Three-Dimensional Speckle-Tracking Echocardiography-Derived Left Ventricular Global Longitudinal Strain and Mitral Annular Plane Systolic Excursion Are Associated in Healthy Adults—Insights from the MAGYAR-Healthy Study
Abstract
1. Introduction
2. Materials and Methods
- -
- Radial (RS) representing the thickening/thinning of the LV;
- -
- Circumferential (CS) representing the narrowing/widening of the LV;
- -
- Longitudinal strain (LS) representing the shortening/lengthening of the LV.
3. Results
4. Discussion
- -
- -
- Although 3DSTE-capable software offers simultaneous assessment of LV rotational parameters, the present study did not aim to determine them due to their complexity. This could be a topic of future investigations.
- -
- -
- -
- The relatively small sample size may raise certain concerns, which we tried to overcome by applying appropriate statistical methods (non-parametric tests, providing median values, etc.).
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nemes, A.; Forster, T. Recent echocardiographic examination of the left ventricle—From M-mode to 3D speckle-tracking imaging. Orv. Hetil. 2015, 156, 1723–1740. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Liu, D.; Herrmann, S.; Niemann, M.; Gaudron, P.D.; Voelker, W.; Ertl, G.; Bijnens, B.; Wiedemann, F. Clinical implication of mitral annular plane systolic excursion for patients with cardiovascular disease. Eur. Heart J. Cardiovasc. Imaging 2013, 14, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Cirin, L.; Crișan, S.; Luca, C.T.; Buzaș, R.; Lighezan, D.F.; Văcărescu, C.; Cozgarea, A.; Tudoran, C.; Cozma, D. Mitral Annular Plane Systolic Excursion (MAPSE): A Review of a Simple and Forgotten Parameter for Assessing Left Ventricle Function. J. Clin. Med. 2024, 13, 5265. [Google Scholar] [CrossRef]
- Hensel, K.O.; Roskopf, M.; Wilke, L.; Heusch, A. Intraobserver and interobserver reproducibility of M-mode and B-mode acquired mitral annular plane systolic excursion (MAPSE) and its dependency on echocardiographic image quality in children. PLoS ONE 2018, 13, e0196614. [Google Scholar] [CrossRef]
