The Impact of Tertiary Lymphoid Structures on Tumor Prognosis and the Immune Microenvironment in Colorectal Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Acquisition and Processing of Public Data
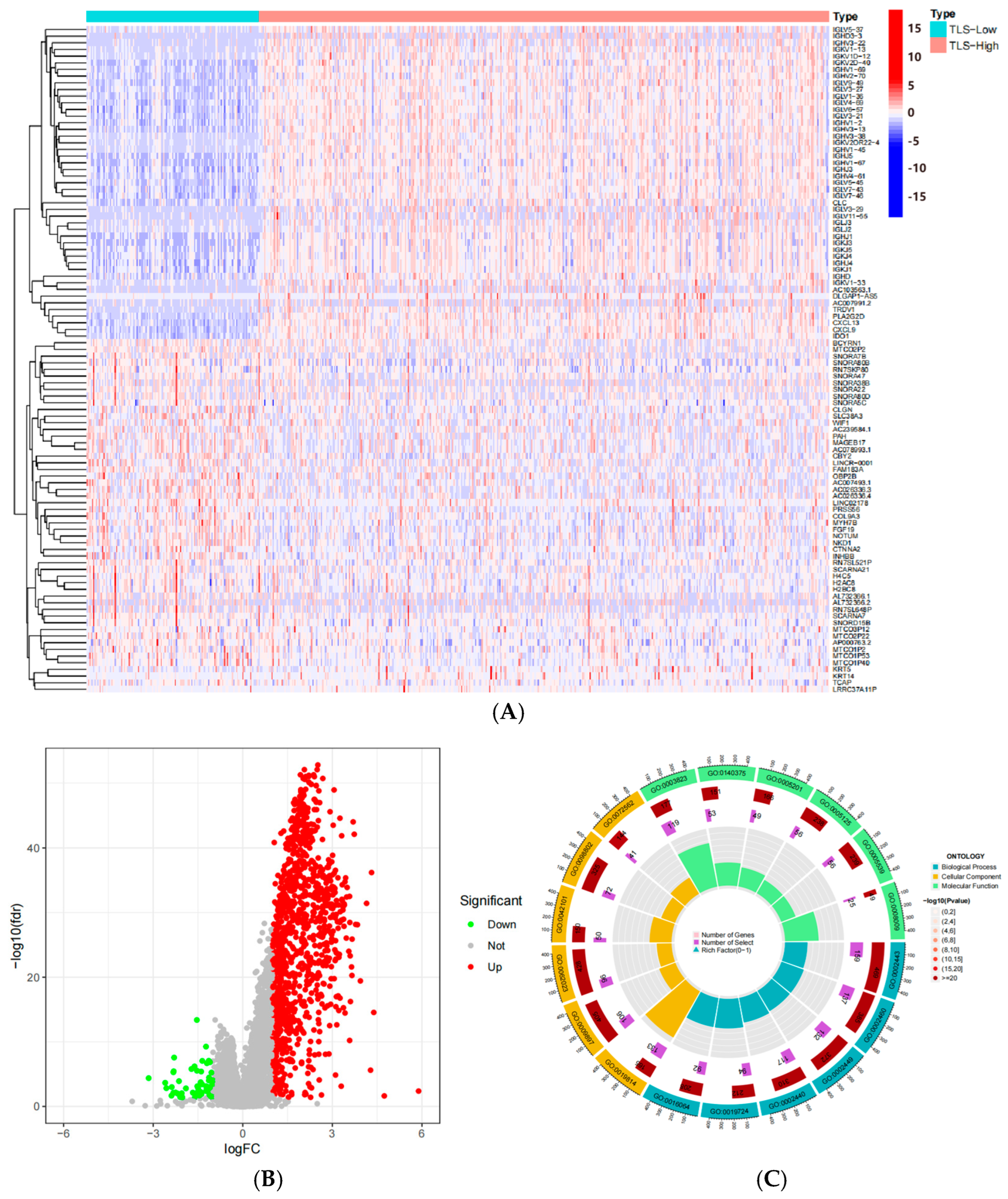
2.2. Identification of TLS-Related Differentially Expressed Genes in CRC
2.3. Enrichment Analysis of TLS-Related Genes
2.4. Prognostic Modelling of TLS-Related Genes
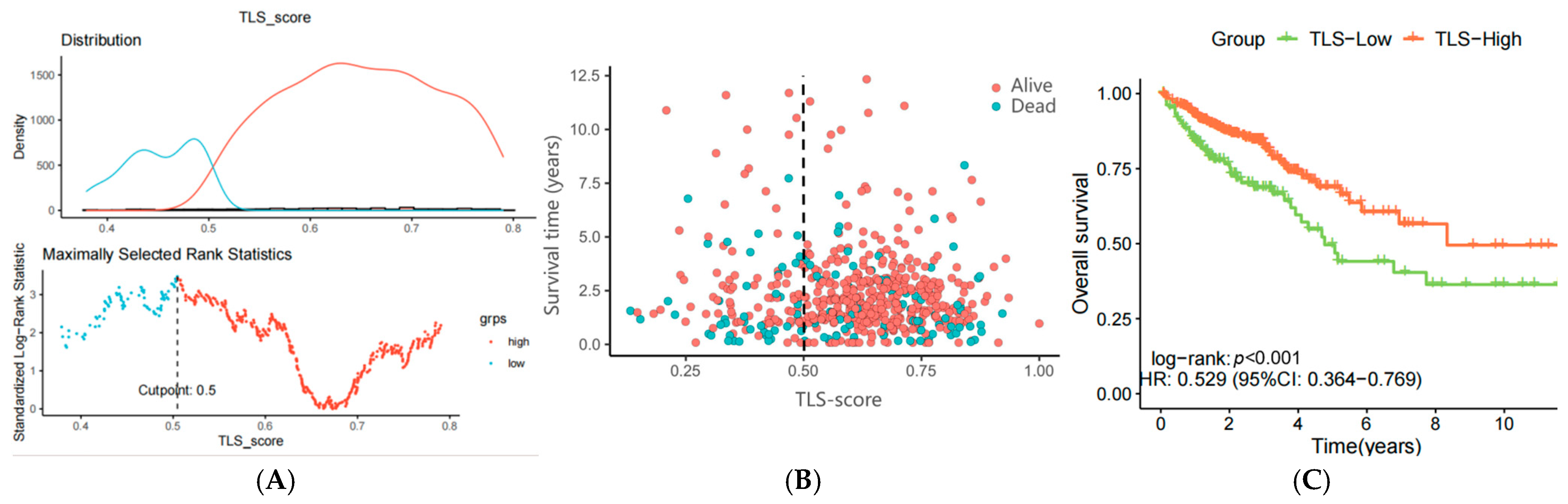
2.5. Calculation of TLS Score
2.6. Validating of Predictive Models
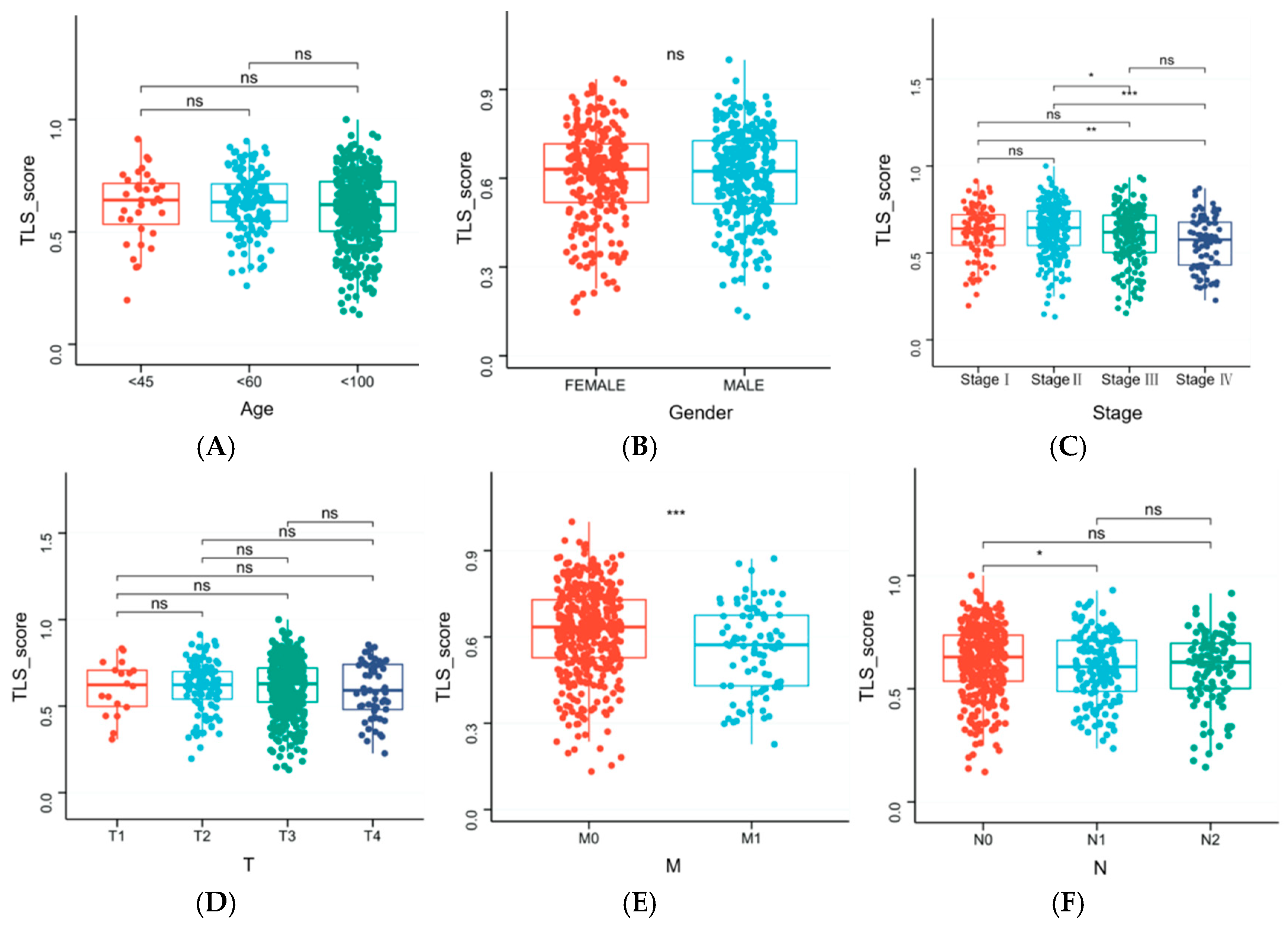
2.7. Correlation Between Clinical Characteristics and TLS Score
2.8. Independent Prognostic Analysis and Nomogram Construction
2.9. Genetic Alterations in TLS Groups
2.10. Immune Cell Infiltration Profiles in TLS Groups
2.11. Immune Checkpoints Analysis
2.12. Statistical Analysis
3. Results
3.1. Identification of TLS-Related Differentially Expressed Genes in CRC
3.2. Construction of a Prognostic Model of TLS-Related Genes in CRC
3.3. Functional Enrichment Analysis of TLS-Related Differential Genes
3.4. Construction of a Prognostic Model Using ssGSEA Enrichment Analysis
3.5. Verification of the TLS Prognostic Model Using the GSE75500 Dataset
3.6. Functional Enrichment Analysis Based on ssGSEA Score
3.7. Clinical Characteristics Influencing TLS Score
3.8. Independent Prognostic Analysis and Nomogram Construction
3.9. Genetic Alterations in TLS Groups
3.10. Immune Cell Infiltration Profiles in TLS Groups
3.11. Association Between TLS Scores and Immune Checkpoints
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CRC | colorectal cancer |
| OS | overall survival |
| TLSs | tertiary lymphoid structures |
| TME | tumor microenvironment |
| DCs | dendritic cells |
| HEVs | high endothelial venules |
| CRLMs | colorectal liver metastases |
| TCGA | The Cancer Genome Atlas |
| GEO | Gene Expression Omnibus |
| RNA-seq | RNA sequencing |
| GO | Gene Ontology |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| KM | Kaplan–Meier |
