Pharmacogenomic Study of SARS-CoV-2 Treatments: Identifying Polymorphisms Associated with Treatment Response in COVID-19 Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Sample Selection
2.1.1. Clinical Sample
2.1.2. Response Phenotype
2.2. SNP Genotyping
Variant Imputation
2.3. Candidate Gene Analysis
2.4. Statistical Analyses
2.5. Pathway Enrichment Analyses
3. Results
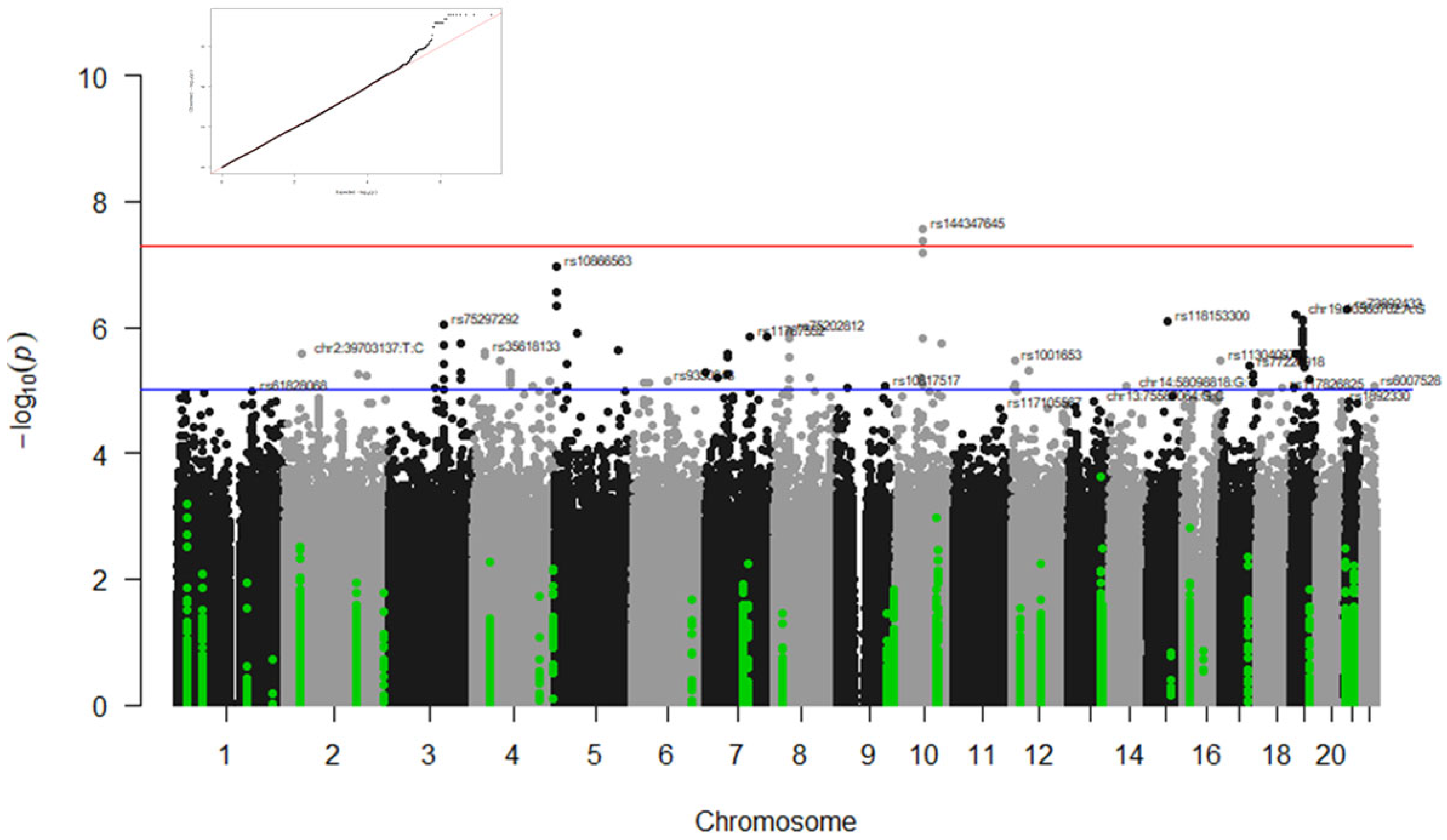
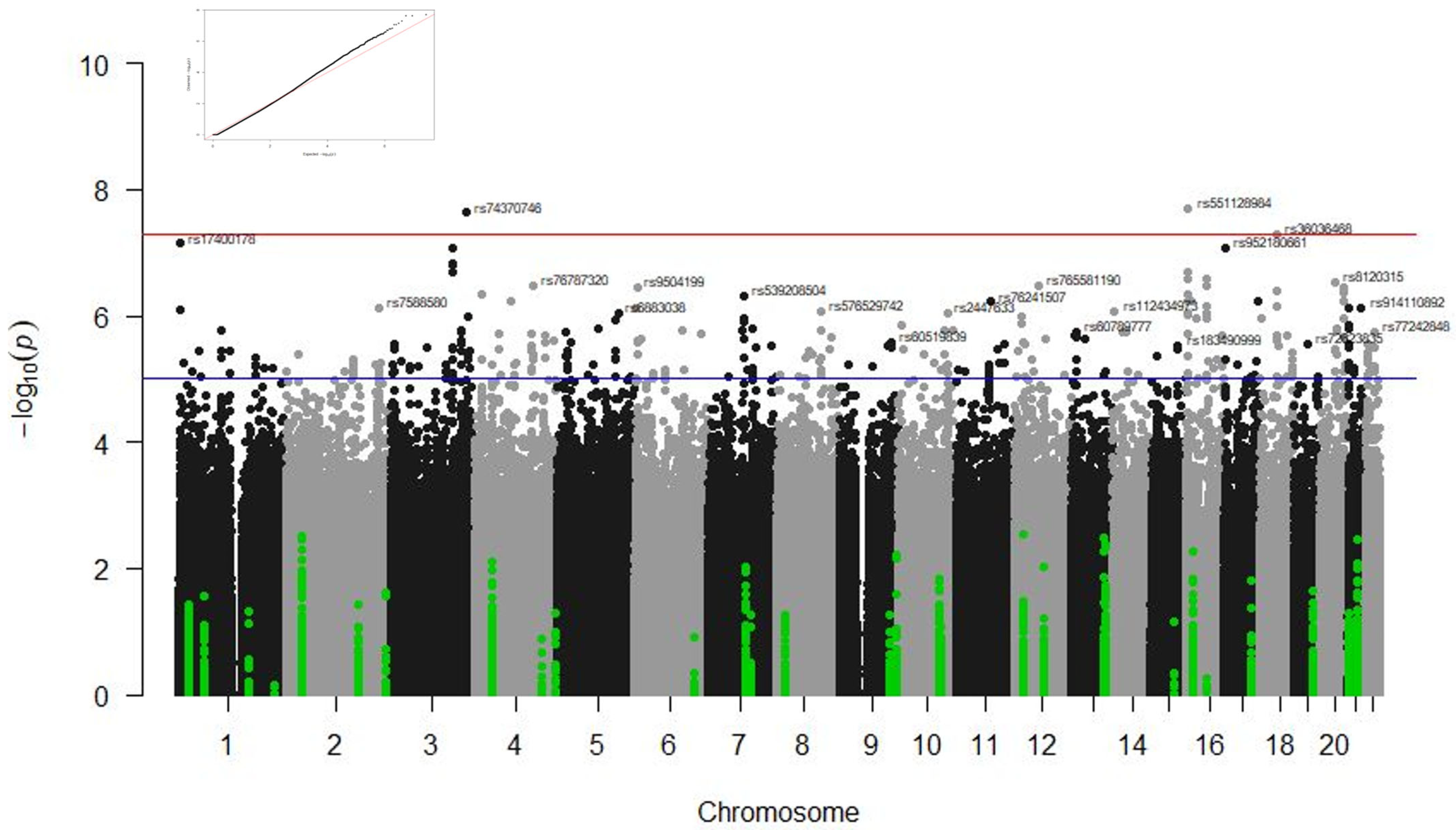
3.1. GWAS Results
3.1.1. Associations with Type of Ventilation
3.1.2. Associations with Radiological Affectation
3.1.3. Associations with ICU and Survival at 90 Days
3.2. Candidate Genes Results
3.2.1. Associations with Survival at 90 Days
3.2.2. Associations with Admission to the Intensive Care Unit (ICU)
3.2.3. Associations with the Type of Ventilation
3.2.4. Associations with Radiological Affectation
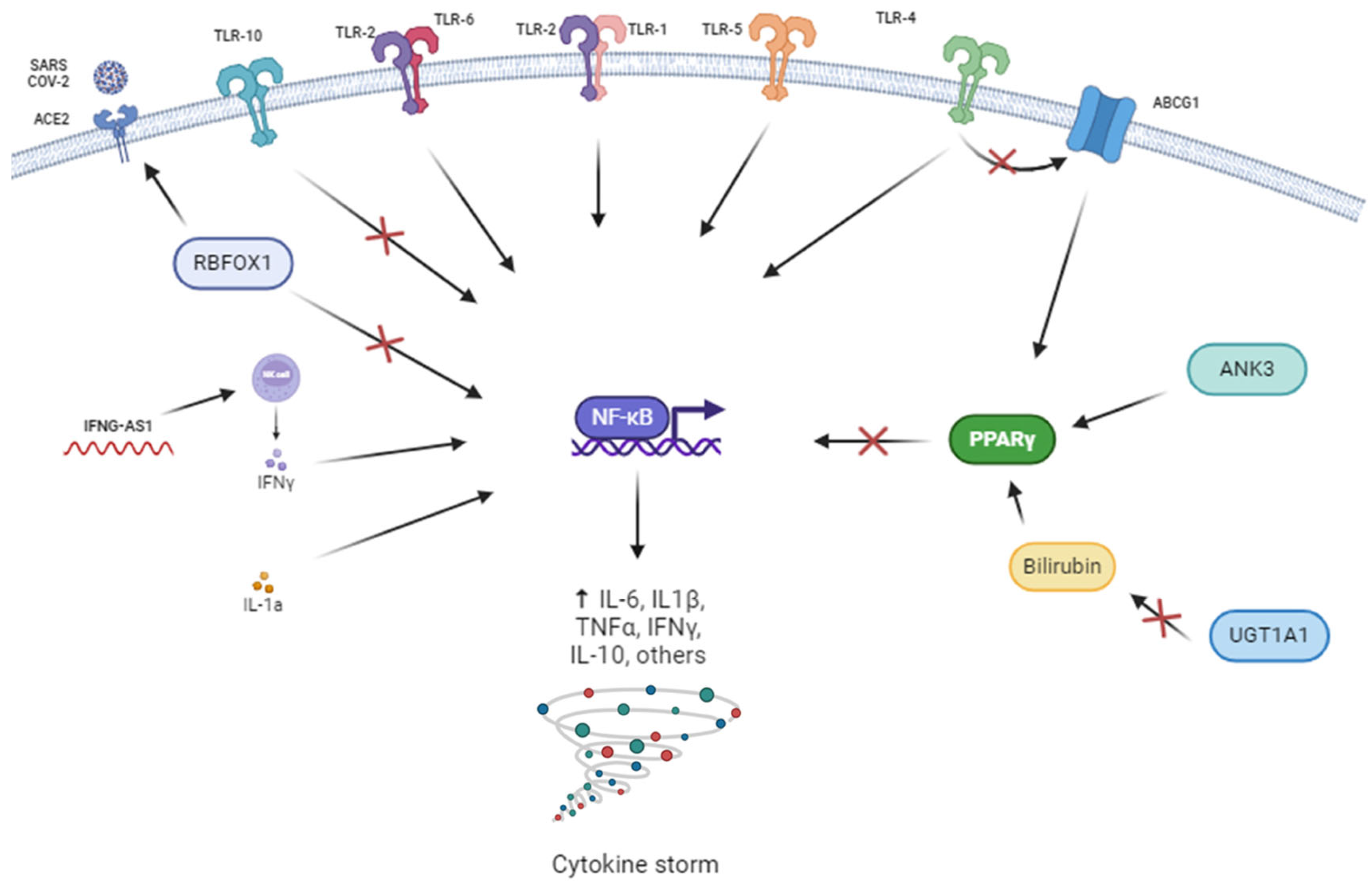
3.3. Functional Enrichment Analyses Results
4. Discussion
4.1. Genes Related to Response to Immunomodulators
4.2. Genes Related to the Corticoid Response
4.3. Genes Related to Response to Corticoids and/or Immunomodulators
4.4. Pathway Enrichment Analysis
4.5. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Molina-Mora, J.A.; González, A.; Jiménez-Morgan, S.; Cordero-Laurent, E.; Brenes, H.; Soto-Garita, C.; Sequeira-Soto, J.; Duarte-Martínez, F. Clinical Profiles at the Time of Diagnosis of SARS-CoV-2 Infection in Costa Rica During the Pre-Vaccination Period Using a Machine Learning Aroach. Phenomics 2022, 2, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Raghav, P.K.; Mann, Z.; Ahluwalia, S.K.; Rajalingam, R. Potential Treatments of COVID-19: Drug Repurposing and Therapeutic Interventions. J. Pharmacol. Sci. 2023, 152, 1–21. [Google Scholar] [CrossRef] [PubMed]
- COVID-19 Vaccinations Have Saved More than 1.4 Million Lives in the WHO European Region, a New Study Finds. Available online: https://www.who.int/europe/news-room/16-01-2024-covid-19-vaccinations-have-saved-more-than-1.4-million-lives-in-the-who-european-region--a-new-study-finds (accessed on 26 February 2024).
- Noureddine, F.Y.; Chakkour, M.; El Roz, A.; Reda, J.; Al Sahily, R.; Assi, A.; Joma, M.; Salami, H.; Hashem, S.J.; Harb, B.; et al. The Emergence of SARS-CoV-2 Variant(s) and Its Impact on the Prevalence of COVID-19 Cases in the Nabatieh Region, Lebanon. Med. Sci. 2021, 9, 40. [Google Scholar] [CrossRef]
- Gong, Z.; Song, T.; Hu, M.; Che, Q.; Guo, J.; Zhang, H.; Li, H.; Wang, Y.; Liu, B.; Shi, N. Natural and Socio-Environmental Factors in the Transmission of COVID-19: A Comprehensive Analysis of Epidemiology and Mechanisms. BMC Public Health 2024, 24, 2196. [Google Scholar] [CrossRef] [PubMed]
- Cruz, R.; Diz-De Almeida, S.; de Heredia, M.L.; Quintela, I.; Ceballos, F.C.; Pita, G.; Lorenzo-Salazar, J.M.; González-Montelongo, R.; Gago-Domínguez, M.; Porras, M.S.; et al. A Novel Genes and Sex Differences in COVID-19 Severity. Hum. Mol. Genet. 2022, 31, 3789. [Google Scholar] [CrossRef] [PubMed]
- Mo, Y.; Fisher, D. A Review of Treatment Modalities for Middle East Respiratory Syndrome. J. Antimicrob. Chemother. 2016, 71, 3340–3350. [Google Scholar] [CrossRef]
