Abstract
Caffeine is commonly used to excess by the general public, and most pregnant women drink caffeine on a daily basis, which can become a habit. Maternal caffeine intake during pregnancy is associated with severe gestational outcomes. Due to its lipophilic nature, caffeine can cross the blood–brain barrier, placental barrier, and even amniotic fluid. It can be found in substantive amounts in breast milk and semen. There has been a reported drop in neonatal anthropometric measurements with increased caffeine consumption in some cohort studies. This narrative review using literature titles and abstracts from the electronic databases of PubMed, Embase, and Scopus investigates the data linking maternal caffeine use to unfavorable pregnancy outcomes. It also evaluates the validity of the recommendations made by health professionals on caffeine consumption by mothers from the available literature. The results of our comprehensive literature search of case–control studies, cohort studies, randomized control trials, and meta-analyses, imply that caffeine use during pregnancy is linked to miscarriage, stillbirth, low birth weight, and babies that are small for gestational age. It was also found that there may be effects on the neurodevelopment of the child and links to obesity and acute leukemia. These effects can even be seen at doses well below the daily advised limit of 200 mg. The genetic variations in caffeine metabolism and epigenetic changes may play a role in the differential response to caffeine doses. It is crucial that women obtain solid, evidence-based guidance regarding the possible risks associated with caffeine.
1. Introduction
There is growing epidemiological evidence that maternal nutrition affects pregnancy outcomes, fetal growth and development, and the chance of developing diseases as an adult [1]. The optimal maternal diet should provide all essential nutrients. However, regardless of content, the maternal diet also contains pollutants and substances with pharmacological actions that may have negative effects. Caffeine is reported to be one of the most common drinks consumed by people worldwide, including pregnant women [2,3]. Caffeine consumption in adults is beneficial for certain cancers (such as prostate, melanoma, liver, and breast cancer), liver diseases (such as liver fibrosis and liver cirrhosis), type 2 diabetes, cardiovascular diseases (such as coronary heart disease and stroke), and neurological diseases (such as Parkinson’s disease and Alzheimer’s disease). However, caffeine use by pregnant women is linked with negative pregnancy outcomes [4,5]. It has also been linked to structural abnormalities and abnormal growth and development of the child [6,7,8]. Guidance from various countries advises women to limit their caffeine consumption to less than 200 mg daily (moderate) [9,10,11,12]. There is no local guidance for caffeine consumption in the UAE. Caffeine can be consumed in multiple forms, including coffee, espresso, tea, soda, dark chocolate, and breakfast cereals [3]. It is also reported that pregnant women consume more than the recommended amount throughout the world [13]. There is a need for comprehensive evidence on the effects of caffeine intake in pregnancy and the further growth and development of the resultant child. Furthermore, the possible mechanisms of these effects need to be understood.
2. Materials and Methods
Objective: This narrative review aims to provide a comprehensive overview of the current understanding of caffeine use in pregnancy and investigates the data linking maternal caffeine use to unfavorable pregnancy outcomes.
Inclusion Criteria:
- Peer-reviewed articles focusing on caffeine use in pregnancy and pregnancy outcomes.
- Studies and reviews published in English that contribute to the understanding of caffeine effects in offspring neonates and children.
Exclusion Criteria:
- Non-peer-reviewed literature, such as editorials and opinion pieces.
- Studies not focused on human neonatal populations, animal studies, or laboratory-based research.
Literature Search Strategy: A comprehensive literature search was conducted using the PubMed, Embase, and Scopus databases. The search incorporated MeSH terms and relevant keywords, including the following: “caffeine”, “pregnancy outcomes”, “fetal impacts”, “spontaneous abortion”, “miscarriage’, ‘small for gestational age’, “low birth weight”, “preterm delivery”, “stillbirth”, “childhood overweight”, “childhood leukemia”, and “fetal neurodevelopment”. The search employed Boolean operators (AND, OR) to ensure comprehensive coverage. Two reviewers independently screened the search results for relevance. They assessed the titles and abstracts, followed by a full-text evaluation of potentially eligible articles. Discrepancies were resolved through discussion or consultation with the third reviewer. Articles published up to 31 December 2023 were considered for review.
Data Extraction and Analysis: Key information was extracted from the selected articles, including author(s) and publication year, study type and design, population characteristics, key findings related to the fetal effect of maternal caffeine consumption during pregnancy, and future directions.
Synthesis of Information: The extracted data were synthesized through thematic analysis, resulting in categorized findings on interindividual differences in the reaction to caffeine, adverse pregnancy outcomes (miscarriage, stillbirth, small for gestational age, low birth weight), child growth (and neurodevelopment), and childhood diseases.
Limitations: This review acknowledges potential biases due to the selected literature and the subjective nature of narrative syntheses.
Ethical Considerations: No ethical approval was required for this review as it utilized published literature.
3. Results and Discussion
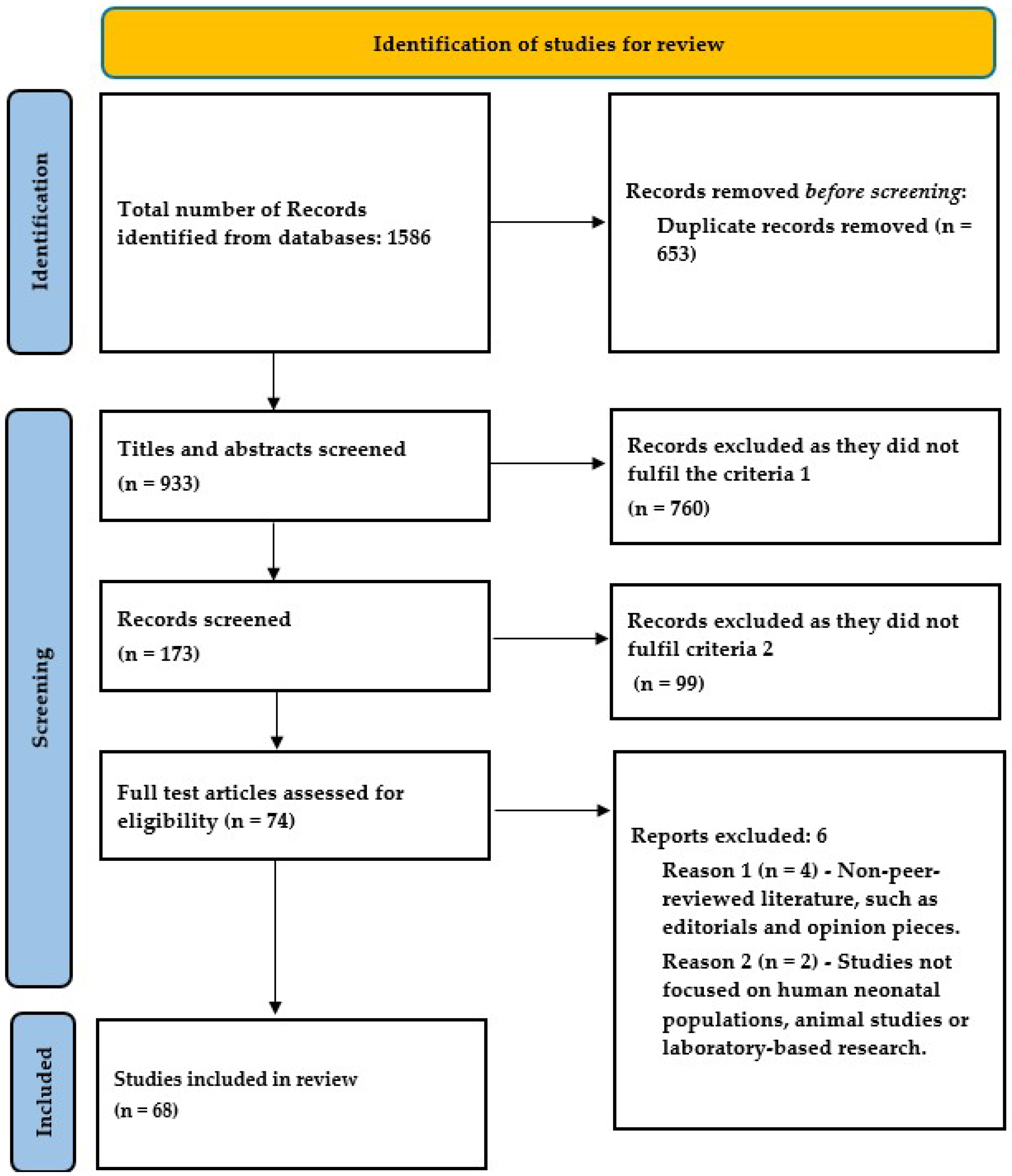
The review of the published literature yielded 54 observational studies, 1 randomized control trial, and 13 meta-analyses focusing on the effect of maternal caffeine use on pregnancy and childhood (Table 1). There are several cohorts and case–control studies from various regions of the world regarding the outcomes of miscarriage, birth weight, and stillbirth, but evidence on preterm birth is limited.

Table 1.
Meta-analyses.
3.1. Caffeine Metabolism and Interindividual Differences in the Reaction to Caffeine
Human studies have demonstrated that the effects of caffeine on pregnancy outcomes vary greatly amongst individuals [27,28,29,30,31]. Over the past 20 years, there has been a major increase in scientific interest in the field of substantial interindividual phenotypic variation and the underlying mechanisms in complex characteristics and disorders. The idea that a person’s susceptibility to disease is a complicated readout of the interactions between their environment, genetic makeup, and epigenetic modifications during development is becoming more and more acknowledged [32,33]. The relative contributions of these several factors to interindividual variation and disease propensity vary from case to case, rely on particular situations, and occasionally exhibit notable synergism. Caffeine exerts its stimulant action through adenosine receptors. Both the sensitivity of adenosine receptors and the metabolism of caffeine contribute to individual variance in response [34]. Specifically, control of Cytochrome P4501A2 (CYP1A2), the enzyme that limits the rate of metabolism of caffeine, is a thoroughly researched instance. CYP1A2 and N-acetyltransferase 2 (NAT2) are key enzymes in the metabolism of caffeine. Glutathione S-transferase alpha1 (GSTA1) conjugates glutathione to aromatic amines and may also be active in the metabolism of caffeine. According to epidemiological research, women with higher CYP1A2 enzyme activity (rapid caffeine metabolism) are more likely to experience pregnancy issues when exposed to the same dosages of caffeine compared to women with lower CYP1A2 enzyme activity [27,28,29,30,31]. Human CYP1A2 mRNA levels show inter-individual variations of over 40 times, and the 3-demethylation of caffeine allows for the investigation of up to 60 times variation in the CYP1A2 enzyme’s in vivo activity [35]. Apart from the hepatic constitutive expression of CYP1A2, various extrinsic and intrinsic factors can control the activity of CYP1A2, including cigarette smoking, excessive coffee consumption, inhibition by oral contraceptives, and co-regulation by other liver-enriched transcription factors [36,37]. In addition to genetic variants, the regulation of CYP1A2 by environmental factors may also entail numerous layers of epigenetic mechanisms. Studies have also shown that a combination of the status and activity of these metabolites may play a key role in the varied outcomes in individuals [38]. This is an attractive topic for future research and could result in individualized precision medicine.
