Hallmarks of Brain Plasticity
Abstract
:1. Glossary
2. Introduction
2.1. Concept of Brain Plasticity
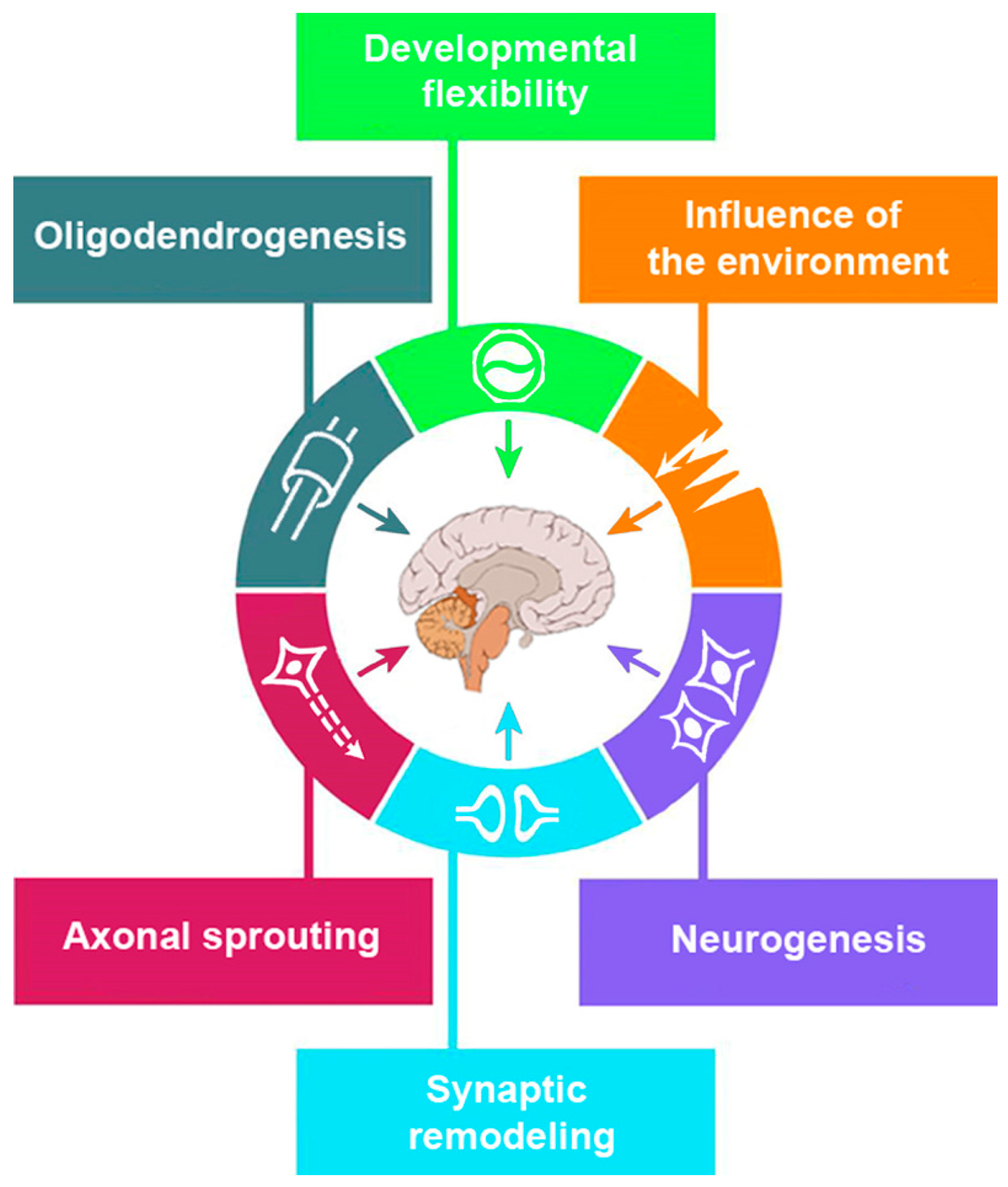
2.2. Different Types of Plasticity
3. Neuroanatomic and Neurophysiologic Bases of Brain Plasticity
3.1. Plasticity in the Periphery and at the Centrum of the Brain
3.2. Natural Plasticity in Different Functional Areas
4. Pathophysiological Mechanisms Underlying Cerebral Plasticity
4.1. Plasticity Mechanisms at the Microlevel
4.2. Plasticity Mechanisms at the Macrolevel
5. Modulation of Experience-Dependent Change
5.1. Modulation by Sex Hormones
5.2. Neurodevelopment and Brain Plasticity in Childhood
5.3. Brain Plasticity in Adulthood
6. Molecular Mechanisms of Brain Plasticity
| No | Name (Acronym) | Molecular Species | References |
|---|---|---|---|
| 1 | Long non-coding RNA (lncRNA) | Gomafu, GAS5, MALAT1, HOTAIR | [131,132,133,134,135] |
| 2 | MicroRNA (miRNA) | miR-9, miR-34, miR-132 | [136,137,138] |
| miR-17-92 cluster | [139,140] | ||
| miR-144-5p, miR-145, miR-153 | [141,142,143] | ||
| hsa-miR-1-3p, hsa-miR-335-5p, hsa-miR-34a-5p | [144] | ||
| 3 | Circular RNA (circRNA) | ciRS-7, circRMST, circFAT3 | [145] |
| circIgfbp2 | [146] | ||
| nearly 1167 cerebral circRNAs | [147] | ||
| cirC_0000400, cirC_0000331, cirC_0000406, cirC_0000798 | [148] | ||
| 4 | Enhancer RNA (eRNA) | Bdnf-Enhg1, Bdnf-Enhg2 | [149] |
| Evf2 | [150] | ||
| 5 | Long intergenic non-coding RNA (lincRNA) | linc-Brn1b | [151] |
| Xist | [152] | ||
| 6 | Piwi-interacting RNA (piRNA) | list of 1251 brain-specific piRNAs; piR-hsa-1281, piR-hsa-1280, piR-hsa-1282, piR-hsa-27492 | [153,154,155] |
| 7 | Y RNA (yRNA) | nELAVL/Y RNA complex hY1, hY4, hY5 | [156,157,158] |
6.1. Long Non-Coding RNAs
6.2. MicroRNAs
6.3. Circular RNAs
6.4. Enhancer RNAs
6.5. Long Intergenic Non-Coding RNAs
6.6. Piwi-Interacting RNAs
6.7. Y RNAs
7. Brain Neuroplasticity from a Network Neuroscience Perspective
7.1. High-Resolution Neuroimaging Systems and Techniques
7.2. Computational Models of Neuronal Dynamics
7.3. Graph Theory Applications
8. Implications for Medical Practice
8.1. Pharmacology
8.2. Transcranial Magnetic Stimulation
8.3. Surgery
8.4. Transplantation
9. Conclusions
- ▪
- The brain is a dynamic construct that changes structurally and/or functionally and constitutes interactive distributed glial–neuro–synaptic networks. The behavioral consequences of these changes may vary as a function of their effective connectivity, but the overall system remains stable due to homeostatic plasticity.
- ▪
- New insight into the concept of brain plasticity and homeostasis may provide additional perspectives on functional recovery following brain damage. Knowledge of this phenomenon will enable physicians to exploit the potential of cerebral plasticity and regulate eloquent networks with timely interventions. Future studies may reveal pathophysiological mechanisms of brain plasticity at microscopic and macroscopic levels, which will advance rehabilitation strategies and improve quality of life in patients with neurological disease.
- ▪
- Non-coding RNAs are optimal candidates for elucidating the molecular pathways underlying the phenomenon of brain plasticity. Candidates may signal the development of various neuropsychiatric disorders comprising schizophrenia, addiction, and fear-related anxiety disorders. The diversity of ncRNAs and their association with neurodegenerative disease renders them particularly interesting targets for new therapeutic approaches. New RNA-based therapeutics may arise from novel data on ncRNA regulation and the downstream effects of their interactions.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| BP | brain plasticity |
| circRNA | circular RNA |
| eRNA | enhancer RNA |
| lincRNA | long intergenic non-coding RNA |
| lncRNA | long non-coding RNA |
| mRNA | messenger RNA |
| miRNA | microRNA |
| ncRNA | non-coding RNA |
| piRNA | Piwi-interacting RNA |
| TMS | transcranial magnetic stimulation |
| yRNA | Y RNA |
References
- Johnston, M.V. Clinical disorders of brain plasticity. Brain Dev. 2004, 26, 73–80. [Google Scholar] [CrossRef]
- Duffau, H. Brain plasticity: From pathophysiological mechanisms to therapeutic applications. J. Clin. Neurosci. 2006, 13, 885–897. [Google Scholar] [CrossRef] [PubMed]
- Kolb, B.; Gibb, R. Brain plasticity and behaviour in the developing brain. J. Can. Acad. Child Adolesc. Psychiatry 2011, 20, 265. [Google Scholar]
- Fischer, T.M.; Blazis, D.E.; Priver, N.A.; Carew, T.J. Metaplasticity at identified inhibitory synapses in Aplysia. Nature 1997, 389, 860–865. [Google Scholar] [CrossRef] [PubMed]
- Forrest, M.P.; Parnell, E.; Penzes, P. Dendritic structural plasticity and neuropsychiatric disease. Nat. Rev. Neurosci. 2018, 19, 215–234. [Google Scholar] [CrossRef] [PubMed]
- Nature Portfolio. Spine Plasticity—Nature, Subjects. 2024. Available online: https://www.nature.com/subjects/spine-plasticity (accessed on 22 June 2024).