- Brault, C.; Zerbib, Y.; Mercado, P.; Diouf, M.; Michaud, A.; Tribouilloy, C.; Maizel, J.; Slama, M. Mitral annular plane systolic excursion for assessing left ventricular systolic dysfunction in patients with septic shock. BJA Open 2023, 7, 100220. [Google Scholar] [CrossRef]
- Jarori, U.; Maatman, T.K.; Maatman, B.; Mastouri, R.; Sawada, S.G.; Khemka, A. Mitral Annular Plane Systolic Excursion: An Early Marker of Mortality in Severe COVID-19. J. Am. Soc. Echocardiogr. 2020, 33, 1411–1413. [Google Scholar] [CrossRef]
- Narang, A.; Addetia, K. An introduction to left ventricular strain. Curr. Opin. Cardiol. 2018, 33, 455–463. [Google Scholar] [CrossRef]
- Ammar, K.A.; Paterick, T.E.; Khandheria, B.K.; Jan, M.F.; Kramer, C.; Umland, M.M.; Tercius, A.J.; Baratta, L.; Tajik, A.J. Myocardial mechanics: Understanding and applying three-dimensional speckle tracking echocardiography in clinical practice. Echocardiography 2012, 29, 861–872. [Google Scholar] [CrossRef]
- Urbano-Moral, J.A.; Patel, A.R.; Maron, M.S.; Arias-Godinez, J.A.; Pandian, N.G. Three-dimensional speckle-tracking echocardiography: Methodological aspects and clinical potential. Echocardiography 2012, 29, 997–1010. [Google Scholar] [CrossRef]
- Muraru, D.; Niero, A.; Rodriguez-Zanella, H.; Cherata, D.; Badano, L. Three-dimensional speckle-tracking echocardiography: Benefits and limitations of integrating myocardial mechanics with three-dimensional imaging. Cardiovasc. Diagn. Ther. 2018, 8, 101–117. [Google Scholar] [CrossRef]
- Gao, L.; Lin, Y.; Ji, M.; Wu, W.; Li, H.; Qian, M.; Zhang, L.; Xie, M.; Li, Y. Clinical Utility of Three-Dimensional Speckle-Tracking Echocardiography in Heart Failure. J. Clin. Med. 2022, 11, 6307. [Google Scholar] [CrossRef] [PubMed]