- Chavda, V.P.; Vuu, S.; Mishra, T.; Kamaraj, S.; Patel, A.B.; Sharma, N.; Chen, Z.S. Recent Review of COVID-19 Management: Diagnosis, Treatment and Vaccination. Pharmacol. Rep. 2022, 74, 1120–1148. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Huang, S.; Yin, L. The Cytokine Storm and COVID-19. J. Med. Virol. 2020, 93, 250. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, O.; Ibáñez, S.; Poli, M.C.; Roessler, P.; Aylwin, M.; Roizen, G.; Iruretagoyena, M.; Agar, V.; Donoso, J.; Fierro, M.; et al. First Report of Tocilizumab Use in a Cohort of Latin American Patients Hospitalized for Severe COVID-19 Pneumonia. Front. Med. 2020, 7, 596916. [Google Scholar] [CrossRef] [PubMed]
- Fu, B.; Xu, X.; Wei, H. Why Tocilizumab Could Be an Effective Treatment for Severe COVID-19? J. Transl. Med. 2020, 18, 164. [Google Scholar] [CrossRef] [PubMed]
- Kotak, S.; Khatri, M.; Malik, M.; Malik, M.; Hassan, W.; Amjad, A.; Malik, F.; Hassan, H.; Ahmed, J.; Zafar, M. Use of Tocilizumab in COVID-19: A Systematic Review and Meta-Analysis of Current Evidence. Cureus 2020, 12, e10869. [Google Scholar] [CrossRef] [PubMed]
- Biswas, M.; Sawajan, N.; Rungrotmongkol, T.; Sanachai, K.; Ershadian, M.; Sukasem, C. Pharmacogenetics and Precision Medicine Aroaches for the Improvement of COVID-19 Therapies. Front. Pharmacol. 2022, 13, 835136. [Google Scholar] [CrossRef] [PubMed]
- Yeh, R.F.; Gaver, V.E.; Patterson, K.B.; Rezk, N.L.; Baxter-Meheux, F.; Blake, M.J.; Eron, J.J.; Klein, C.E.; Rublein, J.C.; Kashuba, A.D.M. Lopinavir/Ritonavir Induces the Hepatic Activity of Cytochrome P450 Enzymes CYP2C9, CYP2C19, and CYP1A2 But Inhibits the Hepatic and Intestinal Activity of CYP3A as Measured by a Phenotyping Drug Cocktail in Healthy Volunteers. JAIDS J. Acquir. Immune Defic. Syndr. 2006, 42, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Rendic, S.; Guengerich, F.P. Metabolism and Interactions of Chloroquine and Hydroxychloroquine with Human Cytochrome P450 Enzymes and Drug Transporters. Curr. Drug Metab. 2020, 21, 1127–1135. [Google Scholar] [CrossRef]
- Tomlinson, E.S.; Maggs, J.L.; Park, B.K.; Back, D.J. Dexamethasone Metabolism in Species Differences. J. Steroid. Biochem. Mol. Biol. 1997, 62, 345–352. [Google Scholar] [CrossRef]
- García-Menaya, J.M.; Cordobés-Durán, C.; García-Martín, E.; Agúndez, J.A.G. Pharmacogenetic Factors Affecting Asthma Treatment Response. Potential Implications for Drug Therapy. Front. Pharmacol. 2019, 10, 520. [Google Scholar] [CrossRef]
- Badary, O.A. Pharmacogenomics and COVID-19: Clinical Implications of Human Genome Interactions with Repurposed Drugs. Pharmacogenomics J. 2021, 21, 275–284. [Google Scholar] [CrossRef]
- Fricke-Galindo, I.; Falfán-Valencia, R. Pharmacogenetics Aroach for the Improvement of COVID-19 Treatment. Viruses 2021, 13, 413. [Google Scholar] [CrossRef]
- Pino-Yanes, M.; Corrales, A.; Casula, M.; Blanco, J.; Muriel, A.; Espinosa, E.; García-Bello, M.; Torres, A.; Ferrer, M.; Zavala, E.; et al. Common Variants of TLR1 Associate with Organ Dysfunction and Sustained Pro-Inflammatory Responses during Sepsis. PLoS ONE 2010, 5, e13759. [Google Scholar] [CrossRef]
- San Gil, A. Pneumònia Aguda de La Comunitat En l’Adult: Etiologia i Biomarcadors Genètics de l’Hoste. Ph.D. Thesis, Universitat Internacional de Catalunya, Barcelona, Spain, 2017. [Google Scholar]