- Wikipedia Contributors. Homeostatic plasticity—Wikipedia, The Free Encyclopedia. 2024. Available online: https://en.wikipedia.org/w/index.php?title=Homeostatic_plasticity&oldid=1216627734 (accessed on 22 June 2024).
- Kujala, T.; Alho, K.; Näätänen, R. Cross-modal reorganization of human cortical functions. Trends Neurosci. 2000, 23, 115–120. [Google Scholar] [CrossRef]
- Shimojo, S.; Shams, L. Sensory modalities are not separate modalities: Plasticity and interactions. Curr. Opin. Neurobiol. 2001, 11, 505–509. [Google Scholar] [CrossRef]
- Bavelier, D.; Neville, H.J. Cross-modal plasticity: Where and how? Nat. Rev. Neurosci. 2002, 3, 443–452. [Google Scholar] [CrossRef]
- Rioult-Pedotti, M.S.; Friedman, D.; Donoghue, J.P. Learning-induced LTP in neocortex. Science 2000, 290, 533–536. [Google Scholar] [CrossRef]
- Stephan, K.E.; Friston, K.J. Analyzing effective connectivity with functional magnetic resonance imaging. Wiley Interdiscip. Rev. 2010, 1, 446–459. [Google Scholar] [CrossRef] [PubMed]
- La Rosa, C.; Parolisi, R.; Bonfanti, L. Brain structural plasticity: From adult neurogenesis to immature neurons. Front. Neurosci. 2020, 14, 512123. [Google Scholar] [CrossRef] [PubMed]
- Sale, A.; Berardi, N.; Maffei, L. Environment and brain plasticity: Towards an endogenous pharmacotherapy. Physiol. Rev. 2014, 94, 189–234. [Google Scholar] [CrossRef] [PubMed]
- Mandolesi, L.; Gelfo, F.; Serra, L.; Montuori, S.; Polverino, A.; Curcio, G.; Sorrentino, G. Environmental factors promoting neural plasticity: Insights from animal and human studies. Neural Plast. 2017, 2017, 7219461. [Google Scholar] [CrossRef]
- Buttelmann, F.; Karbach, J. Development and plasticity of cognitive flexibility in early and middle childhood. Front. Psychol. 2017, 8, 258078. [Google Scholar] [CrossRef] [PubMed]
- Stampanoni Bassi, M.; Iezzi, E.; Gilio, L.; Centonze, D.; Buttari, F. Synaptic plasticity shapes brain connectivity: Implications for network topology. Int. J. Mol. Sci. 2019, 20, 6193. [Google Scholar] [CrossRef]
- Chen, M.; Zheng, B. Axon plasticity in the mammalian central nervous system after injury. Trends Neurosci. 2014, 37, 583–593. [Google Scholar] [CrossRef]
- Marshall, K.L.; Farah, M.H. Axonal regeneration and sprouting as a potential therapeutic target for nervous system disorders. Neural Regen. Res. 2021, 16, 1901–1910. [Google Scholar]
- El Waly, B.; Macchi, M.; Cayre, M.; Durbec, P. Oligodendrogenesis in the normal and pathological central nervous system. Front. Neurosci. 2014, 8, 87323. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, J.L.; Makowiecki, K.; Cullen, C.L.; Young, K.M. Oligodendrogenesis and myelination regulate cortical development, plasticity and circuit function. Semin. Cell Dev. Biol. 2021, 118, 14–23. [Google Scholar] [CrossRef]
- Scott, H. Brain Plasticity Influencing Phantom Limb and Prosthetics. Outstanding Honors Theses, University of South Florida, Tampa, FL, USA, 2011. [Google Scholar]
- Duffau, H. New Insights into Functional Mapping in Cerebral Tumor Surgery: Study of The Dynamic Interactions Between the Lesion and the Brain; Focus on Brain Mapping Research; Nova Science Pub Inc.: Hauppauge, NY, USA, 2006; p. 1. [Google Scholar]
- Kolb, B.; Whishaw, I.Q. Brain plasticity and behavior. Annu. Rev. Psychol. 1998, 49, 43–64. [Google Scholar] [CrossRef]
- Johnston, M.V.; Nishimura, A.; Harum, K.; Pekar, J.; Blue, M.E. Sculpting the developing brain. Adv. Pediatr. 2001, 48, 1–40. [Google Scholar] [CrossRef]
- Gould, E.; Reeves, A.J.; Graziano, M.S.; Gross, C.G. Neurogenesis in the neocortex of adult primates. Science 1999, 286, 548–552. [Google Scholar] [CrossRef]
- Greenough, W.T. Structural correlates of information storage in the mammalian brain: A review and hypothesis. Trends Neurosci. 1984, 7, 229–233. [Google Scholar] [CrossRef]
- Greenough, W.T.; Hwang, H.; Gorman, C. Evidence for active synapse formation or altered postsynaptic metabolism in visual cortex of rats reared in complex environments. Proc. Natl. Acad. Sci. USA 1985, 82, 4549–4552. [Google Scholar] [CrossRef]
- Turner, A.M.; Greenough, W.T. Differential rearing effects on rat visual cortex synapses. I. Synaptic and neuronal density and synapses per neuron. Brain Res. 1985, 329, 195–203. [Google Scholar] [CrossRef]
- Jacobs, B.; Schall, M.; Scheibel, A.B. A quantitative dendritic analysis of Wernicke’s area in humans. II. Gender, hemispheric, and environmental factors. J. Comp. Neurol. 1993, 327, 97–111. [Google Scholar] [CrossRef]
- Purpura, D.P. Dendritic spine” dysgenesis” and mental retardation. Science 1974, 186, 1126–1128. [Google Scholar] [CrossRef] [PubMed]
- Wikipedia Contributors. Neuroplasticity—Wikipedia, The Free Encyclopedia. 2024. Available online: https://en.wikipedia.org/wiki/Neuroplasticity (accessed on 22 June 2024).
- Nudo, R.J.; Wise, B.M.; SiFuentes, F.; Milliken, G.W. Neural substrates for the effects of rehabilitative training on motor recovery after ischemic infarct. Science 1996, 272, 1791–1794. [Google Scholar] [CrossRef]
- Georgopoulos, A.P. News in motor cortical physiology. Physiology 1999, 14, 64–68. [Google Scholar] [CrossRef]
- Sanes, J.N.; Schieber, M.H. Orderly somatotopy in primary motor cortex: Does it exist? NeuroImage 2001, 13, 968–974. [Google Scholar] [CrossRef] [PubMed]
- Karni, A.; Meyer, G.; Rey-Hipolito, C.; Jezzard, P.; Adams, M.M.; Turner, R.; Ungerleider, L.G. The acquisition of skilled motor performance: Fast and slow experience-driven changes in primary motor cortex. Proc. Natl. Acad. Sci. USA 1998, 95, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Hlustik, P.; Solodkin, A.; Gullapalli, R.; Noll, D.C.; Small, S.L. Hand motor skill learning generalizes anatomically and behaviorally. NeuroImage 2000, 11, S866. [Google Scholar] [CrossRef]
- Pesenti, M.; Thioux, M.; Seron, X.; De Volder, A. Neuroanatomical substrates of arabic number processing, numerical comparison, and simple addition: A PET study. J. Cogn. Neurosci. 2000, 12, 461–479. [Google Scholar] [CrossRef]
- Ganis, G.; Keenan, J.P.; Kosslyn, S.M.; Pascual-Leone, A. Transcranial magnetic stimulation of primary motor cortex affects mental rotation. Cereb. Cortex 2000, 10, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Laubach, M.; Wessberg, J.; Nicolelis, M.A. Cortical ensemble activity increasingly predicts behaviour outcomes during learning of a motor task. Nature 2000, 405, 567–571. [Google Scholar] [CrossRef]
- Salenius, S.; Hari, R. Synchronous cortical oscillatory activity during motor action. Curr. Opin. Neurobiol. 2003, 13, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Buchel, C.; Coull, J.; Friston, K.J. The predictive value of changes in effective connectivity for human learning. Science 1999, 283, 1538–1541. [Google Scholar] [CrossRef] [PubMed]
- Andres, F.G.; Gerloff, C. Coherence of sequential movements and motor learning. J. Clin. Neurophysiol. 1999, 16, 520. [Google Scholar] [CrossRef] [PubMed]
- Duffau, H.; Capelle, L.; Sichez, N.; Denvil, D.; Lopes, M.; Sichez, J.P.; Bitar, A.; Fohanno, D. Intraoperative mapping of the subcortical language pathways using direct stimulations: An anatomo-functional study. Brain 2002, 125, 199–214. [Google Scholar] [CrossRef] [PubMed]
- Duffau, H.; Gatignol, P.; Mandonnet, E.; Peruzzi, P.; Tzourio-Mazoyer, N.; Capelle, L. New insights into the anatomo-functional connectivity of the semantic system: A study using cortico-subcortical electrostimulations. Brain 2005, 128, 797–810. [Google Scholar] [CrossRef]
- McClelland, J.L.; Rogers, T.T. The parallel distributed processing approach to semantic cognition. Nat. Rev. Neurosci. 2003, 4, 310–322. [Google Scholar] [CrossRef] [PubMed]
