Low-Burden Oligometastatic Disease of the Lung Treated with Robotic Stereotactic Ablative Radiotherapy: A Retrospective Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Planning and Treatment Details
2.2. Treatment Efficacy and Toxicity Evaluation
2.3. Statistical Analysis
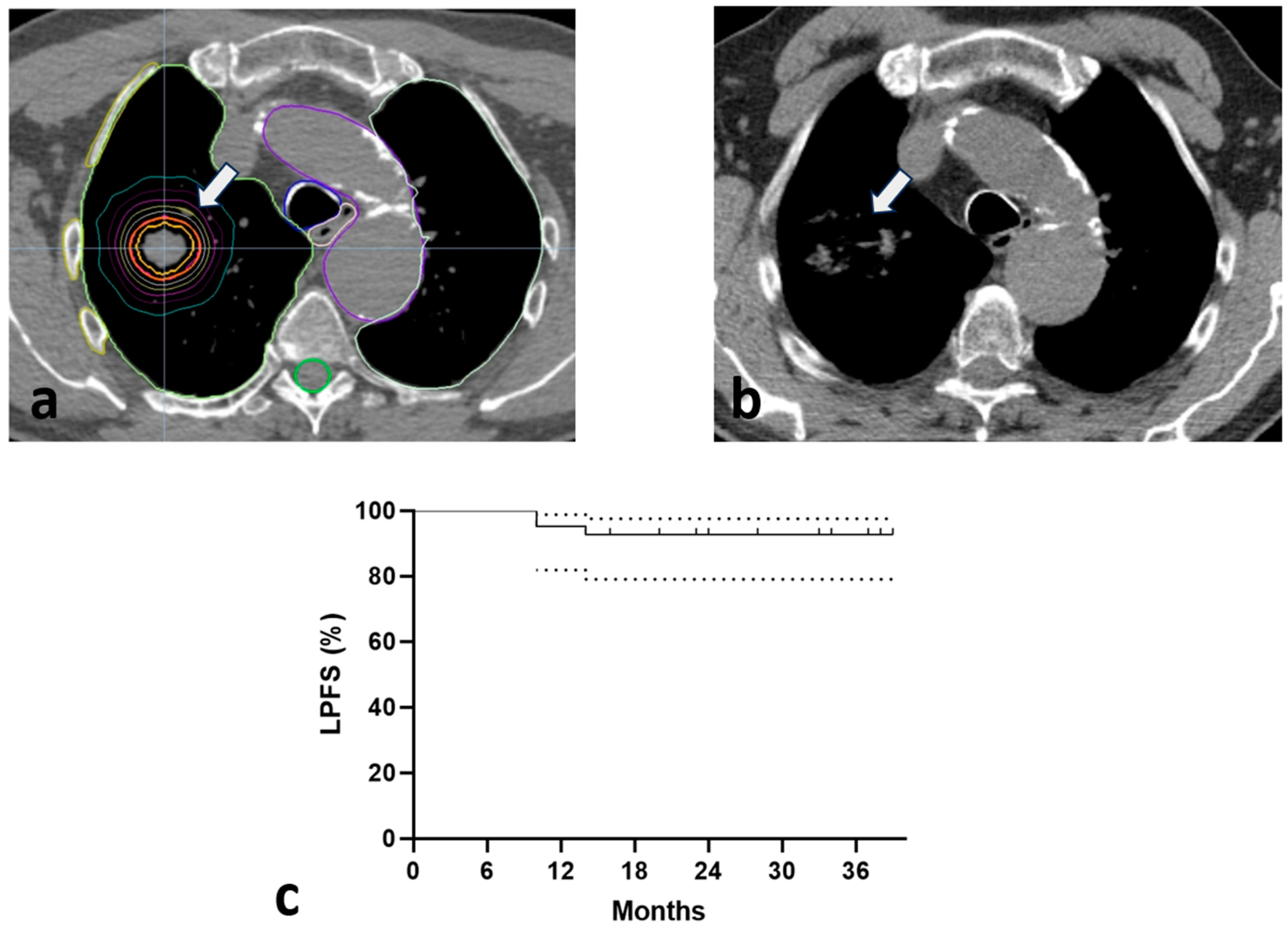
3. Results
3.1. Toxicity
3.2. Response
3.3. Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hudock, N.L.; Mani, K.; Khunsriraksakul, C.; Walter, V.; Nekhlyudov, L.; Wang, M.; Lehrer, E.J.; Hudock, M.R.; Liu, D.J.; Spratt, D.E.; et al. Future trends in incidence and long-term survival of metastatic cancer in the United States. Commun. Med. 2023, 3, 76. [Google Scholar] [CrossRef] [PubMed]
- Sonkin, D.; Thomas, A.; Teicher, B.A. Cancer treatments: Past, present, and future. Cancer Genet. 2024, 286–287, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Pacifico, P.; Colciago, R.R.; De Felice, F.; Boldrini, L.; Salvestrini, V.; Nardone, V.; Desideri, I.; Greco, C.; Arcangeli, S. A critical review on oligometastatic disease: A radiation oncologist’s perspective. Med. Oncol. 2022, 39, 181. [Google Scholar] [CrossRef] [PubMed]
- Harrow, S.; Palma, D.A.; Olson, R.; Gaede, S.; Louie, A.V.; Haasbeek, C.; Mulroy, L.; Lock, M.; Rodrigues, G.B.; Yaremko, B.P.; et al. Stereotactic Radiation for the Comprehensive Treatment of Oligometastases (SABR-COMET): Extended Long-Term Outcomes. Int. J. Radiat. Oncol. Biol. Phys. 2022, 114, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Lievens, Y.; Guckenberger, M.; Gomez, D.; Hoyer, M.; Iyengar, P.; Kindts, I.; Mendez Romero, A.; Nevens, D.; Palma, D.; Park, C.; et al. Defining oligometastatic disease from a radiation oncology perspective: An ESTRO-ASTRO consensus document. Radiother. Oncol. 2020, 148, 157–166. [Google Scholar] [CrossRef]
- Koukourakis, I.M.; Tiniakos, D.; Kouloulias, V.; Zygogianni, A. The molecular basis of immuno-radiotherapy. Int. J. Radiat. Biol. 2023, 99, 715–736. [Google Scholar] [CrossRef] [PubMed]
- Gerull, W.D.; Puri, V.; Kozower, B.D. The epidemiology and biology of pulmonary metastases. J. Thorac. Dis. 2021, 13, 2585–2589. [Google Scholar] [CrossRef]
- Chang, J.Y.; Mehran, R.J.; Feng, L.; Verma, V.; Liao, Z.; Welsh, J.W.; Lin, S.H.; O’Reilly, M.S.; Jeter, M.D.; Balter, P.A.; et al. Stereotactic ablative radiotherapy for operable stage I non-small-cell lung cancer (revised STARS): Long-term results of a single-arm, prospective trial with prespecified comparison to surgery. Lancet Oncol. 2021, 22, 1448–1457. [Google Scholar] [CrossRef]
