Advances in Targeted and Chemotherapeutic Strategies for Colorectal Cancer: Current Insights and Future Directions
Abstract
1. Introduction
2. Chemotherapy
3. Target Therapy
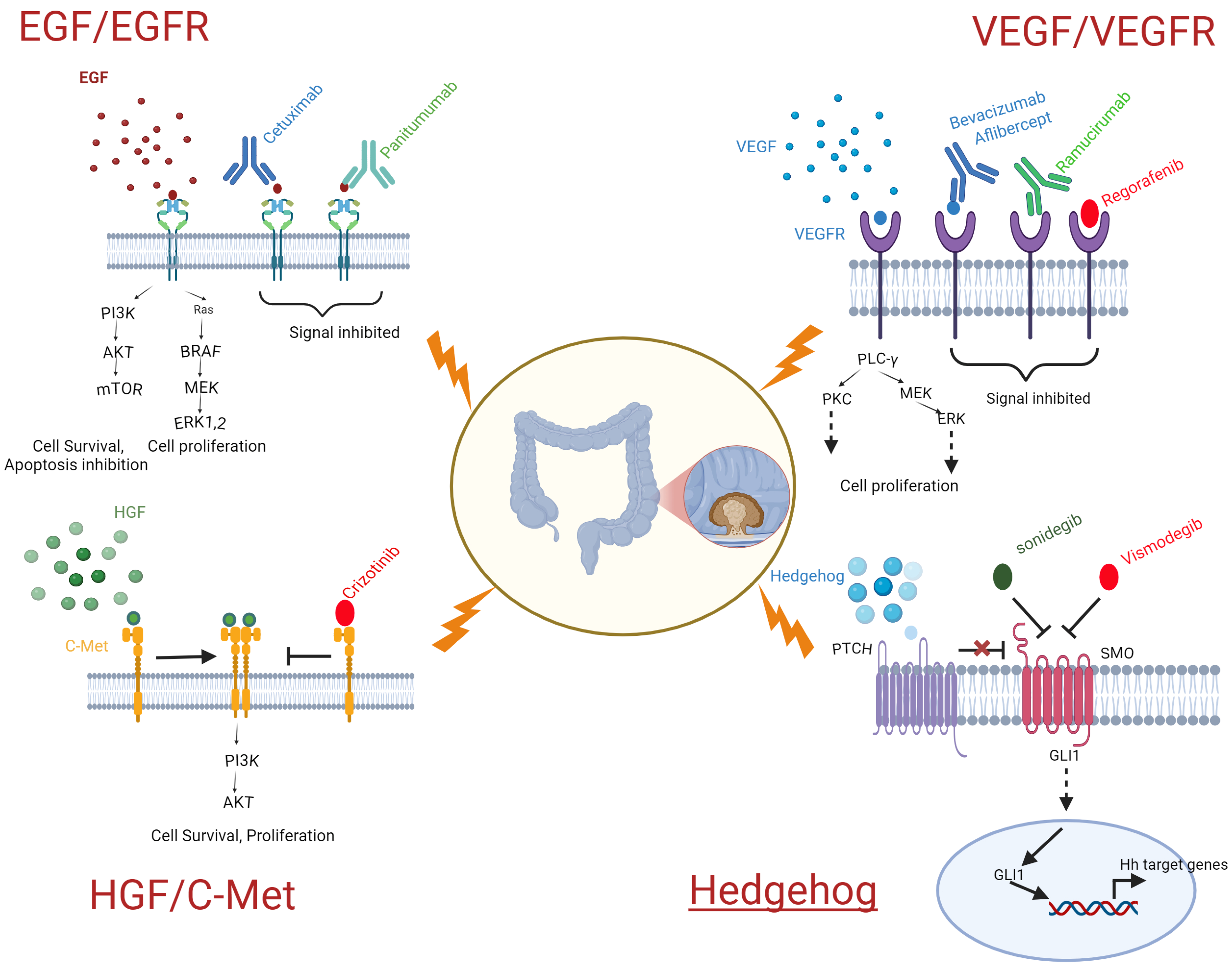
3.1. Ongoing Target Therapy
3.1.1. VEGF/VEGER Target Therapy
| Drug Targeted Against VEGF | Mode of Action | Response Rate | Year of Approval | |
|---|---|---|---|---|
| 1. | Bevacizumab | Bevacizumab is a humanized monoclonal antibody target against VEGF-A to prevent interaction with VEGFR-2. | ~48.89% [46] | 2004 |
| 2. | Aflibercept | Aflibercept inhibits VEGF-A,B and PIGF. | 20.9% [47] | 2011 |
| 3. | Regorafenib | Regorafenib inhibits VEGFR-1, -2, -3, PDGFR, c-Kit, and FGFR. | 33% [48] | 2012 |
| 4. | Ramucirumab | Ramucirumab is a human monoclonal antibody that targets the VEGFR-2 extracellular domain. | 58.3% [27] | 2014 |
3.1.2. EGF/EGFR
| Drug Targeted Against EGFR | Mode of Action | Response Rate | Year of Approval | |
|---|---|---|---|---|
| 1. | Cetuximab | Cetuximab is a chimeric IgG1 monoclonal antibody that specifically targets the extracellular domain of the epidermal growth-factor receptor (EGFR). | 48.7–53.8% [63] | 2004 |
| 2. | Panitumumab | Panitumumab is a human monoclonal antibody that specifically targets the EGFR. | 10% [64] | 2006 |
3.1.3. HGF/CMet
3.1.4. Hedgehog
3.2. Future Target Therapy
3.2.1. IGF/IGF1R
3.2.2. Wnt/β-Catenin
3.2.3. Notch
3.2.4. TGF-β Smad
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef] [PubMed]
- Ohishi, T.; Kaneko, M.K.; Yoshida, Y.; Takashima, A.; Kato, Y.; Kawada, M. Current Targeted Therapy for Metastatic Colorectal Cancer. Int. J. Mol. Sci. 2023, 24, 1702. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Kuipers, E.J.; Grady, W.M.; Lieberman, D.; Seufferlein, T.; Sung, J.J.; Boelens, P.G.; van de Velde, C.J.; Watanabe, T. Colorectal cancer. Nat. Rev. Dis. Primers 2015, 1, 15065. [Google Scholar] [CrossRef]
- Riihimäki, M.; Hemminki, A.; Sundquist, J.; Hemminki, K. Patterns of metastasis in colon and rectal cancer. Sci. Rep. 2016, 6, 29765. [Google Scholar] [CrossRef]
- Biller, L.H.; Schrag, D. Diagnosis and Treatment of Metastatic Colorectal Cancer: A Review. JAMA 2021, 325, 669–685. [Google Scholar] [CrossRef]
- Argilés, G.; Tabernero, J.; Labianca, R.; Hochhauser, D.; Salazar, R.; Iveson, T.; Laurent-Puig, P.; Quirke, P.; Yoshino, T.; Taieb, J.; et al. Localised colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 1291–1305. [Google Scholar] [CrossRef]
- Labianca, R.; Nordlinger, B.; Beretta, G.D.; Mosconi, S.; Mandalà, M.; Cervantes, A.; Arnold, D. Early colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2013, 24, vi64–vi72. [Google Scholar] [CrossRef]
- Modest, D.P.; Pant, S.; Sartore-Bianchi, A. Treatment sequencing in metastatic colorectal cancer. Eur. J. Cancer 2019, 109, 70–83. [Google Scholar] [CrossRef]
- Woo, I.S.; Jung, Y.H. Metronomic chemotherapy in metastatic colorectal cancer. Cancer Lett. 2017, 400, 319–324. [Google Scholar] [CrossRef]
