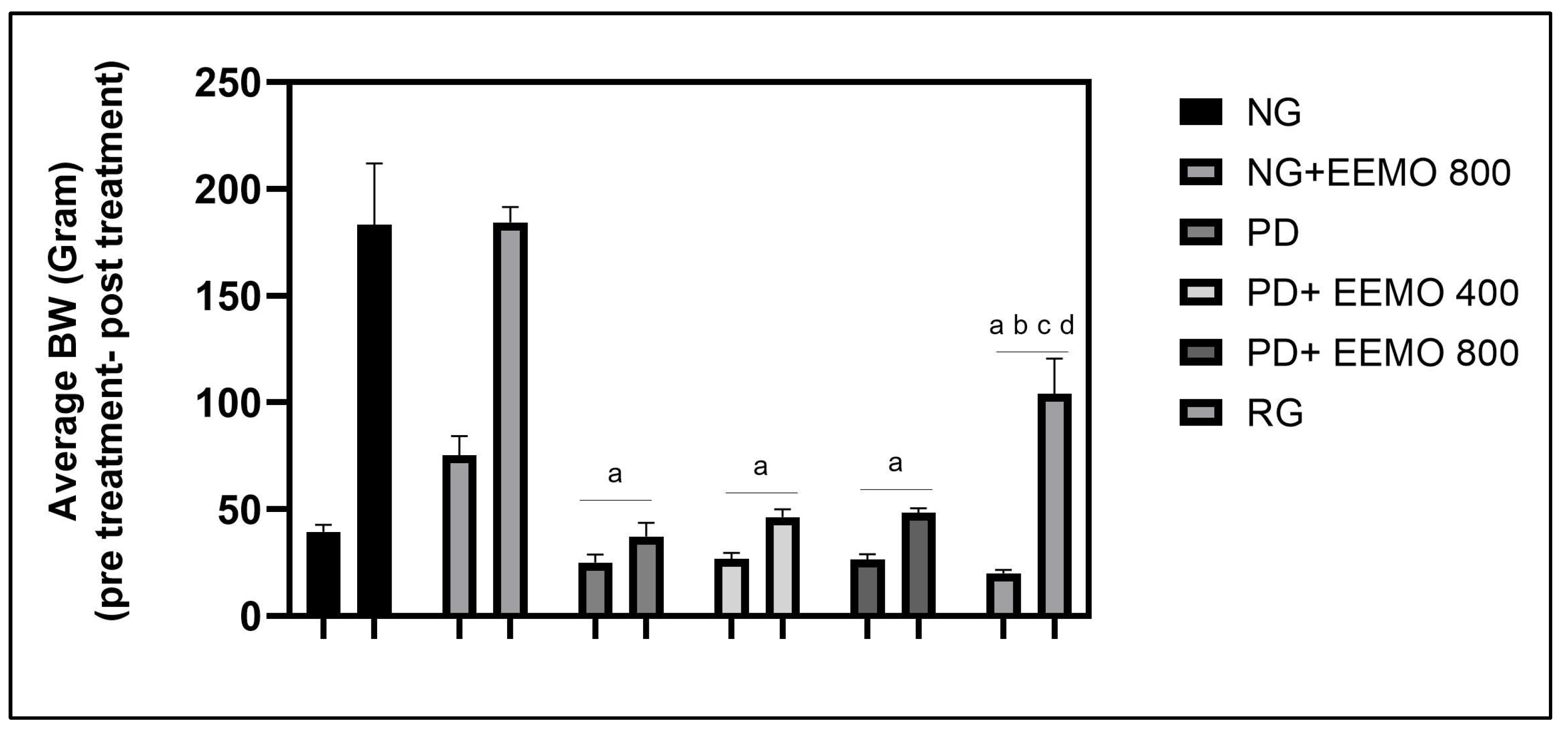
3.2. Body Mass Index, Food Consumption, Energy Intake and Feed Efficiency
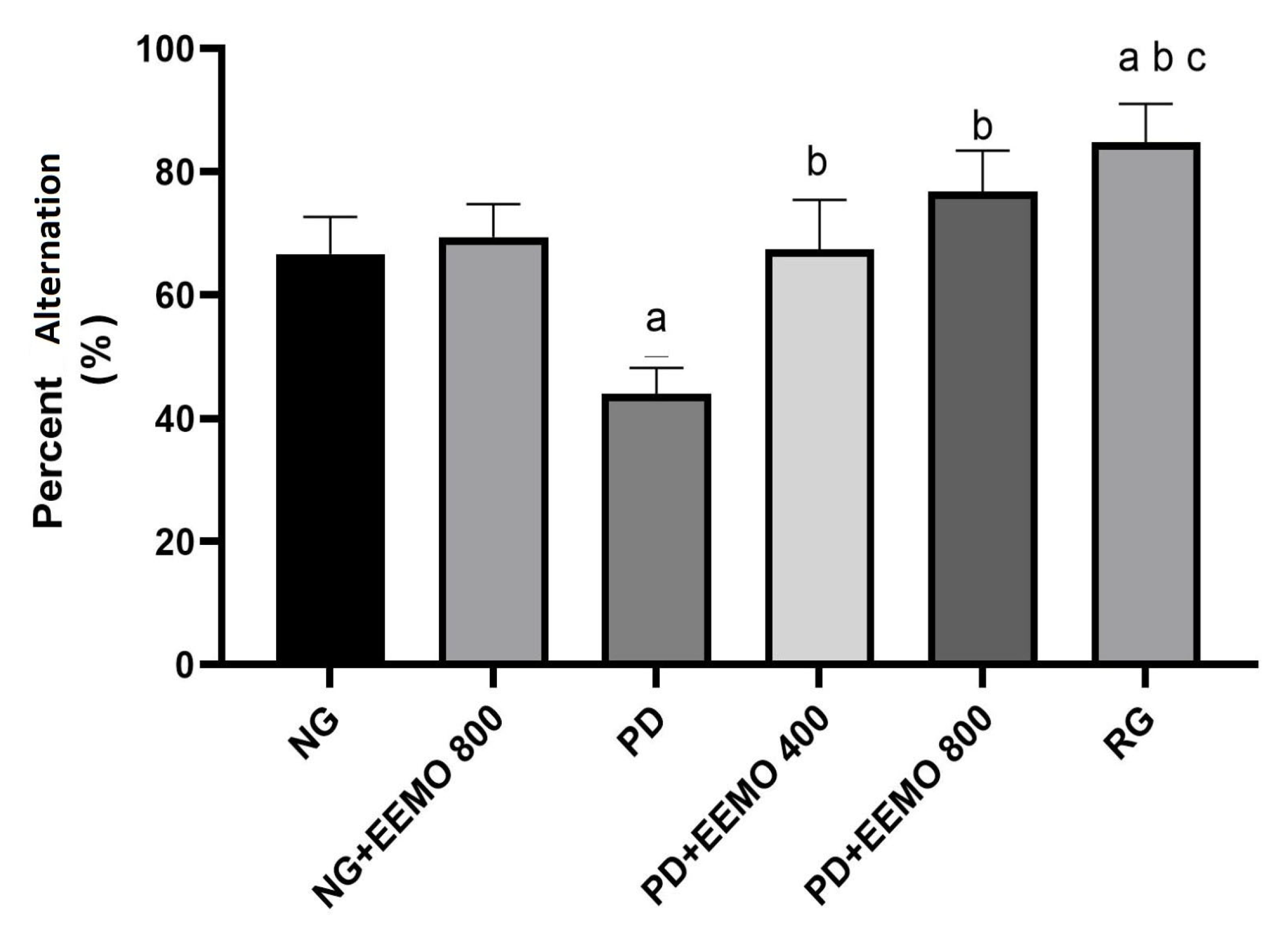
3.3. Cognitive Function/Memory Assessment (Y-Maze Test)
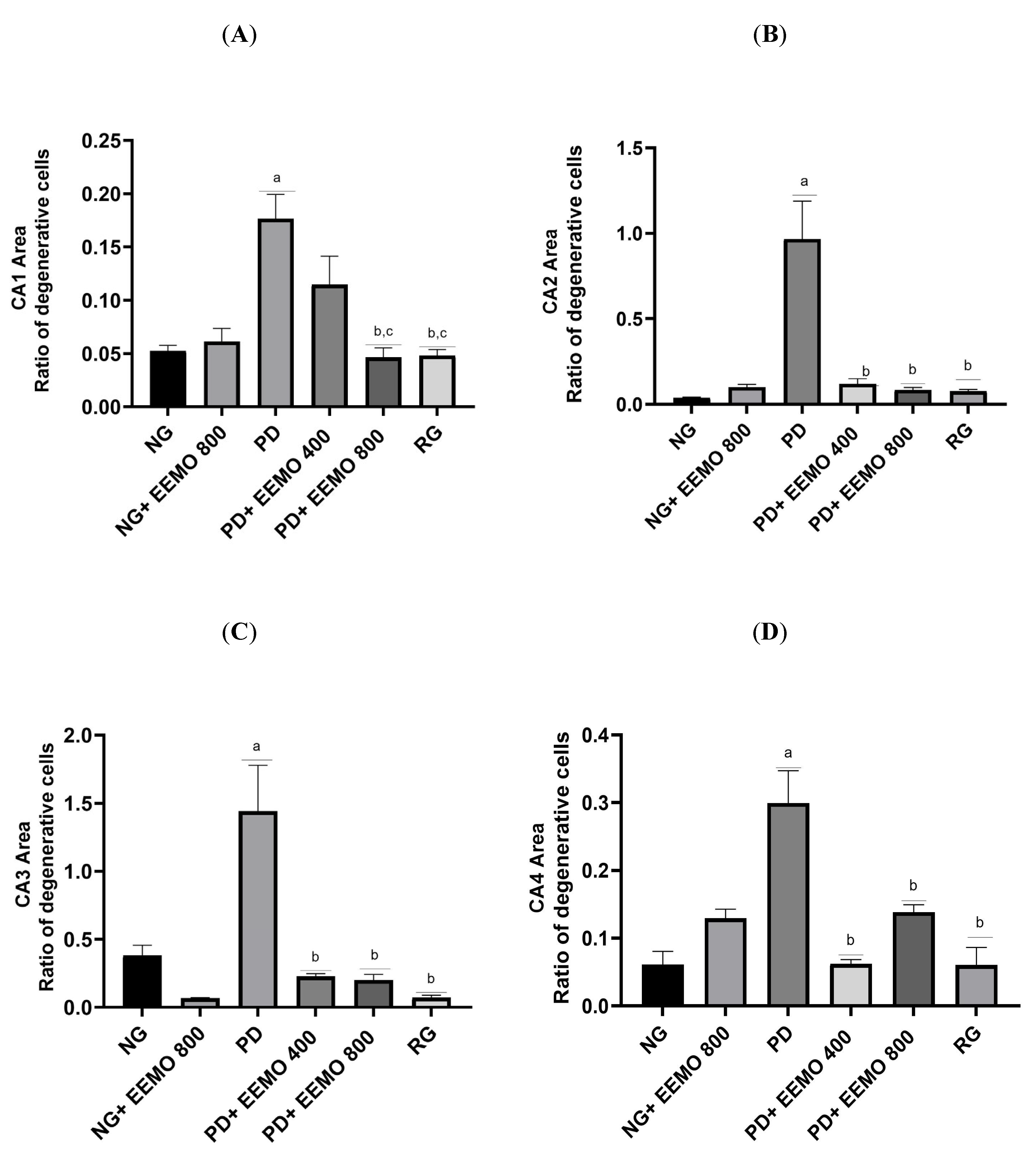
3.4. HE Staining of Hippocampal Tissue
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Global Hunger Index. 2019. Available online: https://www.globalhungerindex.org/pdf/en/2022.pdf (accessed on 10 February 2023).