- AL-Eitan, L.N.; Alahmad, S.Z. Pharmacogenomics of Genetic Polymorphism within the Genes Responsible for SARS-CoV-2 Susceptibility and the Drug-Metabolising Genes Used in Treatment. Rev. Med. Virol. 2021, 31, e2194. [Google Scholar] [CrossRef] [PubMed]
- Franczyk, B.; Rysz, J.; Miłoński, J.; Konecki, T.; Rysz-Górzyńska, M.; Gluba-Brzózka, A. Will the Use of Pharmacogenetics Improve Treatment Efficiency in COVID-19? Pharmaceuticals 2022, 15, 739. [Google Scholar] [CrossRef]
- Fatima, S.; Ratnani, I.; Husain, M.; Surani, S. Radiological Findings in Patients with COVID-19. Cureus 2020, 12, e7651. [Google Scholar] [CrossRef] [PubMed]
- Graffelman, J.; Moreno, V. The Mid P-Value in Exact Tests for Hardy-Weinberg Equilibrium. Stat. Al. Genet. Mol. Biol. 2013, 12, 433–448. [Google Scholar] [CrossRef]
- Taliun, D.; Harris, D.N.; Kessler, M.D.; Carlson, J.; Szpiech, Z.A.; Torres, R.; Taliun, S.A.G.; Corvelo, A.; Gogarten, S.M.; Kang, H.M.; et al. Sequencing of 53,831 Diverse Genomes from the NHLBI TOPMed Program. Nature 2021, 590, 290. [Google Scholar] [CrossRef]
- AL-Taie, A.; Büyük, A.Ş.; Sardas, S. Considerations into Pharmacogenomics of COVID-19 Pharmacotherapy: Hope, Hype and Reality. Pulm. Pharmacol. Ther. 2022, 77, 102172. [Google Scholar] [CrossRef]
- Moreira, R.P.P.; Jorge, A.A.L.; Gomes, L.G.; Kaupert, L.C.; Filho, J.M.; Mendonca, B.B.; Bachega, T.A.S.S. Pharmacogenetics of Glucocorticoid Replacement Could Optimize the Treatment of Congenital Adrenal Hyperplasia Due to 21-Hydroxylase Deficiency. Clinics 2011, 66, 1361. [Google Scholar] [CrossRef] [PubMed]
- Montazersaheb, S.; Hosseiniyan Khatibi, S.M.; Hejazi, M.S.; Tarhriz, V.; Farjami, A.; Ghasemian Sorbeni, F.; Farahzadi, R.; Ghasemnejad, T. COVID-19 Infection: An Overview on Cytokine Storm and Related Interventions. Virol. J. 2022, 19, 92. [Google Scholar] [CrossRef]
- Vohra, M.; Sharma, A.R.; Satyamoorthy, K.; Rai, P.S. Pharmacogenomic Considerations for Repurposing of Dexamethasone as a Potential Drug against SARS-CoV-2 Infection. Per. Med. 2021, 18, 389–398. [Google Scholar] [CrossRef]
- Lee, J.S.; Wang, J.; Martin, M.; Germer, S.; Kenwright, A.; Benayed, R.; Spleiss, O.; Platt, A.; Pilson, R.; Hemmings, A.; et al. Genetic Variation in UGT1A1 Typical of Gilbert Syndrome Is Associated with Unconjugated Hyperbilirubinemia in Patients Receiving Tocilizumab. Pharmacogenet. Genom. 2011, 21, 365–374. [Google Scholar] [CrossRef]
- Fardel, O.; Payen, L.; Courtois, A.; Vernhet, L.; Lecureur, V. Regulation of Biliary Drug Efflux Pump Expression by Hormones and Xenobiotics. Toxicology 2001, 167, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Rubin, L.; Zhou, Z.; Zhang, H.; Su, Q.; Hou, S.T.; Lazarovici, P.; Zheng, W. Pharmacological Therapies and Drug Development Targeting SARS-CoV-2 Infection. Cytokine Growth Factor Rev. 2022, 68, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Jarczak, D.; Nierhaus, A. Cytokine Storm—Definition, Causes, and Implications. Int. J. Mol. Sci. 2022, 23, 11740. [Google Scholar] [CrossRef] [PubMed]
- Hasanvand, A. COVID-19 and the Role of Cytokines in This Disease. Inflammopharmacology 2022, 789–798. [Google Scholar] [CrossRef]