- Vodenkova, S.; Buchler, T.; Cervena, K.; Veskrnova, V.; Vodicka, P.; Vymetalkova, V. 5-fluorouracil and other fluoropyrimidines in colorectal cancer: Past, present and future. Pharmacol. Ther. 2020, 206, 107447. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, S.M.; Al Doghaither, H.A.; Al-Ghafari, A.B.; Pushparaj, P.N. 5-Fluorouracil and capecitabine therapies for the treatment of colorectal cancer (Review). Oncol. Rep. 2023, 50, 175. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.; Kubota, Y.; Ishida, H.; Sasaki, Y. Irinotecan, a key chemotherapeutic drug for metastatic colorectal cancer. World J. Gastroenterol. 2015, 21, 12234–12248. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Rubio, E.; Sastre, J.; Zaniboni, A.; Labianca, R.; Cortés-Funes, H.; de Braud, F.; Boni, C.; Benavides, M.; Dallavalle, G.; Homerin, M. Oxaliplatin as single agent in previously untreated colorectal carcinoma patients: A phase II multicentric study. Ann. Oncol. 1998, 9, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Comella, P.; Casaretti, R.; Sandomenico, C.; Avallone, A.; Franco, L. Role of oxaliplatin in the treatment of colorectal cancer. Ther. Clin. Risk Manag. 2009, 5, 229–238. [Google Scholar] [CrossRef]
- Raedler, L.A. Lonsurf (Trifluridine plus Tipiracil): A New Oral Treatment Approved for Patients with Metastatic Colorectal Cancer. Am. Health Drug Benefits 2016, 9, 97–100. [Google Scholar]
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-Fluorouracil: Mechanisms of action and clinical strategies. Nat. Rev. Cancer 2003, 3, 330–338. [Google Scholar] [CrossRef]
- Ross, P.; Norman, A.; Cunningham, D.; Webb, A.; Iveson, T.; Padhani, A.; Prendiville, J.; Watson, M.; Massey, A.; Popescu, R. A prospective randomised trial of protracted venous infusion 5-fluorouracil with or without mitomycin C in advanced colorectal cancer. Ann. Oncol. 1997, 8, 995–1001. [Google Scholar] [CrossRef]
- Walko, C.M.; Lindley, C. Capecitabine: A review. Clin. Ther. 2005, 27, 23–44. [Google Scholar] [CrossRef]
- Hirsch, B.R.; Zafar, S.Y. Capecitabine in the management of colorectal cancer. Cancer Manag. Res. 2011, 3, 79–89. [Google Scholar] [CrossRef]
- Reyhanoglu, G.; Smith, T. Irinotecan. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar] [PubMed]
- Rothenberg, M.L. Efficacy and toxicity of irinotecan in patients with colorectal cancer. Semin. Oncol. 1998, 25, 39–46. [Google Scholar] [PubMed]
- Arango, D.; Wilson, A.J.; Shi, Q.; Corner, G.A.; Arañes, M.J.; Nicholas, C.; Lesser, M.; Mariadason, J.M.; Augenlicht, L.H. Molecular mechanisms of action and prediction of response to oxaliplatin in colorectal cancer cells. Br. J. Cancer 2004, 91, 1931–1946. [Google Scholar] [CrossRef] [PubMed]
- Rothenberg, M.L. Efficacy of oxaliplatin in the treatment of colorectal cancer. Oncology 2000, 14, 9–14. [Google Scholar]
- Kish, T.; Uppal, P. Trifluridine/Tipiracil (Lonsurf) for the Treatment of Metastatic Colorectal Cancer. Pharm. Ther. 2016, 41, 314–325. [Google Scholar]
- Voutsadakis, I.A. A Systematic Review and Meta-Analysis of Trifluridine/Tipiracil plus Bevacizumab for the Treatment of Metastatic Colorectal Cancer: Evidence from Real-World Series. Curr. Oncol. 2023, 30, 5227–5239. [Google Scholar] [CrossRef]
- Verdaguer, H.; Tabernero, J.; Macarulla, T. Ramucirumab in metastatic colorectal cancer: Evidence to date and place in therapy. Ther. Adv. Med. Oncol. 2016, 8, 230–242. [Google Scholar] [CrossRef]
- Balasubramanian, S.; Priyathersini, N.; Johnson, T. Expression of Vascular Endothelial Growth Factor (VEGF) in Colorectal Adenoma and Carcinoma in a Tertiary Care Center. Cureus 2022, 14, e31393. [Google Scholar] [CrossRef]