3.2. Pregnancy Outcomes and Caffeine Use
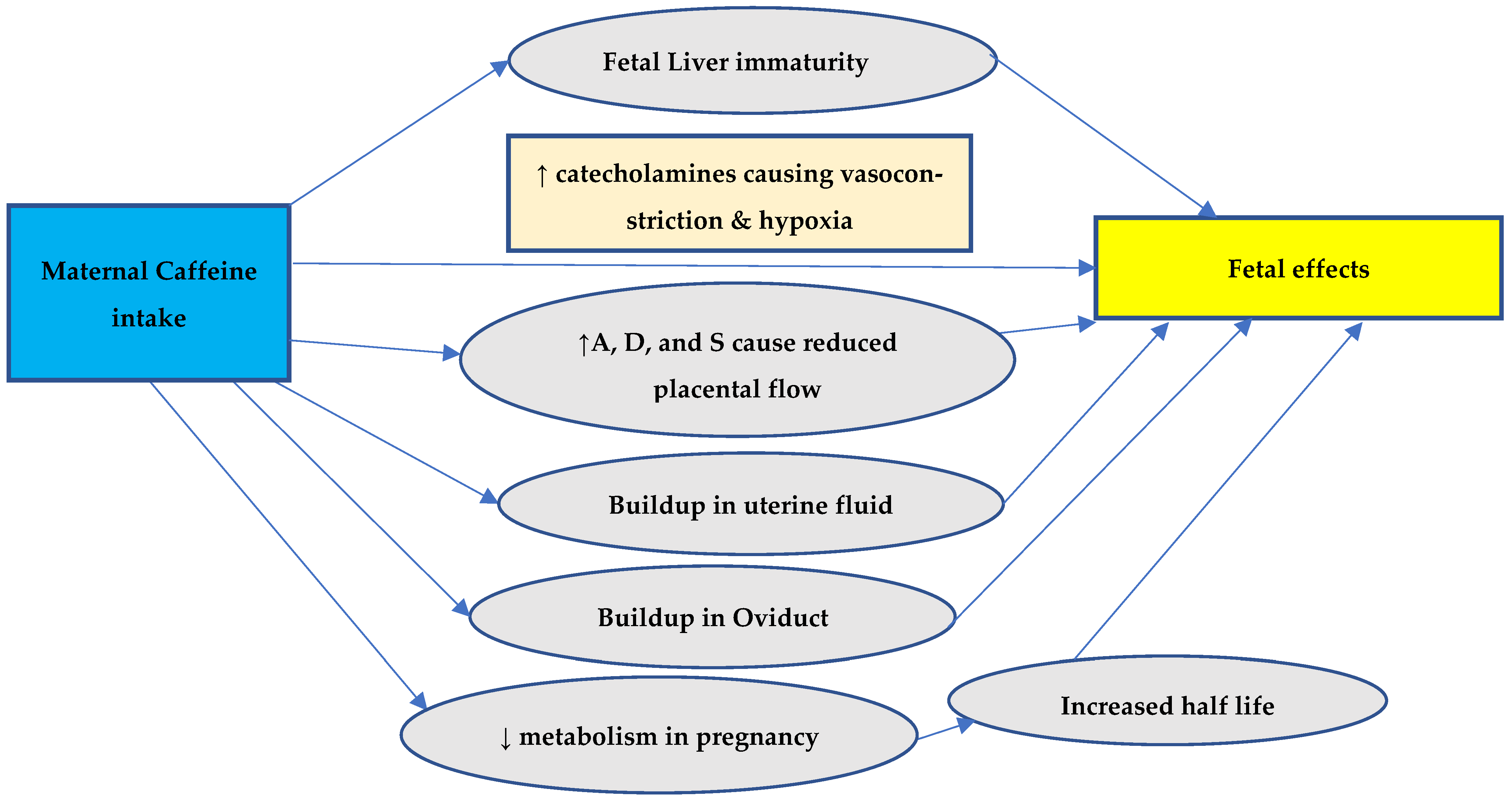
Everyday consumption of caffeine, a xanthine alkaloid, is widespread around the world, and it is largely found in coffee, tea, energy drinks, chocolates, and some soft beverages. Caffeine can cross through the placental barrier. The fetal liver is immature, and primary enzymes that render caffeine inactive are not expressed by the fetus. Thus, caffeine excretion is delayed in the fetus. Caffeine metabolites can accumulate in the fetal brain [39,40,41]. During pregnancy, the metabolism of caffeine is greatly reduced, especially after the first trimester [42]. As the pregnancy progresses, the half-life of caffeine increases from 2.5 to 4.5 h to about 15 h [43]. This is due to the decreased activity of liver enzymes responsible for caffeine metabolism (by one-third in the first trimester and by half in the second) [44]. Mothers’ caffeine absorption may also build up in oviductal or uterine fluid settings [45]; this can further affect the embryonic development of the fetus.
Furthermore, adrenaline, dopamine, and serotonin concentrations rise due to coffee consumption, which affects placental blood flow and the delivery of nutrients to the fetus through the placenta [20,46]. High coffee consumption during pregnancy may increase the fetus’s catecholamine levels, which may promote placental vasoconstriction [47] and raise the fetal heart rate, affecting the oxygenation of the fetus [48]. The symptoms of withdrawal like irritability and arrhythmias are also observed in newborns of mothers consuming caffeine during pregnancy, further substantiating the accumulation in the fetus in utero. Figure 1 summarizes the possible explanation of the effects of maternal caffeine intake on the fetus.

Figure 1.
PRISMA (version 2020) flow diagram for study inclusion.
Up until recently, the majority of specialists recommended that pregnant women consume no more than 300 mg of caffeine each day [16]; however, recent recommendations of the European Food Safety Authority (EFSA) and the American Institute of Medicine have limited the amount to 200 mg/day [12]. The reason for this may be the inter-individual variations in genes and epigenetic modification.
3.3. Adverse Pregnancy Outcomes
A frequent element in the diet of pregnant women, caffeine is the most widely consumed psychoactive substance in the world [49]. Possible mechanisms of adverse pregnancy outcomes are shown in Figure 2. A putative link between caffeine use and perinatal morbidities is of concern since many women continue to drink coffee and other caffeinated drinks while pregnant. Reduced metabolism and increased half-life during pregnancy (15.08 vs. 4.71 h) cause buildup in the body, which crosses the placenta freely and causes fetal effects due to the inability to be metabolized by the fetal liver [50,51,52]. It is important that women receive advice about the negative pregnancy outcomes that can be caused by caffeine consumption [7].

Figure 2.
Possible mechanisms of adverse pregnancy outcomes. A = adenosine, D = dopamine, and S = serotonin.
There are a limited number of studies on structural anomalies associated with caffeine use. These studies have reported either no association or non-significant associations. However, there were considerable biases in both of these studies [13]. One of the unfavorable outcomes of pregnancy is fetal death—defined as the death of the fetus before complete evacuation from its mother—which is responsible for a significant fraction of perinatal mortality [53]. It can be separated into stillbirth and spontaneous abortion [22,54,55].
3.3.1. Spontaneous Abortion/Miscarriage
Spontaneous abortion (SAB) is defined as the involuntary termination of a pregnancy leading to fetal death before 20 weeks of gestation [56].
It has been reported that coffee use alters the levels of endogenous hormones [57,58,59,60]. Caffeine was shown to be favorably correlated with sex hormone-binding globulin [59] and negatively correlated with levels of estradiol and progesterone during the luteal phase of the menstrual cycle [58,59,60]. Thus, it is plausible that hormonal alterations brought on by caffeine use might influence the chances of SAB [57].
Caffeine can cause vasoconstriction in the fetus via increased catecholamine output, resulting in decreased uterine and placental blood flow, leading to fetal hypoxia [46]. There were 30 observational studies and 4 meta-analyses reporting the outcomes of the risk of miscarriage (Table 2). These studies used questionnaire surveys as well as bio-marker levels as evidence of caffeine consumption. Most of the studies adjusted the confounders for SAB as maternal age, education status, economic status, ethnicity, pre-pregnancy body mass index, smoking, alcohol use, and exercise levels. All of the studies reported a significant risk of miscarriage with either pre-pregnancy consumption, during pregnancy, or both. Only one survey reported a suggestive association. This study also reported suspected recall bias [61]. However, the prospective studies were free from recall biases.
A comprehensive and up-to-date meta-analysis investigated the association between maternal coffee and caffeine and the risk of pregnancy loss and confirmed that coffee or caffeine consumption raises the risk of pregnancy loss [58]. In a prospective cohort study, it was shown that caffeine use during pregnancy was related to a higher risk of miscarriage and that there was a dose–response relationship, with the majority of the risk at 200 mg or more of caffeine per day. This observed impact was not affected by a number of possible confounders, such as pregnancy-related symptoms like nausea and vomiting or intolerance to coffee. There was an approximately 80% higher risk of miscarriage linked with caffeine use of 200 mg/day or more, even among women who did not alter their caffeine consumption habits while pregnant. Finally, caffeine intake from sources other than coffee revealed a comparable elevated risk of miscarriage, suggesting that the higher risk of miscarriage was caused by caffeine alone rather than other potential compounds in coffee. Independent of pregnancy-related symptoms, the data showed that consuming large amounts of coffee while pregnant increases the chance of miscarriage [62]. There is considerable agreement between all of the studies regarding the causation of SAB. However, the doses used as the reference were different, and multiple studies reported a non-linear association with the daily dose. A recent study also reported no threshold for safe intake [63]. This needs further research, keeping in mind the interindividual variation in caffeine metabolism.

Table 2.
Caffeine intake and spontaneous abortion/miscarriage. OR: Odds Ratio.
Table 2.