- Kemenkes Hasil Survei Status Gizi Indonesia (SSGI) 2022. Kemenkes 2023, 1–7. Available online: https://layanandata.kemkes.go.id/katalog-data/ssgi/ketersediaan-data/ssgi-2022 (accessed on 11 June 2023).
- Batista, T.H.; Ribeiro, A.C.A.F.; Kalil, B.; Giusti-Paiva, A.; Vilela, F.C. Maternal Protein Malnutrition Prolongs Sickness Behavior in Male Offspring. J. Neuroimmunol. 2020, 341, 577169. [Google Scholar] [CrossRef] [PubMed]
- Pardhe, H.A.; Krishnaveni, N.; Chekraverthy, B.K.; Patel, S.; Naveen, S.; Rashmi, V.; Govinden, P.C. Evaluation of Mineral and Near-Infrared Forecasting of Wheat Yield Varieties Using Spectrophotometric Techniques. Glob. J. Environ. Sci. Manag. 2024, 10, 189–204. [Google Scholar] [CrossRef]
- Wardani, H.K.; Puspitasari, H.P.; Priyandani, Y. Overview of Compliance to Taking Iron-Folic Acid Supplementation at Primary Healthcare Centre during the COVID-19 Pandemic. Pharm. Educ. 2023, 23, 140–144. [Google Scholar] [CrossRef]
- Naik, A.A.; Patro, I.K.; Patro, N. Slow Physical Growth, Delayed Reflex Ontogeny, and Permanent Behavioral as Well as Cognitive Impairments in Rats Following Intra-Generational Protein Malnutrition. Front. Neurosci. 2015, 9, 446. [Google Scholar] [CrossRef]
- Belluscio, L.M.; Berardino, B.G.; Ferroni, N.M.; Ceruti, J.M.; Cánepa, E.T. Early Protein Malnutrition Negatively Impacts Physical Growth and Neurological Reflexes and Evokes Anxiety and Depressive-like Behaviors. Physiol. Behav. 2014, 129, 237–254. [Google Scholar] [CrossRef]
- García-Ruiz, M.; Díaz-Cintra, S.; Cintra, L.; Corkidi, G. Effect of Protein Malnutrition on CA3 Hippocampal Pyramidal Cells in Rats of Three Ages. Brain Res. 1993, 625, 203–212. [Google Scholar] [CrossRef]
- Díaz-Cintra, S.; Cintra, L.; Ortega, A.; Kemper, T.; Morgane, P.J. Effects of Protein Deprivation on Pyramidal Cells of the Visual Cortex in Rats of Three Age Groups. J. Comp. Neurol. 1990, 292, 117–126. [Google Scholar] [CrossRef]
- Noback, C.R.; Eisenman, L.M. Some Effects of Protein-calorie Undernutrition on the Developing Central Nervous System of the Rat. Anat. Rec. 1981, 201, 67–73. [Google Scholar] [CrossRef]
- Mushtaq, B.S.; Hussain, M.B.; Omer, R.; Toor, H.A.; Waheed, M.; Shariati, M.A.; Sergey, P.; Heydari, M. Moringa Oleifera in Malnutrition: A Comprehensive Review. Curr. Drug Discov. Technol. 2021, 18, 235–243. [Google Scholar] [CrossRef]
- Shija, A.E.; Rumisha, S.F.; Oriyo, N.M.; Kilima, S.P.; Massaga, J.J. Effect of Moringa oleifera Leaf Powder Supplementation on Reducing Anemia in Children below Two Years in Kisarawe District, Tanzania. Food Sci. Nutr. 2019, 7, 2584–2594. [Google Scholar] [CrossRef] [PubMed]
- Dhakar, R.; Pooniya, B.; Gupta, M.; Maurya, S.; Bairwa, N. Sanwarmal Moringa: The Herbal Gold to Combat Malnutrition. Chron. Young Sci. 2011, 2, 119. [Google Scholar] [CrossRef]
- Thurber, M.D.; Fahey, J.W. Adoption of Moringa oleifera to Combat Under-Nutrition Viewed through the Lens of the “Diffusion of Innovations” Theory. Ecol. Food Nutr. 2009, 48, 212–225. [Google Scholar] [CrossRef]
- D’Auria, G.; Nitride, C.; Nicolai, M.A.; Mamone, G.; Montesano, D.; Mills, E.N.C.; Ferranti, P. Identification of Allergen Encoding Sequences in a Novel Food Ingredient from Moringa oleifera Leaves. Food Chem. 2023, 401, 134185. [Google Scholar] [CrossRef]
- Wardana, M.S.; Jufri, M.; Mun’im, A. Physicochemical Properties and Nutrition of Moringa oleifera Lam. Leaf Extract: A Preliminary Study on Preparation Phytosomes As Herbal Supplement for Children. Int. J. Appl. Pharm. 2022, 14, 281–287. [Google Scholar] [CrossRef]
- Gopalakrishnan, L.; Doriya, K.; Kumar, D.S. Moringa oleifera: A Review on Nutritive Importance and Its Medicinal Application. Food Sci. Hum. Wellness 2016, 5, 49–56. [Google Scholar] [CrossRef]