- Dakowicz, D.; Zajkowska, M.; Mroczko, B. Relationship between VEGF Family Members, Their Receptors and Cell Death in the Neoplastic Transformation of Colorectal Cancer. Int. J. Mol. Sci. 2022, 23, 3375. [Google Scholar] [CrossRef]
- Lohela, M.; Bry, M.; Tammela, T.; Alitalo, K. VEGFs and receptors involved in angiogenesis versus lymphangiogenesis. Curr. Opin. Cell Biol. 2009, 21, 154–165. [Google Scholar] [CrossRef]
- Sidorkiewicz, I.; Zbucka-Krętowska, M.; Zaręba, K.; Lubowicka, E.; Zajkowska, M.; Szmitkowski, M.; Gacuta, E.; Ławicki, S. Plasma levels of M-CSF and VEGF in laboratory diagnostics and differentiation of selected histological types of cervical cancers. BMC Cancer 2019, 19, 398. [Google Scholar] [CrossRef]
- Ntellas, P.; Mavroeidis, L.; Gkoura, S.; Gazouli, I.; Amylidi, A.-L.; Papadaki, A.; Zarkavelis, G.; Mauri, D.; Karpathiou, G.; Kolettas, E. Old player-new tricks: Non angiogenic effects of the VEGF/VEGFR pathway in cancer. Cancers 2020, 12, 3145. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Ueno, H.; Shibuya, M. VEGF activates protein kinase C-dependent, but Ras-independent Raf-MEK-MAP kinase pathway for DNA synthesis in primary endothelial cells. Oncogene 1999, 18, 2221–2230. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N.; Hillan, K.J.; Novotny, W. Bevacizumab (Avastin), a humanized anti-VEGF monoclonal antibody for cancer therapy. Biochem. Biophys. Res. Commun. 2005, 333, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Saif, M.W. Anti-VEGF agents in metastatic colorectal cancer (mCRC): Are they all alike? Cancer Manag. Res. 2013, 5, 103–115. [Google Scholar] [CrossRef]
- Ferrara, N.; Hillan, K.J.; Gerber, H.P.; Novotny, W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat. Rev. Drug Discov. 2004, 3, 391–400. [Google Scholar] [CrossRef]
- Garcia, J.; Hurwitz, H.I.; Sandler, A.B.; Miles, D.; Coleman, R.L.; Deurloo, R.; Chinot, O.L. Bevacizumab (Avastin®) in cancer treatment: A review of 15 years of clinical experience and future outlook. Cancer Treat. Rev. 2020, 86, 102017. [Google Scholar] [CrossRef]
- Hurwitz, H.; Fehrenbacher, L.; Novotny, W.; Cartwright, T.; Hainsworth, J.; Heim, W.; Berlin, J.; Baron, A.; Griffing, S.; Holmgren, E.; et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N. Engl. J. Med. 2004, 350, 2335–2342. [Google Scholar] [CrossRef]
- Tew, W.P.; Gordon, M.; Murren, J.; Dupont, J.; Pezzulli, S.; Aghajanian, C.; Sabbatini, P.; Mendelson, D.; Schwartz, L.; Gettinger, S.; et al. Phase 1 Study of Aflibercept Administered Subcutaneously to Patients with Advanced Solid Tumors. Clin. Cancer Res. 2010, 16, 358–366. [Google Scholar] [CrossRef]
- Wilhelm, S.M.; Dumas, J.; Adnane, L.; Lynch, M.; Carter, C.A.; Schütz, G.; Thierauch, K.-H.; Zopf, D. Regorafenib (BAY 73-4506): A new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int. J. Cancer 2011, 129, 245–255. [Google Scholar] [CrossRef]
- Arai, H.; Battaglin, F.; Wang, J.; Lo, J.H.; Soni, S.; Zhang, W.; Lenz, H.-J. Molecular insight of regorafenib treatment for colorectal cancer. Cancer Treat. Rev. 2019, 81, 101912. [Google Scholar] [CrossRef]