Caffeine intake and spontaneous abortion/miscarriage. OR: Odds Ratio.
| Author and Year (Reference) | Country | Study Design | Events/Sample Size | Comments and Associate Factors |
|---|---|---|---|---|
| Purdue-Smithe et al. (2019) [63] | USA | Prospective Cohort | Pre-conceptional caffeine consumption is associated with increased risk. Biomarkers confirmed consumption. No safe threshold; miscarriage is not dependent on nausea or vomiting during pregnancy | |
| Gaskin et al. (2018) [64] | USA | Prospective Cohort | 2756/15,950 (17.2%) | Pre-pregnancy intake was associated with increased risk (not during early pregnancy). Factors like the age of the mother, BMI, smoking, alcohol, physical activity, history of infertility, race, and folate intake were considered and adjusted |
| Morales-Suárez-Varela et al. (2018) [65] | Denmark | Cohort | Risk: 1.22 (0.91–1.63) | Compared to no intake, >3 cups/day is associated with a higher risk of miscarriage. Age, parity, socio-economic status, physical exercise, alcohol, and BMI were also considered |
| Hahn et al. (2015) [66] | Denmark | Cohort | 732/5132 (14.3%) | Age, physical activity, parity, BMI, education, smoking, and previous miscarriage |
| Agnesi et al. (2011) [67] | Italy | Case–Control | 123/231 0.53 | Maternal age and education. Focusses on the effect after community education and awareness |
| Stefanidou et al. (2011) [68] | Italy | Case–Control | 52/312 (16.6%) | Maternal caffeine intake is associated with a three-fold increase in recurrent miscarriage per 100 mg daily intake. Not confounded by age, education, and tobacco intake |
| Greenwood et al. (2010) [17] | UK | Prospective Cohort | 28/2635 (1.1%) | Increase in late miscarriage with consumption >300 mg in early pregnancy. Age, parity, smoking, and alcohol |
| Pollack et al. (2010) [69] | USA | Prospective Cohort | 13/66 (19.6%) | Age, alcohol, smoking, and previous miscarriage |
| Zhang et al. (2010) [70] | China | Case–Control | 326/726 (44.9%) | Age, alcohol, smoking, education, BMI, and previous history |
| Weng et al. (2008) [62] | USA | Prospective Cohort | 172/1063 (16.2%) | Increased risk of miscarriage not confounded by age, race, education, income, previous miscarriage, alcohol smoking, pregnancy nausea, or vomiting |
| Savitz et al. (2008) [61] | USA | Cross-Sectional Cohort | 258/2407 (10.7%) | Pre-pregnancy intake associated with increased risk. Age, ethnicity, education, alcohol, nausea, and vomiting were considered. Possibility of recall bias |
| Maconochie et al. (2007) [71] | UK | Case–Control | (603/6719) | No association between caffeine intake and miscarriage after adjusting for confounders. The primary aim was causes of miscarriage |
| Khoury et al. (2004) [72] | USA | Prospective Cohort | 23/191 (12%) | Age, type-1 diabetes, previous SAB, nephropathy, retinopathy, glycemic control, and smoking were considered. Increased risk not confounded by smoking |
| Rasch et al., (2003) [73] | Denmark | Case–Control | OR: 2.21 (330/1498) | Daily intake of >375 mg is associated with increased risk. Age, parity, cigarette, and alcohol considered |
| Giannelli et al. (2003) [74] | UK | Case–Control | 160/474 (33.7%) | Double the risk with consumption for >300 mg compared to ≤150 mg daily. The majority were non-smokers. Age and nausea were considered and adjusted |
| Tolstrup et al. (2003) [75] | Denmark | Case–Control | OR: 1.26 for 75–300 mg (303/1684) | Pre-pregnancy intake has a linear relation with miscarriage. Doses were <75, 75–300, 301–500, 501–900, and >900 mg. Age, smoking, and alcohol were considered as confounders |
| Wen et al. (2001) [76] | USA | Prospective Cohort | 75/650 (11.5%) | Pre-pregnancy and early pregnancy intake. Increased risk not related to nausea and vomiting. Women with nausea had increased risk with intake ≥300 mg daily |
| Cnattingius et al. (2000) [77] | Sweden | Case–Control | 562/1515 (37%) | Increased risk not confounded by age, history of SAB, alcohol, or pregnancy symptoms. Risk persists in non-smokers |
| Parazzini et al. (1998) [78] | Italy | Case–Control | 782/1543 (50.6%) | OR: 1.2, 1.8, and 4.0 for 1, 2 to 3, and ≥4 cups per day, respectively. Age, education, previous miscarriages, alcohol, smoking, and severity of nausea were considered |
| Fenster et al. (1997) [79] | USA | Cohort | 498/5144 (9.6%) | Pre-pregnancy and early pregnancy caffeine intake. Age, smoking, alcohol, obstetric history, and socio-economic status were adjusted |
| Dlugosz et al. (1996) [51] | USA | Cohort | 135/2967 (4.6%) | Maternal age was also included as a risk factor |
| Al-Ansary et al. (1994) [80] | Saudi Arabia | Case–Control | 226/452 | Increased risk in caffeine intake >150 mg daily. Primary inquiry into causes of miscarriage |
| Dominguez- Rojas et al. (1994) [81] | Spain | Cohort | 169/691 (24%) | Age and previous miscarriage |
| Infante-Rivard et al. (1993) [82] | Canada | Case–Control | OR: 2.62 (>321 mg daily during pregnancy) (331/1324) | Increased risk for consumption pre-pregnancy (OR: 1.85) and during pregnancy. Age, education, smoking, alcohol, and uterine malformations were confounders and adjusted. ORs increased by a factor of 1.22 for each 100 mg daily intake |
| Mills et al. (1993) [83] | USA | Cohort | 59/423 (13.9%) | Smoking, alcohol intake, age, parity, education, and previous miscarriage were included as risk factors |
| Armstrong et al. (1992) [84] | Canada | Cohort | 7760/35,848 (21.6%) | Age, education, and ethnicity were the considered risk factors |
| Parazzini et al. (1991) [85] | Italy | Case–Control | 78/212 (36%) | Maternal age |
| Fenster et al. (1991) [86] | USA | Case–Control | OR: 1.22 (607/1891) | Dose dependent. Confounders were adjusted. Heavy consumption (>300 mg daily) with nausea doubled the risk (OR: 2.1) |
| Wilcox et al. (1990) [87] | USA | Cohort | 43/171 (25%) | The association between miscarriage and risk factors was explored. Age, pregnancy history, weight, education, prenatal DES exposure, smoking, alcohol, and marijuana were other variables |
| Axelsson et al. (1989) [88] | Sweden | Cohort | 126/1242 (10.1%) | Age, occupation, and smoking were other risk factors |
3.3.2. Stillbirth
For the birth of a fetus without any sign of life after 20 weeks of pregnancy or when it weighs 14 oz, the term “stillbirth” is used. It can be due to intrauterine demise in pregnancy or during childbirth [56]. There were eight observational studies and two meta-analyses focusing on the outcome of stillbirth or late fetal demise (Table 3).
Coffee consumption during pregnancy is linked to a higher risk of stillbirth [89]. In several ways, caffeine may raise the risk of late fetal mortality. One mechanism by which coffee may cause fetal hypoxia and vasoconstriction in the uteroplacental circulation is by increasing the release of catecholamines from the renal medulla [47,90]. Another possibility is that caffeine may directly affect a developing fetus’s circulatory system, causing tachycardia and other arrhythmias [48]. Compared to pregnant women who drank no coffee, the rate of stillbirth dropped in those who drank one to three cups per day, climbed slightly in those who drank four to seven cups per day, and more than doubled in those who drank eight or more cups per day. These findings seem to point to an impact threshold of four to seven cups per day [89]. Women should be made aware that caffeine use during pregnancy increases the chance of stillbirth, especially at doses higher than those advised by the World Health Organization (WHO) (>300 mg/day) [91]. These studies suggest a definite association of stillbirth with a higher intake of caffeine than the recommended amount. One study explored the gene pleomorphism for caffeine metabolism and reported a combination of the slow oxidizer status of CYP1A2, the slow acetylator status of NAT2, and the low activity of GSTA1 as being significantly associated with stillbirth [38].

Table 3.
Caffeine and stillbirth. aOR: adjusted Odds Ratio. OR: Overall Risk. RR: Relative Risk.
Table 3.
Caffeine and stillbirth. aOR: adjusted Odds Ratio. OR: Overall Risk. RR: Relative Risk.
| Author and Year [Reference] | Country | Study Design | Events/Sample Size | Remarks and Associate Factors |
|---|---|---|---|---|
| Heazell et al. (2021) [91] | UK | Case–Control | aOR: 1.27 (1.14–1.43) for each 100 mg daily increase | 290/1019 (28.4%) of stillbirth The attributable risk for stillbirth associated with >300 mg of caffeine/day was 7.4% Age, BMI, smoking, ethnicity, education, parity, and dietary supplements |
| Morales- Suárez-Varela et al. (2018) [65] | Denmark | Cohort | 1178/90,086 (1.3%) Risk: 1.05 (0.62–1.77) | Compared to no intake, >3 cups/Day is associated with higher risk. Age, parity, socio-economic status, physical exercise, alcohol, and BMI were also considered |
| Gaskin et al. (2018) [64] | USA | Prospective Cohort | 1.24 (0.57–2.69) ≥4 servings compared to never | There is a higher but non-significant risk of stillbirth with pre-pregnancy intake of >400 mg daily. Factors like the age of the mother, BMI, smoking, alcohol, physical activity, history of infertility, race, and folate intake were considered and adjusted |
| Greenwood et al. (2010) [17] | UK | Prospective Cohort | 28/2635 (1.1%) | Increase in adverse outcomes with consumption >300 mg in early pregnancy. Compared to those consuming <100 mg/day, 2.2 for 100–199 mg/day, 1.7 for 200–299 mg/day, and 5.1 for >300 mg/day |
| Matijasevich et al. (2006) [92] | Uruguay | Case–Control | OR: 2.33 (382/1174) | Fetal death significantly more common with ≥300 mg/day. Other factors were education, previous miscarriage, pregnancy symptoms, and regular prenatal care |
| Bech et al. (2006) [38] | Denmark | Case–Control | RR: 2 (142/299) | Increased risk of stillbirth with a combination of slow metabolism genotypes of caffeine metabolism |
| Bech et al. (2005) [93] | Denmark | Prospective Cohort | 1102/88,482 (1.2%) | The risk increased linearly with increased daily intake. Risk: 1.03, 1.33, and 1.59 with 1, 2–3, and >4 cups daily consumption of coffee, respectively. Age, parity, smoking, BMI, and alcohol intake were adjusted as confounders |
| Wisborg et al. (2003) [89] | Denmark | Prospective Cohort | 82/18,478 (0.4%) | Caffeine intake during the first trimester is associated with stillbirth. Smoking, alcohol, parity, age, BMI, and education were other factors included |
3.3.3. Low Birth Weight (LBW)/Small for Gestational Age (SGA)
Low birth weight is defined as a birth weight of less than the 10th centile (−2 standard deviations from the mean). Small for gestational age on the other hand takes into account the gestational age and can represent a constitutionally small fetus or intrauterine growth restriction. Various factors can affect fetal growth in utero, including genetic, environmental, and metabolic, as well as infectious agents [55,94,95,96]. There were seventeen studies and seven meta-analyses that investigated the effect of maternal caffeine intake on the risk of LBW or SGA (Table 4).