- Andrias, D.R.; Fahmida, U.; Adi, A.C. Nutritional Potential of Underutilized Food Crops to Improve Diet Quality of Young Children in Food Insecure Prone Areas of Madura Island, Indonesia. Asia Pac. J. Clin. Nutr. 2019, 28, 826–836. [Google Scholar] [CrossRef]
- Katmawanti, S.; Supriyadi; Mariroh, F. Is Instant Porridge with a High Calcium Content Based on Moringa oleifera as an Alternative Baby Food to Prevent Stunting in Indonesia? J. Public Health Res. 2021, 10, 353–357. [Google Scholar] [CrossRef]
- Zakaria; Sirajuddin; Veni, H.; Burhanuddin, B.; Rosmini; Suryani, A.; Bohari; Siswanto, A.W. Linear Growth of Infants Aged 0–6 Months in Breastfeeding Mothers Who Consume Moringa oleifera Leaf Extract Capsules: Randomized Controlled Doubleblind Design. Food Res. 2022, 6, 135–143. [Google Scholar] [CrossRef]
- Purwoningsih, E.; Arozal, W.; Lee, H.J.; Munim, A. Neuroprotective and Antioxidant Activities of Aqueous Extract Moringa oleifera Leaves. Int. J. Appl. Pharm. 2022, 14, 127–131. [Google Scholar] [CrossRef]
- Bakre, A.G.; Aderibigbe, A.O.; Ademowo, O.G. Studies on Neuropharmacological Profile of Ethanol Extract of Moringa oleifera Leaves in Mice. J. Ethnopharmacol. 2013, 149, 783–789. [Google Scholar] [CrossRef] [PubMed]
- Ingale, S.P.; Gandhi, F.P. Effect of Aqueous Extract of Moringa oleifera Leaves on Pharmacological Models of Epilepsy and Anxiety in Mice. Int. J. Epilepsy 2016, 3, 12–19. [Google Scholar] [CrossRef]
- Hannan, M.A.; Kang, J.Y.; Mohibbullah, M.; Hong, Y.K.; Lee, H.; Choi, J.S.; Choi, I.S.; Moon, I.S. Moringa oleifera with Promising Neuronal Survival and Neurite Outgrowth Promoting Potentials. J. Ethnopharmacol. 2014, 152, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Kambuno, N.; Louisa, M.; Wuyung, P.; Silaen, O.; Supali, T. In Vivo Toxicity Study in Sprague-Dawley Rats Receiving Different Doses of Moringa oleifera Extract. Open Vet. J. 2024, 14, 2294. [Google Scholar] [CrossRef]
- Asiedu-Gyekye, I.J.; Frimpong-Manso, S.; Awortwe, C.; Antwi, D.A.; Nyarko, A.K. Micro-and Macroelemental Composition and Safety Evaluation of the Nutraceutical Moringa oleifera Leaves. J. Toxicol. 2014, 2014, 786979. [Google Scholar] [CrossRef]
- Ganguly, R. Effect of Moringa oleifera in Experimental Model of Alzheimer’s Disease: Role of Antioxidants. Ann. Neurosci. 2005, 12, 33–36. [Google Scholar] [CrossRef]
- Sengupta, P. Review A Scientific Review of Age Determination for a Laboratory Rat: How Old Is It in Comparison with Human Age? Biomed. Int. 2011, 2, 81–89. [Google Scholar]
- Struck, M.B.; Andrutis, K.A.; Ramirez, H.E.; Battles, A.H. Effect of a Short-Term Fast on Ketamine–Xylazine in Rats. J. Am. Assoc. Lab. Anim. Sci. 2011, 50, 344–348. [Google Scholar]
- Novelli, E.L.B.; Diniz, Y.S.; Galhardi, C.M.; Ebaid, G.M.X.; Rodrigues, H.G.; Mani, F.; Fernandes, A.A.H.; Cicogna, A.C.; Filho, J.L.V.B.N. Anthropometrical Parameters and Markers of Obesity in Rats. Lab. Anim. 2007, 41, 111–119. [Google Scholar] [CrossRef]
- Heo, H.; Shin, Y.; Cho, W.; Choi, Y.S.; Kim, H.; Kwon, Y.K. Memory Improvement in Ibotenic Acid Induced Model Rats by Extracts of Scutellaria baicalensis. J. Ethnopharmacol. 2009, 122, 20–27. [Google Scholar] [CrossRef]
- Perry, A.; Brat, D. Practical Surgical Neuropathology: A Diagnostic Approach; Elsevier: Amsterdam, The Netherlands, 2010; ISBN 9780443069826. [Google Scholar]
- Bhasin, K.K.S.; Van Nas, A.; Martin, L.J.; Davis, R.C.; Devaskar, S.U. Maternal Low-Protein Diet or Hypercholesterolemia Reduces Circulating Essential Amino Acids and Leads to Intrauterine Growth Restriction. Diabetes 2009, 58, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Whitaker, K.W.; Totoki, K.; Reyes, T.M. Metabolic Adaptations to Early Life Protein Restriction Differ by Offspring Sex and Post-Weaning Diet in the Mouse. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 1067–1074. [Google Scholar] [CrossRef] [PubMed]