- Britt, R.D.; Thompson, M.A.; Sasse, S.; Pabelick, C.M.; Gerber, A.N.; Prakash, X.Y.S. Th1 Cytokines TNF-and IFN-Promote Corticosteroid Resistance in Developing Human Airway Smooth Muscle. Am. J. Physiol. Lung. Cell. Mol. Physiol. 2019, 316, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.R.; Bender, D.; Maller, J.; Sklar, P.; De Bakker, P.I.W.; Daly, M.J.; et al. PLINK: A Tool Set for Whole-Genome Association and Population-Based Linkage Analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Luzum, J.A.; Nicol, M.R.; Jacobson, P.A. Pharmacogenomics of COVID-19 Therapies. NPJ Genom. Med. 2020, 5, 35. [Google Scholar] [CrossRef]
- Zaidat, O.O.; Erwin Grüter, B.; Cantonal Hospital, A.; Luis Rafael Moscote-Salazar, S.; Yan, J.; Jiang, W.; Liu, J.; Liao, X.; Zhou, J.; Li, B.; et al. A Rare Variant of ANK3 Is Associated With Intracranial Aneurysm. Front. Neurol. 2021, 1, 672570. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, Z.; Hui, L.; Sun, P. Effects of CACNA1C and ANK3 on Cognitive Function in Patients with Bipolar Disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 2024, 133, 111016. [Google Scholar] [CrossRef]
- Kloth, K.; Lozic, B.; Tagoe, J.; Hoffer, M.J.V.; Van der Ven, A.; Thiele, H.; Altmüller, J.; Kubisch, C.; Au, P.Y.B.; Denecke, J.; et al. ANK3 Related Neurodevelopmental Disorders: Expanding the Spectrum of Heterozygous Loss-of-Function Variants. Neurogenetics 2021, 22, 263. [Google Scholar] [CrossRef]
- Thoeni, C.; Waldherr, R.; Scheuerer, J.; Schmitteckert, S.; Roeth, R.; Niesler, B.; Cutz, E.; Flechtenmacher, C.; Goeert, B.; Schirmacher, P.; et al. Expression Analysis of Atp-Binding Cassette Transporters Abcb11 and Abcb4 in Primary Sclerosing Cholangitis and Variety of Pediatric and Adult Cholestatic and Noncholestatic Liver Diseases. Can. J. Gastroenterol. Hepatol. 2019, 2019, 1085717. [Google Scholar] [CrossRef] [PubMed]
- Fore, F.; Indriputri, C.; Mamutse, J.; Nugraha, J. TLR10 and Its Unique Anti-Inflammatory Properties and Potential Use as a Target in Therapeutics. Immune Netw. 2020, 20, e21. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Zhang, L.; Chen, C.; Wang, Q.; Guo, L.; Ma, Q.; Deng, P.; Zhu, G.; Li, B.; Pi, Y.; et al. The Critical Role of ABCG1 and PPARγ/LXRα Signaling in TLR4 Mediates Inflammatory Responses and Lipid Accumulation in Vascular Smooth Muscle Cells. Cell. Tissue Res. 2017, 368, 145–157. [Google Scholar] [CrossRef]
- Bank, S.; Skytt Andersen, P.; Burisch, J.; Pedersen, N.; Roug, S.; Galsgaard, J.; Ydegaard Turino, S.; Broder Brodersen, J.; Rashid, S.; Kaiser Rasmussen, B.; et al. Polymorphisms in the Inflammatory Pathway Genes TLR2, TLR4, TLR9, LY96, NFKBIA, NFKB1, TNFA, TNFRSF1A, IL6R, IL10, IL23R, PTPN22, and PPARG Are Associated with Susceptibility of Inflammatory Bowel Disease in a Danish Cohort. PLoS ONE 2014, 9, e98815. [Google Scholar] [CrossRef]
- Duggan, J.M.; You, D.; Cleaver, J.O.; Larson, D.T.; Garza, R.J.; Guzmán Pruneda, F.A.; Tuvim, M.J.; Zhang, J.; Dickey, B.F.; Evans, S.E. Synergistic Interactions of TLR2/6 and TLR9 Induce a High Level of Resistance to Lung Infection in Mice. J. Immunol. 2011, 186, 5916–5926. [Google Scholar] [CrossRef]