Table 4.
Caffeine and small for GA/LBW. aOR: adjusted Odds Ratio.
The evidence is consistent with a risk of LBW or SGA in women with higher intake of caffeine prior to pregnancy and in early pregnancy. The only randomized trial demonstrated that moderate reduction of intake has no effect on birth weight or length of gestation [93]. A prospective cohort study in Finland included 2007 women in the first and 4362 in the third trimester of pregnancy for a survey on the intake of caffeine during pregnancy. They reported that more than 30% of women exceeded the recommended daily intake during pregnancy. The adjusted odds ratio (aOR) of SGA was 1.87 (1.16–3.02) in women with moderate (51–200 mg/day) and aOR 1.51 (1.08–2.10) in women with high (>200 mg/day) caffeine intake during the first trimester. Caffeine intake in the third trimester of pregnancy was not associated with SGA [97]. This demonstrates that even the recommended daily intake can cause adverse fetal effects. This may be explained by the polymorphisms of the genes that encode enzymes responsible for caffeine metabolism.
3.3.4. Preterm Birth
Preterm birth is defined as the commencement of spontaneous labor before 37 weeks of gestation [16]. Preterm birth increases the risk of neurodevelopmental, pulmonary, and gastrointestinal problems, and, later on, childhood obesity [111,112,113,114,115], as well as hypertension [115] and reduced insulin levels later in life [116]. It is a major cause of infant mortality [38]. One of the prenatal exposures examined for a potential relation to preterm birth is pregnant women’s caffeine use [16].
A meta-analysis of cohort and case–control studies found no evidence of a relationship between caffeine use during pregnancy and the risk of preterm birth. This study, however, was unable to draw any conclusions about caffeine intakes over 300–400 mg/d because the majority of studies used this as their upper limit [16]. Kobayashi et al. (2019) investigated dose-dependent associations between prenatal caffeine consumption and adverse birth outcomes, including preterm birth, using data from the Japan Environment and Children’s Study. The study found that maternal caffeine intake was significantly associated with an increased risk of preterm birth, small for gestational age (SGA), and reduced birth weight. A key finding was that even moderate caffeine consumption (100–200 mg per day) elevated the risk of preterm birth compared to low or no caffeine intake. The study emphasized the need for culturally specific recommendations on caffeine consumption during pregnancy, given differences in dietary habits and caffeine sources [100]. Okubo et al. (2015) focused on maternal caffeine intake from Japanese and Chinese teas, a prevalent source of caffeine in the Japanese population. Their findings indicated a significant association between higher caffeine intake and preterm birth. The study highlighted that tea-based caffeine sources had similar effects to coffee, suggesting that total caffeine intake—regardless of source—is a critical factor in influencing pregnancy outcomes. The authors posited that caffeine’s potential to increase circulating catecholamines and stress hormones could contribute to uterine contractions and preterm labor [104]. Most studies found no significant link between caffeine use during pregnancy and the risk of preterm delivery [93,117]. A Cochrane review also did not find any evidence of benefit from the reduction of caffeine intake in pregnancy on preterm delivery. However, it only included two studies with low-quality evidence [118].
3.4. Effect of Maternal Caffeine Use on Childhood Development and Disease
3.4.1. Neurodevelopment
The placental barrier is easily crossed by caffeine, a potent neuromodulator that is extensively utilized, but little is known about the long-term effects of gestational caffeine exposure (GCE) on neurodevelopment. In a study, magnetic resonance imaging (MRI) data were used to examine the structural results of the brain in 27 key fiber pathways as a function of GCE. The MRI data were obtained from a very large sample of 9157 children, aged 9–10 years, as part of the Adolescent Brain and Cognitive Developments study [58,119]. Using mixed effects binomial regression, significant correlations between GCE and fractional anisotropy (FA) values in the left hemisphere’s inferior fronto-occipito fasciculus and corticospinal tract were found. Further investigations were conducted to study the interaction between these fiber tracts, GCE, cognitive measures (working memory, task efficiency), and psychopathology measures (externalization, internalization, somatization, and neurodevelopment) [119]. GCE was associated with worse results on all psychiatric tests but had no influence on cognitive assessments. Higher FA levels in both fiber tracts were linked to fewer neurodevelopmental issues. Both cognitive tasks resulted in better performance. These findings imply that GCE can contribute to future neurodevelopmental difficulties, which occur in part due to changes in the architecture of key proteins. These findings imply that the current recommendations for reducing caffeine use during pregnancy might need modification [119,120]. A recent study from Japan involving 1199 mother–child pairs showed that higher maternal caffeine consumption during pregnancy was independently associated with a reduced risk of peer problems in the children and was not related to the risk of emotional problems, conduct problems, or hyperactivity in the children [121].
3.4.2. Childhood Obesity/Overweight
Childhood overweight and obesity have become significant public health challenges worldwide, with their origins often tracing back to prenatal and early-life exposure. Maternal caffeine intake during pregnancy has been studied as a potential factor influencing the offspring’s growth patterns, body mass index (BMI), and adiposity. Voerman et al. (2016) conducted a prospective cohort study examining maternal caffeine intake during pregnancy in relation to early growth and body fat distribution in children of school age. It was reported that moderate and high maternal caffeine intake was associated with greater total body fat and abdominal fat in offspring [103]. In another study, the prevalence of overweight rose by 5% at three years, 6% at five years, and 3% at eight years when prenatal caffeine intake increased from low to extremely high. Children born to average-, high-, and very-high-caffeine consumers had 1.05, 1.17, and 1.44 greater adjusted odds of being overweight at three years of age, respectively, than children born to low caffeine consumers. Similar ORs were discovered at the age of five years; however, at eight years of age, the risk was substantial only for the highest caffeine consumption category. The exclusion of pregnant smokers or SGA neonates had no effect on the outcomes [122]. A similar linear relationship between maternal caffeine consumption as a continuous variable and the risk of being overweight at ages three and five, with a greater OR at age three than at age five was observed in another study [123,124].
Yet another study demonstrated that even intake of less than 300 mg/day was strongly linked to an elevated risk of excess infant development and obesity, even after omitting very high users and using non-drinkers as the reference group. Finally, when growth data from actual measures were used to investigate the link between maternal coffee use and overweight at different age stages, comparable trends and relationships were seen [125]. It was also reported that at any amount above 50 mg/day, caffeine was linked to increased BMI from one month to eight years but was associated with faster height gain only up to three months of age [126]. Papadopoulou et al. (2018) utilized data from a large Norwegian cohort to investigate the relationship between maternal caffeine intake and childhood growth trajectories. Their findings corroborated previous studies about the association, notably, the associations across various caffeine sources, including coffee, tea, and soft drinks, emphasizing that total caffeine intake—rather than specific sources—is critical to consider [122].
Chen et al. (2019) examined parental and grandparental caffeine intake in relation to childhood obesity. Their analysis indicated that only maternal caffeine intake was significantly associated with higher childhood BMI and adiposity, proposing a direct influence on the intrauterine environment [22]. When maternal serum paraxanthine (a primary caffeine metabolite) levels were analyzed in association with offspring BMI at ages 4 and 7 years, a weak positive association was observed [127]. Thus, the potential biological mechanisms behind the association between maternal caffeine consumption and childhood obesity are believed to be fetal programming, impaired placental function, epigenetic modifications, and rapid infant growth.
3.4.3. Childhood Leukemia
Childhood acute leukemia (AL), including acute lymphoblastic leukemia (ALL) and acute myeloid leukemia (AML), represents the most common malignancy in children. While its etiology remains multifactorial, maternal exposures during pregnancy, including caffeine consumption, have been investigated for their potential role in influencing leukemia risk. Menegaux et al. (2007) conducted a case–control study in France examining maternal coffee and alcohol consumption, alongside parental smoking, as risk factors for childhood leukemia. Their findings suggested a potential association between high maternal coffee intake (defined as three or more cups per day) and an increased risk of AL [128]. The study noted that caffeine may influence fetal development through its effects on DNA replication and repair, potentially leading to oncogenic mutations. However, the authors emphasized the need for further research to corroborate these findings and explore underlying mechanisms. Building on prior research, Bonaventure et al. (2013) explored the interplay between maternal caffeine consumption and genetic predispositions in a case–control study. They observed an increased risk of ALL in children born to mothers with higher coffee intake during pregnancy, particularly among those with specific metabolic polymorphisms affecting caffeine metabolism. These findings highlight the potential role of gene-environment interactions in modulating leukemia risk. The study underscored the importance of considering individual metabolic differences when assessing the impact of maternal caffeine consumption [129]. Milne et al. (2011) reported an elevated risk of ALL associated with higher maternal coffee intake, with a dose–response relationship. Tea consumption, however, did not demonstrate a significant association. This study reinforced the hypothesis that caffeine exposure may disrupt fetal hematopoiesis or immune system development, potentially increasing leukemia susceptibility [64]. In a subsequent pooled analysis involving data from multiple international studies, Milne et al. (2018) further confirmed a modest but statistically significant increase in ALL risk with higher coffee consumption during pregnancy [23,130]. Importantly, this study addressed potential confounders, such as socioeconomic status and parental smoking, to strengthen the reliability of its findings. The authors proposed that caffeine’s potential to induce oxidative stress and impair DNA integrity might contribute to leukemogenesis. Focusing specifically on AML, Karalexi et al. (2019) utilized data from the Childhood Leukemia International Consortium to investigate maternal coffee and tea consumption during pregnancy. Unlike studies on ALL, they did not identify a significant association between caffeine intake and AML risk. The authors suggested that differences in leukemia subtypes and their underlying pathophysiology might explain the lack of correlation. They also noted the limited sample size for AML cases, which could affect the statistical power of the analysis [131]. The ESTELLE study by Orsi et al. (2015) examined maternal caffeine consumption in relation to childhood AL within a broader context of parental lifestyle factors. While the study did not find a definitive association between moderate coffee or tea intake and AL risk, it suggested that very high levels of caffeine consumption (e.g., five or more cups per day) might contribute to a slight increase in risk. The study also highlighted the complexity of disentangling caffeine’s effects from other maternal and environmental exposures [132].