- Sarris, J.; Logan, A.C.; Akbaraly, T.N.; Amminger, G.P.; Balanzá-martínez, V.; Freeman, M.P.; Hibbeln, J. Nutritional Medicine as Mainstream in Psychiatry. Lancet Psychiatry 2015, 2, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.S.; Susser, E.; Lin, S.P.; Gorman, J.M. Increased Risk of Affective Disorders in Males after Second Trimester Prenatal Exposure to the Dutch Hunger Winter of 1944–45. Br. J. Psychiatry 1995, 166, 601–606. [Google Scholar] [CrossRef]
- Graaff, J.S.; Tiemeier, H.; Steegers-theunissen, R.P.M.; Hofman, A.; Jaddoe, V.W.V.; Verhulst, F.C.; Roza, S.J. Maternal Dietary Patterns during Pregnancy and Child Internalising and Externalising Problems. The Generation R Study. Clin. Nutr. 2014, 33, 115–121. [Google Scholar] [CrossRef]
- Katmawanti, S.; Yunus, M.; Kurniawan, A.W.; Sulistyorini, A.; Fauzi, R.; Supriyadi, S.; Amanda, D.; Samah, D.A.; Wahyuni, S.; Azizah, A.G.; et al. Effect of Glutamine, Glucose Unhidrate and Moringa oleifera on Blood Lymphocytes in White Mice (Rattus novergicus) Wistar Strain, Following Induction of a Proteinenergy-Deficient Diet. J. Public Health Afr. 2022, 13, 87–91. [Google Scholar] [CrossRef]
- Brar, S.; Haugh, C.; Robertson, N.; Owuor, P.M.; Waterman, C.; Fuchs, G.J.; Attia, S.L. The Impact of Moringa oleifera Leaf Supplementation on Human and Animal Nutrition, Growth, and Milk Production: A Systematic Review. Phytother. Res. 2022, 36, 1600–1615. [Google Scholar] [CrossRef]
- Sinha, S.; Patro, N.; Patro, I.K. Maternal Protein Malnutrition: Current and Future Perspectives of Spirulina Supplementation in Neuroprotection. Front. Neurosci. 2018, 12, 966. [Google Scholar] [CrossRef]
- Sinha, S.; Patro, N.; Patro, I.K. Amelioration of Neurobehavioral and Cognitive Abilities of F1 Progeny Following Dietary Supplementation with Spirulina to Protein Malnourished Mothers. Brain Behav. Immun. 2020, 85, 69–87. [Google Scholar] [CrossRef]
- Sinha, S.; Patro, N.; Tiwari, P.K.; Patro, I.K. Maternal Spirulina Supplementation during Pregnancy and Lactation Partially Prevents Oxidative Stress, Glial Activation and Neuronal Damage in Protein Malnourished F1 Progeny. Neurochem. Int. 2020, 141, 104877. [Google Scholar] [CrossRef]
- Merih, I.; Comunoglu, N.U.; Comunoglu, C.; Eren, B.; Ekici, I.D.; Ozkan, F. Age-Related Changes in the Rat Hippocampus. J. Clin. Neurosci. 2008, 15, 568–574. [Google Scholar] [CrossRef]
- Zammit, A.R.; Ezzati, A.; Zimmerman, M.E.; Lipton, R.B.; Lipton, M.L.; Katz, M.J. Roles of Hippocampal Subfields in Verbal and Visual Episodic Memory. Behav. Brain Res. 2017, 317, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Takeda, A.; Suzuki, M.; Tempaku, M.; Ohashi, K.; Tamano, H. Influx Of Extracellular Zn2+ Into The Hippocampal Ca1 Neurons Is Required for Cognitive Performance via Long-Term Potentiation. Neurosci. Lett. 2015, 304, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Zeineh, M.M.; Engel, S.A.; Thompson, P.M.; Bookheimer, S.Y. Dynamics of the Hippocampus during Encoding and Retrieval of Face-Name Pairs. Science 2003, 299, 577–580. [Google Scholar] [CrossRef]
- Nauer, R.K.; Whiteman, A.S.; Dunne, M.F.; Stern, C.E.; Schon, K. Hippocampal Subfield and Medial Temporal Cortical Persistent Activity during Working Memory Reflects Ongoing Encoding. Front. Syst. Neurosci. 2015, 9, 30. [Google Scholar] [CrossRef]
- Wang, S.Y.; Xue, X.; Duan, R.; Gong, P.Y.; Yan, E.; Jiang, T.; Zhang, Y.D. A TREML2 Missense Variant Influences Specific Hippocampal Subfield Volumes in Cognitively Normal Elderly Subjects. Brain Behav. 2020, 10, e01573. [Google Scholar] [CrossRef]
