Visualization of Atherosclerotic Plaques Paired with Joheksol 350 (Omnipaque)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Plaque Tissue Sample Preparation for MRI Measurement
2.2.2. MRI Examination
2.2.3. Optical Coherence Tomography
2.2.4. Statistical Analysis
3. Results
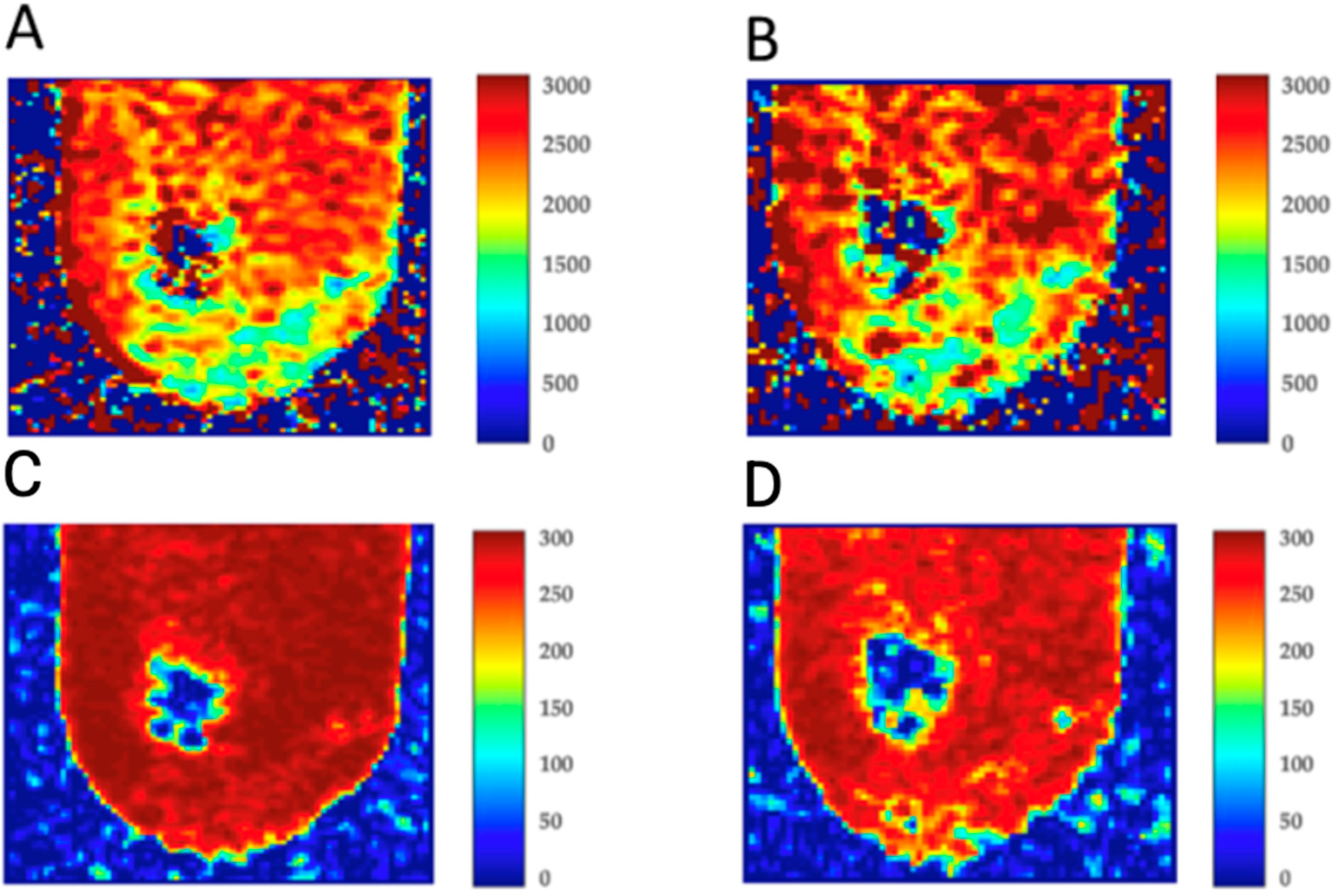
3.1. MRI Results
3.2. Imaging of Coronary Artery In Vivo
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CT | Computed tomography |
| MRI | Magnetic resonance imaging |
| OCT | Optical coherence tomography |
| ms | Milliseconds |
References
- Demina, E.P.; Miroshnikova, V.V.; Schwarzman, A.L. Role of the ABC transporters A1 and G1, key reverse cholesterol transport proteins, in atherosclerosis. Mol. Biol. 2016, 50, 223–230. (In Russian) [Google Scholar] [CrossRef]
- Luthi, A.J.; Patel, P.C.; Ko, C.H.; Mutharasan, R.K.; Mirkin, C.A.; Thaxton, C.S. Nanotechnology for synthetic high-density lipoproteins. Trends Mol. Med. 2010, 16, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Leaphart, D.; Waring, A.; Suranyi, P.; Fernandes, V. Call a Spade a Spade: Missed Diagnosis of Apical Hypertrophic Cardiomyopathy. Am. J. Med. Sci. 2019, 358, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Li, K.; Yang, K. Adipose-Derived Stem Cell Exosomes and Related microRNAs in Atherosclerotic Cardiovascular Disease. J. Cardiovasc. Transl. Res. 2023, 16, 453–462. [Google Scholar] [CrossRef]
- Almohtasib, Y.; Fancher, A.J.; Sawalha, K. Emerging Trends in Atherosclerosis: Time to Address Atherosclerosis From a Younger Age. Cureus 2024, 16, e56635. [Google Scholar] [CrossRef]
- Arbab-Zadeh, A.; Nakano, M.; Virmani, R.; Fuster, V. Acute coronary events. Circulation 2012, 125, 1147–1156. [Google Scholar] [CrossRef] [PubMed]
- Luca, A.C.; David, S.G.; David, A.G.; Țarcă, V.; Pădureț, I.A.; Mîndru, D.E.; Roșu, S.T.; Roșu, E.V.; Adumitrăchioaiei, H.; Bernic, J.; et al. Atherosclerosis from Newborn to Adult-Epidemiology, Pathological Aspects, and Risk Factors. Life 2023, 13, 2056. [Google Scholar] [CrossRef] [PubMed]
- Jebari-Benslaiman, S.; Galicia-García, U.; Larrea-Sebal, A.; Olaetxea, J.R.; Alloza, I.; Vandenbroeck, K.; Benito-Vicente, A.; Martín, C. Pathophysiology of Atherosclerosis. Int. J. Mol. Sci. 2022, 23, 3346. [Google Scholar] [CrossRef] [PubMed]
- Gusev, E.; Sarapultsev, A. Atherosclerosis and Inflammation: Insights from the Theory of General Pathological Processes. Int. J. Mol. Sci. 2023, 24, 7910. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Rodrigues, C.F.; Sharopov, F.; Docea, A.O.; Can Karaca, A.; Sharifi-Rad, M.; Kahveci Karıncaoglu, D.; Gülseren, G.; Şenol, E.; Demircan, E.; et al. Diet, Lifestyle and Cardiovascular Diseases: Linking Pathophysiology to Cardioprotective Effects of Natural Bioactive Compounds. Int. J. Environ. Res. Public Health 2020, 17, 2326. [Google Scholar] [CrossRef] [PubMed]
- Ziółkiewicz, A.; Kasprzak-Drozd, K.; Rusinek, R.; Markut-Miotła, E.; Oniszczuk, A. The Influence of Polyphenols on Atherosclerosis Development. Int. J. Mol. Sci. 2023, 24, 7146. [Google Scholar] [CrossRef] [PubMed]