- Niibe, Y.; Yamamoto, T.; Onishi, H.; Yamashita, H.; Katsui, K.; Matsumoto, Y.; Oh, R.J.; Aoki, M.; Shintani, T.; Yamada, K.; et al. Pulmonary Oligometastases Treated by Stereotactic Body Radiation Therapy: A Nationwide Survey of 1,378 Patients. Anticancer. Res. 2020, 40, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Horner-Rieber, J.; Bernhardt, D.; Blanck, O.; Duma, M.; Eich, H.T.; Gerum, S.; Gkika, E.; Hass, P.; Henkenberens, C.; Herold, H.U.; et al. Long-term Follow-up and Patterns of Recurrence of Patients With Oligometastatic NSCLC Treated With Pulmonary SBRT. Clin. Lung Cancer 2019, 20, e667–e677. [Google Scholar] [CrossRef]
- Ricco, A.; Davis, J.; Rate, W.; Yang, J.; Perry, D.; Pablo, J.; D’Ambrosio, D.; Sharma, S.; Sundararaman, S.; Kolker, J.; et al. Lung metastases treated with stereotactic body radiotherapy: The RSSearch(R) patient Registry’s experience. Radiat. Oncol. 2017, 12, 35. [Google Scholar] [CrossRef]
- Zygogianni, A.; Koukourakis, I.M.; Georgakopoulos, J.; Armpilia, C.; Liakouli, Z.; Desse, D.; Ntoumas, G.; Simopoulou, F.; Nikoloudi, M.; Kouloulias, V. Robotic Stereotactic Ablative Radiotherapy for Patients with Early-Stage Lung Cancer: Results of an Interim Analysis. Cancers 2024, 16, 3227. [Google Scholar] [CrossRef]
- Diez, P.; Hanna, G.G.; Aitken, K.L.; van As, N.; Carver, A.; Colaco, R.J.; Conibear, J.; Dunne, E.M.; Eaton, D.J.; Franks, K.N.; et al. UK 2022 Consensus on Normal Tissue Dose-Volume Constraints for Oligometastatic, Primary Lung and Hepatocellular Carcinoma Stereotactic Ablative Radiotherapy. Clin. Oncol. 2022, 34, 288–300. [Google Scholar] [CrossRef]
- Hanna, G.G.; Murray, L.; Patel, R.; Jain, S.; Aitken, K.L.; Franks, K.N.; van As, N.; Tree, A.; Hatfield, P.; Harrow, S.; et al. UK Consensus on Normal Tissue Dose Constraints for Stereotactic Radiotherapy. Clin. Oncol. 2018, 30, 5–14. [Google Scholar] [CrossRef]
- Fowler, J.F. The linear-quadratic formula and progress in fractionated radiotherapy. Br. J. Radiol. 1989, 62, 679–694. [Google Scholar] [CrossRef] [PubMed]
- Bentzen, S.M.; Skoczylas, J.Z.; Bernier, J. Quantitative clinical radiobiology of early and late lung reactions. Int. J. Radiat. Biol. 2000, 76, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- O, J.H.; Lodge, M.A.; Wahl, R.L. Practical PERCIST: A Simplified Guide to PET Response Criteria in Solid Tumors 1.0. Radiology 2016, 280, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0, Cancer therapy Evaluation Program, Division of Cancer Treatment and Diagnosis, NIH/National Cancer Institute. Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf (accessed on 5 January 2025).