- Hatten, M.E. New directions in neuronal migration. Science 2002, 297, 1660–1663. [Google Scholar] [CrossRef]
- Gandolfo, F.; Li, C.S.; Benda, B.; Schioppa, C.P.; Bizzi, E. Cortical correlates of learning in monkeys adapting to a new dynamical environment. Proc. Natl. Acad. Sci. USA 2000, 97, 2259–2263. [Google Scholar] [CrossRef] [PubMed]
- Jercog, D.; Winke, N.; Sung, K.; Fernandez, M.M.; Francioni, C.; Rajot, D.; Courtin, J.; Chaudun, F.; Jercog, P.E.; Valerio, S.; et al. Dynamical prefrontal population coding during defensive behaviours. Nature 2021, 595, 690–694. [Google Scholar] [CrossRef]
- Lamprecht, R.; LeDoux, J. Structural plasticity and memory. Nat. Rev. Neurosci. 2004, 5, 45–54. [Google Scholar] [CrossRef]
- Byrne, J.H. Synapses Plastic plasticity. Nature 1997, 389, 791–792. [Google Scholar] [CrossRef]
- Buonomano, D.V.; Merzenich, M.M. Cortical plasticity: From synapses to maps. Annu. Rev. Neurosci. 1998, 21, 149–186. [Google Scholar] [CrossRef] [PubMed]
- Aroniadou, V.A.; Keller, A. Mechanisms of LTP induction in rat motor cortex in vitro. Cereb. Cortex 1995, 5, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Braunewell, K.H.; Manahan-Vaughan, D. Long-term depression: A cellular basis for learning? Rev. Neurosci. 2001, 12, 121–140. [Google Scholar] [CrossRef]
- Schulz, A.; Miehl, C.; Berry, M.J., II; Gjorgjieva, J. The generation of cortical novelty responses through inhibitory plasticity. eLife 2021, 10, e65309. [Google Scholar] [CrossRef]
- Martin, S.J.; Grimwood, P.D.; Morris, R.G. Synaptic plasticity and memory: An evaluation of the hypothesis. Annu. Rev. Neurosci. 2000, 23, 649–711. [Google Scholar] [CrossRef] [PubMed]
- Khona, M.; Fiete, I.R. Attractor and integrator networks in the brain. Nat. Rev. Neurosci. 2022, 23, 744–766. [Google Scholar] [CrossRef]
- Widrow, B.; Kim, Y.; Park, D.; Perin, J.K. Nature’s learning rule: The Hebbian-LMS algorithm. In Artificial Intelligence in the Age of Neural Networks and Brain Computing; Elsevier: Amsterdam, The Netherlands, 2024; pp. 11–40. [Google Scholar]
- Cruikshank, S.J.; Weinberger, N.M. Evidence for the Hebbian hypothesis in experience-dependent physiological plasticity of neocortex: A critical review. Brain Res. Rev. 1996, 22, 191–228. [Google Scholar] [CrossRef]
- Kilgard, M.P.; Merzenich, M.M. Cortical map reorganization enabled by nucleus basalis activity. Science 1998, 279, 1714–1718. [Google Scholar] [CrossRef] [PubMed]
- Innocenti, G.M.; Schmidt, K.; Milleret, C.; Fabri, M.; Knyazeva, M.G.; Battaglia-Mayer, A.; Aboitiz, F.; Ptito, M.; Caleo, M.; Marzi, C.A.; et al. The functional characterization of callosal connections. Prog. Neurobiol. 2022, 208, 102186. [Google Scholar] [CrossRef] [PubMed]
- Blitz, D.M.; Foster, K.A.; Regehr, W.G. Short-term synaptic plasticity: A comparison of two synapses. Nat. Rev. Neurosci. 2004, 5, 630–640. [Google Scholar] [CrossRef] [PubMed]
- Malenka, R.C.; Nicoll, R.A. Silent synapses speak up. Neuron 1997, 19, 473–476. [Google Scholar] [CrossRef]
- Ridding, M.; Brouwer, B.; Miles, T.; Pitcher, J.; Thompson, P. Changes in muscle responses to stimulation of the motor cortex induced by peripheral nerve stimulation in human subjects. Exp. Brain Res. 2000, 131, 135–143. [Google Scholar] [CrossRef]
- Fields, R.D.; Stevens-Graham, B. New insights into neuron-glia communication. Science 2002, 298, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Haydon, P.G. GLIA: Listening and talking to the synapse. Nat. Rev. Neurosci. 2001, 2, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Trachtenberg, J.T.; Chen, B.E.; Knott, G.W.; Feng, G.; Sanes, J.R.; Welker, E.; Svoboda, K. Long-term in vivo imaging of experience-dependent synaptic plasticity in adult cortex. Nature 2002, 420, 788–794. [Google Scholar] [CrossRef] [PubMed]
- Ivanco, T.L.; Greenough, W.T. Physiological consequences of morphologically detectable synaptic plasticity: Potential uses for examining recovery following damage. Neuropharmacology 2000, 39, 765–776. [Google Scholar] [CrossRef] [PubMed]
- by Synaptic, H.D.I. Rapid Dendritic Morphogenesis in CA1. Cold Spring Harb. Symp. Quant. Biol. 1987, 52, 825. [Google Scholar]
- Poo, M.m. Neurotrophins as synaptic modulators. Nat. Rev. Neurosci. 2001, 2, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Turrigiano, G.G.; Nelson, S.B. Homeostatic plasticity in the developing nervous system. Nat. Rev. Neurosci. 2004, 5, 97–107. [Google Scholar] [CrossRef]
- Selzer, M.E. Promotion of axonal regeneration in the injured CNS. Lancet Neurol. 2003, 2, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Ullian, E.M.; Sapperstein, S.K.; Christopherson, K.S.; Barres, B.A. Control of synapse number by glia. Science 2001, 291, 657–661. [Google Scholar] [CrossRef]
- Shao, Y.; McCarthy, K.D. Plasticity of astrocytes. Glia 1994, 11, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Langle, S.L.; Poulain, D.A.; Theodosis, D.T. Neuronal–glial remodeling: A structural basis for neuronal–glial interactions in the adult hypothalamus. J. Physiol. 2002, 96, 169–175. [Google Scholar] [CrossRef]
- Zhang, W.; Couldwell, W.T.; Simard, M.F.; Song, H.; Lin, J.H.; Nedergaard, M. Direct gap junction communication between malignant glioma cells and astrocytes. Cancer Res. 1999, 59, 1994–2003. [Google Scholar]
- Steindler, D.A.; Pincus, D.W. Stem cells and neuropoiesis in the adult human brain. Lancet 2002, 359, 1047–1054. [Google Scholar] [CrossRef] [PubMed]
- Sanai, N.; Tramontin, A.D.; Quinones-Hinojosa, A.; Barbaro, N.M.; Gupta, N.; Kunwar, S.; Lawton, M.T.; McDermott, M.W.; Parsa, A.T.; Manuel-García Verdugo, J.; et al. Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature 2004, 427, 740–744. [Google Scholar] [CrossRef] [PubMed]
- Pincus, D.; Harrison-Restelli, C.; Barry, J.; Goodman, R.; Fraser, R.; Nedergaard, M.; Goldman, S. In vitro neurogenesis by adult human epileptic temporal neocortex. Clin. Neurosurg. 1997, 44, 17–25. [Google Scholar]
- Roy, N.S.; Wang, S.; Jiang, L.; Kang, J.; Benraiss, A.; Harrison-Restelli, C.; Fraser, R.A.; Couldwell, W.T.; Kawaguchi, A.; Okano, H.; et al. In vitro neurogenesis by progenitor cells isolated from the adult human hippocampus. Nat. Med. 2000, 6, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Nunes, M.C.; Roy, N.S.; Keyoung, H.M.; Goodman, R.R.; McKhann, G.; Jiang, L.; Kang, J.; Nedergaard, M.; Goldman, S.A. Identification and isolation of multipotential neural progenitor cells from the subcortical white matter of the adult human brain. Nat. Med. 2003, 9, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Gross, C.G. Neurogenesis in the adult brain: Death of a dogma. Nat. Rev. Neurosci. 2000, 1, 67–73. [Google Scholar] [CrossRef]
- Magavi, S.S.; Macklis, J.D. Induction of neuronal type-specific neurogenesis in the cerebral cortex of adult mice: Manipulation of neural precursors in situ. Dev. Brain Res. 2002, 134, 57–76. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.K.; Botez, M. Diaschisis and neurobehavior. Can. J. Neurol. Sci. 1998, 25, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Seitz, R.J.; Azari, N.P.; Knorr, U.; Binkofski, F.; Herzog, H.; Freund, H.J. The role of diaschisis in stroke recovery. Stroke 1999, 30, 1844–1850. [Google Scholar] [CrossRef]
- Duffau, H.; Sichez, J.P.; Lehéricy, S. Intraoperative unmasking of brain redundant motor sites during resection of a precentral angioma: Evidence using direct cortical stimulation. Ann. Neurol. 2000, 47, 132–135. [Google Scholar] [CrossRef]
- Duffau, H. Acute functional reorganisation of the human motor cortex during resection of central lesions: A study using intraoperative brain mapping. J. Neurol. Neurosurg. Psychiatry 2001, 70, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Rijntjes, M.; Weiller, C. Recovery of motor and language abilities after stroke: The contribution of functional imaging. Prog. Neurobiol. 2002, 66, 109–122. [Google Scholar] [CrossRef]