The potential mechanisms linking maternal caffeine consumption to childhood leukemia involve caffeine’s known biological effects on cell proliferation, DNA repair, and oxidative stress. High levels of caffeine intake may disrupt normal fetal development by inducing DNA damage or impairing immune system maturation, which are critical processes in preventing malignancies like leukemia. Additionally, genetic polymorphisms affecting caffeine metabolism may modulate individual susceptibility. For example, slow caffeine metabolizers may experience prolonged exposure to caffeine’s metabolites, potentially amplifying its adverse effects on fetal development. Studies such as those by Bonaventure et al. (2013) highlight the importance of considering these genetic factors in understanding the risk associated with maternal caffeine intake [130].
Despite the suggestive evidence, several limitations warrant caution in interpreting these findings. First, most studies rely on self-reported dietary intake, which is subject to recall bias and inaccuracies. Second, confounding factors such as maternal diet, stress levels, and other lifestyle behaviors may influence the observed associations. While many studies attempt to adjust for these variables, residual confounding cannot be ruled out. Moreover, the heterogeneity of findings across studies underscores the complexity of this relationship. For instance, while some studies identify a dose–response relationship, others find no significant association, particularly for AML. Differences in study populations, sample sizes, and exposure assessment methods may contribute to these discrepancies. The relationship between maternal caffeine consumption during pregnancy and childhood acute leukemia remains an area of active investigation. Evidence suggests a modest association, particularly for ALL, with higher maternal coffee intake. However, the findings are not uniform, and further research is needed to elucidate the underlying mechanisms, account for genetic variability, and refine exposure assessment methods. Pregnant individuals may benefit from adhering to current recommendations to limit caffeine intake as a precautionary measure while future studies continue to explore this important public health question.
4. Conclusions
It is well acknowledged that prolonged chemical exposure during pregnancy should be taken seriously. Caution is important when the chemical under investigation is caffeine, which is a highly addictive and non-nutritional drug that is taken by almost everyone. There is a strong body of cumulative data linking caffeine usage by mothers to a variety of unfavorable pregnancy outcomes. Significant dose–response relationships indicative of causation are frequently reported in observational studies and meta-analyses, as well as numerous reports of no intake threshold below which associations are absent. As a result, the data available now do not support medical advice that suggests “moderate” caffeine use during pregnancy is safe. Contrarily, a growing body of scientific research suggests that women who are pregnant or considering becoming pregnant should steer clear of caffeine. Studies have produced experimental and epidemiological data indicating that examining the processes of caffeine response and genetic variations in more detail might open up new possibilities for precision medicine. Creating a quick and easy way to assess a person’s sensitivity to caffeine would help females monitor their own health as well as that of the fetus during pregnancy.
Author Contributions
Conceptualization, Y.M.A., A.K., R.A.F.Z., O.E.B.S., R.D., S.S.K. and M.G.B.K.; methodology, S.S.K., R.D. and M.G.B.K.; software, M.G.B.K. and S.S.K.; validation, S.S.K., R.D., M.G.B.K., Y.M.A., A.K., R.A.F.Z. and O.E.B.S.; formal analysis, S.S.K., R.D., M.G.B.K. and A.K.; investigation, Y.M.A., A.K., R.A.F.Z., O.E.B.S., R.D. and M.G.B.K.; resources, S.N.M.B., H.Z., S.S., H.C.G., S.S.K., R.D. and M.G.B.K.; data curation, S.S.K., R.D., M.G.B.K. and R.A.F.Z.; writing—original draft preparation, Y.M.A., A.K., R.A.F.Z., O.E.B.S., S.S.K. and R.D.; writing—review and editing, S.N.M.B., H.Z., S.S., H.C.G., S.S.K., R.D., M.G.B.K. and O.E.B.S.; visualization, S.N.M.B., H.Z., S.S., H.C.G., S.S.K. and R.D.; supervision, S.S.K., R.D., M.G.B.K., S.N.M.B., H.Z., S.S., H.C.G. and Y.M.A.; project administration, R.D. and S.S.K.; funding acquisition, none. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Barker, D.J. The fetal and infant origins of adult disease. BMJ 1990, 301, 1111. [Google Scholar] [CrossRef] [PubMed]
- Hoyt, A.T.; Browne, M.; Richardson, S.; Druschel, C.; National Birth Defects Prevention Study. Maternal caffeine consumption and small for gestational age births: Results from a population-based case-control study. Matern. Child. Health J. 2014, 18, 1540–1551. [Google Scholar] [CrossRef] [PubMed]
- Gahr, M. Caffeine, the most frequently consumed psychostimulant: A narrative review article. Fortschr. Neurol. Psychiatr. 2020, 88, 318–330. (In German) [Google Scholar] [CrossRef] [PubMed]
- Poole, R.; Kennedy, O.J.; Roderick, P.; Fallowfield, J.A.; Hayes, P.C.; Parkes, J. Coffee consumption and health: Umbrella review of meta-analyses of multiple health outcomes. BMJ 2017, 359, j5024. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Godos, J.; Galvano, F.; Giovannucci, E.L. Coffee, Caffeine, and Health Outcomes: An Umbrella Review. Annu. Rev. Nutr. 2017, 37, 131–156. [Google Scholar] [CrossRef] [PubMed]
- Galéra, C.; Bernard, J.Y.; van der Waerden, J.; Bouvard, M.P.; Lioret, S.; Forhan, A.; De Agostini, M.; Melchior, M.; Heude, B.; EDEN Mother-Child Cohort Study Group. Prenatal caffeine exposure and child IQ at age 5.5 years: The EDEN mother-child cohort. Biol. Psychiatry 2016, 80, 720–726. [Google Scholar] [CrossRef] [PubMed]
- James, J.E. Maternal caffeine consumption and pregnancy outcomes: A narrative review with implications for advice to mothers and mothers-to-be. BMJ Evid. Based Med. 2021, 26, 114–115. [Google Scholar] [CrossRef] [PubMed]
- Rohweder, R.; de Oliveira Schmalfuss, T.; Dos Santos Borniger, D.; Ferreira, C.Z.; Zanardini, M.K.; Lopes, G.P.T.F.; Barbosa, C.P.; Moreira, T.D.; Schuler-Faccini, L.; Sanseverino, M.T.V.; et al. Caffeine intake during pregnancy and adverse outcomes: An integrative review. Reprod. Toxicol. 2024, 123, 108518. [Google Scholar] [CrossRef] [PubMed]
- American College of Obstetricians and Gynecologists (ACOG). Committee Opinion No. 462: Moderate caffeine consumption during pregnancy. Obstet. Gynecol. 2010, 116, 467–468. [Google Scholar] [CrossRef] [PubMed]
- Millen, B.E.; Abrams, S.; Adams-Campbell, L.; Anderson, C.A.; Brenna, J.T.; Campbell, W.W.; Clinton, S.; Hu, F.; Nelson, M.; Neuhouser, M.L.; et al. The 2015 Dietary Guidelines Advisory Committee Scientific Report: Development and Major Conclusions. Adv. Nutr. 2016, 7, 438–444. [Google Scholar] [CrossRef] [PubMed]
- National Health Service. Should I Limit Caffeine During Pregnancy? 2018. Available online: https://www.nhs.uk/pregnancy/keeping-well/foods-to-avoid/ (accessed on 1 November 2024).
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies). Scientific Opinion on the safety of caffeine. EFSA J. 2015, 13, 4102. [Google Scholar] [CrossRef]
- Lakin, H.; Sheehan, P.; Soti, V. Maternal Caffeine Consumption and Its Impact on the Fetus: A Review. Cureus 2023, 15, e48266. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fernandes, O.; Sabharwal, M.; Smiley, T.; Pastuszak, A.; Koren, G.; Einarson, T. Moderate to heavy caffeine consumption during pregnancy and relationship to spontaneous abortion and abnormal fetal growth: A meta-analysis. Reprod. Toxicol. 1998, 12, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Santos, I.S.; Victora, C.G.; Huttly, S.; Carvalhal, J.B. Caffeine intake and low birth weight: A population-based case-control study. Am. J. Epidemiol. 1998, 147, 620–627. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Maslova, E.; Bhattacharya, S.; Lin, S.W.; Michels, K.B. Caffeine consumption during pregnancy and risk of preterm birth: A meta-analysis. Am. J. Clin. Nutr. 2010, 92, 1120–1132. [Google Scholar] [CrossRef]
- Greenwood, D.C.; Thatcher, N.J.; Ye, J.; Garrard, L.; Keogh, G.; King, L.G.; Cade, J.E. Caffeine intake during pregnancy and adverse birth outcomes: A systematic review and dose-response meta-analysis. Eur. J. Epidemiol. 2014, 29, 725–734. [Google Scholar] [CrossRef]
- Cheng, J.; Su, H.; Zhu, R.; Wang, X.; Peng, M.; Song, J.; Fan, D. Maternal coffee consumption during pregnancy and risk of childhood acute leukemia: A metaanalysis. Am. J. Obstet. Gynecol. 2014, 210, 151.e1–151.e10. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhao, H.; Song, J.-M.; Zhang, J.; Tang, Y.L.; Xin, C.M. A meta-analysis of risk of pregnancy loss and caffeine and coffee consumption during pregnancy. Int. J. Gynaecol. Obstet. 2015, 130, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Rhee, J.; Kim, R.; Kim, Y.; Tam, M.; Lai, Y.; Keum, N.; Oldenburg, C.E. Maternal caffeine consumption during pregnancy and risk of low birth weight: A dose-response meta-analysis of observational studies. PLoS ONE 2015, 10, e0132334. [Google Scholar] [CrossRef]