- Kronenberg, F.; Mora, S.; Stroes, E.S.G.; Ference, B.A.; Arsenault, B.J.; Berglund, L.; Dweck, M.R.; Koschinsky, M.; Lambert, G.; Mach, F.; et al. Lipoprotein(a) in atherosclerotic cardiovascular disease and aortic stenosis: A European Atherosclerosis Society consensus statement. Eur. Heart J. 2022, 43, 3925–3946. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.B.; Mengi, S.A.; Xu, Y.J.; Arneja, A.S.; Dhalla, N.S. Pathogenesis of atherosclerosis: A multifactorial process. Exp. Clin. Cardiol. 2002, 7, 40–53. [Google Scholar]
- Rafieian-Kopaei, M.; Setorki, M.; Doudi, M.; Baradaran, A.; Nasri, H. Atherosclerosis: Process, indicators, risk factors and new hopes. Int. J. Prev. Med. 2014, 5, 927–946. [Google Scholar]
- Madaudo, C.; Coppola, G.; Parlati, A.L.M.; Corrado, E. Discovering Inflammation in Atherosclerosis: Insights from Pathogenic Pathways to Clinical Practice. Int. J. Mol. Sci. 2024, 25, 6016. [Google Scholar] [CrossRef] [PubMed]
- Lusis, A.J. Atherosclerosis. Nature 2000, 407, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Poznyak, A.V.; Sukhorukov, V.N.; Eremin, I.I.; Nadelyaeva, I.I.; Orekhov, A.N. Diagnostics of atherosclerosis: Overview of the existing methods. Front. Cardiovasc. Med. 2023, 10, 1134097. [Google Scholar] [CrossRef]
- Verjans, J.W.; Jaffer, F.A. Biological imaging of atherosclerosis: Moving beyond anatomy. J. Cardiovasc. Transl. Res. 2013, 6, 681–694. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.J.; Huang, Y.S.; An, L.N.; Han, X.Q.; Zhang, J.G.; Wang, Q.L.; Sun, J.; Wang, S.R. Effect of ozone produced from antibody-catalyzed water oxidation on pathogenesis of atherosclerosis. Acta Biochim. Biophys. Sin. 2006, 38, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Vinegoni, C.; Botnaru, I.; Aikawa, E.; Calfon, M.A.; Iwamoto, Y.; Folco, E.J.; Ntziachristos, V.; Weissleder, R.; Libby, P.; Jaffer, F.A. Indocyanine green enables near-infrared fluorescence imaging of lipid-rich, inflamed atherosclerotic plaques. Sci. Transl. Med. 2011, 3, 84ra45. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Khalafi, S.; Botero Fonnegra, C.; Reyes, A.; Hui, V.W. Developments in the Use of Indocyanine Green (ICG) Fluorescence in Colorectal Surgery. J. Clin. Med. 2024, 13, 4003. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Daghem, M.; Bing, R.; Fayad, Z.A.; Dweck, M.R. Noninvasive Imaging to Assess Atherosclerotic Plaque Composition and Disease Activity: Coronary and Carotid Applications. JACC Cardiovasc. Imaging 2020, 13, 1055–1068. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shishikura, D. Noninvasive imaging modalities to visualize atherosclerotic plaques. Cardiovasc. Diagn. Ther. 2016, 6, 340–353. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fujimoto, J.G.; Pitris, C.; Boppart, S.A.; Brezinski, M.E. Optical coherence tomography: An emerging technology for biomedical imaging and optical biopsy. Neoplasia 2000, 2, 9–25. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Spaide, R.F.; Fujimoto, J.G.; Waheed, N.K.; Sadda, S.R.; Staurenghi, G. Optical coherence tomography angiography. Prog. Retin. Eye Res. 2018, 64, 1–55. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Araki, M.; Park, S.J.; Dauerman, H.L.; Uemura, S.; Kim, J.S.; Di Mario, C.; Johnson, T.W.; Guagliumi, G.; Kastrati, A.; Joner, M.; et al. Optical coherence tomography in coronary atherosclerosis assessment and intervention. Nat. Rev. Cardiol. 2022, 19, 684–703, Erratum in: Nat. Rev. Cardiol. 2024, 21, 348. https://doi.org/10.1038/s41569-023-00982-z. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Coletta, J.; Suzuki, N.; Nascimento, B.R.; Bezerra, H.G.; Rosenthal, N.; Guagliumi, G.; Rollins, A.M.; Costa, M.A. Use of optical coherence tomography for accurate characterization of atherosclerosis. Arq. Bras. Cardiol. 2010, 94, 250–254, In Portuguese version pp. 268-272 and in Spanish version pp. 254-259. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Li, Z.; Wang, J.; Huang, Y.; Yin, Y.; Li, Z. Atherosclerotic Plaque Tissue Characterization: An OCT-Based Machine Learning Algorithm With ex vivo Validation. Front. Bioeng. Biotechnol. 2020, 8, 749. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cao, J.J.; Shen, L.; Nguyen, J.; Rapelje, K.; Porter, C.; Shlofmitz, E.; Jeremias, A.; Cohen, D.J.; Ali, Z.A.; Shlofmitz, R. Accuracy and limitation of plaque detection by coronary CTA: A section-to-section comparison with optical coherence tomography. Sci. Rep. 2023, 13, 11845. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pang, W.; Yuan, C.; Zhong, T.; Huang, X.; Pan, Y.; Qu, J.; Nie, L.; Zhou, Y.; Lai, P. Diagnostic and therapeutic optical imaging in cardiovascular diseases. iScience 2024, 27, 111216. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Popescu, D.P.; Choo-Smith, L.P.; Flueraru, C.; Mao, Y.; Chang, S.; Disano, J.; Sherif, S.; Sowa, M.G. Optical coherence tomography: Fundamental principles, instrumental designs and biomedical applications. Biophys. Rev. 2011, 3, 155. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Matcher, S.J. Practical aspects of OCT imaging in tissue engineering. Methods Mol. Biol. 2011, 695, 261–280. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yuan, S.; Qi, J.; Zhang, Q.; Ji, Z. Advantages and prospects of optical coherence tomography in interventional therapy of coronary heart disease (Review). Exp. Ther. Med. 2022, 23, 255. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Terashima, M.; Kaneda, H.; Suzuki, T. The role of optical coherence tomography in coronary intervention. Korean J. Intern. Med. 2012, 27, 1–12. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, A.; Qi, W.; Gao, T.; Tang, X. Molecular Contrast Optical Coherence Tomography and Its Applications in Medicine. Int. J. Mol. Sci. 2022, 23, 3038. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pierce, M.C.; Javier, D.J.; Richards-Kortum, R. Optical contrast agents and imaging systems for detection and diagnosis of cancer. Int. J. Cancer 2008, 123, 1979–1990. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, W.; Schilperoort, M.; Cao, Y.; Shi, J.; Tabas, I.; Tao, W. Macrophage-targeted nanomedicine for the diagnosis and treatment of atherosclerosis. Nat. Rev. Cardiol. 2022, 19, 228–249. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bagalkot, V.; Badgeley, M.A.; Kampfrath, T.; Deiuliis, J.A.; Rajagopalan, S.; Maiseyeu, A. Hybrid nanoparticles improve targeting to inflammatory macrophages through phagocytic signals. J. Control Release 2015, 217, 243–255. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wei, X.; Runnels, J.M.; Lin, C.P. Selective uptake of indocyanine green by reticulocytes in circulation. Investig. Ophthalmol. Vis. Sci. 2003, 44, 4489–4496. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alander, J.T.; Kaartinen, I.; Laakso, A.; Pätilä, T.; Spillmann, T.; Tuchin, V.V.; Venermo, M.; Välisuo, P. A review of indocyanine green fluorescent imaging in surgery. Int. J. Biomed. Imaging 2012, 2012, 940585. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Langer, H.F.; Haubner, R.; Pichler, B.J.; Gawaz, M. Radionuclide imaging: A molecular key to the atherosclerotic plaque. J. Am. Coll. Cardiol. 2008, 52, 1–12. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Varga-Szemes, A.; Maurovich-Horvat, P.; Schoepf, U.J.; Zsarnoczay, E.; Pelberg, R.; Stone, G.W.; Budoff, M.J. Computed Tomography Assessment of Coronary Atherosclerosis: From Threshold-Based Evaluation to Histologically Validated Plaque Quantification. J. Thorac. Imaging 2023, 38, 226–234. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Goel, S.; Miller, A.; Agarwal, C.; Zakin, E.; Acholonu, M.; Gidwani, U.; Sharma, A.; Kulbak, G.; Shani, J.; Chen, O. Imaging Modalities to Identity Inflammation in an Atherosclerotic Plaque. Radiol. Res. Pract. 2015, 2015, 410967. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fernández-Alvarez, V.; Linares-Sánchez, M.; Suárez, C.; López, F.; Guntinas-Lichius, O.; Mäkitie, A.A.; Bradley, P.J.; Ferlito, A. Novel Imaging-Based Biomarkers for Identifying Carotid Plaque Vulnerability. Biomolecules 2023, 13, 1236. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alonso-Herranz, L.; Albarrán-Juárez, J.; Bentzon, J.F. Mechanisms of fibrous cap formation in atherosclerosis. Front. Cardiovasc. Med. 2023, 10, 1254114. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stefanadis, C.; Antoniou, C.K.; Tsiachris, D.; Pietri, P. Coronary Atherosclerotic Vulnerable Plaque: Current Perspectives. J. Am. Heart Assoc. 2017, 6, e005543. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chamié, D.; Wang, Z.; Bezerra, H.; Rollins, A.M.; Costa, M.A. Optical Coherence Tomography and Fibrous Cap Characterization. Curr. Cardiovasc. Imaging Rep. 2011, 4, 276–283. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Toutouzas, K.; Karanasos, A.; Tousoulis, D. Optical Coherence Tomography For the Detection of the Vulnerable Plaque. Eur. Cardiol. 2016, 11, 90–95. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, Y.K.; Kwak, H.S.; Chung, G.H.; Hwang, S.B. Lipid-Rich Necrotic Core of Basilar Artery Atherosclerotic Plaque: Contrast-Enhanced Black Blood Imaging on Vessel Wall Imaging. Diagnostics 2019, 9, 69. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xia, J.; Yin, A.; Li, Z.; Liu, X.; Peng, X.; Xie, N. Quantitative Analysis of Lipid-Rich Necrotic Core in Carotid Atherosclerotic Plaques by In Vivo Magnetic Resonance Imaging and Clinical Outcomes. Med. Sci. Monit. 2017, 23, 2745–2750. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Onea, H.L.; Olinic, M.; Lazar, F.L.; Homorodean, C.; Ober, M.C.; Spinu, M.; Achim, A.; Tataru, D.A.; Olinic, D.M. A Review Paper on Optical Coherence Tomography Evaluation of Coronary Calcification Pattern: Is It Relevant Today? J. Cardiovasc. Dev. Dis. 2024, 11, 231. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Summary of Product Characteristics for CONTRAPAQUE 350. Available online: http://www.efda.gov.et/wp-content/uploads/2023/08/Iohexol-Injection-USP-350-mg-I-mL-CONTRAPAQUE-350-UNIQUE-PHARMACEUTICAL-LABORATORIES.pdf (accessed on 22 December 2024).