- Krainik, A.; Duffau, H.; Capelle, L.; Cornu, P.; Boch, A.L.; Mangin, J.F.; Le Bihan, D.; Marsault, C.; Chiras, J.; Lehéricy, S. Role of the healthy hemisphere in recovery after resection of the supplementary motor area. Neurology 2004, 62, 1323–1332. [Google Scholar] [CrossRef] [PubMed]
- Levänen, S.; Jousmäki, V.; Hari, R. Vibration-induced auditory-cortex activation in a congenitally deaf adult. Curr. Biol. 1998, 8, 869–872. [Google Scholar] [CrossRef]
- Lee, D.S.; Lee, J.S.; Oh, S.H.; Kim, S.K.; Kim, J.W.; Chung, J.K.; Lee, M.C.; Kim, C.S. Cross-modal plasticity and cochlear implants. Nature 2001, 409, 149–150. [Google Scholar] [CrossRef]
- Rossini, P.M.; Dal Forno, G. Integrated technology for evaluation of brain function and neural plasticity. Phys. Med. Rehabil. Clin. North Am. 2004, 15, 263–306. [Google Scholar] [CrossRef]
- Luders, E.; Gaser, C.; Jancke, L.; Schlaug, G. A voxel-based approach to gray matter asymmetries. Neuroimage 2004, 22, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Josse, G.; Mazoyer, B.; Crivello, F.; Tzourio-Mazoyer, N. Left planum temporale: An anatomical marker of left hemispheric specialization for language comprehension. Cogn. Brain Res. 2003, 18, 1–14. [Google Scholar] [CrossRef]
- Sluming, V.; Barrick, T.; Howard, M.; Cezayirli, E.; Mayes, A.; Roberts, N. Voxel-based morphometry reveals increased gray matter density in Broca’s area in male symphony orchestra musicians. Neuroimage 2002, 17, 1613–1622. [Google Scholar] [CrossRef]
- Hutchinson, S.; Lee, L.H.L.; Gaab, N.; Schlaug, G. Cerebellar volume of musicians. Cereb. Cortex 2003, 13, 943–949. [Google Scholar] [CrossRef]
- Mackay, C.E.; Roberts, N.; Mayes, A.R.; Downes, J.J.; Foster, J.K.; Mann, D. An exploratory study of the relationship between face recognition memory and the volume of medial temporal lobe structures in healthy young males. Behav. Neurol. 1998, 11, 3–20. [Google Scholar] [CrossRef]
- Maguire, E.A.; Gadian, D.G.; Johnsrude, I.S.; Good, C.D.; Ashburner, J.; Frackowiak, R.S.; Frith, C.D. Navigation-related structural change in the hippocampi of taxi drivers. Proc. Natl. Acad. Sci. USA 2000, 97, 4398–4403. [Google Scholar] [CrossRef]
- Paus, T.; Zijdenbos, A.; Worsley, K.; Collins, D.L.; Blumenthal, J.; Giedd, J.N.; Rapoport, J.L.; Evans, A.C. Structural maturation of neural pathways in children and adolescents: In vivo study. Science 1999, 283, 1908–1911. [Google Scholar] [CrossRef]
- Draganski, B.; Gaser, C.; Busch, V.; Schuierer, G.; Bogdahn, U.; May, A. Changes in grey matter induced by training. Nature 2004, 427, 311–312. [Google Scholar] [CrossRef]
- Juraska, J.M. Sex differences in dendritic response to differential experience in the rat visual cortex. Brain Res. 1984, 295, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Juraska, J.M. Sex differences in developmental plasticity of behavior and the brain. In Developmental Neuropsychobiology; Academic Press: Cambridge, MA, USA, 1986; pp. 409–422. [Google Scholar]
- Juraska, J.M.; Fitch, J.M.; Henderson, C.; Rivers, N. Sex differences in the dendritic branching of dentate granule cells following differential experience. Brain Res. 1985, 333, 73–80. [Google Scholar] [CrossRef]
- Juraska, J.M. The structure of the rat cerebral cortex: Effects of gender and the environment. In The Cerebral Cortex of the Rat; Kolb, B., Tees, R.C., Eds.; The MIT Press: Cambridge, MA, USA, 1990. [Google Scholar]
- Coleman, P.; Buell, S. Regulation of dendritic extent in developing and aging brain. In Synaptic Plasticity; The Guilford Press: New York, NY, USA, 1985; pp. 311–333. [Google Scholar]
- Duque, A.; Arellano, J.I.; Rakic, P. An assessment of the existence of adult neurogenesis in humans and value of its rodent models for neuropsychiatric diseases. Mol. Psychiatry 2022, 27, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Raff, M.C.; Barres, B.A.; Burne, J.F.; Coles, H.S.; Ishizaki, Y.; Jacobson, M.D. Programmed cell death and the control of cell survival: Lessons from the nervous system. Science 1993, 262, 695–700. [Google Scholar] [CrossRef]
- Johansson, B.B. Brain plasticity in health and disease. Keio J. Med. 2004, 53, 231–246. [Google Scholar] [CrossRef]
- Statsenko, Y.; Habuza, T.; Charykova, I.; Gorkom, K.N.V.; Zaki, N.; Almansoori, T.M.; Baylis, G.; Ljubisavljevic, M.; Belghali, M. Predicting age from behavioral test performance for screening early onset of cognitive decline. Front. Aging Neurosci. 2021, 13, 661514. [Google Scholar] [CrossRef] [PubMed]
- Statsenko, Y.; Habuza, T.; Gorkom, K.N.V.; Zaki, N.; Almansoori, T.M.; Al Zahmi, F.; Ljubisavljevic, M.R.; Belghali, M. Proportional changes in cognitive subdomains during normal brain aging. Front. Aging Neurosci. 2021, 13, 673469. [Google Scholar] [CrossRef]
- Statsenko, Y.; Habuza, T.; Smetanina, D.; Simiyu, G.L.; Uzianbaeva, L.; Neidl-Van Gorkom, K.; Zaki, N.; Charykova, I.; Al Koteesh, J.; Almansoori, T.M.; et al. Brain morphometry and cognitive performance in normal brain aging: Age-and sex-related structural and functional changes. Front. Aging Neurosci. 2022, 13, 713680. [Google Scholar] [CrossRef]
- Belghali, M.; Statsenko, Y.; Laver, V. Stroop switching card test: Brief screening of executive functions across the lifespan. Aging Neuropsychol. Cogn. 2022, 29, 14–33. [Google Scholar] [CrossRef]
- Statsenko, Y.; Habuza, T.; Uzianbaeva, L.; Gorkom, K.; Belghali, M.; Charykova, I. Correlation between lifelong dynamics of psychophysiological performance and brain morphology. ESNR 2021. Neuroradiology 2021, 63, 41–42. [Google Scholar]
- Habuza, T.; Statsenko, Y.; Uzianbaeva, L.; Gorkom, K.; Zaki, N.; Belghali, M. Models of brain cognitive and morphological changes across the life: Machine learning-based approach. ESNR 2021. Neuroradiology 2021, 63, 10–26226. [Google Scholar]
- Uzianbaeva, L.; Statsenko, Y.; Habuza, T.; Gorkom, K.; Belghali, M.; Charykova, I. Effects of sex age-related changes in brain morphology. ESNR 2021. Neuroradiology 2021, 63, 42–43. [Google Scholar]
- Gorkom, K.; Statsenko, Y.; Habuza, T.; Uzianbaeva, L.; Belghali, M.; Charykova, I. Comparison of brain volumetric changes with functional outcomes in physiologic brain aging. ESNR 2021. Neuroradiology 2021, 63, 43–44. [Google Scholar]
- Statsenko, Y.; Habuza, T.; Charykova, I.; Gorkom, K.; Zaki, N.; Almansoori, T.; Ljubisavljevic, M.; Szolics, M.; Al Koteesh, J.; Ponomareva, A.; et al. AI models of age-associated changes in CNS composition identified by MRI. J. Neurol. Sci. 2021, 429, 118303. [Google Scholar] [CrossRef]
- Habuza, T.; Zaki, N.; Statsenko, Y.; Elyassami, S. MRI and cognitive tests-based screening tool for dementia. J. Neurol. Sci. 2021, 429, 118964. [Google Scholar] [CrossRef]
- Statsenko, Y.; Habuza, T.; Gorkom, K.N.V.; Zaki, N.; Almansoori, T.M. Applying the inverse efficiency score to visual–motor task for studying speed-accuracy performance while aging. Front. Aging Neurosci. 2020, 12, 574401. [Google Scholar] [CrossRef]
- Habuza, T.; Zaki, N.; Statsenko, Y.; Alnajjar, F.; Elyassami, S. Predicting the diagnosis of dementia from MRI data: Added value to cognitive tests. In Proceedings of the 7th Annual International Conference on Arab Women in Computing in Conjunction with the 2nd Forum of Women in Research, Sharjah, United Arab Emirates, 25–26 August 2021; pp. 1–7. [Google Scholar]
- Habuza, T.; Zaki, N.; Mohamed, E.A.; Statsenko, Y. Deviation from model of normal aging in Alzheimer’s disease: Application of deep learning to structural MRI data and cognitive tests. IEEE Access 2022, 10, 53234–53249. [Google Scholar] [CrossRef]