| ssGSEA | single-sample gene set enrichment analysis |
| stage | tumor stage |
| T | tumor size and local invasion |
| N | lymph node involvement |
| M | metastasis |
| ANOVA | analysis of variance |
| CC | cellular component |
| BP | biological process |
| MF | molecular function |
| TMB | tumor mutational burden |
| NK | natural killer |
| ICB | immune checkpoint blockade |
References
- Barkhatov, L.; Aghayan, D.L.; Scuderi, V.; Cipriani, F.; Fretland, Å.A.; Kazaryan, A.M.; Ratti, F.; Armstrong, T.; Belli, A.; Dagher, I.; et al. Long-term oncological outcomes after laparoscopic parenchyma-sparing redo liver resections for patients with metastatic colorectal cancer: A European multi-center study. Surg. Endosc. 2022, 36, 3374–3381. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fedewa, S.A.; Ahnen, D.J.; Meester, R.G.; Barzi, A.; Jemal, A. Colorectal cancer statistics, 2017. CA Cancer J. Clin. 2017, 67, 177–193. [Google Scholar] [CrossRef]
- Kong, W.S.; Li, J.J.; Deng, Y.Q.; Ju, H.Q.; Xu, R.H. Immunomodulatory molecules in colorectal cancer liver metastasis. Cancer Lett. 2024, 598, 217113. [Google Scholar] [CrossRef]
- Stewart, C.L.; Warner, S.; Ito, K.; Raoof, M.; Wu, G.X.; Lu, W.P.; Kessler, J.; Kim, J.Y.; Fong, Y. Cytoreduction for colorectal metastases: Liver, lung, peritoneum, lymph nodes, bone, brain. When does it palliate, prolong survival, and potentially cure? Curr. Probl. Surg. 2018, 55, 330–379. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, G.; Zeng, Q.; Wu, W.; Lei, K.; Zhang, C.; Tang, M.; Zhang, Y.; Xiang, X.; Tan, L.; et al. CCL19-producing fibroblasts promote tertiary lymphoid structure formation enhancing anti-tumor IgG response in colorectal cancer liver metastasis. Cancer Cell 2024, 42, 1370–1385.e9. [Google Scholar] [CrossRef] [PubMed]
- Sautès-Fridman, C.; Petitprez, F.; Calderaro, J.; Fridman, W.H. Tertiary lymphoid structures in the era of cancer immunotherapy. Nat. Rev. Cancer 2019, 19, 307–325. [Google Scholar] [CrossRef]