- Yau, J.L.W.; Hibberd, C.; Noble, J.; Seckl, J.R. The Effect of Chronic Fluoxetine Treatment on Brain Corticosteroid Receptor MRNA Expression and Spatial Memory in Young and Aged Rats. Mol. Brain Res. 2002, 106, 117–123. [Google Scholar] [CrossRef]
- Mulugeta, E.; Chandranath, I.; Karlsson, E.; Winblad, B.; Adem, A. Temporal and Region-Dependent Changes in Muscarinic M4 Receptors in the Hippocampus and Entorhinal Cortex of Adrenalectomized Rats. Exp. Brain Res. 2006, 173, 309–317. [Google Scholar] [CrossRef]
- Léonhardt, M.; Lesage, J.; Dufourny, L.; Dickès-Coopman, A.; Montel, V.; Dupouy, J.P. Perinatal Maternal Food Restriction Induces Alterations in Hypothalamo-Pituitary-Adrenal Axis Activity and in Plasma Corticosterone-Binding Globulin Capacity of Weaning Rat Pups. Neuroendocrinology 2002, 75, 45–54. [Google Scholar] [CrossRef]
- Lister, J.P.; Blatt, G.J.; Kemper, T.L.; Tonkiss, J.; Debassio, W.A.; Galler, J.R.; Rosene, D.L. Prenatal Protein Malnutrition Alters the Proportion but Not Numbers of Parvalbuminimmunoreactive Interneurons in the Hippocampus of the Adult Sprague-Dawley Rat. Nutr. Neurosci. 2011, 14, 165–178. [Google Scholar] [CrossRef]
- Lukoyanov, N.V.; Andrade, J.P. Behavioral Effects of Protein Deprivation and Rehabilitation in Adult Rats: Relevance to Morphological Alterations in the Hippocampal Formation. Behav. Brain Res. 2000, 112, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Lister, J.P.; Tonkiss, J.; Blatt, G.J.; Kemper, T.L.; DeBassio, W.A.; Galler, J.R.; Rosene, D.L. Asymmetry of Neuron Numbers in the Hippocampal Formation of Prenatally Malnourished and Normally Nourished Rats: A Stereological Investigation. Hippocampus 2006, 16, 946–958. [Google Scholar] [CrossRef] [PubMed]
- Lister, J.P.; Blatt, G.J.; DeBassio, W.A.; Kemper, T.L.; Tonkiss, J.; Galler, J.R.; Rosene, D.L. Effect of Prenatal Protein Malnutrition on Numbers of Neurons in the Principal Cell Layers of the Adult Rat Hippocampal Formation. Hippocampus 2005, 15, 393–403. [Google Scholar] [CrossRef]
- Georgieff, M.K. Nutrition and the Developing Brain: Nutrient Priorities and Measurement. Am. J. Clin. Nutr. 2007, 85, 614S–620S. [Google Scholar] [CrossRef]
- Omotoso, G.O.; Gbadamosi, I.T.; Afolabi, T.T.; Abdulwahab, A.B.; Akinlolu, A.A. Ameliorative Effects of Moringa on Cuprizone- Induced Memory Decline in Rat Model of Multiple Sclerosis. Anat. Cell Biol. 2018, 51, 119–127. [Google Scholar] [CrossRef]
- Kirisattayakul, W.; Wattanathorn, J.; Tong-un, T.; Muchimapura, S.; Wannanon, P.; Jittiwat, J. Cerebroprotective Effect of Moringa oleifera against Focal Ischemic Stroke Induced by Middle Cerebral Artery Occlusion. Oxidative Med. Cell. Longev. 2013, 2013, 10–13. [Google Scholar] [CrossRef]
- Ndlovu, S.S.; Ghazi, T.; Chuturgoon, A.A. The Potential of Moringa oleifera to Ameliorate HAART-Induced Pathophysiological Complications. Cells 2022, 11, 2981. [Google Scholar] [CrossRef]
- Almeida, F.; Silva, G.A.; Fiúza, A.T.; Chianca, D.; Ferreira, A.J.; Chiarini-Garcia, H. Gestational and Postnatal Protein Deficiency Affects Postnatal Development and Histomorphometry of Liver, Kidneys, and Ovaries of Female Rats’ Offspring. Appl. Physiol. Nutr. Metab. 2012, 2, 293–300. [Google Scholar] [CrossRef]
- Reyes-Castro, L.A.; Rodriguez, J.S.; Charco, R.; Bautista, C.J.; Larrea, F.; Nathanielsz, P.W.; Zambrano, E. Maternal Protein Restriction in the Rat during Pregnancy and/or Lactation Alters Cognitive and Anxiety Behaviors of Female Offspring. Int. J. Dev. Neurosci. 2012, 30, 39–45. [Google Scholar] [CrossRef]