- Statsenko, Y.; Meribout, S.; Habuza, T.; Almansoori, T.M.; Gorkom, K.N.V.; Gelovani, J.G.; Ljubisavljevic, M. Patterns of structure- function association in normal aging and in Alzheimer’s disease: Screening for mild cognitive impairment and dementia with ML regression and classification models. Front. Aging Neurosci. 2023, 14, 943566. [Google Scholar] [CrossRef] [PubMed]
- Smetanina, D.; Meribout, S.; Habuza, T.; Simiyu, G.; Ismail, F.; Zareba, K.; Gorkom, K.; Almansoori, T.; Gelovani, J.; Ljubisavljevic, M.; et al. Reference curves of age-related volumetric changes in hippocampus and brain ventricles in healthy population. J. Neurol. Sci. 2023, 455, 121995. [Google Scholar] [CrossRef]
- Meribout, S.; Habuza, T.; Smetanina, D.; Simiyu, G.; Ismail, F.; Gorkom, K.; Almansoori, T.; Gelovani, J.; Statsenko, Y.; Ljubisavljevic, M. Rate and onset of cognitive decline and cortical atrophy in normal and pathological ageing. J. Neurol. Sci. 2023, 455, 121426. [Google Scholar] [CrossRef]
- Meribout, S.; Habuza, T.; Smetanina, D.; Simiyu, G.; Ismail, F.; Gorkom, K.; Almansoori, T.; Gelovani, J.; Statsenko, Y.; Ljubisavljevic, M. Functional changes in age-related neurocognitive slowing and disease-related cognitive decline: Evidence from global cognitive tests and psychophysiological tests. J. Neurol. Sci. 2023, 455, 121424. [Google Scholar] [CrossRef]
- Statsenko, Y.; Meribout, S.; Habuza, T.; Smetanina, D.; Simiyu, G.; Ismail, F.; Gorkom, K.; Almansoori, T.; Gelovani, J.; Ljubisavljevic, M. Structure-function association patterns of the brain in individuals with different level of cognitive impairment. J. Neurol. Sci. 2023, 455, 121462. [Google Scholar] [CrossRef]
- Spadaro, P.A.; Bredy, T.W. Emerging role of non-coding RNA in neural plasticity, cognitive function, and neuropsychiatric disorders. Front. Genet. 2012, 3, 132. [Google Scholar] [CrossRef]
- Kyzar, E.J.; Bohnsack, J.P.; Pandey, S.C. Current and future perspectives of noncoding RNAs in brain function and neuropsychiatric disease. Biol. Psychiatry 2022, 91, 183–193. [Google Scholar] [CrossRef]
- Earls, L.R.; Westmoreland, J.J.; Zakharenko, S.S. Non-coding RNA regulation of synaptic plasticity and memory: Implications for aging. Ageing Res. Rev. 2014, 17, 34–42. [Google Scholar] [CrossRef]
- Keihani, S.; Kluever, V.; Fornasiero, E.F. Brain long noncoding RNAs: Multitask regulators of neuronal differentiation and function. Molecules 2021, 26, 3951. [Google Scholar] [CrossRef]
- Ip, J.Y.; Sone, M.; Nashiki, C.; Pan, Q.; Kitaichi, K.; Yanaka, K.; Abe, T.; Takao, K.; Miyakawa, T.; Blencowe, B.J.; et al. Gomafu lncRNA knockout mice exhibit mild hyperactivity with enhanced responsiveness to the psychostimulant methamphetamine. Sci. Rep. 2016, 6, 27204. [Google Scholar] [CrossRef]
- Zhao, H.; Jin, T.; Cheng, X.; Qin, J.; Zhang, L.; He, H.; Xue, J.; Jin, G. GAS5 which is regulated by Lhx8 promotes the recovery of learning and memory in rats with cholinergic nerve injury. Life Sci. 2020, 260, 118388. [Google Scholar] [CrossRef]
- Li, L.; Zhuang, Y.; Zhao, X.; Li, X. Long non-coding RNA in neuronal development and neurological disorders. Front. Genet. 2019, 9, 436598. [Google Scholar] [CrossRef]
- Bhan, A.; Soleimani, M.; Mandal, S.S. Long noncoding RNA (lncRNA): Functions in health and disease. In Gene Regulation, Epigenetics and Hormone Signaling; Wiley-Blackwell: Hoboken, NJ, USA, 2017; pp. 169–208. [Google Scholar]
- Khalifa, F.N.; Hussein, R.F.; Mekawy, D.M.; Elwi, H.M.; Alsaeed, S.A.; Elnawawy, Y.; Shaheen, S.H. Potential role of the lncRNA “HOTAIR”/miRNA “206”/BDNF network in the alteration in expression of synaptic plasticity gene arc and BDNF level in sera of patients with heroin use disorder through the PI3K/AKT/mTOR pathway compared to the controls. Mol. Biol. Rep. 2024, 51, 293. [Google Scholar] [CrossRef]
- Sim, S.E.; Lim, C.S.; Kim, J.I.; Seo, D.; Chun, H.; Yu, N.K.; Lee, J.; Kang, S.J.; Ko, H.G.; Choi, J.H.; et al. The brain-enriched microRNA miR-9-3p regulates synaptic plasticity and memory. J. Neurosci. 2016, 36, 8641–8652. [Google Scholar] [CrossRef]
- McNeill, E.M.; Warinner, C.; Alkins, S.; Taylor, A.; Heggeness, H.; DeLuca, T.F.; Fulga, T.A.; Wall, D.P.; Griffith, L.C.; Van Vactor, D. The conserved microRNA miR-34 regulates synaptogenesis via coordination of distinct mechanisms in presynaptic and postsynaptic cells. Nat. Commun. 2020, 11, 1092. [Google Scholar] [CrossRef]
- Qian, Y.; Song, J.; Ouyang, Y.; Han, Q.; Chen, W.; Zhao, X.; Xie, Y.; Chen, Y.; Yuan, W.; Fan, C. Advances in roles of miR-132 in the nervous system. Front. Pharmacol. 2017, 8, 770. [Google Scholar] [CrossRef]
- Xin, H.; Katakowski, M.; Wang, F.; Qian, J.Y.; Liu, X.S.; Ali, M.M.; Buller, B.; Zhang, Z.G.; Chopp, M. MicroRNA-17–92 cluster in exosomes enhance neuroplasticity and functional recovery after stroke in rats. Stroke 2017, 48, 747–753. [Google Scholar] [CrossRef]
- Jin, J.; Kim, S.N.; Liu, X.; Zhang, H.; Zhang, C.; Seo, J.S.; Kim, Y.; Sun, T. miR-17-92 cluster regulates adult hippocampal neurogenesis, anxiety, and depression. Cell Rep. 2016, 16, 1653–1663. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, Y.; Wang, P.; Li, X.; Song, Z.; Wei, C.; Zhang, Q.; Luo, B.; Liu, Z.; Yang, Y.; et al. Clinical and preclinical evaluation of miR-144-5p as a key target for major depressive disorder. CNS Neurosci. Ther. 2023, 29, 3598–3611. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Yin, F.; Li, R.; Ruan, Q.; Meng, C.; Zhao, K.; Zhu, Q. MicroRNA-145-Mediated KDM6A Downregulation Enhances Neural Repair after Spinal Cord Injury via the NOTCH2/Abcb1a Axis. Oxidative Med. Cell. Longev. 2021, 2021, 2580619. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; Zhao, J.; Chang, S.; Sun, Q.; Liu, N.; Dong, J.; Chen, Y.; Yang, D.; Ye, D.; Liu, X.; et al. MicroRNA-153 improves the neurogenesis of neural stem cells and enhances the cognitive ability of aged mice through the notch signaling pathway. Cell Death Differ. 2020, 27, 808–825. [Google Scholar] [CrossRef] [PubMed]
- Del Val, C.; Díaz de la Guardia-Bolívar, E.; Zwir, I.; Mishra, P.P.; Mesa, A.; Salas, R.; Poblete, G.F.; de Erausquin, G.; Raitoharju, E.; Kähönen, M.; et al. Gene expression networks regulated by human personality. Mol. Psychiatry 2024, 29, 2241–2260. [Google Scholar] [CrossRef]
- Seeler, S.; Andersen, M.S.; Sztanka-Toth, T.; Rybiczka-Tešulov, M.; van den Munkhof, M.H.; Chang, C.C.; Maimaitili, M.; Venø, M.T.; Hansen, T.B.; Pasterkamp, R.J.; et al. A circular RNA expressed from the FAT3 locus regulates neural development. Mol. Neurobiol. 2023, 60, 3239–3260. [Google Scholar] [CrossRef]
- Du, M.; Wu, C.; Yu, R.; Cheng, Y.; Tang, Z.; Wu, B.; Fu, J.; Tan, W.; Zhou, Q.; Zhu, Z.; et al. A novel circular RNA, circIgfbp2, links neural plasticity and anxiety through targeting mitochondrial dysfunction and oxidative stress-induced synapse dysfunction after traumatic brain injury. Mol. Psychiatry 2022, 27, 4575–4589. [Google Scholar] [CrossRef]
- Bao, N.; Liu, J.; Peng, Z.; Zhang, R.; Ni, R.; Li, R.; Wu, J.; Liu, Z.; Pan, B. Identification of circRNA-miRNA-mRNA networks to explore the molecular mechanism and immune regulation of postoperative neurocognitive disorder. Aging 2022, 14, 8374. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, E.; Fitzsimmons, C.; Geaghan, M.P.; Shannon Weickert, C.; Atkins, J.R.; Wang, X.; Cairns, M.J. Circular RNA biogenesis is decreased in postmortem cortical gray matter in schizophrenia and may alter the bioavailability of associated miRNA. Neuropsychopharmacology 2019, 44, 1043–1054. [Google Scholar] [CrossRef]
- Brookes, E.; Alan Au, H.Y.; Varsally, W.; Barrington, C.; Hadjur, S.; Riccio, A. A novel enhancer that regulates Bdnf expression in developing neurons. bioRxiv 2021. [Google Scholar] [CrossRef]