- Zhao, L.; Jin, S.; Wang, S.; Zhang, Z.; Wang, X.; Chen, Z.; Wang, X.; Huang, S.; Zhang, D.; Wu, H. Tertiary lymphoid structures in diseases: Immune mechanisms and therapeutic advances. Signal Transduct. Target. Ther. 2024, 9, 225. [Google Scholar] [CrossRef]
- Li, H.; Ding, J.Y.; Zhang, M.J.; Yu, H.J.; Sun, Z.J. Tertiary lymphoid structures and cytokines interconnections: The implication in cancer immunotherapy. Cancer Lett. 2023, 568, 216293. [Google Scholar] [CrossRef] [PubMed]
- Helmink, B.A.; Reddy, S.M.; Gao, J.; Zhang, S.; Basar, R.; Thakur, R.; Yizhak, K.; Sade-Feldman, M.; Blando, J.; Han, G.; et al. B cells and tertiary lymphoid structures promote immunotherapy response. Nature 2020, 577, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, G.; Luo, Y.; Xu, J.; He, J. The molecular subtypes and clinical prognosis characteristic of tertiary lymphoid structures-related gene of cutaneous melanoma. Sci. Rep. 2023, 13, 23097. [Google Scholar] [CrossRef] [PubMed]
- Vanhersecke, L.; Brunet, M.; Guégan, J.P.; Rey, C.; Bougouin, A.; Cousin, S.; Moulec, S.L.; Besse, B.; Loriot, Y.; Larroquette, M.; et al. Mature tertiary lymphoid structures predict immune checkpoint inhibitor efficacy in solid tumors independently of PD-L1 expression. Nat. Cancer 2021, 2, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Cabrita, R.; Lauss, M.; Sanna, A.; Donia, M.; Skaarup Larsen, M.; Mitra, S.; Johansson, I.; Phung, B.; Harbst, K.; Vallon-Christersson, J.; et al. Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature 2020, 577, 561–565. [Google Scholar] [CrossRef] [PubMed]
- Tietscher, S.; Wagner, J.; Anzeneder, T.; Langwieder, C.; Rees, M.; Sobottka, B.; de Souza, N.; Bodenmiller, B. A comprehensive single-cell map of T cell exhaustion-associated immune environments in human breast cancer. Nat. Commun. 2023, 14, 98. [Google Scholar] [CrossRef] [PubMed]