- Thomopoulos, T.P.; Ntouvelis, E.; Diamantaras, A.-A.; Tzanoudaki, M.; Baka, M.; Hatzipantelis, E.; Kourti, M.; Polychronopoulou, S.; Sidi, V.; Stiakaki, E.; et al. Maternal and childhood consumption of coffee, tea and cola beverages in association with childhood leukemia: A meta-analysis. Cancer Epidemiol. 2015, 39, 1047–1059. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.W.; Wu, Y.; Neelakantan, N.; Chong, M.F.; Pan, A.; van Dam, R.M. Maternal caffeine intake during pregnancy and risk of pregnancy loss: A categorical and dose-response meta-analysis of prospective studies. Public. Health Nutr. 2016, 19, 1233–1244. [Google Scholar] [CrossRef] [PubMed]
- Milne, E.; Greenop, K.R.; Petridou, E.; Bailey, H.D.; Orsi, L.; Kang, A.Y.; Baka, M.; Bonaventure, A.; Kourti, M.; Metayer, C.; et al. Maternal consumption of coffee and tea during pregnancy and risk of childhood all: A pooled analysis from the Childhood Leukemia International Consortium. Cancer Causes Control 2018, 29, 539–550. [Google Scholar] [CrossRef] [PubMed]
- Jin, F.; Qiao, C. Association of maternal caffeine intake during pregnancy with low birth weight, childhood overweight, and obesity: A meta-analysis of cohort studies. Int. J. Obes. 2021, 45, 279–287. [Google Scholar] [CrossRef]
- Soltani, S.; Salari-Moghaddam, A.; Saneei, P.; Askari, M.; Larijani, B.; Azadbakht, L.; Esmaillzadeh, A. Maternal caffeine consumption during pregnancy and risk of low birth weight: A dose–response meta-analysis of cohort studies. Crit. Rev. Food Sci. Nutr. 2021, 63, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Askari, M.; Bazshahi, E.; Payande, N.; Mobaderi, T.; Fahimfar, N.; Azadbakht, L. Relationship between caffeine intake and small for gestational age and preterm birth: A dose-response meta-analysis. Crit. Rev. Food Sci. Nutr. 2023, 64, 6942–6952. [Google Scholar] [CrossRef] [PubMed]
- CARE Study Group. Maternal caffeine intake during pregnancy and risk of fetal growth restriction: A large prospective observational study. BMJ 2008, 337, a2332, Erratum in: BMJ 2010, 340, c2331. https://doi.org/10.1136/bmj.c2331. [Google Scholar] [CrossRef] [PubMed]
- Signorello, L.B.; Nordmark, A.; Granath, F.; Blot, W.J.; McLaughlin, J.K.; Annerén, G.; Lundgren, S.; Ekbom, A.; Rane, A.; Cnattingius, S. Caffeine metabolism and the risk of spontaneous abortion of normal karyotype fetuses. Obstet. Gynecol. 2001, 98, 1059–1066. [Google Scholar] [CrossRef] [PubMed]
- Grosso, L.M.; Triche, E.W.; Belanger, K.; Benowitz, N.L.; Holford, T.R.; Bracken, M.B. Caffeine metabolites in umbilical cord blood, cytochrome P-450 1A2 activity, and intrauterine growth restriction. Am. J. Epidemiol. 2006, 163, 1035–1041. [Google Scholar] [CrossRef] [PubMed]
- Sata, F.; Yamada, H.; Suzuki, K.; Saijo, Y.; Kato, E.H.; Morikawa, M.; Minakami, H.; Kishi, R. Caffeine intake, CYP1A2 polymorphism and the risk of recurrent pregnancy loss. Mol. Hum. Reprod. 2005, 11, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, S.; Limpar, M.; Sata, F.; Kobayashi, S.; Kishi, R. Interaction between maternal caffeine intake during pregnancy and CYP1A2 C164A polymorphism affects infant birth size in the Hokkaido study. Pediatr. Res. 2017, 82, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Cavalli, G.; Heard, E. Advances in epigenetics link genetics to the environment and disease. Nature 2019, 571, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Panzeri, I.; Pospisilik, J.A. Epigenetic control of variation and stochasticity in metabolic disease. Mol. Metab. 2018, 14, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Nehlig, A. Interindividual Differences in Caffeine Metabolism and Factors Driving Caffeine Consumption. Pharmacol. Rev. 2018, 70, 384–411. [Google Scholar] [CrossRef]
- Gunes, A.; Dahl, M.L. Variation in CYP1A2 activity and its clinical implications: Influence of environmental factors and genetic polymorphisms. Pharmacogenomics 2008, 9, 625–637. [Google Scholar] [CrossRef]
- Zhou, S.F.; Wang, B.; Yang, L.P.; Liu, J.P. Structure, function, regulation and polymorphism and the clinical significance of human cytochrome P450 1A2. Drug Metab Rev. 2010, 42, 268–354. [Google Scholar] [CrossRef]
- Klein, K.; Winter, S.; Turpeinen, M.; Schwab, M.; Zanger, U.M. Pathway-Targeted Pharmacogenomics of CYP1A2 in Human Liver. Front. Pharmacol. 2010, 1, 129. [Google Scholar] [CrossRef] [PubMed]
- Bech, B.H.; Autrup, H.; Nohr, E.A.; Henriksen, T.B.; Olsen, J. Stillbirth and slow metabolizers of caffeine: Comparison by genotypes. Int. J. Epidemiol. 2006, 35, 948–953. [Google Scholar] [CrossRef] [PubMed]
- Copenhagen: Nordic Council of Ministers. (TemaNord). Intake of Caffeine and Other Methylxanthines During Pregnancy and Risk for Adverse Effects in Pregnant Women and their Foetuses. Available online: https://urn.kb.se/resolve?urn=urn:nbn:se:norden:org:diva-1860 (accessed on 1 March 2024).
- Aldridge, A.; Bailey, J.; Neims, A.H. The disposition of caffeine during and after pregnancy. Semin. Perinatol. 1981, 5, 310–314. [Google Scholar]
- Temple, J.L.; Bernard, C.; Lipshultz, S.E.; Czachor, J.D.; Westphal, J.A.; Mestre, M.A. The Safety of Ingested Caffeine: A Comprehensive Review. Front. Psychiatry 2017, 8, 80. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Campbell, S.C.; Stockmann, C.; Tak, C.; Schoen, K.; Clark, E.A.; Varner, M.W.; Spigarelli, M.G.; Sherwin, C.M. Pregnancy-induced changes in the pharmacokinetics of caffeine and its metabolites. J. Clin. Pharmacol. 2016, 56, 590–596. [Google Scholar] [CrossRef]
- Fredholm, B.B.; Bättig, K.; Holmén, J.; Nehlig, A.; Zvartau, E.E. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol. Rev. 1999, 51, 83–133. [Google Scholar] [CrossRef] [PubMed]
- Tsutsumi, K.; Kotegawa, T.; Matsuki, S.; Tanaka, Y.; Ishii, Y.; Kodama, Y.; Kuranari, M.; Miyakawa, I.; Nakano, S. The effect of pregnancy on cytochrome P4501A2, xanthine oxidase, and N-acetyltransferase activities in humans. Clin. Pharmacol. Ther. 2001, 70, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Q.; Wang, H.; Duan, E. Uterine Fluid in Pregnancy: A Biological and Clinical Outlook. Trends Mol. Med. 2017, 23, 604–614. [Google Scholar] [CrossRef] [PubMed]
- Grosso, L.M.; Bracken, M.B. Caffeine metabolism, genetics, and perinatal outcomes: A review of exposure assessment considerations during pregnancy. Ann. Epidemiol. 2005, 15, 460–466. [Google Scholar] [CrossRef]
- Kirkinen, P.; Jouppila, P.; Koivula, A.; Vuori, J.; Puukka, M. The effect of caffeine on placental and fetal blood flow in human pregnancy. Am. J. Obstet. Gynecol. 1983, 147, 939–942. [Google Scholar] [CrossRef]
- Resch, B.A.; Papp, J.G.; Gyöngyösi, J.; Széll, S.J. Die Wirkung des Koffeins auf die fetale Herzfrequenz und die Koffeinkonsum-Gewohnheiten der Schwangeren [Effect of caffeine on fetal heart rate and caffeine consumption habits in pregnant patients]. Zentralbl. Gynakol. 1985, 107, 1249–1253. (In German) [Google Scholar]
- Wierzejska, R. Caffeine--common ingredient in a diet and its influence on human health. Rocz. Panstw. Zakl. Hig. 2012, 63, 141–147. [Google Scholar]
- Jarosz, M.; Wierzejska, R.; Siuba, M. Maternal caffeine intake and its effect on pregnancy outcomes. Eur. J. Obstet. Gynecol. Reprod. Biol. 2012, 160, 156–160. [Google Scholar] [CrossRef]
- Dlugosz, L.; Bracken, M.B. Reproductive effects of caffeine: A review and theoretical analysis. Epidemiol. Rev. 1992, 14, 83–100. [Google Scholar] [CrossRef] [PubMed]
- Brazier, J.L.; Ritter, J.; Berland, M.; Khenfer, D.; Faucon, G. Pharmacokinetics of caffeine during and after pregnancy. Dev. Pharmacol. Ther. 1983, 6, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Sims, M.A.; Collins, K.A. Fetal death: A 10-year retrospective study. Am. J. Forensic Med. Pathol. 2001, 22, 261–265. [Google Scholar] [CrossRef]
- Feresu, S.A.; Harlow, S.D.; Welch, K.; Gillespie, B.W. Incidence of and socio-demographic risk factors for stillbirth, preterm birth and low birthweight among Zimbabwean women. Paediatr. Perinat. Epidemiol. 2004, 18, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Dube, R.; Al-Zuheiri, S.T.S.; Syed, M.; Harilal, L.; Zuhaira, D.A.L.; Kar, S.S. Prevalence, Clinico-Bacteriological Profile, and Antibiotic Resistance of Symptomatic Urinary Tract Infections in Pregnant Women. Antibiotics 2022, 12, 33. [Google Scholar] [CrossRef]
- Robinson, G.E. Pregnancy loss. Best. Pract. Res. Clin. Obstet. Gynaecol. 2014, 28, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Lucero, J.; Harlow, B.L.; Barbieri, R.L.; Sluss, P.; Cramer, D.W. Early follicular phase hormone levels in relation to patterns of alcohol, tobacco, and coffee use. Fertil. Steril. 2001, 76, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Lawson, C.C.; LeMasters, G.K.; Levin, L.S.; Liu, J.H. Pregnancy hormone metabolite patterns, pregnancy symptoms, and coffee consumption. Am. J. Epidemiol. 2002, 156, 428–437. [Google Scholar] [CrossRef] [PubMed]
- Kotsopoulos, J.; Eliassen, A.H.; Missmer, S.A.; Hankinson, S.E.; Tworoger, S.S. Relationship between caffeine intake and plasma sex hormone concentrations in premenopausal and postmenopausal women. Cancer 2009, 115, 2765–2774. [Google Scholar] [CrossRef] [PubMed]