- Chakraborty, C.; Sharma, A.R.; Bhattacharya, M.; Sharma, G.; Lee, S.S.; Agoramoorthy, G. Consider TLR5 for New Therapeutic Development against COVID-19. J. Med. Virol. 2020, 2314–2315. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, D.; Bhandari, R.; Kuhad, A. TLR4 as a Therapeutic Target for Respiratory and Neurological Complications of SARS-CoV-2. Expert Opin. Ther. Targets 2021, 491–508. [Google Scholar] [CrossRef]
- Gao, C.; Ren, S.; Lee, J.H.; Qiu, J.; Chapski, D.J.; Rau, C.D.; Zhou, Y.; Abdellatif, M.; Nakano, A.; Vondriska, T.M.; et al. RBFox1-Mediated RNA Splicing Regulates Cardiac Hypertrophy and Heart Failure. J. Clin. Investig. 2016, 126, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Dery, K.J.; Wong, Z.; Wei, M.; Kupiec-Weglinski, J.W. Mechanistic Insights into Alternative Gene Splicing in Oxidative Stress and Tissue Injury. Antioxid. Redox. Signal. 2023, 41, 890–909. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Cheng, L.; Gao, Z.; Li, J.; Ding, Y.; Shi, R.; Xiang, Q.; Chen, X. Investigation of the Shared Molecular Mechanisms and Hub Genes between Myocardial Infarction and Depression. Front. Cardiovasc. Med. 2023, 10, 1203168. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Long, Q.X.; Ren, J.H.; Chen, H.; Li, M.M.; Cheng, Z.; Chen, J.; Zhou, L.; Huang, A.L. Genome-Wide Association Study of SARS-CoV-2 Infection in Chinese Population. Eur. J. Clin. Microbiol. Infect. Dis. 2022, 41, 1155–1163. [Google Scholar] [CrossRef]
- Alors-Pérez, E.; Pedraza-Arevalo, S.; Blázquez-Encinas, R.; García-Vioque, V.; Agraz-Doblas, A.; Yubero-Serrano, E.M.; Sánchez-Frías, M.E.; Serrano-Blanch, R.; Gálvez-Moreno, M.Á.; Gracia-Navarro, F.; et al. Altered CELF4 Splicing Factor Enhances Pancreatic Neuroendocrine Tumors Aggressiveness Influencing MTOR and Everolimus Response. Mol. Ther. Nucleic Acids 2023, 35, 102090. [Google Scholar] [CrossRef]
- Engel, J.J.; van der Made, C.I.; Keur, N.; Setiabudiawan, T.; Röring, R.J.; Damoraki, G.; Dijkstra, H.; Lemmers, H.; Ioannou, S.; Poulakou, G.; et al. Dexamethasone Attenuates Interferon-Related Cytokine Hyperresponsiveness in COVID-19 Patients. Front. Immunol. 2023, 14, 1233318. [Google Scholar] [CrossRef]
- Botton, M.R.; Whirl-Carrillo, M.; Del Tredici, A.L.; Sangkuhl, K.; Cavallari, L.H.; Agúndez, J.A.G.; Duconge, J.; Lee, M.T.M.; Woodahl, E.L.; Claudio-Campos, K.; et al. PharmVar GeneFocus: CYP2C19. Clin. Pharmacol. Ther. 2020, 109, 352. [Google Scholar] [CrossRef]
- Matoulková, P.; Pávek, P.; Malý, J.; Vlček, J. Cytochrome P450 Enzyme Regulation by Glucocorticoids and Consequences in Terms of Drug Interaction. Expert Opin. Drug Metab. Toxicol. 2014, 10, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Scott, S.A.; Sangkuhl, K.; Shuldiner, A.R.; Hulot, J.S.; Thorn, C.F.; Altman, R.B.; Klein, T.E. PharmGKB Summary: Very Important Pharmacogene Information for Cytochrome P450, Family 2, Subfamily C, Polypeptide 19. Pharmacogenet. Genom. 2012, 22, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Creeden, J.F.; Gordon, D.M.; Stec, D.E.; Hinds, T.D. Bilirubin as a Metabolic Hormone: The Physiological Relevance of Low Levels. Am. J. Physiol. -Endocrinol. Metab. 2021, 320, 191–207. [Google Scholar] [CrossRef]