- Propper, D.J.; Balkwill, F.R. Harnessing cytokines and chemokines for cancer therapy. Nat. Rev. Clin. Oncol. 2022, 19, 237–253. [Google Scholar] [CrossRef]
- Nagarsheth, N.; Wicha, M.S.; Zou, W. Chemokines in the cancer microenvironment and their relevance in cancer immunotherapy. Nat. Rev. Immunol. 2017, 17, 559–572. [Google Scholar] [CrossRef]
- Rouanne, M.; Arpaia, N.; Marabelle, A. CXCL13 shapes tertiary lymphoid structures and promotes response to immunotherapy in bladder cancer. Eur. J. Cancer 2021, 151, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Balkwill, F. Cancer and the chemokine network. Nat. Rev. Cancer 2004, 4, 540–550. [Google Scholar] [CrossRef] [PubMed]
- Zou, W. Immunosuppressive networks in the tumor environment and their therapeutic relevance. Nat. Rev. Cancer 2005, 5, 263–274. [Google Scholar] [CrossRef]
- Zou, W.; Restifo, N.P. T(H)17 cells in tumor immunity and immunotherapy. Nat. Rev. Immunol. 2010, 10, 248–256, Erratum in Nat. Rev. Immunol. 2011, 11, 565. [Google Scholar] [CrossRef]
- Yamamoto, T.; Kawada, K.; Obama, K. Inflammation-Related Biomarkers for the Prediction of Prognosis in Colorectal Cancer Patients. Int. J. Mol. Sci. 2021, 22, 8002. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.N.; Xiao, W.; OuYang, P.Y.; You, K.; Zeng, Z.F.; Ding, P.R.; Pan, Z.Z.; Xu, R.H.; Gao, Y.H. The prognostic impact of preoperative blood monocyte count in pathological T3N0M0 rectal cancer without neoadjuvant chemoradiotherapy. Tumor Biol. 2015, 36, 8213–8219. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, A.; Kai, S.; Endo, Y.; Iwaki, K.; Uchida, H.; Tominaga, M.; Okunaga, R.; Shibata, K.; Ohta, M.; Kitano, S. Prognostic value of preoperative peripheral blood monocyte count in patients with colorectal liver metastasis after liver resection. J. Gastrointest. Surg. 2007, 11, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zeng, J.; Luo, B.; Mao, Y.; Liang, Y.; Zhao, W.; Hu, N.; Chen, G.; Zheng, X. High expression of activated CD4 memory T cells and CD8 T cells and low expression of M0 macrophage are associated with better clinical prognosis in bladder cancer patients. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 2020, 36, 97–103. (In Chinese) [Google Scholar] [PubMed]