- Abou-Elkacem, L.; Arns, S.; Brix, G.; Gremse, F.; Zopf, D.; Kiessling, F.; Lederle, W. Regorafenib Inhibits Growth, Angiogenesis, and Metastasis in a Highly Aggressive, Orthotopic Colon Cancer Model. Mol. Cancer Ther. 2013, 12, 1322–1331. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, X.; Lu, D.; Brennan, L.; Persaud, K.; Liu, M.; Miao, H.; Witte, L.; Zhu, Z. A recombinant, fully human, bispecific antibody neutralizes the biological activities mediated by both vascular endothelial growth factor receptors 2 and 3. Mol. Cancer Ther. 2005, 4, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Goede, V.; Coutelle, O.; Neuneier, J.; Reinacher-Schick, A.; Schnell, R.; Koslowsky, T.C.; Weihrauch, M.R.; Cremer, B.; Kashkar, H.; Odenthal, M.; et al. Identification of serum angiopoietin-2 as a biomarker for clinical outcome of colorectal cancer patients treated with bevacizumab-containing therapy. Br. J. Cancer 2010, 103, 1407–1414. [Google Scholar] [CrossRef] [PubMed]
- Itatani, Y.; Kawada, K.; Yamamoto, T.; Sakai, Y. Resistance to Anti-Angiogenic Therapy in Cancer—Alterations to Anti-VEGF Pathway. Int. J. Mol. Sci. 2018, 19, 1232. [Google Scholar] [CrossRef]
- Xiong, L.; Lou, Y.; Wang, L. Effect of bevacizumab combined with first-line chemotherapy on metastatic colorectal cancer. Am. J. Transl. Res. 2021, 13, 3609–3617. [Google Scholar]
- Nakatsumi, H.; Komatsu, Y.; Harada, K.; Kawamoto, Y.; Yuki, S.; Sawada, K.; Ishiguro, A.; Sogabe, S.; Ando, T.; Sasaki, Y.; et al. A multicenter, prospective, phase II trial of second-line aflibercept plus FOLFIRI in patients with metastatic colorectal cancer refractory to an anti-EGFR antibody: HGCSG1801. Int. J. Cancer 2024, 155, 2223–2231. [Google Scholar] [CrossRef]
- Xu, D.; Liu, Y.; Tang, W.; Xu, L.; Liu, T.; Jiang, Y.; Zhou, S.; Qin, X.; Li, J.; Zhao, J.; et al. Regorafenib in Refractory Metastatic Colorectal Cancer: A Multi-Center Retrospective Study. Front. Oncol. 2022, 12, 838870. [Google Scholar] [CrossRef]
- Piawah, S.; Venook, A.P. Targeted therapy for colorectal cancer metastases: A review of current methods of molecularly targeted therapy and the use of tumor biomarkers in the treatment of metastatic colorectal cancer. Cancer 2019, 125, 4139–4147. [Google Scholar] [CrossRef]
- Janani, B.; Vijayakumar, M.; Priya, K.; Kim, J.H. EGFR-Based Targeted Therapy for Colorectal Cancer-Promises and Challenges. Vaccines 2022, 10, 499. [Google Scholar] [CrossRef]
- Tabana, Y.; Dahham, S.; Shah, A.; Majid, A. Major signaling pathways of colorectal carcinogenesis. Recent. Adv. Colon. Cancer 2016, 1, 1–2. [Google Scholar]
- Jeong, W.-J.; Ro, E.J.; Choi, K.-Y. Interaction between Wnt/β-catenin and RAS-ERK pathways and an anti-cancer strategy via degradations of β-catenin and RAS by targeting the Wnt/β-catenin pathway. npj Precis. Oncol. 2018, 2, 5. [Google Scholar] [CrossRef] [PubMed]
- Saletti, P.; Molinari, F.; De Dosso, S.; Frattini, M. EGFR signaling in colorectal cancer: A clinical perspective. Gastrointest. Cancer Targets Ther. 2015, 5, 21–38. [Google Scholar]
- Hutchinson, R.A.; Adams, R.A.; McArt, D.G.; Salto-Tellez, M.; Jasani, B.; Hamilton, P.W. Epidermal growth factor receptor immunohistochemistry: New opportunities in metastatic colorectal cancer. J. Transl. Med. 2015, 13, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Mellstedt, H. Monoclonal antibodies in human cancer. Drugs Today 2003, 39, 1–16. [Google Scholar]
- Mendelsohn, J.; Baselga, J. The EGF receptor family as targets for cancer therapy. Oncogene 2000, 19, 6550–6565. [Google Scholar] [CrossRef]