- Gammal, R.S.; Court, M.H.; Haidar, C.E.; Iwuchukwu, O.F.; Gaur, A.H.; Alvarellos, M.; Guillemette, C.; Lennox, J.L.; Whirl-Carrillo, M.; Brummel, S.S.; et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for UGT1A1 and Atazanavir Prescribing. Clin. Med. Ther. 2016, 99, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, S.; Tanimoto, K.; Kohno, K.; Kadowaki, M.; Takase, K.; Kondo, S.; Kubota, A.; Takeshita, M.; Okamura, S. UGT1A1 *6 Polymorphism Predicts Outcome in Elderly Patients with Relapsed or Refractory Diffuse Large B-Cell Lymphoma Treated with Carboplatin, Dexamethasone, Etoposide and Irinotecan. Ann. Hematol. 2015, 94, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Scialo, F.; Daniele, A.; Amato, F.; Pastore, L.; Matera, M.G.; Cazzola, M.; Castaldo, G.; Bianco, A. ACE2: The Major Cell Entry Receptor for SARS-CoV-2. Lung 2020, 867–877. [Google Scholar] [CrossRef]
- Finney, L.J.; Glanville, N.; Farne, H.; Aniscenko, J.; Fenwick, P.; Kemp, S.V.; Trujillo-Torralbo, M.B.; Loo, S.L.; Calderazzo, M.A.; Wedzicha, J.A.; et al. Inhaled Corticosteroids Downregulate the SARS-CoV-2 Receptor ACE2 in COPD through Suression of Type I Interferon. J. Allergy Clin. Immunol. 2021, 147, 510–519.e5. [Google Scholar] [CrossRef] [PubMed]
- O’Beirne, S.L.; Salit, J.; Kaner, R.J.; Crystal, R.G.; Strulovici-Barel, Y. Up-Regulation of ACE2, the SARS-CoV-2 Receptor, in Asthmatics on Maintenance Inhaled Corticosteroids. Respir. Res. 2021, 22, 200. [Google Scholar] [CrossRef] [PubMed]
- Stein, N.; Berhani, O.; Schmiedel, D.; Duev-Cohen, A.; Seidel, E.; Kol, I.; Tsukerman, P.; Hecht, M.; Reches, A.; Gamliel, M.; et al. IFNG-AS1 Enhances Interferon Gamma Production in Human Natural Killer Cells. iScience 2019, 11, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Oram, J.F.; Vaughan, A.M. ATP-Binding Cassette Cholesterol Transporters and Cardiovascular Disease. Circ. Res. 2006, 99, 1031–1043. [Google Scholar] [CrossRef] [PubMed]
- McPeek, M.; Malur, A.; Tokarz, D.A.; Lertpiriyapong, K.; Gowdy, K.M.; Murray, G.; Wingard, C.J.; Fessler, M.B.; Barna, B.P.; Thomassen, M.J. Alveolar Macrophage ABCG1 Deficiency Promotes Pulmonary Granulomatous Inflammation. Am. J. Respir. Cell Mol. Biol. 2019, 61, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Castañeda, A.; Lu, P.; Geraghty, A.C.; Song, E.; Lee, M.H.; Wood, J.; O’Dea, M.R.; Dutton, S.; Shamardani, K.; Nwangwu, K.; et al. Mild Respiratory COVID Can Cause Multi-Lineage Neural Cell and Myelin Dysregulation. Cell 2022, 185, 2452–2468.e16. [Google Scholar] [CrossRef]
- Battaglini, D.; Lopes-Pacheco, M.; Castro-Faria-Neto, H.C.; Pelosi, P.; Rocco, P.R.M. Laboratory Biomarkers for Diagnosis and Prognosis in COVID-19. Front. Immunol. 2022, 13, 857573. [Google Scholar] [CrossRef] [PubMed]

| Treatment | Phenotype | n (%) |
|---|---|---|
|
Immunomodulators (IMM) | Survival at 90 days | 96 (14) did not survive 580 (86) survived |
| ICU | 346 (50) ICU 350 (50) No ICU | |
| Type of Ventilation * | 1 = 59 (9); 2 = 286 (41); 3 = 351 (50) | |
| Radiological affectation | 647 (99) affected 9 (1) unaffected | |
|
Corticoids (CORT) | Survival at 90 days | 315 (17) did not survive 1501 (83) survived |
| ICU | 449 (23) ICU 1495 (77) No ICU | |