- Cajigas, I.; Chakraborty, A.; Swyter, K.R.; Luo, H.; Bastidas, M.; Nigro, M.; Morris, E.R.; Chen, S.; VanGompel, M.J.; Leib, D.; et al. The Evf2 ultraconserved enhancer lncRNA functionally and spatially organizes megabase distant genes in the developing forebrain. Mol. Cell 2018, 71, 956–972. [Google Scholar] [CrossRef]
- Sauvageau, M.; Goff, L.A.; Lodato, S.; Bonev, B.; Groff, A.F.; Gerhardinger, C.; Sanchez-Gomez, D.B.; Hacisuleyman, E.; Li, E.; Spence, M.; et al. Multiple knockout mouse models reveal lincRNAs are required for life and brain development. eLife 2013, 2, e01749. [Google Scholar] [CrossRef]
- Chanda, K.; Mukhopadhyay, D. LncRNA Xist, X-chromosome instability and Alzheimer’s disease. Curr. Alzheimer Res. 2020, 17, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Rajasethupathy, P.; Antonov, I.; Sheridan, R.; Frey, S.; Sander, C.; Tuschl, T.; Kandel, E.R. A role for neuronal piRNAs in the epigenetic control of memory-related synaptic plasticity. Cell 2012, 149, 693–707. [Google Scholar] [CrossRef]
- Zuo, L.; Wang, Z.; Tan, Y.; Chen, X.; Luo, X. piRNAs and their functions in the brain. Int. J. Hum. Genet. 2016, 16, 53–60. [Google Scholar] [CrossRef]
- Qiu, W.; Guo, X.; Lin, X.; Yang, Q.; Zhang, W.; Zhang, Y.; Zuo, L.; Zhu, Y.; Li, C.S.R.; Ma, C.; et al. Transcriptome-wide piRNA profiling in human brains of Alzheimer’s disease. Neurobiol. Aging 2017, 57, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Scheckel, C.; Drapeau, E.; Frias, M.A.; Park, C.Y.; Fak, J.; Zucker-Scharff, I.; Kou, Y.; Haroutunian, V.; Ma’ayan, A.; Buxbaum, J.D.; et al. Regulatory consequences of neuronal ELAV-like protein binding to coding and non-coding RNAs in human brain. eLife 2016, 5, e10421. [Google Scholar] [CrossRef]
- Wei, Z.; Batagov, A.O.; Schinelli, S.; Wang, J.; Wang, Y.; El Fatimy, R.; Rabinovsky, R.; Balaj, L.; Chen, C.C.; Hochberg, F.; et al. Coding and noncoding landscape of extracellular RNA released by human glioma stem cells. Nat. Commun. 2017, 8, 1145. [Google Scholar] [CrossRef] [PubMed]
- Haack, F.; Trakooljul, N.; Gley, K.; Murani, E.; Hadlich, F.; Wimmers, K.; Ponsuksili, S. Deep sequencing of small non-coding RNA highlights brain-specific expression patterns and RNA cleavage. RNA Biol. 2019, 16, 1764–1774. [Google Scholar] [CrossRef]
- Zimmer-Bensch, G. Emerging roles of long non-coding RNAs as drivers of brain evolution. Cells 2019, 8, 1399. [Google Scholar] [CrossRef]
- Bernard, D.; Prasanth, K.V.; Tripathi, V.; Colasse, S.; Nakamura, T.; Xuan, Z.; Zhang, M.Q.; Sedel, F.; Jourdren, L.; Coulpier, F.; et al. A long nuclear-retained non-coding RNA regulates synaptogenesis by modulating gene expression. EMBO J. 2010, 29, 3082–3093. [Google Scholar] [CrossRef]
- Tripathi, V.; Ellis, J.D.; Shen, Z.; Song, D.Y.; Pan, Q.; Watt, A.T.; Freier, S.M.; Bennett, C.F.; Sharma, A.; Bubulya, P.A.; et al. The nuclear-retained noncoding RNA MALAT1 regulates alternative splicing by modulating SR splicing factor phosphorylation. Mol. Cell 2010, 39, 925–938. [Google Scholar] [CrossRef]
- Liau, W.S.; Zhao, Q.; Bademosi, A.; Gormal, R.S.; Gong, H.; Marshall, P.R.; Periyakaruppiah, A.; Madugalle, S.U.; Zajaczkowski, E.L.; Leighton, L.J.; et al. Fear extinction is regulated by the activity of long noncoding RNAs at the synapse. Nat. Commun. 2023, 14, 7616. [Google Scholar] [CrossRef]
- Lu, T.X.; Rothenberg, M.E. MicroRNA. J. Allergy Clin. Immunol. 2018, 141, 1202–1207. [Google Scholar] [CrossRef]
- Starega-Roslan, J.; Krol, J.; Koscianska, E.; Kozlowski, P.; Szlachcic, W.J.; Sobczak, K.; Krzyzosiak, W.J. Structural basis of microRNA length variety. Nucleic Acids Res. 2011, 39, 257–268. [Google Scholar] [CrossRef]
- Fiore, R.; Siegel, G.; Schratt, G. MicroRNA function in neuronal development, plasticity and disease. Biochim. Biophys. Acta (BBA)-Gene Regul. Mech. 2008, 1779, 471–478. [Google Scholar] [CrossRef]
- Martins, H.C.; Schratt, G. MicroRNA-dependent control of neuroplasticity in affective disorders. Transl. Psychiatry 2021, 11, 263. [Google Scholar] [CrossRef] [PubMed]
- Constantin, L. Circular RNAs and neuronal development. In Circular RNAs: Biogenesis and Functions; Springer Nature: Singapore, 2018; pp. 205–213. [Google Scholar]
- Kim, T.K.; Hemberg, M.; Gray, J.M.; Costa, A.M.; Bear, D.M.; Wu, J.; Harmin, D.A.; Laptewicz, M.; Barbara-Haley, K.; Kuersten, S.; et al. Widespread transcription at neuronal activity-regulated enhancers. Nature 2010, 465, 182–187. [Google Scholar] [CrossRef]
- Arner, E.; Daub, C.O.; Vitting-Seerup, K.; Andersson, R.; Lilje, B.; Drabløs, F.; Lennartsson, A.; Rönnerblad, M.; Hrydziuszko, O.; Vitezic, M.; et al. Transcribed enhancers lead waves of coordinated transcription in transitioning mammalian cells. Science 2015, 347, 1010–1014. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.M.; Kim, T.K.; West, A.E.; Nord, A.S.; Markenscoff-Papadimitriou, E.; Lomvardas, S. Genomic views of transcriptional enhancers: Essential determinants of cellular identity and activity-dependent responses in the CNS. J. Neurosci. 2015, 35, 13819–13826. [Google Scholar] [CrossRef] [PubMed]
- Roberts, T.C.; Morris, K.V.; Wood, M.J. The role of long non-coding RNAs in neurodevelopment, brain function and neurological disease. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130507. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Xie, R.; Liu, X.; Shou, J.; Gu, W.; Che, X. Long coding RNA XIST contributes to neuronal apoptosis through the downregulation of AKT phosphorylation and is negatively regulated by miR-494 in rat spinal cord injury. Int. J. Mol. Sci. 2017, 18, 732. [Google Scholar] [CrossRef] [PubMed]
- Aravin, A.; Gaidatzis, D.; Pfeffer, S.; Lagos-Quintana, M.; Landgraf, P.; Iovino, N.; Morris, P.; Brownstein, M.J.; Kuramochi-Miyagawa, S.; Nakano, T.; et al. A novel class of small RNAs bind to MILI protein in mouse testes. Nature 2006, 442, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Girard, A.; Sachidanandam, R.; Hannon, G.J.; Carmell, M.A. A germline-specific class of small RNAs binds mammalian Piwi proteins. Nature 2006, 442, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Betel, D.; Sheridan, R.; Marks, D.S.; Sander, C. Computational analysis of mouse piRNA sequence and biogenesis. PLoS Comput. Biol. 2007, 3, e222. [Google Scholar] [CrossRef]
- Paillard, J. Réflexions sur l’usage du concept de plasticité en neurobiology. J. Psychol. 1976, 1, 33–47. [Google Scholar]
- Alexandre, F. A global framework for a systemic view of brain modeling. Brain Inform. 2021, 8, 3. [Google Scholar] [CrossRef] [PubMed]
- Hille, M.; Kühn, S.; Kempermann, G.; Bonhoeffer, T.; Lindenberger, U. From animal models to human individuality: Integrative approaches to the study of brain plasticity. Neuron 2024, 112, 3522–3541. [Google Scholar] [CrossRef]
- De Yoe, E.A.; Bandettini, P.; Neitz, J.; Miller, D.; Winans, P. Functional magnetic resonance imaging (FMRI) of the human brain. J. Neurosci. Methods 1994, 54, 171–187. [Google Scholar] [CrossRef] [PubMed]
- Bandettini, P.A. Twenty years of functional MRI: The science and the stories. Neuroimage 2012, 62, 575–588. [Google Scholar] [CrossRef] [PubMed]
- Poldrack, R.A.; Mumford, J.A.; Nichols, T.E. Handbook of Functional MRI Data Analysis; Cambridge University Press: Cambridge, UK, 2024. [Google Scholar]
- Mechelli, A.; Price, C.J.; Friston, K.J.; Ashburner, J. Voxel-based morphometry of the human brain: Methods and applications. Curr. Med. Imaging 2005, 1, 105–113. [Google Scholar] [CrossRef]
- Scarpazza, C.; Stefania De Simone, M. Voxel-based morphometry: Current perspectives. Neurosci. Neuroecon. 2016, 5, 19–35. [Google Scholar] [CrossRef]