- Sun, Y.; Liu, L.; Fu, Y.; Liu, Y.; Gao, X.; Xia, X.; Zhu, D.; Wang, X.; Zhou, X. Metabolic reprogramming involves in transition of activated/resting CD4+ memory T cells and prognosis of gastric cancer. Front. Immunol. 2023, 14, 1275461. [Google Scholar] [CrossRef]
- Wang, W.; Li, T.; Xie, Z.; Zhao, J.; Zhang, Y.; Ruan, Y.; Han, B. Integrating single-cell and bulk RNA sequencing data unveils antigen presentation and process-related CAFS and establishes a predictive signature in prostate cancer. J. Transl. Med. 2024, 22, 57. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Craft, J.; Joshi, N.S. T follicular helper cells in cancer, tertiary lymphoid structures, and beyond. Semin. Immunol. 2023, 69, 101797. [Google Scholar] [CrossRef]
- Chaurio, R.A.; Anadon, C.M.; Lee Costich, T.; Payne, K.K.; Biswas, S.; Harro, C.M.; Moran, C.; Ortiz, A.C.; Cortina, C.; Rigolizzo, K.E.; et al. TGF-β-mediated silencing of genomic organizer SATB1 promotes Tfh cell differentiation and formation of intra-tumoral tertiary lymphoid structures. Immunity 2022, 55, 115–128.e9. [Google Scholar] [CrossRef]
- Anderson, N.R.; Minutolo, N.G.; Gill, S.; Klichinsky, M. Macrophage-Based Approaches for Cancer Immunotherapy. Cancer Res. 2021, 81, 1201–1208. [Google Scholar] [CrossRef]
- Mantovani, A.; Sica, A.; Sozzani, S.; Allavena, P.; Vecchi, A.; Locati, M. The Chemokine System in Diverse Forms of Macrophage Activation and Polarization. Trends Immunol. 2004, 25, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Liang, Y.; Wang, L. Shaping Polarization Of Tumor-Associated Macrophages In Cancer Immunotherapy. Front. Immunol. 2022, 13, 888713. [Google Scholar] [CrossRef] [PubMed]