- Ghodeshwar, G.K.; Dube, A.; Khobragade, D. Impact of Lifestyle Modifications on Cardiovascular Health: A Narrative Review. Cureus 2023, 15, e42616. [Google Scholar] [CrossRef] [PubMed]
- Rippe, J.M. Lifestyle Strategies for Risk Factor Reduction, Prevention, and Treatment of Cardiovascular Disease. Am. J. Lifestyle Med. 2018, 13, 204–212. [Google Scholar] [CrossRef]
- Sizar, O.; Khare, S.; Patel, P.; Talati, R. Statin Medications. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Yachmaneni, A., Jr.; Jajoo, S.; Mahakalkar, C.; Kshirsagar, S.; Dhole, S. A Comprehensive Review of the Vascular Consequences of Diabetes in the Lower Extremities: Current Approaches to Management and Evaluation of Clinical Outcomes. Cureus 2023, 15, e47525. [Google Scholar] [CrossRef] [PubMed]
- Chhabra, L.; Zain, M.A.; Siddiqui, W.J. Angioplasty. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Jongsma, H.; Bekken, J.; Ayez, N.; Hoogewerf, C.J.; Van Weel, V.; Fioole, B. Angioplasty versus stenting for iliac artery lesions. Cochrane Database Syst. Rev. 2020, 12, CD007561. [Google Scholar] [PubMed]
- Harris, R.; Croce, B.; Tian, D.H. Coronary artery bypass grafting. Ann. Cardiothorac. Surg. 2013, 2, 579. [Google Scholar]
- DaCosta, M.; Tadi, P.; Surowiec, S.M. Carotid Endarterectomy. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Rerkasem, A.; Orrapin, S.; Howard, D.P.; Rerkasem, K. Carotid endarterectomy for symptomatic carotid stenosis. Cochrane Database Syst. Rev. 2020, 9, CD001081. [Google Scholar]
- Tarkin, J.M.; Dweck, M.R.; Evans, N.R.; Takx, R.A.; Brown, A.J.; Tawakol, A.; Fayad, Z.A.; Rudd, J.H. Imaging Atherosclerosis. Circ. Res. 2016, 118, 750–769. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Truong, M.; Lennartsson, F.; Bibic, A.; Sundius, L.; Persson, A.; Siemund, R.; In’t Zandt, R.; Goncalves, I.; Wassélius, J. Classifications of atherosclerotic plaque components with T1 and T2* mapping in 11.7 T MRI. Eur. J. Radiol. Open 2021, 8, 100323. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Berger, A. Magnetic resonance imaging. BMJ 2002, 324, 35. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Babaniamansour, P.; Mohammadi, M.; Babaniamansour, S.; Aliniagerdroudbari, E. The Relation between Atherosclerosis Plaque Composition and Plaque Rupture. J. Med. Signals Sens. 2020, 10, 267–273. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Benson, J.C.; Saba, L.; Bathla, G.; Brinjikji, W.; Nardi, V.; Lanzino, G. MR Imaging of Carotid Artery Atherosclerosis: Updated Evidence on High-Risk Plaque Features and Emerging Trends. AJNR Am. J. Neuroradiol. 