- Saltz, L.B.; Meropol, N.J.; Loehrer Sr, P.J.; Needle, M.N.; Kopit, J.; Mayer, R.J. Phase II trial of cetuximab in patients with refractory colorectal cancer that expresses the epidermal growth factor receptor. J. Clin. Oncol. 2004, 22, 1201–1208. [Google Scholar] [CrossRef]
- Jonker, D.J.; O’Callaghan, C.J.; Karapetis, C.S.; Zalcberg, J.R.; Tu, D.; Au, H.-J.; Berry, S.R.; Krahn, M.; Price, T.; Simes, R.J. Cetuximab for the treatment of colorectal cancer. N. Engl. J. Med. 2007, 357, 2040–2048. [Google Scholar] [CrossRef]
- Saoudi González, N.; Ros, J.; Baraibar, I.; Salvà, F.; Rodríguez-Castells, M.; Alcaraz, A.; García, A.; Tabernero, J.; Élez, E. Cetuximab as a Key Partner in Personalized Targeted Therapy for Metastatic Colorectal Cancer. Cancers 2024, 16, 412. [Google Scholar] [CrossRef]
- Gemmete, J.J.; Mukherji, S.K. Panitumumab (vectibix). AJNR. Am. J. Neuroradiol. 2011, 32, 1002–1003. [Google Scholar] [CrossRef]
- Dubois, E.A.; Cohen, A.F. Panitumumab. Br. J. Clin. Pharmacol. 2009, 68, 482–483. [Google Scholar] [CrossRef]
- Parseghian, C.; Eluri, M.; Kopetz, S.; Raghav, K. Mechanisms of resistance to EGFR-targeted therapies in colorectal cancer: More than just genetics. Front. Cell Dev. Biol. 2023, 11, 1176657. [Google Scholar] [CrossRef] [PubMed]
- Tanioka, H.; Asano, M.; Yoshida, R.; Waki, N.; Uno, F.; Ishizaki, M.; Yamashita, K.; Morishita, Y.; Nagasaka, T. Cetuximab retreatment in patients with metastatic colorectal cancer who exhibited a clinical benefit in response to prior cetuximab: A retrospective study. Oncol. Lett. 2018, 16, 3674–3680. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, E.; Morgillo, F.; Troiani, T.; Tortora, G.; Ciardiello, F. Panitumumab: The evidence of its therapeutic potential in metastatic colorectal cancer care. Core Evid. 2007, 2, 81–88. [Google Scholar] [PubMed]
- Granito, A.; Guidetti, E.; Gramantieri, L. c-MET receptor tyrosine kinase as a molecular target in advanced hepatocellular carcinoma. J. Hepatocell. Carcinoma 2015, 2, 29–38. [Google Scholar]
- Boromand, N.; Hasanzadeh, M.; ShahidSales, S.; Farazestanian, M.; Gharib, M.; Fiuji, H.; Behboodi, N.; Ghobadi, N.; Hassanian, S.M.; Ferns, G.A. Clinical and prognostic value of the C-Met/HGF signaling pathway in cervical cancer. J. Cell. Physiol. 2018, 233, 4490–4496. [Google Scholar] [CrossRef]
- Farrell, J.; Kelly, C.; Rauch, J.; Kida, K.; Garcia-Munoz, A.; Monsefi, N.; Turriziani, B.; Doherty, C.; Mehta, J.P.; Matallanas, D. HGF induces epithelial-to-mesenchymal transition by modulating the mammalian hippo/MST2 and ISG15 pathways. J. Proteome Res. 2014, 13, 2874–2886. [Google Scholar] [CrossRef]
- Cheng, Y.; Song, Y.; Qu, J.; Che, X.; Song, N.; Fan, Y.; Wen, T.; Xu, L.; Gong, J.; Wang, X. The chemokine receptor CXCR4 and c-MET cooperatively promote epithelial-mesenchymal transition in gastric cancer cells. Transl. Oncol. 2018, 11, 487–497. [Google Scholar] [CrossRef]
- Konstorum, A.; Lowengrub, J.S. Activation of the HGF/c-Met axis in the tumor microenvironment: A multispecies model. J. Theor. Biol. 2018, 439, 86–99. [Google Scholar] [CrossRef]
- Lam, B.Q.; Dai, L.; Qin, Z. The role of HGF/c-MET signaling pathway in lymphoma. J. Hematol. Oncol. 2016, 9, 135. [Google Scholar] [CrossRef]
- Arnold, L.; Enders, J.; Thomas, S.M. Activated HGF-c-Met axis in head and neck cancer. Cancers 2017, 9, 169. [Google Scholar] [CrossRef]