- Matos, J.D.; Balachandran, I.; Heidinger, B.H.; Mohebali, D.; Feldman, S.A.; McCormick, I.; Litmanovich, D.; Manning, W.J.; Carroll, B.J. Mitral annular plane systolic excursion and tricuspid annular plane systolic excursion for risk stratification of acute pulmonary embolism. Echocardiography 2020, 37, 1008–1013. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, T.; Dulgheru, R.; Bernard, A.; Ilardi, F.; Contu, L.; Addetia, K.; Caballero, L.; Akhaladze, N.; Athanassopoulos, G.D.; Barone, D.; et al. Echocardiographic reference ranges for normal left ventricular 2D strain: Results from the EACVI NORRE study. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Silbiger, J.J. Anatomy, mechanics, and pathophysiology of the mitral annulus. Am. Heart J. 2012, 164, 163–176. [Google Scholar] [CrossRef]
- Nesser, H.J.; Mor-Avi, V.; Gorissen, W.; Weinert, L.; Steringer-Mascherbauer, R.; Niel, J.; Sugeng, L.; Lang, R.M. Quantification of left ventricular volumes using three-dimensional echocardiographic speckle tracking: Comparison with MRI. Eur. Heart J. 2009, 30, 1565–1573. [Google Scholar] [CrossRef]
- Kleijn, S.A.; Brouwer, W.P.; Aly, M.F.A.; Russel, I.K.; de Roest, G.J.; Beek, A.M.; van Rossum, A.C.; Kamp, O. Comparison between three-dimensional speckle-tracking echocardiography and cardiac magnetic resonance imaging for quantification of left ventricular volumes and function. Eur. Heart J. Cardiovasc. Imaging 2012, 13, 834–839. [Google Scholar] [CrossRef]
- Kleijn, S.A.; Aly, M.F.A.; Terwee, C.B.; van Rossum, A.C.; Kamp, O. Reliability of left ventricular volumes and function measurements using three-dimensional speckle tracking echocardiography. Eur. Heart J. Cardiovasc. Imaging 2012, 13, 159–168. [Google Scholar] [CrossRef]
- Ahmad, H.; Gayat, E.; Yodwut, C.; Abduch, M.C.; Patel, A.R.; Weinert, L.; Desai, A.; Tsang, W.; Garcia, J.G.N.; Lang, R.M.; et al. Evaluation of myocardial deformation in patients with sickle cell disease and preserved ejection fraction using three-dimensional speckle tracking echocardiography. Echocardiography 2012, 29, 962–969. [Google Scholar] [CrossRef]