- Kouloulias, V.; Zygogianni, A.; Efstathopoulos, E.; Victoria, O.; Christos, A.; Pantelis, K.; Koutoulidis, V.; Kouvaris, J.; Sandilos, P.; Varela, M.; et al. Suggestion for a new grading scale for radiation induced pneumonitis based on radiological findings of computerized tomography: Correlation with clinical and radiotherapeutic parameters in lung cancer patients. Asian Pac. J. Cancer Prev. 2013, 14, 2717–2722. [Google Scholar] [CrossRef]
- Tanguturi, S.K.; George, S.; Marcus, K.J.; Demetri, G.D.; Baldini, E.H. Whole Lung Irradiation in Adults with Metastatic Ewing Sarcoma: Practice Patterns and Implications for Treatment. Sarcoma 2015, 2015, 591698. [Google Scholar] [CrossRef] [PubMed]
- Fleming, C.; Rimner, A.; Foster, A.; Woo, K.M.; Zhang, Z.; Wu, A.J. Palliative efficacy and local control of conventional radiotherapy for lung metastases. Ann. Palliat. Med. 2017, 6, S21–S27. [Google Scholar] [CrossRef] [PubMed]
- Phillips, R.; Shi, W.Y.; Deek, M.; Radwan, N.; Lim, S.J.; Antonarakis, E.S.; Rowe, S.P.; Ross, A.E.; Gorin, M.A.; Deville, C.; et al. Outcomes of Observation vs Stereotactic Ablative Radiation for Oligometastatic Prostate Cancer: The ORIOLE Phase 2 Randomized Clinical Trial. JAMA Oncol. 2020, 6, 650–659. [Google Scholar] [CrossRef] [PubMed]
- Iyengar, P.; Wardak, Z.; Gerber, D.E.; Tumati, V.; Ahn, C.; Hughes, R.S.; Dowell, J.E.; Cheedella, N.; Nedzi, L.; Westover, K.D.; et al. Consolidative Radiotherapy for Limited Metastatic Non-Small-Cell Lung Cancer: A Phase 2 Randomized Clinical Trial. JAMA Oncol. 2018, 4, e173501. [Google Scholar] [CrossRef]
- Siva, S.; MacManus, M.; Ball, D. Stereotactic radiotherapy for pulmonary oligometastases: A systematic review. J. Thorac. Oncol. 2010, 5, 1091–1099. [Google Scholar] [CrossRef] [PubMed]
- Rieber, J.; Streblow, J.; Uhlmann, L.; Flentje, M.; Duma, M.; Ernst, I.; Blanck, O.; Wittig, A.; Boda-Heggemann, J.; Krempien, R.; et al. Stereotactic body radiotherapy (SBRT) for medically inoperable lung metastases-A pooled analysis of the German working group “stereotactic radiotherapy”. Lung Cancer 2016, 97, 51–58. [Google Scholar] [CrossRef]
- Ricardi, U.; Filippi, A.R.; Guarneri, A.; Ragona, R.; Mantovani, C.; Giglioli, F.; Botticella, A.; Ciammella, P.; Iftode, C.; Buffoni, L.; et al. Stereotactic body radiation therapy for lung metastases. Lung Cancer 2012, 75, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Schulz, A.; Meyer, F.; Dubrovska, A.; Borgmann, K. Cancer Stem Cells and Radioresistance: DNA Repair and Beyond. Cancers 2019, 11, 862. [Google Scholar] [CrossRef] [PubMed]
- Vitale, I.; Manic, G.; De Maria, R.; Kroemer, G.; Galluzzi, L. DNA Damage in Stem Cells. Mol. Cell 2017, 66, 306–319. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Han, J.; Lin, K.; Wang, J.; Li, G.; Li, X.; Gao, Y. PTEN/AKT and Wnt/beta-catenin signaling pathways regulate the proliferation of Lgr5+ cells in liver cancer. Biochem. Biophys. Res. Commun. 2023, 683, 149117. [Google Scholar] [CrossRef]