- Schliep, K.C.; Schisterman, E.F.; Mumford, S.L.; Pollack, A.Z.; Zhang, C.; Ye, A.; Stanford, J.B.; Hammoud, A.O.; Porucznik, C.A.; Wactawski-Wende, J. Caffeinated beverage intake and reproductive hormones among premenopausal women in the BioCycle Study. Am. J. Clin. Nutr. 2012, 95, 488–497. [Google Scholar] [CrossRef] [PubMed]
- Savitz, D.A.; Chan, R.L.; Herring, A.H.; Howards, P.P.; Hartmann, K.E. Caffeine and miscarriage risk. Epidemiology 2008, 19, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Weng, X.; Odouli, R.; Li, D.K. Maternal caffeine consumption during pregnancy and the risk of miscarriage: A prospective cohort study. Am. J. Obstet. Gynecol. 2008, 198, 279. [Google Scholar] [CrossRef]
- Purdue-Smithe, A.; Kim, K.; Schisterman, E.; Schliep, K.; Perkins, N.; Sjaarda, L.; Mills, J.; Silver, R.; Andriessen, V.; Alkhalaf, Z.; et al. Caffeinated beverage intake and serum caffeine metabolites and risk of pregnancy loss (OR17-04-19). Curr. Dev. Nutrit. 2019, 3, 19. [Google Scholar] [CrossRef]
- Gaskins, A.J.; Rich-Edwards, J.W.; Williams, P.L.; Toth, T.L.; Missmer, S.A.; Chavarro, J.E. Pre-pregnancy caffeine and caffeinated beverage intake and risk of spontaneous abortion. Eur. J. Nutr. 2018, 57, 107–117. [Google Scholar] [CrossRef]
- Morales-Suárez-Varela, M.; Nohr, E.A.; Olsen, J.; Bech, B.H. Potential combined effects of maternal smoking and coffee intake on foetal death within the Danish National Birth Cohort. Eur. J. Public Health 2018, 28, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Hahn, K.A.; Wise, L.A.; Rothman, K.J.; Mikkelsen, E.M.; Brogly, S.B.; Sørensen, H.T.; Riis, A.H.; Hatch, E.E. Caffeine and caffeinated beverage consumption and risk of spontaneous abortion. Hum. Reprod. 2015, 30, 1246–1255. [Google Scholar] [CrossRef] [PubMed]
- Agnesi, R.; Valentini, F.; Fedeli, U.; Rylander, R.; Meneghetti, M.; Fadda, E.; Buja, A.; Mastrangelo, G. Maternal exposures and risk of spontaneous abortion before and after a community oriented health education campaign. Eur. J. Public Health 2011, 21, 282–285. [Google Scholar] [CrossRef]
- Stefanidou, E.M.; Caramellino, L.; Patriarca, A.; Menato, G. Maternal caffeine consumption and sine causa recurrent miscarriage. Eur. J. Obstet. Gynecol. Reprod. Biol. 2011, 158, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Pollack, A.Z.; Buck Louis, G.M.; Sundaram, R.; Lum, K.J. Caffeine consumption and miscarriage: A prospective cohort study. Fertil. Steril. 2010, 93, 304–306. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.Y.; Wei, Y.S.; Niu, J.M.; Li, Y.; Miao, Z.L.; Wang, Z.N. Risk factors for unexplained recurrent spontaneous abortion in a population from southern China. Int. J. Gynaecol. Obstet. 2010, 108, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Maconochie, N.; Doyle, P.; Prior, S.; Simmons, R. Risk factors for first trimester miscarriage–results from a UK-population-based case-control study. BJOG 2007, 114, 170–186. [Google Scholar] [CrossRef]
- Khoury, J.C.; Miodovnik, M.; Buncher, C.R.; Kalkwarf, H.; McElvy, S.; Khoury, P.R.; Sibai, B. Consequences of smoking and caffeine consumption during pregnancy in women with type 1 diabetes. J. Matern. Fetal Neonatal Med. 2004, 15, 44–50. [Google Scholar] [CrossRef]
- Rasch, V. Cigarette, alcohol, and caffeine consumption: Risk factors for spontaneous abortion. Acta Obstet. Gynecol. Scand. 2003, 82, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Giannelli, M.; Doyle, P.; Roman, E.; Pelerin, M.; Hermon, C. The effect of caffeine consumption and nausea on the risk of miscarriage. Paediatr. Perinatal. Epidemiol. 2003, 17, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Tolstrup, J.S.; Kjaer, S.K.; Munk, C.; Madsen, L.B.; Ottesen, B.; Bergholt, T.; Grønbaek, M. Does caffeine and alcohol intake before pregnancy predict the occurrence of spontaneous abortion? Hum. Reprod. 2003, 18, 2704–2710. [Google Scholar] [CrossRef] [PubMed]
- Wen, W.; Shu, X.O.; Jacobs, D.R., Jr.; Brown, J.E. The associations of maternal caffeine consumption and nausea with spontaneous abortion. Epidemiology 2001, 12, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Cnattingius, S.; Signorello, L.B.; Annerén, G.; Clausson, B.; Ekbom, A.; Ljunger, E.; Blot, W.J.; McLaughlin, J.K.; Petersson, G.; Rane, A.; et al. Caffeine intake and the risk of first-trimester spontaneous abortion. N. Engl. J. Med. 2000, 343, 1839–1845. [Google Scholar] [CrossRef] [PubMed]
- Parazzini, F.; Chatenoud, L.; Di Cintio, E.; Mezzopane, R.; Surace, M.; Zanconato, G.; Fedele, L.; Benzi, G. Coffee consumption and risk of hospitalized miscarriage before 12 weeks of gestation. Hum. Reprod. 1998, 13, 2286–2291. [Google Scholar] [CrossRef][Green Version]
- Fenster, L.; Hubbard, A.E.; Swan, S.H.; Windham, G.C.; Waller, K.; Hiatt, R.A.; Benowitz, N. Caffeinated beverages, decaffeinated coffee, and spontaneous abortion. Epidemiology 1997, 8, 515–523. [Google Scholar] [CrossRef] [PubMed]
- al-Ansary, L.A.; Babay, Z.A. Risk factors for spontaneous abortion: A preliminary study on Saudi women. J. R. Soc. Health. 1994, 114, 188–193. [Google Scholar] [CrossRef]
- Domínguez-Rojas, V.; de Juanes-Pardo, J.R.; Astasio-Arbiza, P.; Ortega-Molina, P.; Gordillo-Florencio, E. Spontaneous abortion in a hospital population: Are tobacco and coffee intake risk factors? Eur. J. Epidemiol. 1994, 10, 665–668. [Google Scholar] [CrossRef]
- Infante Rivard, C.; Fernández, A.; Gauthier, R.; David, M.; Rivard, G.E. Fetal loss associated with caffeine intake before and during pregnancy. JAMA 1993, 270, 2940–2943. [Google Scholar] [CrossRef]
- Mills, J.L.; Holmes, L.B.; Aarons, J.H.; Simpson, J.L.; Brown, Z.A.; Jovanovic-Peterson, L.G.; Conley, M.R.; Graubard, B.I.; Knopp, R.H.; Metzger, B.E. Moderate caffeine use and the risk of spontaneous abortion and intrauterine growth retardation. JAMA 1993, 269, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, B.G.; McDonald, A.D.; Sloan, M. Cigarette, alcohol, and coffee consumption and spontaneous abortion. Am. J. Public Health 1992, 82, 85–87. [Google Scholar] [CrossRef]
- Parazzini, F.; Bocciolone, L.; Fedele, L.; Negri, E.; La Vecchia, C.; Acaia, B. Risk factors for spontaneous abortion. Int. J. Epidemiol. 1991, 20, 157–161. [Google Scholar] [CrossRef]
- Fenster, L.; Eskenazi, B.; Windham, G.C.; Swan, S.H. Caffeine consumption during pregnancy and spontaneous abortion. Epidemiology 1991, 2, 168–174. [Google Scholar] [CrossRef]
- Wilcox, A.J.; Weinberg, C.R.; Baird, D.D. Risk factors for early pregnancy loss. Epidemiology 1990, 1, 382–385. [Google Scholar] [CrossRef] [PubMed]
- Axelsson, G.; Rylander, R.; Molin, I. Outcome of pregnancy in relation to irregular and inconvenient work schedules. Br. J. Ind. Med. 1989, 46, 393–398. [Google Scholar] [CrossRef]
- Wisborg, K.; Kesmodel, U.; Bech, B.H.; Hedegaard, M.; Henriksen, T.B. Maternal consumption of coffee during pregnancy and stillbirth and infant death in first year of life: Prospective study. BMJ 2003, 326, 420. [Google Scholar] [CrossRef]
- Weathersbee, P.S.; Lodge, J.R. Caffeine: Its direct and indirect influence on reproduction. J. Reprod. Med. 1977, 19, 55–63. [Google Scholar] [PubMed]
- Heazell, A.E.P.; Timms, K.; Scott, R.E.; Rockliffe, L.; Budd, J.; Li, M.; Cronin, R.; McCowan, L.M.E.; Mitchell, E.A.; Stacey, T.; et al. Associations between consumption of coffee and caffeinated soft drinks and late stillbirth-Findings from the Midland and North of England stillbirth case-control study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 256, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Matijasevich, A.; Barros, F.C.; Santos, I.S.; Yemini, A. Maternal caffeine consumption and fetal death: A case-control study in Uruguay. Paediatr. Perinat. Epidemiol. 2006, 20, 100–109. [Google Scholar] [CrossRef]
- Bech, B.H.; Obel, C.; Henriksen, T.B.; Olsen, J. Effect of reducing caffeine intake on birth weight and length of gestation: Randomized controlled trial. BMJ 2007, 334, 409. [Google Scholar] [CrossRef]
- Dube, R.; Bambani, T.; Saif, S.; Hashmi, N.; Patni, M.A.M.F.; Kedia, N.R. The Prevalence of Gestational Diabetes Mellitus in Polycystic Ovary Disease—A Systematic Review, Meta-Analysis, and Exploration of Associated Risk Factors. Diabetology 2024, 5, 430–446. [Google Scholar] [CrossRef]
- AlZuheiri, S.T.; Dube, R.; Menezes, G.; Qasem, S. Clinical profile and outcome of Group B streptococcal colonization in mothers and neonates in Ras Al Khaimah, United Arab Emirates: A prospective observational study. Saudi J. Med. Med. Sci. 2021, 9, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Fatima, A.; Abuhijleh, S.A.; Fatah, A.; Mohsin, M.M.; Kar, S.S.; Dube, R.; George, B.T.; Kuruba, M.G.B. Infantile Neuroaxonal Dystrophy: Case Report and Review of Literature. Medicina 2024, 60, 1322. [Google Scholar] [CrossRef]