- Hedderich, D.M.; Opfer, R.; Krüger, J.; Spies, L.; Yakushev, I.; Buchert, R. Clinical validation of artificial intelligence-based single-subject morphometry without normative reference database. J. Alzheimer’s Dis. 2025, 103, 13872877241304607. [Google Scholar] [CrossRef]
- Tournier, J.D.; Mori, S.; Leemans, A. Diffusion tensor imaging and beyond. Magn. Reson. Med. 2011, 65, 1532. [Google Scholar] [CrossRef]
- Van Hecke, W.; Emsell, L.; Sunaert, S. (Eds.) Diffusion Tensor Imaging: A Practical Handbook; Springer: New York, NY, USA, 2016. [Google Scholar]
- Dell’Acqua, F.; Leemans, A.; Dawson, M.; Descoteaux, M. Single-shell diffusion models: From DTI to HARDI. In Handbook of Diffusion MR Tractography; Academic Press: Cambridge, MA, USA, 2025; pp. 177–200. [Google Scholar]
- Craik, A.; He, Y.; Contreras-Vidal, J.L. Deep learning for electroencephalogram (EEG) classification tasks: A review. J. Neural Eng. 2019, 16, 031001. [Google Scholar] [CrossRef] [PubMed]
- Michel, C.M.; Brunet, D. EEG source imaging: A practical review of the analysis steps. Front. Neurol. 2019, 10, 325. [Google Scholar] [CrossRef]
- Müller-Putz, G.R. Electroencephalography. Handbook of clinical neurology 2020, 168, 249–262. [Google Scholar] [PubMed]
- Baillet, S. Magnetoencephalography for brain electrophysiology and imaging. Nat. Neurosci. 2017, 20, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Brickwedde, M.; Anders, P.; Kühn, A.A.; Lofredi, R.; Holtkamp, M.; Kaindl, A.M.; Grent-‘t-Jong, T.; Krüger, P.; Sander, T.; Uhlhaas, P.J. Applications of OPM-MEG for translational neuroscience: A perspective. Transl. Psychiatry 2024, 14, 341. [Google Scholar] [CrossRef]
- Rhodes, N.; Sato, J.; Safar, K.; Amorim, K.; Taylor, M.J.; Brookes, M.J. Paediatric magnetoencephalography and its role in neurodevelopmental disorders. Br. J. Radiol. 2024, 97, 1591–1601. [Google Scholar] [CrossRef]
- Boppart, S.A. Optical coherence tomography: Technology and applications for neuroimaging. Psychophysiology 2003, 40, 529–541. [Google Scholar] [CrossRef]
- Subhash, H.M. Full-Field and Single-Shot Full-Field Optical Coherence Tomography: A Novel Technique for Biomedical Imaging Applications. Adv. Opt. Technol. 2012, 2012, 435408. [Google Scholar] [CrossRef]
- Yang, L.; Chen, P.; Wen, X.; Zhao, Q. Optical coherence tomography (OCT) and OCT angiography: Technological development and applications in brain science. Theranostics 2025, 15, 122. [Google Scholar] [CrossRef] [PubMed]
- Poldrack, R.A. Imaging brain plasticity: Conceptual and methodological issues—A theoretical review. Neuroimage 2000, 12, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Tai, Y.F.; Piccini, P. Applications of positron emission tomography (PET) in neurology. J. Neurol. Neurosurg. Psychiatry 2004, 75, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Veldsman, M.; Egorova, N. Advances in neuroimaging for neurodegenerative disease. In Neurodegenerative Diseases: Pathology, Mechanisms, and Potential Therapeutic Targets; Springer: Cham, Switzerland, 2017; pp. 451–478. [Google Scholar]
- Hricak, H.; Mayerhoefer, M.E.; Herrmann, K.; Lewis, J.S.; Pomper, M.G.; Hess, C.P.; Riklund, K.; Scott, A.M.; Weissleder, R. Advances and challenges in precision imaging. Lancet Oncol. 2025, 26, e34–e45. [Google Scholar] [CrossRef] [PubMed]
- Lapicque, L. Recherches quantitatives sur l’excitation electrique des nerfs. J. Physiol 1907, 9, 620–635. [Google Scholar]
- Abbott, L.F. Lapicque’s introduction of the integrate-and-fire model neuron (1907). Brain Res. Bull. 1999, 50, 303–304. [Google Scholar] [CrossRef] [PubMed]
- Hodgkin, A.; Huxley, A. A quantitative description of membrane current and its application to conduction and excitation in nerve. J. Physiol. 1952, 117, 500–544. [Google Scholar] [CrossRef] [PubMed]
- Neher, E.; Sakmann, B. Single-channel currents recorded from membrane of denervated frog muscle fibres. Nature 1976, 260, 799–802. [Google Scholar] [CrossRef] [PubMed]
- Ibarz, B.; Casado, J.M.; Sanjuán, M.A. Map-based models in neuronal dynamics. Phys. Rep. 2011, 501, 1–74. [Google Scholar] [CrossRef]
- Buesing, L.; Bill, J.; Nessler, B.; Maass, W. Neural dynamics as sampling: A model for stochastic computation in recurrent networks of spiking neurons. PLoS Comput. Biol. 2011, 7, e1002211. [Google Scholar] [CrossRef] [PubMed]
- Cuppini, C.; Magosso, E.; Ursino, M. Organization, maturation, and plasticity of multisensory integration: Insights from computational modeling studies. Front. Psychol. 2011, 2, 77. [Google Scholar] [CrossRef] [PubMed]
- Shine, J.M.; Müller, E.J.; Munn, B.; Cabral, J.; Moran, R.J.; Breakspear, M. Computational models link cellular mechanisms of neuromodulation to large-scale neural dynamics. Nat. Neurosci. 2021, 24, 765–776. [Google Scholar] [CrossRef] [PubMed]
- Ramaswamy, S. Data-driven multiscale computational models of cortical and subcortical regions. Curr. Opin. Neurobiol. 2024, 85, 102842. [Google Scholar] [CrossRef]
- Po, H.F.; Houben, A.M.; Haeb, A.C.; Jenkins, D.R.; Hill, E.J.; Parri, H.R.; Soriano, J.; Saad, D. Inferring structure of cortical neuronal networks from activity data: A statistical physics approach. Proc. Natl. Acad. Sci. Nexus 2025, 4, pgae565. [Google Scholar] [CrossRef] [PubMed]
- Sporns, O. Contributions and challenges for network models in cognitive neuroscience. Nat. Neurosci. 2014, 17, 652–660. [Google Scholar] [CrossRef] [PubMed]
- Sporns, O.; Betzel, R.F. Modular brain networks. Annu. Rev. Psychol. 2016, 67, 613–640. [Google Scholar] [CrossRef] [PubMed]
- Sporns, O. Graph theory methods: Applications in brain networks. Dialogues Clin. Neurosci. 2018, 20, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Ranasinghe, P.V.; Mapa, M.S. Functional connectivity and cognitive decline: A review of rs-fMRI, EEG, MEG, and graph theory approaches in aging and dementia. Explor. Med. 2024, 5, 797–821. [Google Scholar] [CrossRef]
- Xerri, C.; Zennou-Azogui, Y. Influence of the postlesion environment and chronic piracetam treatment on the organization of the somatotopic map in the rat primary somatosensory cortex after focal cortical injury. Neuroscience 2003, 118, 161–177. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, L.B. Neuropharmacology of TBI-induced plasticity. Brain Inj. 2003, 17, 685–694. [Google Scholar] [CrossRef]
- Plewnia, C.; Hoppe, J.; Cohen, L.G.; Gerloff, C. Improved motor skill acquisition after selective stimulation of central nore-pinephrine. Neurology 2004, 62, 2124–2126. [Google Scholar] [CrossRef] [PubMed]
- Loubinoux, I.; Pariente, J.; Boulanouar, K.; Carel, C.; Manelfe, C.; Rascol, O.; Celsis, P.; Chollet, F. A single dose of the serotonin neurotransmission agonist paroxetine enhances motor output: Double-blind, placebo-controlled, fMRI study in healthy subjects. NeuroImage 2002, 15, 26–36. [Google Scholar] [CrossRef]
- Dam, M.; Tonin, P.; De Boni, A.; Pizzolato, G.; Casson, S.; Ermani, M.; Freo, U.; Piron, L.; Battistin, L. Effects of fluoxetine and maprotiline on functional recovery in poststroke hemiplegic patients undergoing rehabilitation therapy. Stroke 1996, 27, 1211–1214. [Google Scholar] [CrossRef] [PubMed]
- Sawaki, L.; Boroojerdi, B.; Kaelin-Lang, A.; Burstein, A.H.; Bütefisch, C.M.; Kopylev, L.; Davis, B.; Cohen, L.G. Cholinergic influences on use-dependent plasticity. J. Neurophysiol. 2002, 87, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Ziemann, U.; Lönnecker, S.; Steinhoff, B.J.; Paulus, W. The effect of lorazepam on the motor cortical excitability in man. Exp. Brain Res. 1996, 109, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Walker-Batson, D.; Curtis, S.; Natarajan, R.; Ford, J.; Dronkers, N.; Salmeron, E.; Lai, J.; Unwin, D.H. A double-blind, placebo- controlled study of the use of amphetamine in the treatment of aphasia. Stroke 2001, 32, 2093–2098. [Google Scholar] [CrossRef]