- Kormányos, Á.; Kalapos, A.; Domsik, P.; Gyenes, N.; Lengyel, C.; Nemes, A. Normal reference values of left ventricular volumetric parameters in healthy adults-real-life single-center experience from the three-dimensional speckle-tracking echocardiographic MAGYAR-Healthy Study. Quant. Imaging Med. Surg. 2021, 11, 1496–1503. [Google Scholar] [CrossRef]
- Nemes, A.; Kormányos, Á.; Kalapos, A.; Domsik, P.; Gyenes, N.; Ambrus, N.; Lengyel, C. Normal reference values of left ventricular strain parameters in healthy adults: Real-life experience from the single-center three-dimensional speckle-tracking echocardiographic MAGYAR-Healthy Study. J. Clin. Ultrasound 2021, 49, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Nemes, A.; Kormányos, Á.; Ruzsa, Z.; Achim, A.; Ambrus, N.; Lengyel, C. Complexity of left ventricular strains in response to elevated volumes in healthy adults—Detailed analysis from the three-dimensional speckle-tracking echocardiographic MAGYAR-Healthy Study. Int. J. Cardiol Heart. Vasc. 2023, 47, 101236. [Google Scholar] [CrossRef] [PubMed]
- Nemes, A.; Kormányos, Á.; Ambrus, N.; Lengyel, C. Left Ventricular Strains and Right Ventricular Longitudinal Shortening Are Associated in Healthy Adults—A Detailed Analysis from the Three-Dimensional Speckle-Tracking Echocardiographic MAGYAR-Healthy Study. Life 2024, 14, 1422. [Google Scholar] [CrossRef]
- Negishi, T.; Negishi, K.; Thavendiranathan, P.; Cho, G.Y.; Popescu, B.A.; Vinereanu, D.; Kurosawa, K.; Penicka, M.; Marwick, T.H.; SUCCOUR Investigators. Effect of Experience and Training on the Concordance and Precision of Strain Measurements. JACC Cardiovasc. Imaging 2017, 10, 518–522. [Google Scholar] [CrossRef]
- Rösner, A.; Barbosa, D.; Aarsæther, E.; Kjønås, D.; Schirmer, H.; D’hooge, J. The influence of frame rate on two-dimensional speckle-tracking strain measurements: A study on silico-simulated models and images recorded in patients. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 1137–1147. [Google Scholar] [CrossRef]