- Blomberg, O.S.; Spagnuolo, L.; Garner, H.; Voorwerk, L.; Isaeva, O.I.; van Dyk, E.; Bakker, N.; Chalabi, M.; Klaver, C.; Duijst, M.; et al. IL-5-producing CD4+ T cells and eosinophils cooperate to enhance response to immune checkpoint blockade in breast cancer. Cancer Cell 2023, 41, 106–123.e10. [Google Scholar] [CrossRef] [PubMed]
- Gardner, A.; Ruffell, B. Dendritic Cells and Cancer Immunity. Trends Immunol. 2016, 37, 855–865. [Google Scholar] [CrossRef]
- Xiong, S.; Dong, L.; Cheng, L. Neutrophils in cancer carcinogenesis and metastasis. J. Hematol. Oncol. 2021, 14, 173. [Google Scholar] [CrossRef]
- Li, P.; Lu, M.; Shi, J.; Hua, L.; Gong, Z.; Li, Q.; Shultz, L.D.; Ren, G. Dual roles of neutrophils in metastatic colonization are governed by the host NK cell status. Nat. Commun. 2020, 11, 4387. [Google Scholar] [CrossRef] [PubMed]
- Granot, Z.; Henke, E.; Comen, E.A.; King, T.A.; Norton, L.; Benezra, R. Tumor entrained neutrophils inhibit seeding in the premetastatic lung. Cancer Cell 2011, 20, 300–314. [Google Scholar] [CrossRef] [PubMed]
- Matlung, H.L.; Babes, L.; Zhao, X.W.; van Houdt, M.; Treffers, L.W.; van Rees, D.J.; Franke, K.; Schornagel, K.; Verkuijlen, P.; Janssen, H.; et al. Neutrophils kill antibody-opsonized cancer cells by trogoptosis. Cell Rep. 2018, 23, 3946–3959.e3946. [Google Scholar] [CrossRef] [PubMed]
- Calderaro, J.; Petitprez, F.; Becht, E.; Laurent, A.; Hirsch, T.Z.; Rousseau, B.; Luciani, A.; Amaddeo, G.; Derman, J.; Charpy, C.; et al. Intra-tumoral tertiary lymphoid structures are associated with a low risk of early recurrence of hepatocellular carcinoma. J. Hepatol. 2019, 70, 58–65. [Google Scholar] [CrossRef]
- Liu, X.; Tsang, J.Y.S.; Hlaing, T.; Hu, J.; Ni, Y.B.; Chan, S.K.; Cheung, S.Y.; Tse, G.M. Distinct Tertiary Lymphoid Structure Associations and Their Prognostic Relevance in HER2 Positive and Negative Breast Cancers. Oncologist 2017, 22, 1316–1324. [Google Scholar] [CrossRef] [PubMed]
- Dieu-Nosjean, M.C.; Goc, J.; Giraldo, N.A.; Sautès-Fridman, C.; Fridman, W.H. Tertiary lymphoid structures in cancer and beyond. Trends Immunol. 2014, 35, 571–580. [Google Scholar] [CrossRef]