- Bragoni, M.; Altieri, M.; Di Piero, V.; Padovani, A.; Mostardini, C.; Lenzi, G.L. Bromocriptine and speech therapy in non-fluent chronic aphasia after stroke. Neurol. Sci. 2000, 21, 19–22. [Google Scholar] [CrossRef]
- Kessler, J.; Thiel, A.; Karbe, H.; Heiss, W.D. Piracetam improves activated blood flow and facilitates rehabilitation of poststroke aphasic patients. Stroke 2000, 31, 2112–2116. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; Rossini, P.M. TMS in cognitive plasticity and the potential for rehabilitation. Trends Cogn. Sci. 2004, 8, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Stefan, K.; Kunesch, E.; Cohen, L.G.; Benecke, R.; Classen, J. Induction of plasticity in the human motor cortex by paired associative stimulation. Brain 2000, 123 Pt 3, 572–584. [Google Scholar] [CrossRef] [PubMed]
- Oliveri, M.; Bisiach, E.; Brighina, F.; Piazza, A.; La Bua, V.; Buffa, D.; Fierro, B. rTMS of the unaffected hemisphere transiently reduces contralesional visuospatial hemineglect. Neurology 2001, 57, 1338–1340. [Google Scholar] [CrossRef] [PubMed]
- Brighina, F.; Bisiach, E.; Oliveri, M.; Piazza, A.; La Bua, V.; Daniele, O.; Fierro, B. 1 Hz repetitive transcranial magnetic stimulation of the unaffected hemisphere ameliorates contralesional visuospatial neglect in humans. Neurosci. Lett. 2003, 336, 131–133. [Google Scholar] [CrossRef] [PubMed]
- Mottaghy, F.M.; Hungs, M.; Brügmann, M.; Sparing, R.; Boroojerdi, B.; Foltys, H.; Huber, W.; Töpper, R. Facilitation of picture naming after repetitive transcranial magnetic stimulation. Neurology 1999, 53, 1806–1812. [Google Scholar] [CrossRef] [PubMed]
- Grafman, J.; Wassermann, E. Transcranial magnetic stimulation can measure and modulate learning and memory. Neuropsychologia 1999, 37, 159–167. [Google Scholar] [CrossRef]
- Boylan, L.S. Enhancing analogic reasoning with rTMS over the left prefrontal cortex. Neurology 2001, 57, 1349. [Google Scholar] [CrossRef] [PubMed]
- Evers, S.; Böckermann, I.; Nyhuis, P.W. The impact of transcranial magnetic stimulation on cognitive processing: An event-related potential study. Neuroreport 2001, 12, 2915–2918. [Google Scholar] [CrossRef]
- Lee, L.; Siebner, H.R.; Rowe, J.B.; Rizzo, V.; Rothwell, J.C.; Frackowiak, R.S.J.; Friston, K.J. Acute remapping within the motor system induced by low-frequency repetitive transcranial magnetic stimulation. J. Neurosci. 2003, 23, 5308–5318. [Google Scholar] [CrossRef] [PubMed]
- Canavero, S.; Bonicalzi, V.; Paolotti, R.; Castellano, G.; Greco-Crasto, S.; Rizzo, L.; Davini, O.; Maina, R. Therapeutic extradural cortical stimulation for movement disorders: A review. Neurol. Res. 2003, 25, 118–122. [Google Scholar] [CrossRef]
- Tsubokawa, T.; Katayama, Y.; Yamamoto, T.; Hirayama, T.; Koyama, S. Chronic motor cortex stimulation in patients with thalamic pain. J. Neurosurg. 1993, 78, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Canavero, S.; Bonicalzi, V. Extradural cortical stimulation for central pain. In Operative Neuromodulation; Acta Neurochirurgica Supplements; Springer: Vienna, Austria, 2007; Volume 97, pp. 27–36. [Google Scholar]
- Benabid, A. Deep brain stimulation for Parkinson’s disease. Curr. Opin. Neurobiol. 2003, 13, 696–706. [Google Scholar] [CrossRef] [PubMed]
- Lozano, A.M.; Dostrovsky, J.; Chen, R.; Ashby, P. Deep brain stimulation for Parkinson’s disease: Disrupting the disruption. Lancet Neurol. 2002, 1, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Duffau, H.; Capelle, L.; Lopes, M.; Faillot, T.; Sichez, J.P.; Fohanno, D. The insular lobe: Physiopathological and surgical considerations. Neurosurgery 2000, 47, 801–810, discussion 810–811. [Google Scholar] [CrossRef]
- Duffau, H.; Bauchet, L.; Lehéricy, S.; Capelle, L. Functional compensation of the left dominant insula for language. Neuroreport 2001, 12, 2159–2163. [Google Scholar] [CrossRef] [PubMed]
- Duffau, H.; Denvil, D.; Capelle, L. Absence of movement disorders after surgical resection of glioma invading the right striatum. J. Neurosurg. 2002, 97, 363–369. [Google Scholar] [CrossRef]
- Duffau, H.; Capelle, L.; Denvil, D.; Sichez, N.; Gatignol, P.; Lopes, M.; Mitchell, M.C.; Sichez, J.P.; Van Effenterre, R. Functional recovery after surgical resection of low grade gliomas in eloquent brain: Hypothesis of brain compensation. J. Neurol. Neurosurg. Psychiatry 2003, 74, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Duffau, H.; Capelle, L.; Denvil, D.; Gatignol, P.; Sichez, N.; Lopes, M.; Sichez, J.P.; Van Effenterre, R. The role of dominant premotor cortex in language: A study using intraoperative functional mapping in awake patients. NeuroImage 2003, 20, 1903–1914. [Google Scholar] [CrossRef]
- Duffau, H.; Khalil, I.; Gatignol, P.; Denvil, D.; Capelle, L. Surgical removal of corpus callosum infiltrated by low-grade glioma: Functional outcome and oncological considerations. J. Neurosurg. 2004, 100, 431–437. [Google Scholar] [CrossRef]
- Duffau, H.; Denvil, D.; Capelle, L. Long term reshaping of language, sensory, and motor maps after glioma resection: A new parameter to integrate in the surgical strategy. J. Neurol. Neurosurg. Psychiatry 2002, 72, 511–516. [Google Scholar]
- Naeser, M.A.; Palumbo, C.L.; Helm-Estabrooks, N.; Stiassny-Eder, D.; Albert, M.L. Severe nonfluency in aphasia. Role of the medial subcallosal fasciculus and other white matter pathways in recovery of spontaneous speech. Brain 1989, 112 Pt 1, 1–38. [Google Scholar] [CrossRef]
- Holodny, A.I.; Schwartz, T.H.; Ollenschleger, M.; Liu, W.C.; Schulder, M. Tumor involvement of the corticospinal tract: Diffusion magnetic resonance tractography with intraoperative correlation. J. Neurosurg. 2001, 95, 1082. [Google Scholar] [CrossRef] [PubMed]
- Peraud, A.; Meschede, M.; Eisner, W.; Ilmberger, J.; Reulen, H.J. Surgical resection of grade II astrocytomas in the superior frontal gyrus. Neurosurgery 2002, 50, 966–975, discussion 975–977. [Google Scholar] [PubMed]
- Duffau, H. Intraoperative cortico–subcortical stimulations in surgery of low-grade gliomas. Expert Rev. Neurother. 2005, 5, 473–485. [Google Scholar] [CrossRef] [PubMed]
- Döbrössy, M.D.; Dunnett, S.B. The influence of environment and experience on neural grafts. Nat. Rev. Neurosci. 2001, 2, 871–879. [Google Scholar] [CrossRef]
- Bachoud-Lévi, A.C.; Rémy, P.; Nguyen, J.P.; Brugières, P.; Lefaucheur, J.P.; Bourdet, C.; Baudic, S.; Gaura, V.; Maison, P.; Haddad, B.; et al. Motor and cognitive improvements in patients with Huntington’s disease after neural transplantation. Lancet 2000, 356, 1975–1979. [Google Scholar] [CrossRef] [PubMed]
- Lindvall, O. Stem cells for cell therapy in Parkinson’s disease. Pharmacol. Res. 2003, 47, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Buchan, A.M.; Warren, D.; Burnstein, R. Transplantation of cultured human neuronal cells for patients with stroke. Neurology 2001, 56, 821–822. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Statsenko, Y.; Kuznetsov, N.V.; Ljubisaljevich, M. Hallmarks of Brain Plasticity. Biomedicines 2025, 13, 460. https://doi.org/10.3390/biomedicines13020460
Statsenko Y, Kuznetsov NV, Ljubisaljevich M. Hallmarks of Brain Plasticity. Biomedicines. 2025; 13(2):460. https://doi.org/10.3390/biomedicines13020460
Chicago/Turabian StyleStatsenko, Yauhen, Nik V. Kuznetsov, and Milos Ljubisaljevich. 2025. "Hallmarks of Brain Plasticity" Biomedicines 13, no. 2: 460. https://doi.org/10.3390/biomedicines13020460
APA StyleStatsenko, Y., Kuznetsov, N. V., & Ljubisaljevich, M. (2025). Hallmarks of Brain Plasticity. Biomedicines, 13(2), 460. https://doi.org/10.3390/biomedicines13020460