- Sonaglioni, A.; Nicolosi, G.L.; Granato, A.; Bonanomi, A.; Rogamonti, E.; Lombardo, M. Influence of chest wall conformation on reproducibility of main echocardiographic indices of left ventricular systolic function. Minerva Cardiol. Angiol. 2024, 72, 111–124. [Google Scholar] [CrossRef]
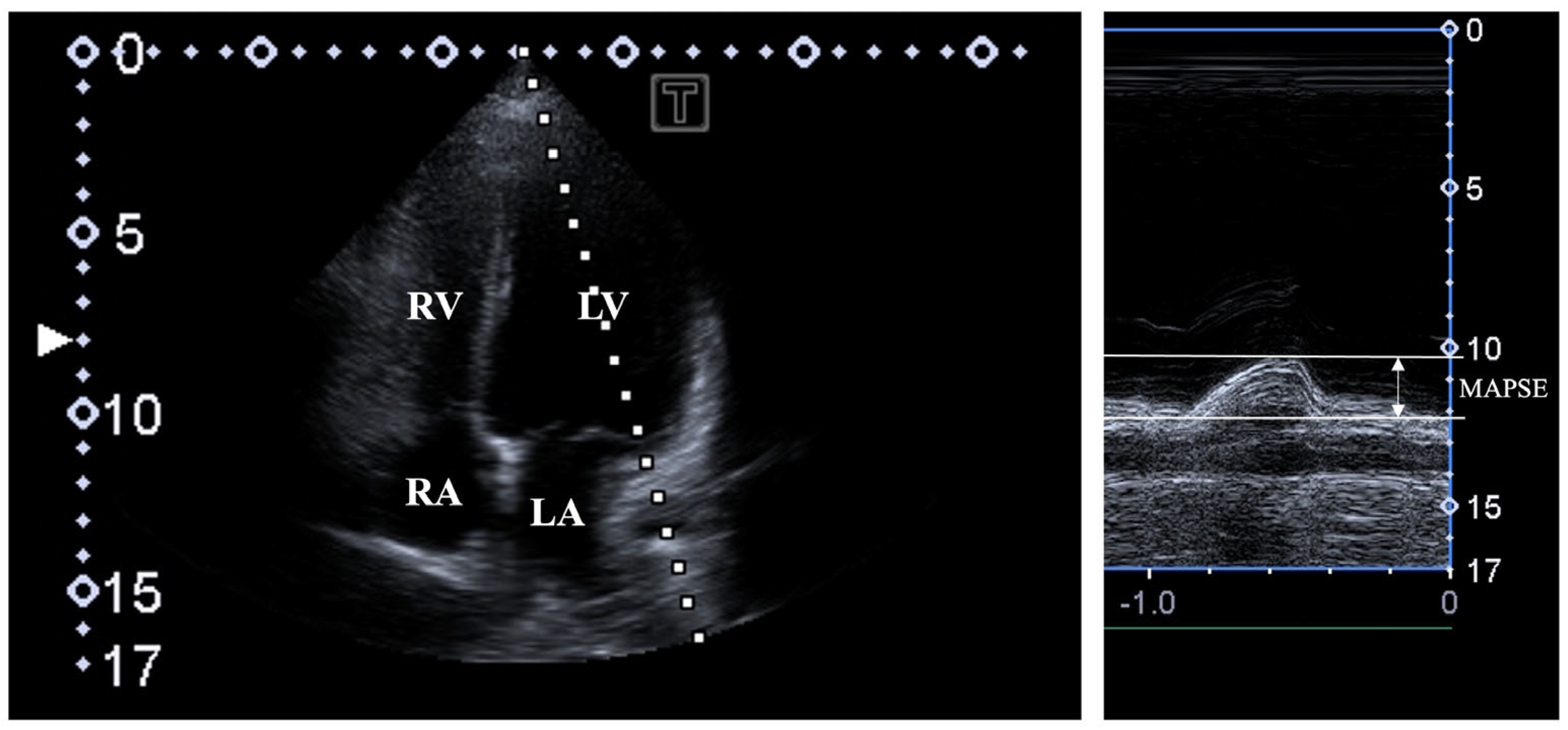
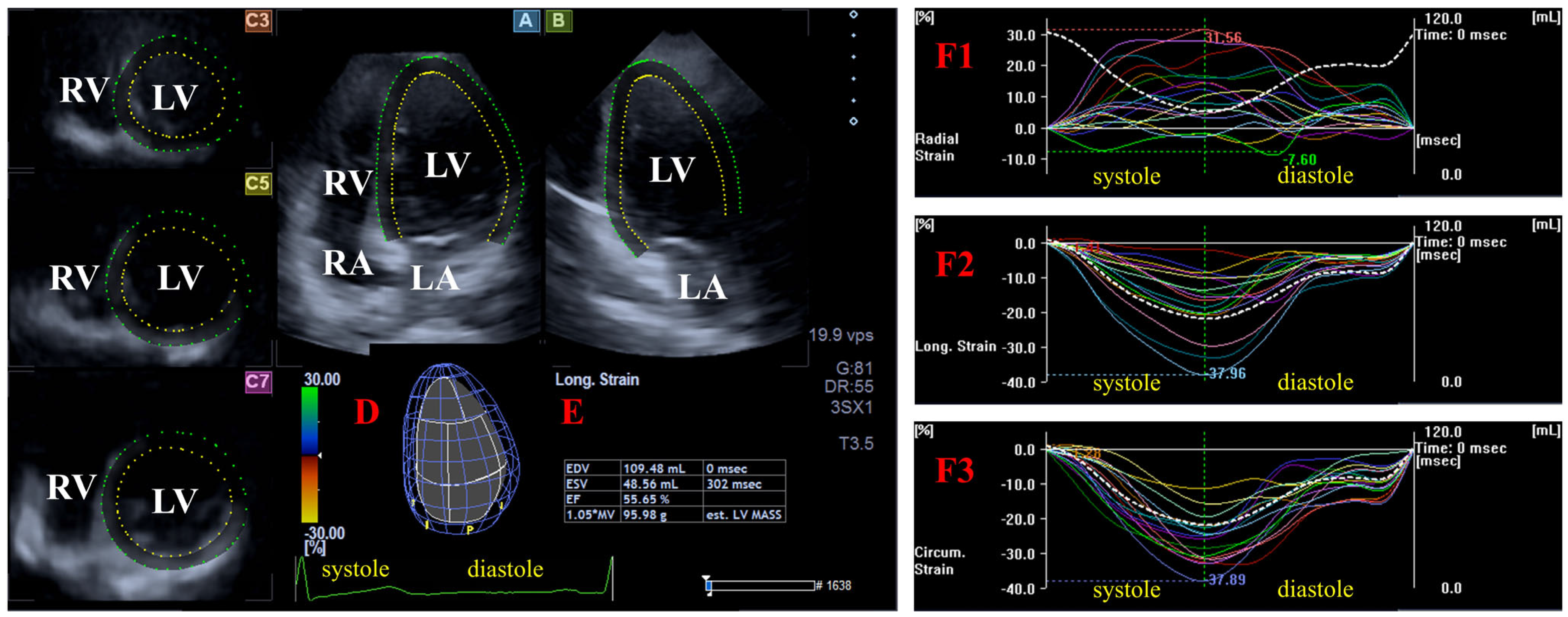

| All Subjects (n = 106) | MAPSE ≤ 11 mm (n = 15) | 11 mm < MAPSE < 17 mm (n = 70) | MAPSE ≥ 17 mm (n = 21) | |
|---|---|---|---|---|
| LV-EDV (mL) | 83.9 ± 21.3 (83.2) | 90.9 ± 26.0 (84.4) | 80.5 ± 19.4 (80.8) | 90.0 ± 21.0 † (90.5) |
| LV-ESV (mL) | 36.0 ± 9.5 (35.1) | 39.9 ± 12.2 (38.3) | 34.9 ± 8.4 (34.9) * | 36.8 ± 10.2 (32.2) |
| LV-EF (%) | 57.4 ± 5.3 (56.5) | 56.3 ± 3.5 (56.0) | 57.1 ± 5.4 (56.4) | 59.1 ± 5.7 (58.1) |
| LV mass (g) | 162.2 ± 31.7 (166.0) | 166.9 ± 35.3 (179.0) | 161.7 ± 28.9 (169.3) | 160.5 ± 37.1 (154) |
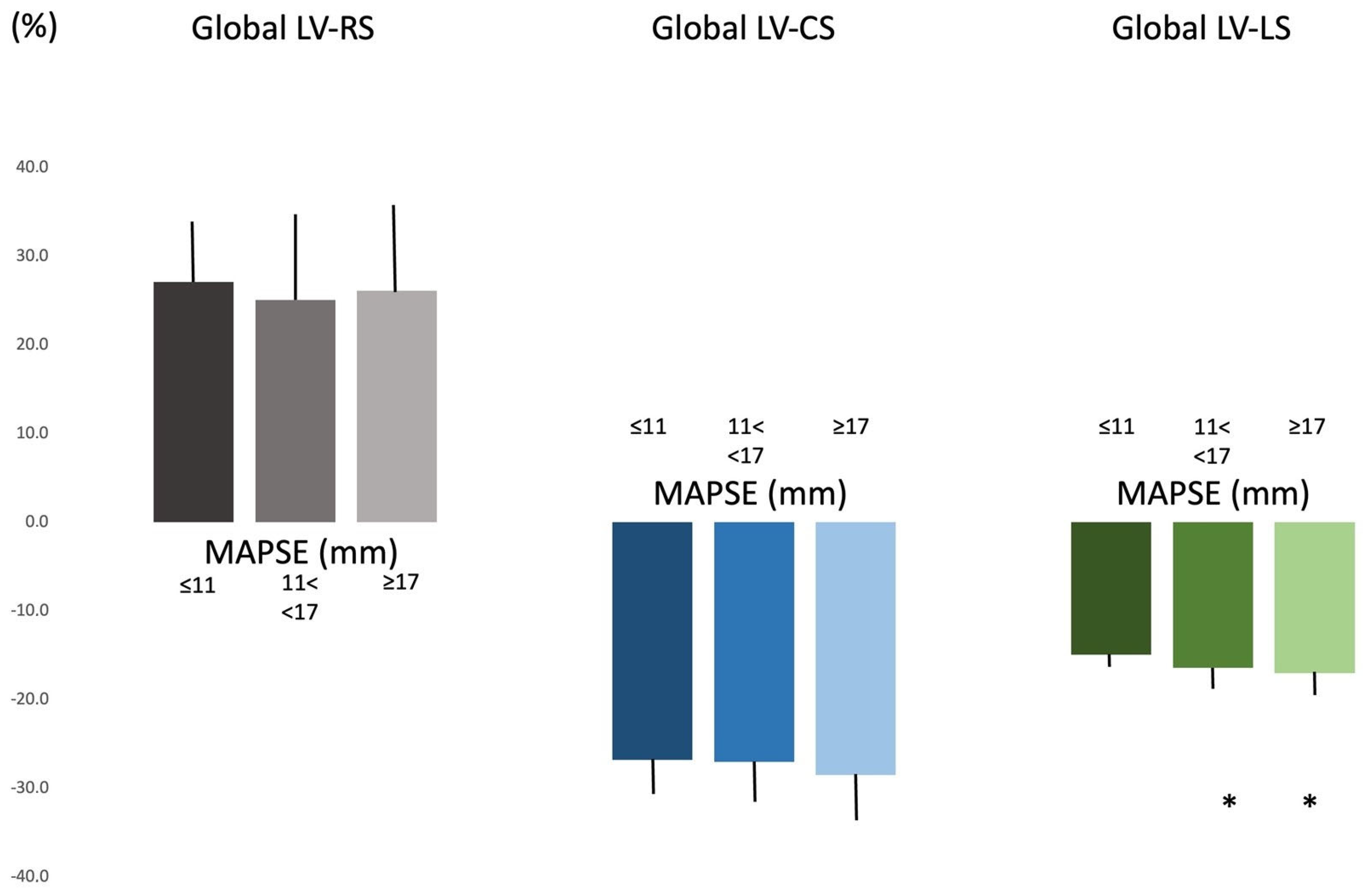
| global LV-RS (%) | 25.6 ± 9.9 (24.7) | 27.1 ± 7.1 (28.8) | 25.1 ± 10.3 (24.2) | 26.1 ± 9.9 (23.0) |
| basal LV-RS (%) | 32.2 ± 12.9 (32.7) | 34.6 ± 8.2 (36.5) | 31.5 ± 12.9 (32.8) | 32.6 ± 15.2 (30.2) |
| midventricular LV-RS (%) | 29.8 ± 11.2 (28.1) | 30.5 ± 8.7 (28.8) | 29.5 ± 12.2 (27.7) | 30.5 ± 8.7 (28.1) |
| apical LV-RS (%) | 17.9 ± 9.2 (16.3) | 17.5 ± 9.3 (17.8) | 17.7 ± 9.4 (16.2) | 18.9 ± 8.1 (17.2) |
| global LV-CS (%) | −27.3 ± 4.8 (−26.6) | −26.8 ± 4.2 (−26.5) | −27.0 ± 4.8 (−26.6) | −28.5 ± 5.2 (−27.0) |
| basal LV-CS (%) | −25.3 ± 4.6 (−25.6) | −26.8 ± 3.9 (−28.5) | −25.1 ± 4.8 (−25.0) | −25.1 ± 3.8 (−25.6) |
| midventricular LV-CS (%) | −29.2 ± 5.6 (−28.5) | −28.8 ± 3.9 (−28.7) | −28.8 ± 5.7 (−28.0) | −30.7 ± 6.0 (−30.4) |
| apical LV-CS (%) | −31.3 ± 10.3 (−30.9) | −28.2 ±11.1 (−28.6) | −31.2 ± 10.0 (−30.9) | −33.8 ± 10.0 (−36.4) |
| global LV-LS (%) | −16.3 ± 2.4 (−16.3) | −14.9 ± 1.6 (−14.6) | −16.4 ± 2.3 (−16.3) * | −17.0 ± 2.6 (−17.3) * |