- Batista, T.H.; Veronesi, V.B.; Ribeiro, A.C.; Giusti-Paiva, A.; Vilela, F.C. Protein Malnutrition during Pregnancy Alters Maternal Behavior and Anxiety-like Behavior in Offspring. Nutr. Neurosci. 2017, 20, 437–442. [Google Scholar] [CrossRef]
- Zongo, U.; Zoungrana, S.L.; Savadogo, A.; Traoré, A.S. Nutritional and Clinical Rehabilitation of Severely Malnourished Children with Moringa oleifera Lam. Leaf Powder in Ouagadougou (Burkina Faso). Food Nutr. Sci. 2013, 04, 991–997. [Google Scholar] [CrossRef]
- Soliman, A.; Yasin, M.; Kassem, A. Leptin in Pediatrics: A Hormone from Adipocyte That Wheels Several Functions in Children. Indian J. Endocrinol. Metab. 2012, 16, 577. [Google Scholar] [CrossRef] [PubMed]
- Klok, M.D.; Jakobsdottir, S.; Drent, M.L. The Role of Leptin and Ghrelin in the Regulation of Food Intake and Body Weight in Humans: A Review. Obes. Rev. 2007, 8, 21–34. [Google Scholar] [CrossRef] [PubMed]
- El-Hodhod, M.A.; Emam, E.K.; Zeitoun, Y.A.; El-Araby, A.M. Serum Ghrelin in Infants with Protein-Energy Malnutrition. Clin. Nutr. 2009, 28, 173–177. [Google Scholar] [CrossRef]
- Altinkaynak, S.; Selimoglu, M.A.; Ertekin, V.; Kilicarslan, B. Serum Ghrelin Levels in Children with Primary Protein-Energy Malnutrition. Pediatr. Int. 2008, 50, 429–431. [Google Scholar] [CrossRef]
- Iorember, F.M. Malnutrition in Chronic Kidney Disease. Front. Pediatr. 2018, 6, 161. [Google Scholar] [CrossRef]
- Montazerifar, F.; Karajibani, M.; Gorgij, F.; Akbari, O. Malnutrition Markers and Serum Ghrelin Levels in Hemodialysis Patients. Int. Sch. Res. Not. 2014, 2014, 765895. [Google Scholar] [CrossRef]
- Semba, R.D.; Trehan, I.; Gonzalez-Friere, M.; Kraemer, K.; Moaddel, R.; Ordiz, M.I.; Ferrucci, L.; Manary, M.J. Perspective: The Potential Role of Essential Amino Acids and the Mechanistic Target of Rapamycin Complex 1. Adv. Nutr. 2016, 7, 853–865. [Google Scholar] [CrossRef]
- Glenn, J.M.; Madero, E.N.; Bott, N.T. Dietary Protein and Amino Acid Intake: Links to the Maintenance of Cognitive Health. Nutrients 2019, 11, 1315. [Google Scholar] [CrossRef]
- Semba, R.D.; Shardell, M.; Sakr Ashour, F.A.; Moaddel, R.; Trehan, I.; Maleta, K.M.; Ordiz, M.I.; Kraemer, K.; Khadeer, M.A.; Ferrucci, L.; et al. Child Stunting Is Associated with Low Circulating Essential Amino Acids. EBioMedicine 2016, 6, 246–252. [Google Scholar] [CrossRef]
- Townsend, D.M.; Tew, K.D.; Tapiero, H. Sulfur Containing Amino Acids and Human Disease. Biomed. Pharmacother. 2004, 58, 47–55. [Google Scholar] [CrossRef] [PubMed]


| No | Amino Acid | EEMO (mg/g) | Standard Diet (mg/g) | Low Protein Diet (mg/g) |
|---|---|---|---|---|
| 1 | Histidine | 5.23 | 6.20 | 3.00 |
| 2 | Isoleucine | 4.99 | 8.10 | 2.90 |
| 3 | Leucine | 5.84 | 17.10 | 8.10 |
| 4 | Lysine | 3.00 | 8.00 | 2.80 |
| 5 | Phenylalanine | 22.25 | 10.40 | 4.00 |
| 6 | Threonine | 6.41 | 7.90 | 3.10 |
| 7 | Tryptophan | 2.04 | 1.80 | 0.70 |
| 8 | Valine | 13.09 | 10.00 | 4.00 |
| 9 | Glutamic Acid | 60.03 | 33.80 | 15.20 |
| 10 | Aspartic acid | 40.33 | 14.40 | 4.80 |
| 11 | Arginine | 14.69 | 13.00 | 5.00 |
| 12 | Alanine | 13.50 | 10.60 | 5.80 |
| 13 | Serine | 8.12 | 10.70 | 4.20 |
| 14 | Proline | 7.07 | 13.50 | 6.90 |
| 15 | Glycine | 5.22 | 10.60 | 4.90 |
| 16 | Tyrosine | 3.07 | 5.40 | 2.40 |
| 17 | Methionine | 0.00 | 0.30 | 0.10 |
| 18 | Cysteine | 0.00 | 7.50 | 5.40 |
| NG | NG+EEMO 800 | PD | PD+EEMO 400 | PD+EEMO 800 | RG | |
|---|---|---|---|---|---|---|
| Before treatment (3 weeks old) | 39.33 ± 3.327 | 43.67 ± 2.733 | 25.00 ± 3.688 | 26.67 ± 2.733 | 26.33 ± 2.503 | 20.67 ± 1.033 |
| After treatment (8 weeks old) | 183.20 ± 28.690 | 184.30 ± 7.062 | 37.17 ± 6.401 | 46.17 ± 3.764 | 48.17 ± 2.229 | 103.8 ± 16.65 |
| p value | <0.001 | <0.001 | 0.007 | 0.002 | <0.001 | <0.001 |