- Delitto, D.; Vertes-George, E.; Hughes, S.J.; Behrns, K.E.; Trevino, J.G. c-Met signaling in the development of tumorigenesis and chemoresistance: Potential applications in pancreatic cancer. World J. Gastroenterol. 2014, 20, 8458–8470. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Feng, Q.; Chen, W.D.; Wang, Y.D. HGF/c-MET: A Promising Therapeutic Target in the Digestive System Cancers. Int. J. Mol. Sci. 2018, 19, 3295. [Google Scholar] [CrossRef] [PubMed]
- Lacy, S.A.; Miles, D.R.; Nguyen, L.T. Clinical pharmacokinetics and pharmacodynamics of cabozantinib. Clin. Pharmacokinet. 2017, 56, 477–491. [Google Scholar] [CrossRef] [PubMed]
- ML, B.P.; Miksad, R.A. Cabozantinib in the treatment of hepatocellular carcinoma. Future Oncol. 2017, 13, 1915–1929. [Google Scholar] [CrossRef]
- Mansfield, A.S.; Wei, Z.; Mehra, R.; Shaw, A.T.; Lieu, C.H.; Forde, P.M.; Drilon, A.E.; Mitchell, E.P.; Wright, J.J.; Takebe, N.; et al. Crizotinib in patients with tumors harboring ALK or ROS1 rearrangements in the NCI-MATCH trial. npj Precis. Oncol. 2022, 6, 13. [Google Scholar] [CrossRef]
- Sun, Y.; Sun, L.; An, Y.; Shen, X. Cabozantinib, a Novel c-Met Inhibitor, Inhibits Colorectal Cancer Development in a Xenograft Model. Med. Sci. Monit. 2015, 21, 2316–2321. [Google Scholar] [CrossRef]
- Saeed, A.; Park, R.; Pathak, H.; Al-Bzour, A.N.; Dai, J.; Phadnis, M.; Al-Rajabi, R.; Kasi, A.; Baranda, J.; Sun, W.; et al. Clinical and biomarker results from a phase II trial of combined cabozantinib and durvalumab in patients with chemotherapy-refractory colorectal cancer (CRC): CAMILLA CRC cohort. Nat. Commun. 2024, 15, 1533. [Google Scholar] [CrossRef]
- Choi, H.Y.; Chang, J.-E. Targeted Therapy for Cancers: From Ongoing Clinical Trials to FDA-Approved Drugs. Int. J. Mol. Sci. 2023, 24, 13618. [Google Scholar] [CrossRef]
- Briscoe, J.; Thérond, P.P. The mechanisms of Hedgehog signalling and its roles in development and disease. Nat. Rev. Mol. Cell Biol. 2013, 14, 416–429. [Google Scholar] [CrossRef]
- Fuccillo, M.; Joyner, A.L.; Fishell, G. Morphogen to mitogen: The multiple roles of hedgehog signalling in vertebrate neural development. Nat. Rev. Neurosci. 2006, 7, 772–783. [Google Scholar] [CrossRef]
- Groves, I.; Placzek, M.; Fletcher, A.G. Of mitogens and morphogens: Modelling Sonic Hedgehog mechanisms in vertebrate development. Philos. Trans. R. Soc. B 2020, 375, 20190660. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Thorne, J.; Grabel, L. Hedgehog serves as a mitogen and survival factor during embryonic stem cell neurogenesis. Stem Cells 2008, 26, 1097–1108. [Google Scholar] [CrossRef] [PubMed]
- Charron, F.; Tessier-Lavigne, M. The Hedgehog, TGF-β/BMP and Wnt families of morphogens in axon guidance. In Axon Growth and Guidance; Springer: New York, NY, USA, 2007; pp. 116–133. [Google Scholar]
- Tyagi, A.; Sharma, A.K.; Damodaran, C. A Review on Notch Signaling and Colorectal Cancer. Cells 2020, 9, 1549. [Google Scholar] [CrossRef] [PubMed]
- Hori, K.; Sen, A.; Artavanis-Tsakonas, S. Notch signaling at a glance. J. Cell Sci. 2013, 126, 2135–2140. [Google Scholar] [CrossRef]
- Rodilla, V.; Villanueva, A.; Obrador-Hevia, A.; Robert-Moreno, A.; Fernández-Majada, V.; Grilli, A.; López-Bigas, N.; Bellora, N.; Albà, M.M.; Torres, F.; et al. Jagged1 is the pathological link between Wnt and Notch pathways in colorectal cancer. Proc. Natl. Acad. Sci. USA 2009, 106, 6315–6320. [Google Scholar] [CrossRef]