| Type of Ventilation | 1 = 346 (18); 2 = 1034 (53); 3 = 565 (29) | |
| Radiological affectation | 1744 (94) affected 105 (6) unaffected | |
|
Immunomodulators and/or Corticoids (COMB) | Survival at 90 days | 321 (16) did not survive 1662 (84) survived |
| ICU | 556 (26) ICU 1621 (74) No ICU | |
| Type of Ventilation | 1 = 346 (16); 2 = 1138 (53); 3 = 669 (31) | |
| Radiological affectation | 1938 (95) affected 110 (5) unaffected |
| Treatment related genes | Type of Drug | Drug | Main Targets and Metabolic Pathways |
| Corticoids | Dexamethasone | CYP3A4, CYP3A5, CYP2B6, CYP2C19, CYP2C8, ABCB1, ABCB11, ABCC2 | |
| Hydrocortisone | CYP3A4, CYP3A5, CYP11B2, CYP2C8, CYP1B1, CYP2B6, CYP2C9, CYP2C19, ABCB1 | ||
| Prednisone | CYP3A5, CYP2B6, CYP2C19, CYP2C8, ABCB1, ABCB11, ABCC2 | ||
| Methylprednisolone | CYP3A4, CYP1B1, CYP2B6, CYP2C8, CYP2C19, CYP2C9, ABCB1 | ||
| Cortisone | CYP3A4, CYP3A5 | ||
| Immuno-modulators | OTHERS | IL1R1, IL1A, IL1B, IL2, IL4, IL6, IL6R, IL10, TNFA, IFNAR1 | |
| Tocilizumab | IL6R, CYP3A4, FCGR3A, UGT1A1, FCGR3A | ||
| Interferon | Interferons | IFNAR1, IFNAR2, CYP1A2, IFITM3, IFNG, IFNGR1, IFNGR2, IFNLR1, IFNA16, IRF7 | |
| Infection related genes | Related Function | Genes Involved | |
| Entry point | ACE, ACE2, ABO | ||
| Cytokine storm | TLR10, TLR8, TLR7, TLR5, TLR4, TLR3, TLR2, TLR1, IL1R1, IL-1A, IL1B, IL2, IL4, IL6, IL6R, IL10 & TNF-α, IFNAR1, IFNAR2, IFITM3, IFNG, IFNGR1, IFNGR2, IFNLR1, IFNA16, IRF7 | ||
| Treatment | Phenotype | Gene | SNP | Allele | OR/ | p-Value |
|---|---|---|---|---|---|---|
| BETA | ||||||
| Immunomodulators cohort (IMM) | Type of Ventilation | ANK3 | rs1443476451 | T | −0.64 | 2.68 × 10−8 |
| rs169153541 | T | −0.64 | 2.68 × 10−8 | |||
| rs1452991491 | A | −0.64 | 2.68 × 10−8 | |||
| rs1427405851 | T | −0.64 | 2.68 × 10−8 | |||
| rs1157012661 | A | −0.64 | 2.68 × 10−8 | |||
| rs1161657341 | C | −0.64 | 2.68 × 10−8 | |||
| rs169153591 | G | −0.64 | 2.68 × 10−8 | |||
| rs169153611 | C | −0.64 | 2.68 × 10−8 | |||
| rs1498470981 | G | −0.64 | 4.27 × 10−8 | |||
| rs1448067831 | G | −0.64 | 4.27 × 10−8 | |||
| Corticoids cohort (CORT) | Radiological affectation | MIR924HG | rs360364681 | T | 0.21 | 4.99 × 10−8 |
| RBFOX1 | rs5511289841 | C | 0.24 | 2.01 × 10 −8 | ||
| ZMAT3 | rs743707464 | A | 0.05 | 2.33 × 10 −8 | ||
| rs784516713 | C | 0.05 | 2.33 × 10 −8 | |||
| Combined cohort (COMB) | Radiological affectation | RBFOX1 | rs5511289841 | C | 0.23 | 3.00 × 10−9 |
| rs727651291 | G | 0.28 | 4.75 × 10−8 | |||
| ABCG1 | rs9141108922 | A | 0.14 | 1.38 × 10−8 | ||
| rs1123026203 | C | 0.14 | 1.38 × 10−8 |
| Treatment | Phenotype | Gene | SNP | Allele | OR/Beta | p-Value |
|---|---|---|---|---|---|---|
| Immunomodulators cohort (IMM) | Survival at 90 Days | TLR5 | rs558663121 | T | 13.91 | 4.39 × 10−3 |
| rs5427414101 | T | 9.82 | 5.39 × 10−3 | |||
| ICU | ABCB11 | rs37705851 | A | 1.53 | 2.55 × 10−4 | |
| Corticoids cohort (CORT) | Survival at 90 Days | IFNG-AS1 | rs123068991 | C | 0.65 | 4.05 × 10−5 |
| rs123007161 | C | 0.65 | 4.05 × 10−5 | |||
| TLR1, TLR6 | rs1115307901 | dupT | 1.54 | 2.86 × 10−4 | ||
| rs68494001 | A | 1.54 | 2.86 × 10−4 | |||
| rs119334551 | G | 1.55 | 2.56 × 10−4 | |||
| rs1464685881 | T | 1.54 | 3.49 × 10−4 | |||