- Arnold, C.R.; Mangesius, J.; Skvortsova, I.-I.; Ganswindt, U. The Role of Cancer Stem Cells in Radiation Resistance. Front. Oncol. 2020, 10, 164. [Google Scholar] [CrossRef]
- Lee, Y.H.; Kang, K.M.; Choi, H.S.; Ha, I.B.; Jeong, H.; Song, J.H.; Jang, I.S.; Kim, S.H.; Lee, J.W.; Rhee, D.Y.; et al. Comparison of stereotactic body radiotherapy versus metastasectomy outcomes in patients with pulmonary metastases. Thorac. Cancer 2018, 9, 1671–1679. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Exposito, N.; Ramos, R.; Navarro-Perez, V.; Molina, K.; Arnaiz, M.D.; Padrones, S.; Ruffinelli, J.C.; Santos, C.; Guedea, F.; Navarro-Martin, A. Stereotactic Body Radiotherapy versus Surgery for Lung Metastases from Colorectal Cancer: Single-Institution Results. Cancers 2023, 15, 1195. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dong, X.; Yan, S.; Liu, B.; Li, X.; Li, S.; Lv, C.; Cui, X.; Tao, Y.; Yu, R.; et al. Comparison of the Long-term Survival Outcome of Surgery versus Stereotactic Body Radiation Therapy as Initial Local Treatment for Pulmonary Oligometastases from Colorectal Cancer: A Propensity Score Analysis. Int. J. Radiat. Oncol. Biol. Phys. 2025, 121, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Vanpouille-Box, C.; Alard, A.; Aryankalayil, M.J.; Sarfraz, Y.; Diamond, J.M.; Schneider, R.J.; Inghirami, G.; Coleman, C.N.; Formenti, S.C.; Demaria, S. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat. Commun. 2017, 8, 15618. [Google Scholar] [CrossRef] [PubMed]
- Britschgi, C.; Riesterer, O.; Burger, I.A.; Guckenberger, M.; Curioni-Fontecedro, A. Report of an abscopal effect induced by stereotactic body radiotherapy and nivolumab in a patient with metastatic non-small cell lung cancer. Radiat. Oncol. 2018, 13, 102. [Google Scholar] [CrossRef]
- Chang, J.Y.; Lin, S.H.; Dong, W.; Liao, Z.; Gandhi, S.J.; Gay, C.M.; Zhang, J.; Chun, S.G.; Elamin, Y.Y.; Fossella, F.V.; et al. Stereotactic ablative radiotherapy with or without immunotherapy for early-stage or isolated lung parenchymal recurrent node-negative non-small-cell lung cancer: An open-label, randomised, phase 2 trial. Lancet 2023, 402, 871–881. [Google Scholar] [CrossRef]
| No pts | 41 | % |
| PS | ||
| 0 | 15 | 36.6 |
| 1 | 19 | 46.4 |
| 2 | 6 | 14.6 |
| 3 | 1 | 2.4 |
| Gender | ||
| Male | 19 | 46.4 |
| Female | 22 | 53.6 |
| Age | ||
| Median | 72 | |
| Range | 40–84 | |
| Primary tumor | ||
| Breast cancer | 10 | 24.5 |
| Colorectal cancer | 8 | 19.5 |
| Head and neck cancer | 1 | 2.4 |
| Non-small cell lung cancer | 14 | 34.1 |
| Sarcoma | 7 | 17.1 |
| Gastric cancer | 1 | 2.4 |
| Number of lung metastases treated (total) | 51 | |
| 1 (per patient) | 31 | 75.6% |
| 2 (per patient) | 10 | 24.4% |
| Size (mm) | ||
| Median | 17 | |
| Range | 7–30 | |
| 33rd Percentile | 16 | |
| 66th Percentile | 18 | |
| Volume (cc) | ||
| Median | 22 | |
| Range | 3–29 | |
| 33rd Percentile | 19 | |
| 66th Percentile | 23 | |
| Location | ||
| Peripheral | 51 | 100% |
| Concurrent systemic therapy | ||
| None | 3 | 7.3 |