- Wu, W.K.; Wang, X.J.; Cheng, A.S.; Luo, M.X.; Ng, S.S.; To, K.F.; Chan, F.K.; Cho, C.H.; Sung, J.J.; Yu, J. Dysregulation and crosstalk of cellular signaling pathways in colon carcinogenesis. Crit. Rev. Oncol./Hematol. 2013, 86, 251–277. [Google Scholar] [CrossRef]
- Serafin, V.; Persano, L.; Moserle, L.; Esposito, G.; Ghisi, M.; Curtarello, M.; Bonanno, L.; Masiero, M.; Ribatti, D.; Stürzl, M. Notch3 signalling promotes tumour growth in colorectal cancer. J. Pathol. 2011, 224, 448–460. [Google Scholar] [CrossRef]
- Aditya, S.; Rattan, A. Vismodegib: A smoothened inhibitor for the treatment of advanced basal cell carcinoma. Indian Dermatol. Online J. 2013, 4, 365–368. [Google Scholar] [CrossRef]
- Cirrone, F.; Harris, C.S. Vismodegib and the Hedgehog Pathway: A New Treatment for Basal Cell Carcinoma. Clin. Ther. 2012, 34, 2039–2050. [Google Scholar] [CrossRef]
- Berlin, J.; Bendell, J.C.; Hart, L.L.; Firdaus, I.; Gore, I.; Hermann, R.C.; Mulcahy, M.F.; Zalupski, M.M.; Mackey, H.M.; Yauch, R.L.; et al. A randomized phase II trial of vismodegib versus placebo with FOLFOX or FOLFIRI and bevacizumab in patients with previously untreated metastatic colorectal cancer. Clin. Cancer Res. 2013, 19, 258–267. [Google Scholar] [CrossRef]
- Dummer, R.; Guminksi, A.; Gutzmer, R.; Lear, J.T.; Lewis, K.D.; Chang, A.L.S.; Combemale, P.; Dirix, L.; Kaatz, M.; Kudchadkar, R.; et al. Long-term efficacy and safety of sonidegib in patients with advanced basal cell carcinoma: 42-month analysis of the phase II randomized, double-blind BOLT study. Br. J. Dermatol. 2020, 182, 1369–1378. [Google Scholar] [CrossRef] [PubMed]
- Dummer, R.; Ascierto, P.A.; Basset-Seguin, N.; Dréno, B.; Garbe, C.; Gutzmer, R.; Hauschild, A.; Krattinger, R.; Lear, J.T.; Malvehy, J.; et al. Sonidegib and vismodegib in the treatment of patients with locally advanced basal cell carcinoma: A joint expert opinion. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 1944–1956. [Google Scholar] [CrossRef] [PubMed]
- Brancaccio, G.; Pea, F.; Moscarella, E.; Argenziano, G. Sonidegib for the Treatment of Advanced Basal Cell Carcinoma. Front. Oncol. 2020, 10, 582866. [Google Scholar] [CrossRef] [PubMed]
- Nakae, J.; Kido, Y.; Accili, D. Distinct and overlapping functions of insulin and IGF-I receptors. Endocr. Rev. 2001, 22, 818–835. [Google Scholar] [CrossRef]
- Vigneri, P.G.; Tirrò, E.; Pennisi, M.S.; Massimino, M.; Stella, S.; Romano, C.; Manzella, L. The Insulin/IGF System in Colorectal Cancer Development and Resistance to Therapy. Front. Oncol. 2015, 5, 230. [Google Scholar] [CrossRef]
- Codony-Servat, J.; Cuatrecasas, M.; Asensio, E.; Montironi, C.; Martínez-Cardús, A.; Marín-Aguilera, M.; Horndler, C.; Martínez-Balibrea, E.; Rubini, M.; Jares, P.; et al. Nuclear IGF-1R predicts chemotherapy and targeted therapy resistance in metastatic colorectal cancer. Br. J. Cancer 2017, 117, 1777–1786. [Google Scholar] [CrossRef]
- Pollak, M. Insulin and insulin-like growth factor signalling in neoplasia. Nat. Rev. Cancer 2008, 8, 915–928. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Y.; Zhu, J.; Zheng, H.; Chen, S.; Chen, L.; Yang, H.-S. IGF-1R inhibition induces MEK phosphorylation to promote survival in colon carcinomas. Signal Transduct. Target. Ther. 2020, 5, 153. [Google Scholar] [CrossRef]