| rs3765232141 | G | 1.54 | 3.49 × 10−4 | |||
| rs1119809961 | C | 1.54 | 3.49 × 10−4 | |||
| rs1136680691 | G | 1.54 | 3.49 × 10−4 | |||
| rs1480351171 | A | 1.54 | 3.49 × 10−4 | |||
| TLR10 | rs1498958722 | C | 4.56 | 9.05 × 10−4 | ||
| ICU | CYP2C19 | rs122582431 | A | 2.65 | 3.19 × 10−4 | |
| ACE2 | rs625789171 | A | 3.51 | 3.26 × 10−4 | ||
| Type of Ventilation | TLR4 | rs123776321 | C | 0.08 | 3.45 × 10−4 | |
| rs78688591 | G | −0.09 | 6.35 × 10−4 | |||
| UGT1A1 | rs67420781 | T | 0.17 | 5.12 × 10−4 | ||
| Radiological affectation | IL-1α | rs37835851 | T | 0.07 | 7.83 × 10−5 | |
| rs20713751 | T | 0.44 | 5.63 × 10−4 | |||
| rs6971 | T | 0.44 | 5.63 × 10−4 | |||
| Combined cohort (COMB) | Survived 90 Days | IFNG-AS1 | rs123007161 | C | 0.64 | 8.18 × 10−6 |
| rs123068991 | C | 0.65 | 1.18 × 10−5 | |||
| rs108787471 | A | 0.65 | 1.78 × 10−5 | |||
| rs108787491 | T | 0.65 | 2.51 × 10−5 | |||
| rs73017971 | G | 0.66 | 2.54 × 10−5 | |||
| rs73064401 | G | 0.66 | 2.54 × 10−5 | |||
| rs28709551 | T | 0.66 | 3.60 × 10−5 | |||
| rs71331711 | C | 0.66 | 3.60 × 10−5 | |||
| rs71371581 | C | 0.66 | 3.60 × 10−5 | |||
| rs111770591 | T | 0.66 | 4.74 × 10−5 | |||
| TLR10 | rs1498958721 | C | 4.07 | 1.05 × 10−3 | ||
| Ventilation | UGT1A1 | rs67420781 | T | 0.16 | 6.73 × 10−4 | |
| Radiological affectation | IL-1α | rs20713751 | T | 0.47 | 1.13 × 10−3 | |
| rs6971 | T | 0.47 | 1.13 × 10−3 | |||
| rs37835851 | T | 0.08 | 6.41 × 10−5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serra-Llovich, A.; Cullell, N.; Maroñas, O.; José Herrero, M.; Cruz, R.; Almoguera, B.; Ayuso, C.; López-Rodríguez, R.; Domínguez-Garrido, E.; Ortiz-Lopez, R.; et al. Pharmacogenomic Study of SARS-CoV-2 Treatments: Identifying Polymorphisms Associated with Treatment Response in COVID-19 Patients. Biomedicines 2025, 13, 553. https://doi.org/10.3390/biomedicines13030553
Serra-Llovich A, Cullell N, Maroñas O, José Herrero M, Cruz R, Almoguera B, Ayuso C, López-Rodríguez R, Domínguez-Garrido E, Ortiz-Lopez R, et al. Pharmacogenomic Study of SARS-CoV-2 Treatments: Identifying Polymorphisms Associated with Treatment Response in COVID-19 Patients. Biomedicines. 2025; 13(3):553. https://doi.org/10.3390/biomedicines13030553
Chicago/Turabian StyleSerra-Llovich, Alexandre, Natalia Cullell, Olalla Maroñas, María José Herrero, Raquel Cruz, Berta Almoguera, Carmen Ayuso, Rosario López-Rodríguez, Elena Domínguez-Garrido, Rocio Ortiz-Lopez, and et al. 2025. "Pharmacogenomic Study of SARS-CoV-2 Treatments: Identifying Polymorphisms Associated with Treatment Response in COVID-19 Patients" Biomedicines 13, no. 3: 553. https://doi.org/10.3390/biomedicines13030553
APA StyleSerra-Llovich, A., Cullell, N., Maroñas, O., José Herrero, M., Cruz, R., Almoguera, B., Ayuso, C., López-Rodríguez, R., Domínguez-Garrido, E., Ortiz-Lopez, R., Barreda-Sánchez, M., Corton, M., Dalmau, D., Calbo, E., Boix-Palop, L., Dietl, B., Sangil, A., Gil-Rodriguez, A., Guillén-Navarro, E., ... SCOURGE COHORT GROUP. (2025). Pharmacogenomic Study of SARS-CoV-2 Treatments: Identifying Polymorphisms Associated with Treatment Response in COVID-19 Patients. Biomedicines, 13(3), 553. https://doi.org/10.3390/biomedicines13030553