| Chemotherapy | 20 | 48.7 |
| Immunotherapy ± Chemo | 18 | 44 |
| Critical Structure | Max Critical Volume Above Threshold | Threshold Dose (Gy) | Max Point Dose (Gy) |
|---|---|---|---|
| Spinal cord | <0.35 cc | 18 | 21.9 |
| Esophagus | <5 cc | 17.7 | 25.2 |
| Heart | <15 cc | 24 | 30 |
| Great vessels | <10 cc | 39 | 45 |
| Trachea and large bronchus | <4 cc | 15 | 30 |
| Lungs (Right and Left) | <1500 cc | 10.5 Gy | - |
| Rib | <1 cc | 28.8 | 36.9 |
| <30 cc | 30 | - |
| Fractions/ Dose Per Fraction | No. Patients | BEDα/β=3 (Gy) | BEDα/β=10 (Gy) |
|---|---|---|---|
| 1/30 Gy | 1 | 330 | 120 |
| 3/20 Gy | 1 | 460 | 180 |
| 3/18 Gy | 8 | 378 | 151.2 |
| 3/17 Gy | 5 | 340 | 137.7 |
| 3/15 Gy | 5 | 270 | 112.5 |
| 3/12 Gy | 6 | 180 | 79.2 |
| 5/11 Gy | 3 | 256.7 | 115.5 |
| 5/10 Gy | 11 | 216.7 | 100 |
| 15/3.5 Gy | 1 | 113.5 | 70.9 |
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Covariate | R (95% CI) | P | R (95% CI) | P |
| Primary | 0.23 (0.11–0.72) | 0.018 | 0.44(0.21–0.67) | 0.012 |
| Systemic treatment | 0.05 | 0.78 | ||
| Volume | −0.29 | 0.073 | - | |
| BEDα/β=10 | 0.33 | 0.091 | - | |
| No pts | 41 | % |
| Lung Toxicitiy | ||
| Early | 0 | 0 |
| Late | ||
| Grade 0 | 12 | 29.3 |
| Grade 1 | 25 | 61 |
| Grade 2 | 3 | 7.3 |
| Grade 3 | 1 | 2.4 |
| Response | ||
| Complete response | 18 | 43.9% |
| Partial response (>70%) | 23 | 56.1% |
| Survival rates (3-year) | ||
| Overall survival | 37 | 90.2% |
| Local progression-free survival | 38 | 92.6% |
| Metastasis-free survival | 38 | 92.6% |
| Progression-free survival | 36 | 87.8% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zygogianni, A.; Koukourakis, I.M.; Liakouli, Z.; Desse, D.; Georgakopoulos, I.; Armpilia, C.; Lymperopoulou, G.; Kouloulias, V. Low-Burden Oligometastatic Disease of the Lung Treated with Robotic Stereotactic Ablative Radiotherapy: A Retrospective Study. Biomedicines 2025, 13, 517. https://doi.org/10.3390/biomedicines13020517
Zygogianni A, Koukourakis IM, Liakouli Z, Desse D, Georgakopoulos I, Armpilia C, Lymperopoulou G, Kouloulias V. Low-Burden Oligometastatic Disease of the Lung Treated with Robotic Stereotactic Ablative Radiotherapy: A Retrospective Study. Biomedicines. 2025; 13(2):517. https://doi.org/10.3390/biomedicines13020517
Chicago/Turabian StyleZygogianni, Anna, Ioannis M. Koukourakis, Zoi Liakouli, Dimitra Desse, Ioannis Georgakopoulos, Christina Armpilia, Georgia Lymperopoulou, and Vasileios Kouloulias. 2025. "Low-Burden Oligometastatic Disease of the Lung Treated with Robotic Stereotactic Ablative Radiotherapy: A Retrospective Study" Biomedicines 13, no. 2: 517. https://doi.org/10.3390/biomedicines13020517
APA StyleZygogianni, A., Koukourakis, I. M., Liakouli, Z., Desse, D., Georgakopoulos, I., Armpilia, C., Lymperopoulou, G., & Kouloulias, V. (2025). Low-Burden Oligometastatic Disease of the Lung Treated with Robotic Stereotactic Ablative Radiotherapy: A Retrospective Study. Biomedicines, 13(2), 517. https://doi.org/10.3390/biomedicines13020517