- Amisaki, M.; Zebboudj, A.; Yano, H.; Zhang, S.L.; Payne, G.; Chandra, A.K.; Yu, R.; Guasp, P.; Sethna, Z.M.; Ohmoto, A.; et al. IL-33-activated ILC2s induce tertiary lymphoid structures in pancreatic cancer. Nature, 2025; Epub ahead of print. [Google Scholar] [CrossRef]
- Yu, A.; Cao, M.; Zhang, K.; Yang, Y.; Ma, L.; Zhang, X.; Zhao, Y.; Ma, X.; Fan, Z.; Han, Z.; et al. The prognostic value of the tertiary lymphoid structure in gastrointestinal cancers. Front. Immunol. 2023, 14, 1256355. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Lin, X.; Xie, M.; Yao, J.; Song, J.; Ma, X.; Zhang, X.; Zhang, Y.; Liu, Y.; Han, W.; et al. Mature tertiary lymphoid structures: Important contributors to anti-tumor immune efficacy. Front. Immunol. 2024, 15, 1413067. [Google Scholar] [CrossRef] [PubMed]










Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, L.; Xi, L.; Liu, Y.; Wang, G.; Zong, M.; Xue, P.; Zhu, S. The Impact of Tertiary Lymphoid Structures on Tumor Prognosis and the Immune Microenvironment in Colorectal Cancer. Biomedicines 2025, 13, 539. https://doi.org/10.3390/biomedicines13030539
Zhao L, Xi L, Liu Y, Wang G, Zong M, Xue P, Zhu S. The Impact of Tertiary Lymphoid Structures on Tumor Prognosis and the Immune Microenvironment in Colorectal Cancer. Biomedicines. 2025; 13(3):539. https://doi.org/10.3390/biomedicines13030539
Chicago/Turabian StyleZhao, Leyi, Lingze Xi, Yani Liu, Guoliang Wang, Mingtong Zong, Peng Xue, and Shijie Zhu. 2025. "The Impact of Tertiary Lymphoid Structures on Tumor Prognosis and the Immune Microenvironment in Colorectal Cancer" Biomedicines 13, no. 3: 539. https://doi.org/10.3390/biomedicines13030539
APA StyleZhao, L., Xi, L., Liu, Y., Wang, G., Zong, M., Xue, P., & Zhu, S. (2025). The Impact of Tertiary Lymphoid Structures on Tumor Prognosis and the Immune Microenvironment in Colorectal Cancer. Biomedicines, 13(3), 539. https://doi.org/10.3390/biomedicines13030539