| basal LV-LS (%) | −20.3 ± 4.4 (−20.0) | −19.5 ± 3.6 (−19.6) | −20.2 ± 4.5 (−20.0) | −21.4 ± 4.3 (22.0) |
| midventricular LV-LS (%) | −13.7 ± 3.5 (−13.5) | −12.5 ± 3.0 (−11.9) | −13.9 ± 3.8 (−13.79 | −14.0 ± 2.9 (−13.9) |
| apical LV-LS (%) | −17.1 ± 5.8 (−16.8) | −15.2 ± 5.4 (−15.5) | −17.4 ± 5.5 (−16.7) | −17.8 ± 6.5 (−17.9) |
| MAPSE (mm) | 14.2 ± 3.0 (14.4) | 9.3 ± 1.8 (10.3) | 14.0 ± 1.5 (14.1) * | 18.4 ± 1.3 (18.1) *† |
| global LV-RS ≤ 15.7% (n = 14) | 15.7% < global LV-RS < 35.5% (n = 76) | global LV-RS ≥35.5% (n = 16) | global LV-CS≤ −22.5% (n = 8) | −22.5% < global LV-CS < −32.1% (n = 81) | global LV-CS ≥−32.1% (n = 17) | global LV-LS ≤ −13.9% (n = 18) | −13.9% < global LV-LS < −18.7% (n = 72) | global LV-LS ≥−18.7% (n = 16) | |
|---|---|---|---|---|---|---|---|---|---|
| LV-EDV (mL) | 71.9 ± 11.8 (72.1) | 84.2 ± 21.9 (84.0) | 92.0 ± 21.0 (91.7) * | 73.7 ± 12.5 (76.2) | 85.4 ± 22.3 (84.5) | 81.6 ± 17.8 (82.8) | 86.5 ± 24.0 (79.6) | 85.1 ± 19.4 (85.6) | 75.4 ± 24.1 (78.4) |
| LV-ESV (mL) | 33.9 ± 5.1 (33.0) | 36.4 ± 9.5 (35.9) | 35.8 ± 12.2 (33.1) | 37.0 ± 5.2 (37.5) | 37.7 ± 9.4 (34.0) | 27.5 ± 6.9 (25.8) †/†† | 38.6 ± 11.4 (34.9) | 36.4 ± 8.9 (36.8) | 31.1 ± 8.3 (27.5) ‡/‡‡ |
| LV-EF (%) | 53.2 ± 4.6 (52.7) | 57.3 ± 4.4 (56.5) * | 61.6 ± 6.4 (60.2) */** | 49.5 ± 2.9 (50.8) | 56.3 ± 3.0 † (56.2) | 66.3 ± 4.1 (66.4) †/†† | 55.3 ± 4.6 (55.2) | 57.1 ± 5.1 (56.5) | 61.0 ± 5.2 (61.2) ‡/‡‡ |
| LV mass (g) | 149.1 ± 29.5 (157.0) | 163.5 ± 31.0 (167.5) | 167.6 ± 33.7 (179.0) | 164.2 ± 25.4 (171.0) | 163.3 ± 31.5 (166.0) | 155.7 ± 32.2 (162.0) | 162.2 ± 33.3 (169.8) | 163.9 ± 32.1 (170.0) | 154.7 ± 26.6 (156.5) |
| global LV-RS (%) | 11.9 ± 2.9 (12.2) | 24.5 ± 5.0 (24.5) * | 42.8 ± 7.0 (40.6) */** | 18.3 ± 6.5 (20.4) | 24.8 ± 8.3† (24.6) | 32.7 ± 13.6 (31.0) †/†† | 24.5 ± 11.7 (20.7) | 25.2 ± 9.0 (25.0) | 28.4 ± 10.8 (25.7) |