| 5-week mean change | 143.80 ± 26.060 | 140.70 ± 8.406 | 12.17 ± 4.070 a | 19.50 ± 5.167 a | 21.83 ± 2.229 a | 83.17 ± 16.340 a,b,c,d |
| BW increase (%) | 364 ± 50 | 381 ± 50 | 49 ± 17 a | 75 ± 25 a | 84 ± 15 a | 418 ± 59 b,c,d |
| Parameter | NG | NG+EEMO 800 | PD | PD+EEMO 400 | PD+EEMO 800 | RG |
|---|---|---|---|---|---|---|
| a. Anthropometric | ||||||
| Final body weight (g) | 183.20 ± 28.690 | 184.30 ± 7.062 | 37.17 ± 6.401 | 46.17 ± 3,764 | 48.17 ± 2.229 | 103.8 ± 16.65 |
| Body length (cm) | 18.50 ± 0.894 | 18.67 ± 1.807 | 11.42 ± 0.376 | 14.00 ± 0.894 | 12.17 ± 0.8165 | 15.00 ± 0.894 |
| Body mass index (g/cm2) | 0.54 ± 0.084 | 0.54 ± 0.100 | 0.29 ± 0.055 a. | 0.24 ± 0.047 a. | 0.33 ± 0.041 a. | 0.47 ± 0.100 b,c,d |
| Daily weight gain (g/day) Food efficiency (%) | 13.00 ± 1.200 24 (20–26) | 20 ± 1.6 a 30 (26–34) | 5.4 ± 0.38 a,b 26 (22–33) | 5.4 ± 0.54 a,b 21 (21–30) | 4.7 ± 0.80 a,b 20 (16–25) | 8 ± 0.54 a,b,c,d 26 (24–29) |
| b. Food consumption | ||||||
| Food intake (g/BW) | 0.17 ± 0.046 | 0,16 ± 0.035 | 0.19 ± 0.044 | 0.22 ± 0.052 | 0.22 ± 0.039 | 0.20 ± 0.082 |
| Water intake/day (mL/BW) | 0.34 ± 0.150 | 0.70 ± 0.200 a | 1.6 ± 0.19 a | 0.77 ± 0.190 a,b | 0.72 ± 0.210 a,b | 0.54 ± 0.220 b |
| Energy intake (Kcal/g)/BW | 0.64 ± 0.180 | 0.77 ± 0.160 | 0.88 ± 0.190 | 0.85 ± 0.140 | 0.56 ± 0.140 | 0.71 ± 0.290 |
| Carbohydrate intake (g/BW) | 0.11 ± 0.029 | 0.09 ± 0.024 | 0.15 ± 0.030 | 0.17 ± 0.034 a | 0.16 ± 0.026 a | 0.12 ± 0.048 c |
| Protein intake (g/BW) | 0.03 (0.02–0.05) | 0.03 (0.02–0.04) | 0.02 (0.01–0.03)a | 0.02 (0.02–0.03) | 0.02 (0.02–0.03) | 0.03 (0.02–0.06)b |
| Fat energy intake (Kcal/g)/BW | 0.84 ± 0.240 | 0.76 ± 0.170 | 0.9 ± 0.220 | 1.00 ± 0.230 | 1.00 ± 0.180 | 0.97 ± 0.400 |
| c. Nutrition determination | ||||||
| Hemoglobin (Hb) (g/dL) | 14.10 ± 1.265 | 12.97 ± 0.781 | 8.08 ± 0.640 a | 11.27 ± 1.203 a,b | 10.15 ± 1.540 a.b | 13.83 ± 0.662 b,c,d |
| Albumin (mg/dL) | 4.60 ± 0.460 | 6.63 ± 0.620 a | 2.99 ± 0.170 a | 3.57 ± 0.240 a | 4.95 ± 0.830 b,c | 5.7 ± 0.15 a,b,c.d |
| BUN (mmol/mL) | 0.16 ± 0.030 | 0.15 ± 0.730 | 0.07 ± 0.052 | 0.17 ± 0.073 | 0.12 ± 0.050 | 0.05 ± 0.019 a,c |
| Ghrelin | 5.6 (5.4–7.9) | 6.4 (5.0–9.7) | 4.4 (3.4–4.6) | 4.2 (4.0–4.8)a | 3.1 (2.3–3.9) | 6.5 (6.0–7.9)d |
| Leptin | 74 (20–116) | 66 (28–81) | 139 (120–193) | 193 (150–759)a | 174 (139–951)a | 147 (116–545) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kambuno, N.T.; Putra, A.G.A.; Louisa, M.; Wuyung, P.E.; Timan, I.S.; Silaen, O.S.M.; Sukria, H.A.; Supali, T. Moringa oleifera Leaf Extract Improves Cognitive Function in Rat Offspring Born to Protein-Deficient Mothers. Biomedicines 2025, 13, 346. https://doi.org/10.3390/biomedicines13020346
Kambuno NT, Putra AGA, Louisa M, Wuyung PE, Timan IS, Silaen OSM, Sukria HA, Supali T. Moringa oleifera Leaf Extract Improves Cognitive Function in Rat Offspring Born to Protein-Deficient Mothers. Biomedicines. 2025; 13(2):346. https://doi.org/10.3390/biomedicines13020346
Chicago/Turabian StyleKambuno, Norma Tiku, Achmad Gigih Andy Putra, Melva Louisa, Puspita Eka Wuyung, Ina Susianti Timan, Otto Sahat Martua Silaen, Heri Ahmad Sukria, and Taniawati Supali. 2025. "Moringa oleifera Leaf Extract Improves Cognitive Function in Rat Offspring Born to Protein-Deficient Mothers" Biomedicines 13, no. 2: 346. https://doi.org/10.3390/biomedicines13020346
APA StyleKambuno, N. T., Putra, A. G. A., Louisa, M., Wuyung, P. E., Timan, I. S., Silaen, O. S. M., Sukria, H. A., & Supali, T. (2025). Moringa oleifera Leaf Extract Improves Cognitive Function in Rat Offspring Born to Protein-Deficient Mothers. Biomedicines, 13(2), 346. https://doi.org/10.3390/biomedicines13020346