- Kukkonen, A.; Hantunen, S.; Voutilainen, A.; Ruusunen, A.; Backman, K.; Kirjavainen, P.V.; Ylilauri, M.; Voutilainen, R.; Pasanen, M.; Keski-Nisula, L. Maternal caffeine intake during pregnancy and the risk of delivering a small for gestational age baby: Kuopio Birth Cohort. Arch. Gynecol. Obstet. 2024, 310, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Gleason, J.L.; Tekola-Ayele, F.; Sundaram, R.; Hinkle, S.N.; Vafai, Y.; Buck Louis, G.M.; Gerlanc, N.; Amyx, M.; Bever, A.M.; Smarr, M.M.; et al. Association Between Maternal Caffeine Consumption and Metabolism and Neonatal Anthropometry: A Secondary Analysis of the NICHD Fetal Growth Studies–Singletons. JAMA Netw. Open. 2021, 4, e213238. [Google Scholar] [CrossRef]
- Modzelewska, D.; Bellocco, R.; Elfvin, A.; Brantsæter, A.L.; Meltzer, H.M.; Jacobsson, B.; Sengpiel, V. Caffeine exposure during pregnancy, small for gestational age birth and neonatal outcome—Results from the Norwegian Mother and Child Cohort Study. BMC Pregnancy Childbirth 2019, 19, 80. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Sata, F.; Murata, K.; Saijo, Y.; Araki, A.; Miyashita, C.; Itoh, S.; Minatoya, M.; Yamazaki, K.; Ait Bamai, Y.; et al. Dose-dependent associations between prenatal caffeine consumption and small for gestational age, preterm birth, and reduced birthweight in the Japan Environment and Children’s Study. Paediatr. Perinat. Epidemiol. 2019, 33, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-W.; Fitzgerald, R.; Murrin, C.M.; Mehegan, J.; Kelleher, C.C.; Phillips, C.M.; Lifeways Cross Generation Cohort Study. Associations of maternal caffeine intake with birth outcomes: Results from the Lifeways cross generation cohort study. Am. J. Clin. Nutr. 2018, 108, 1301–1308. [Google Scholar] [CrossRef]
- van der Hoeven, T.; Browne, J.L.; Uiterwaal, C.S.P.M.; van der Ent, C.K.; Grobbee, D.E.; Dalmeijer, G.W. Antenatal coffee and tea consumption and the effect on birth outcome and hypertensive pregnancy disorders. PLoS ONE 2017, 12, e0177619. [Google Scholar] [CrossRef]
- Voerman, E.; Jaddoe, V.W.V.; Gishti, O.; Hofman, A.; Franco, O.H.; Gaillard, R. Maternal caffeine intake during pregnancy, early growth, and body fat distribution at school age. Obesity 2016, 24, 1170–1177. [Google Scholar] [CrossRef] [PubMed]
- Okubo, H.; Miyake, Y.; Tanaka, K.; Sasaki, S.; Hirota, Y. Maternal total caffeine intake, mainly from Japanese and Chinese tea, during pregnancy was associated with risk of preterm birth: The Osaka Maternal and Child Health Study. Nutr. Res. 2015, 35, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Bech, B.H.; Frydenberg, M.; Henriksen, T.B.; Obel, C.; Olsen, J. Coffee consumption during pregnancy and birth weight: Does smoking modify the association? J. Caffeine Res. 2015, 5, 65–72. [Google Scholar] [CrossRef]
- Sengpiel, V.; Elind, E.; Bacelis, J.; Nilsson, S.; Grove, J.; Myhre, R.; Haugen, M.; Meltzer, H.M.; Alexander, J.; Jacobsson, B.; et al. Maternal caffeine intake during pregnancy is associated with birth weight but not with gestational length: Results from a large prospective observational cohort study. BMC Med. 2013, 11, 42. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bakker, R.; Steegers, E.A.P.; Obradov, A.; Raat, H.; Hofman, A.; Jaddoe, V.W. Maternal caffeine intake from coffee and tea, fetal growth, and the risks of adverse birth outcomes: The generation R study. Am. J. Clin. Nutr. 2010, 91, 1691–1698. [Google Scholar] [CrossRef]
- Bracken, M.B.; Triche, E.W.; Belanger, K.; Hellenbrand, K.; Leaderer, B.P. Association of maternal caffeine consumption with decrements in fetal growth. Am. J. Epidemiol. 2003, 157, 456–466. [Google Scholar] [CrossRef] [PubMed]
- Clausson, B.; Granath, F.; Ekbom, A.; Lundgren, S.; Nordmark, A.; Signorello, L.B.; Cnattingius, S. Effect of caffeine exposure during pregnancy on birth weight and gestational age. Am. J. Epidemiol. 2002, 155, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Klebanoff, M.A.; Levine, R.J.; Clemens, J.D.; Wilkins, D.G. Maternal serum caffeine metabolites and small-for-gestational age birth. Am. J. Epidemiol. 2002, 155, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Dube, R.; Kar, S.S. Genital Microbiota and Outcome of Assisted Reproductive Treatment-A Systematic Review. Life 2022, 12, 1867. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kar, S.S.; Kar, S.S. Prevention of childhood obesity in India: Way forward. J. Nat. Sci. Biol. Med. 2015, 6, 12–17. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dube, R.; Kar, S.S. COVID-19 in pregnancy: The foetal perspective—A systematic review. BMJ Paediatr. Open 2020, 4, e000859. [Google Scholar] [CrossRef] [PubMed]
- Saigal, S.; Doyle, L.W. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet 2008, 371, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Irving, R.J.; Belton, N.R.; Elton, R.A.; Walker, B.R. Adult cardiovascular risk factors in premature babies. Lancet 2000, 355, 2135–2136. [Google Scholar] [CrossRef] [PubMed]
- Hofman, P.L.; Regan, F.; Jackson, W.E.; Jefferies, C.; Knight, D.B.; Robinson, E.M.; Cutfield, W.S. Premature birth and later insulin resistance. N. Engl. J. Med. 2004, 351, 2179–2186, Erratum in: N. Engl. J. Med. 2004, 351, 2888. [Google Scholar] [CrossRef]
- Chiaffarino, F.; Parazzini, F.; Chatenoud, L.; Ricci, E.; Tozzi, L.; Chiantera, V.; Maffioletti, C.; Fedele, L. Coffee drinking and risk of preterm birth. Eur. J. Clin. Nutr. 2006, 60, 610–613. [Google Scholar] [CrossRef]
- Jahanfar, S.; Jaafar, S.H. Effects of restricted caffeine intake by mother on fetal, neonatal and pregnancy outcomes. Cochr Datab Syst Rev. 2015, 2015, Cd006965. [Google Scholar] [CrossRef] [PubMed]
- Christensen, Z.P.; Freedman, E.G.; Foxe, J.J. Caffeine exposure in utero is associated with structural brain alterations and deleterious neurocognitive outcomes in 9-10 year old children. Neuropharmacology 2021, 186, 108479. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.F.; Eltzschig, H.K.; Fredholm, B.B. Adenosine receptors as drug targets--what are the challenges? Nat. Rev. Drug Discov. 2013, 12, 265–286. [Google Scholar] [CrossRef]
- Miyake, Y.; Tanaka, K.; Okubo, H.; Sasaki, S.; Arakawa, M. Maternal caffeine intake in pregnancy is inversely related to childhood peer problems in Japan: The Kyushu Okinawa Maternal and Child Health Study. Nutr. Neurosci. 2018, 22, 817–824. [Google Scholar] [CrossRef]
- Papadopoulou, E.; Botton, J.; Brantsæter, A.; Haugen, M.; Alexander, J.; Meltzer, H.M.; Bacelis, J.; Elfvin, A.; Jacobsson, B.; Sengpiel, V. Maternal caffeine intake during pregnancy and childhood growth and overweight: Results from a large Norwegian prospective observational cohort study. BMJ Open 2018, 8, e018895. [Google Scholar] [CrossRef] [PubMed]
- Gleason, J.L.; Sundaram, R.; Mitro, S.D.; Hinkle, S.N.; Gilman, S.E.; Zhang, C.; Newman, R.B.; Hunt, K.J.; Skupski, D.W.; Grobman, W.A.; et al. Association of Maternal Caffeine Consumption During Pregnancy with Child Growth. JAMA Netw. Open 2022, 5, e2239609, Erratum in: JAMA Netw. Open 2022, 5, e2247489. [Google Scholar] [CrossRef]
- Li, D.K.; Ferber, J.R.; Odouli, R. Maternal caffeine intake during pregnancy and risk of obesity in offspring: A prospective cohort study. Int. J. Obes. 2015, 39, 658–664. [Google Scholar] [CrossRef] [PubMed]
- Morgan, S.; Koren, G.; Bozzo, P. Is caffeine consumption safe during pregnancy? Can. Fam. Phys. 2013, 59, 361–362. [Google Scholar]
- Qian, J.; Chen, Q.; Ward, S.M.; Duan, E.; Zhang, Y. Impacts of Caffeine during Pregnancy. Trends Endocrinol. Metab. 2020, 31, 218–227. [Google Scholar] [CrossRef]
- Klebanoff, M.A.; Keim, S.A. Maternal serum paraxanthine during pregnancy and offspring body mass index at ages 4 and 7 years. Epidemiology 2015, 26, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Menegaux, F.; Ripert, M.; Hemon, D.; Clavel, J. Maternal alcohol and coffee drinking, parental smoking and childhood leukaemia: A French population-based case-control study. Paediatr. Perinat. Epidemiol. 2007, 21, 293–299. [Google Scholar] [CrossRef]
- Bonaventure, A.; Rudant, J.; Goujon-Bellec, S.; Orsi, L.; Leverger, G.; Baruchel, A.; Bertrand, Y.; Nelken, B.; Pasquet, M.; Michel, G.; et al. Childhood acute leukemia, maternal beverage intake during pregnancy, and metabolic polymorphisms. Cancer Causes Control 2013, 24, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Milne, E.; Royle, J.A.; Bennett, L.C.; de Klerk, N.H.; Bailey, H.D.; Bower, C.; Miller, M.; Attia, J.; Scott, R.J.; Kirby, M.; et al. Maternal consumption of coffee and tea during pregnancy and risk of childhood ALL: Results from an Australian case-control study. Cancer Causes Control 2011, 22, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Karalexi, M.A.; Dessypris, N.; Clavel, J.; Metayer, C.; Erdmann, F.; Orsi, L.; Kang, A.Y.; Schüz, J.; Bonaventure, A.; Greenop, K.R.; et al. Coffee and tea consumption during pregnancy and risk of childhood acute myeloid leukemia: A Childhood Leukemia International Consortium (CLIC) study. Cancer Epidemiol. 2019, 62, 101581. [Google Scholar] [CrossRef] [PubMed]
- Orsi, L.; Rudant, J.; Ajrouche, R.; Leverger, G.; Baruchel, A.; Nelken, B.; Pasquet, M.; Michel, G.; Bertrand, Y.; Ducassou, S.; et al. Parental smoking, maternal alcohol, coffee and tea consumption during pregnancy, and childhood acute leukemia: The ESTELLE study. Cancer Causes Control 2015, 26, 1003–1017. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).