| global LV-CS (%) | −24.4 ± 4.7 (−23.8) | −27.3 ± 4.4 (−26.7) * | −29.9 ± 5.6 (−28.3) */** | −18.8 ± 2.7 (−18.8) | −26.4 ± 2.5 (−26.4) † | −35.4 ± 2.7 (−34.9) †/†† | −26.0 ± 4.5 (−24.8) | −27.1 ± 4.6 (−26.6) | −29.6 ± 4.8 (−28.1) ‡ |
| global LV-LS (%) | −15.7 ± 2.2 (−15.3) | −16.2 ± 2.4 (−16.5) | −16.2 ± 3.4 (−14.9) | −15.8 ± 1.7 (−15.8) | −16.1 ± 2.2 (−16.2) | −17.4 ± 2.9 (−17.9) † | −13.0 ± 1.1 (−13.4) | −16.3 ± 1.3 ‡ (−16.3) | −20.1 ± 1.2 (−19.7) ‡/‡‡ |
| MAPSE (mm) | 14.2 ± 2.4 (14.0) | 14.1 ± 3.2 (14.4) | 14.9 ± 2.6 (14.3) | 14.0 ± 2.9 (14.4) | 13.9 ± 3.1 (14.0) | 15.6 ± 2.5 (15.5) | 12.7 ± 3.1 (12.3) | 14.3 ± 3.1 (14.3) ‡ | 15.4 ± 1.9 (15.6) ‡ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nemes, A.; Ambrus, N.; Lengyel, C. Three-Dimensional Speckle-Tracking Echocardiography-Derived Left Ventricular Global Longitudinal Strain and Mitral Annular Plane Systolic Excursion Are Associated in Healthy Adults—Insights from the MAGYAR-Healthy Study. Biomedicines 2025, 13, 625. https://doi.org/10.3390/biomedicines13030625
Nemes A, Ambrus N, Lengyel C. Three-Dimensional Speckle-Tracking Echocardiography-Derived Left Ventricular Global Longitudinal Strain and Mitral Annular Plane Systolic Excursion Are Associated in Healthy Adults—Insights from the MAGYAR-Healthy Study. Biomedicines. 2025; 13(3):625. https://doi.org/10.3390/biomedicines13030625
Chicago/Turabian StyleNemes, Attila, Nóra Ambrus, and Csaba Lengyel. 2025. "Three-Dimensional Speckle-Tracking Echocardiography-Derived Left Ventricular Global Longitudinal Strain and Mitral Annular Plane Systolic Excursion Are Associated in Healthy Adults—Insights from the MAGYAR-Healthy Study" Biomedicines 13, no. 3: 625. https://doi.org/10.3390/biomedicines13030625
APA StyleNemes, A., Ambrus, N., & Lengyel, C. (2025). Three-Dimensional Speckle-Tracking Echocardiography-Derived Left Ventricular Global Longitudinal Strain and Mitral Annular Plane Systolic Excursion Are Associated in Healthy Adults—Insights from the MAGYAR-Healthy Study. Biomedicines, 13(3), 625. https://doi.org/10.3390/biomedicines13030625