2023, 44, 880–888. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dong, L.; Kerwin, W.S.; Ferguson, M.S.; Li, R.; Wang, J.; Chen, H.; Canton, G.; Hatsukami, T.S.; Yuan, C. Cardiovascular magnetic resonance in carotid atherosclerotic disease. J. Cardiovasc. Magn. Reson. 2009, 11, 53. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wu, Z.; Mittal, S.; Kish, K.; Yu, Y.; Hu, J.; Haacke, E.M. Identification of calcification with MRI using susceptibility-weighted imaging: A case study. J. Magn. Reson. Imaging 2009, 29, 177–182. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mushenkova, N.V.; Summerhill, V.I.; Zhang, D.; Romanenko, E.B.; Grechko, A.V.; Orekhov, A.N. Current Advances in the Diagnostic Imaging of Atherosclerosis: Insights into the Pathophysiology of Vulnerable Plaque. Int. J. Mol. Sci. 2020, 21, 2992. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Saba, L.; Loewe, C.; Weikert, T.; Williams, M.C.; Galea, N.; Budde, R.P.J.; Vliegenthart, R.; Velthuis, B.K.; Francone, M.; Bremerich, J.; et al. State-of-the-art CT and MR imaging and assessment of atherosclerotic carotid artery disease: Standardization of scanning protocols and measurements-a consensus document by the European Society of Cardiovascular Radiology (ESCR). Eur. Radiol. 2023, 33, 1063–1087, Erratum in: Eur. Radiol. 2023, 33, 1497–1498. https://doi.org/10.1007/s00330-022-09246-9. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kramer, C.M.; Anderson, J.D. MRI of atherosclerosis: Diagnosis and monitoring therapy. Expert. Rev. Cardiovasc. Ther. 2007, 5, 69–80. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Andelovic, K.; Winter, P.; Jakob, P.M.; Bauer, W.R.; Herold, V.; Zernecke, A. Evaluation of Plaque Characteristics and Inflammation Using Magnetic Resonance Imaging. Biomedicines 2021, 9, 185. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhou, P.; Wang, Y.; Sun, J.; Yu, Y.; Mossa-Basha, M.; Zhu, C. Assessment of Therapeutic Response to Statin Therapy in Patients With Intracranial or Extracranial Carotid Atherosclerosis by Vessel Wall MRI: A Systematic Review and Updated Meta-Analysis. Front. Cardiovasc. Med. 2021, 8, 742935. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Huang, J.; Liu, C.; Jiao, S.; Chen, Y.; Xu, L.; Gong, T.; Zhu, C.; Song, Y. Application of high-resolution MRI in evaluating statin efficacy on symptomatic intracranial atherosclerosis. Eur. Radiol. 2025, 35, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Counseller, Q.; Aboelkassem, Y. Recent technologies in cardiac imaging. Front. Med. Technol. 2023, 4, 984492. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jung, W.; Boppart, S.A. Optical coherence tomography for rapid tissue screening and directed histological sectioning. Anal. Cell Pathol. 2012, 35, 129–143. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Viscusi, M.M.; La Porta, Y.; Migliaro, G.; Gargano, G.M.; Nusca, A.; Gatto, L.; Budassi, S.; Paolucci, L.; Mangiacapra, F.; Ricottini, E.; et al. Current Applications and New Perspectives in Optical Coherence Tomography (OCT) Coronary Atherosclerotic Plaque Assessment: From PCI Optimization to Pharmacological Treatment Guidance. Photonics 2023, 10, 158. [Google Scholar] [CrossRef]
- Jang, I.K.; Tearney, G.J.; MacNeill, B.; Takano, M.; Moselewski, F.; Iftima, N.; Shishkov, M.; Houser, S.; Aretz, H.T.; Halpern, E.F.; et al. In vivo characterization of coronary atherosclerotic plaque by use of optical coherence tomography. Circulation 2005, 111, 1551–1555. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sinclair, H.; Bourantas, C.; Bagnall, A.; Mintz, G.S.; Kunadian, V. OCT for the identification of vulnerable plaque in acute coronary syndrome. JACC Cardiovasc. Imaging 2015, 8, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Yang, F.; Gutiérrez-Chico, J.L.; Xu, T.; Wu, J.; Wang, L.; Lv, R.; Lai, Y.; Liu, X.; Onuma, Y.; et al. Optical Coherence Tomography-Derived Changes in Plaque Structural Stress Over the Cardiac Cycle: A New Method for Plaque Biomechanical Assessment. Front. Cardiovasc. Med. 2021, 8, 715995. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Longobardo, L.; Mattesini, A.; Valente, S.; Di Mario, C. OCT-guided Percutaneous Coronary Intervention in Bifurcation Lesions. Interv. Cardiol. 2019, 14, 5–9. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tearney, G.J.; Jang, I.K.; Bouma, B.E. Optical coherence tomography for imaging the vulnerable plaque. J. Biomed. Opt. 2006, 11, 021002. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]

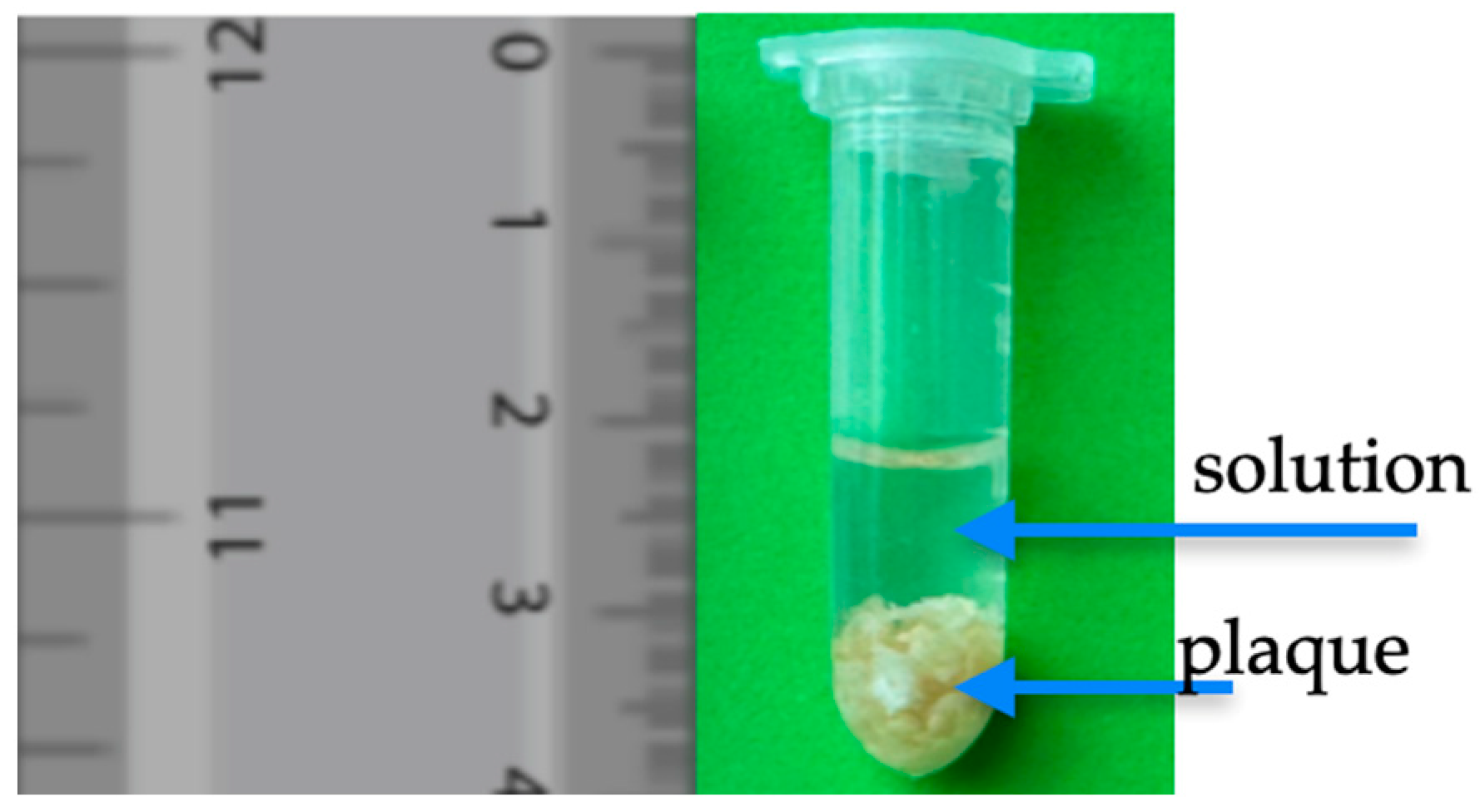



| Disease or Case | n/N (%) |
|---|---|
| Age, year (mean, range) | 76 (69–81) |
| Male/female gender | 5/2 |
| Cardiovascular risk factors | |
| Hypertension | 7/7 (100%) |
| Diabetes mellitus | 5/7 (71.4%) |
| Dyslipidemia | 7/7 (100%) |
| Smoking history | 5/7 (71.4%) |
| Chronic kidney disease | 3/7 (42.8%) |
| Cardiovascular comorbidities | |
| Previous myocardial infarction | 2/7 (28.6%) |
| Previous percutaneous coronary intervention | 5/7 (71.4%) |
| Previous coronary artery bypass surgery | 1/7 (14.3%) |
| History of stroke | 7/7 (100%) |
| Normal sinus rythm | 7/7 (100%) |
| Medications | |
| Statin | 7/7 (100%) |
| Beta-blocker (B-blocker) | 6/7 (85.7%) |
| Aspirin (ASA) | 7/7 (100%) |
| Clopidogrel | 2/7 (28.6%) |
| ACE inhibitors (ACE-i) | 5/7 (71.4%) |
| Angiotensin II receptor blockers (ARB) | 1/7 (14.3%) |
| SGLT2 inhibitors (Flozin) | 2/7 (28.6%) |
| Mean ± SD | ||
|---|---|---|
| Relaxation Time | T1 [ms] | T2 [ms] |
| Before Omnipaque 350 implementation | 1231 ± 12 * | 63 ± 23 * |
| 1 h after | 673 ± 24 * | 45 ± 6 * |
| 48 h after | 460 ± 45 * | 33 ± 4 * |
| Atherosclerotic Plaque Samples (n = 14) Before Omnipaque 350 Implementation | Atherosclerotic Plaque Samples (n = 14) 1 h After Omnipaque 350 Implementation | Atherosclerotic Plaque Samples (n = 14) 24 h After Omnipaque 350 Implementation | ||||
|---|---|---|---|---|---|---|
| No. | T1 [ms] | T2 [ms] | T1 [ms] | T2 [ms] | T1 [ms] | T2 [ms] |
| 1 | 1163 | 58 | 674 | 45 | 461 | 33 |
| 2 | 1271 | 54 | 675 | 47 | 455 | 32 |
| 3 | 989 | 76 | 679 | 43 | 472 | 28 |
| 4 | 1234 | 79 | 585 | 45 | 467 | 31 |
| 5 | 1263 | 67 | 684 | 47 | 460 | 29 |
| 6 | 1370 | 68 | 673 | 51 | 467 | 35 |
| 7 | 1191 | 45 | 677 | 43 | 457 | 28 |
| 8 | 1278 | 67 | 679 | 40 | 467 | 35 |
| 9 | 1324 | 64 | 698 | 44 | 435 | 38 |
| 10 | 1297 | 65 | 701 | 45 | 460 | 45 |
| 11 | 1298 | 67 | 785 | 45 | 474 | 27 |
| 12 | 1341 | 57 | 654 | 41 | 449 | 33 |
| 13 | 989 | 56 | 658 | 48 | 469 | 34 |
| 14 | 1239 | 64 | 662 | 47 | 464 | 34 |
| Mean ± SD | 1231 ± 12 * | 63 ± 23 * | 673 ± 24 * | 45 ± 6 * | 460 ± 45 * | 33 ± 4 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wańczura, P.; Mytych, W.; Bartusik-Aebisher, D.; Leksa, D.; Truszkiewicz, A.; Aebisher, D. Visualization of Atherosclerotic Plaques Paired with Joheksol 350 (Omnipaque). Biomedicines 2025, 13, 399. https://doi.org/10.3390/biomedicines13020399
Wańczura P, Mytych W, Bartusik-Aebisher D, Leksa D, Truszkiewicz A, Aebisher D. Visualization of Atherosclerotic Plaques Paired with Joheksol 350 (Omnipaque). Biomedicines. 2025; 13(2):399. https://doi.org/10.3390/biomedicines13020399
Chicago/Turabian StyleWańczura, Piotr, Wiktoria Mytych, Dorota Bartusik-Aebisher, Dawid Leksa, Adrian Truszkiewicz, and David Aebisher. 2025. "Visualization of Atherosclerotic Plaques Paired with Joheksol 350 (Omnipaque)" Biomedicines 13, no. 2: 399. https://doi.org/10.3390/biomedicines13020399
APA StyleWańczura, P., Mytych, W., Bartusik-Aebisher, D., Leksa, D., Truszkiewicz, A., & Aebisher, D. (2025). Visualization of Atherosclerotic Plaques Paired with Joheksol 350 (Omnipaque). Biomedicines, 13(2), 399. https://doi.org/10.3390/biomedicines13020399