- Yuan, J.; Yin, Z.; Tao, K.; Wang, G.; Gao, J. Function of insulin-like growth factor 1 receptor in cancer resistance to chemotherapy. Oncol. Lett. 2018, 15, 41–47. [Google Scholar] [CrossRef]
- Jentzsch, V.; Osipenko, L.; Scannell, J.W.; Hickman, J.A. Costs and causes of oncology drug attrition with the example of insulin-like growth factor-1 receptor inhibitors. JAMA Netw. Open 2023, 6, e2324977. [Google Scholar] [CrossRef]
- Clevers, H. Wnt/β-Catenin Signaling in Development and Disease. Cell 2006, 127, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Wray, J.; Hartmann, C. WNTing embryonic stem cells. Trends Cell Biol. 2012, 22, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Veeman, M.T.; Axelrod, J.D.; Moon, R.T. A Second Canon: Functions and Mechanisms of β-Catenin-Independent Wnt Signaling. Dev. Cell 2003, 5, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xiao, Q.; Xiao, J.; Niu, C.; Li, Y.; Zhang, X.; Zhou, Z.; Shu, G.; Yin, G. Wnt/β-catenin signalling: Function, biological mechanisms, and therapeutic opportunities. Signal Transduct. Target. Ther. 2022, 7, 3. [Google Scholar] [CrossRef]
- MacDonald, B.T.; Tamai, K.; He, X. Wnt/β-Catenin Signaling: Components, Mechanisms, and Diseases. Dev. Cell 2009, 17, 9–26. [Google Scholar] [CrossRef]
- Disoma, C.; Zhou, Y.; Li, S.; Peng, J.; Xia, Z. Wnt/β-catenin signaling in colorectal cancer: Is therapeutic targeting even possible? Biochimie 2022, 195, 39–53. [Google Scholar] [CrossRef]
- Pai, S.G.; Carneiro, B.A.; Mota, J.M.; Costa, R.; Leite, C.A.; Barroso-Sousa, R.; Kaplan, J.B.; Chae, Y.K.; Giles, F.J. Wnt/β-catenin pathway: Modulating anticancer immune response. J. Hematol. Oncol. 2017, 10, 101. [Google Scholar] [CrossRef]
- Cheng, X.; Xu, X.; Chen, D.; Zhao, F.; Wang, W. Therapeutic potential of targeting the Wnt/β-catenin signaling pathway in colorectal cancer. Biomed. Pharmacother. 2019, 110, 473–481. [Google Scholar] [CrossRef]
- Polakis, P.; Hart, M.; Rubinfeld, B. Defects in the regulation of β-catenin in colorectal cancer. In Colon Cancer Prevention: Dietary Modulation of Cellular and Molecular Mechanisms; Springer: New York, NY, USA, 1999; pp. 23–32. [Google Scholar]
- Polakis, P. The oncogenic activation of β-catenin. Curr. Opin. Genet. Dev. 1999, 9, 15–21. [Google Scholar] [CrossRef]
- Baldus, S.E.; Mönig, S.P.; Huxel, S.; Landsberg, S.; Hanisch, F.-G.; Engelmann, K.; Schneider, P.M.; Thiele, J.; Hölscher, A.H.; Dienes, H.P. MUC1 and nuclear β-catenin are coexpressed at the invasion front of colorectal carcinomas and are both correlated with tumor prognosis. Clin. Cancer Res. 2004, 10, 2790–2796. [Google Scholar] [CrossRef]
- Mojarad, E.N.; Kashfi, S.M.H.; Mirtalebi, H.; Almasi, S.; Chaleshi, V.; Farahani, R.K.; Tarban, P.; Molaei, M.; Zali, M.R.; Kuppen, P.J. Prognostic Significance of Nuclear β-Catenin Expression in Patients with Colorectal Cancer from Iran. Iran. Red. Crescent Med. J. 2015, 17, e22324. [Google Scholar] [CrossRef][Green Version]
- He, T.-C.; Sparks, A.B.; Rago, C.; Hermeking, H.; Zawel, L.; Da Costa, L.T.; Morin, P.J.; Vogelstein, B.; Kinzler, K.W. Identification of c-MYC as a target of the APC pathway. Science 1998, 281, 1509–1512. [Google Scholar] [CrossRef] [PubMed]
- Tetsu, O.; McCormick, F. β-Catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature 1999, 398, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Brabletz, T.; Jung, A.; Dag, S.; Hlubek, F.; Kirchner, T. β-catenin regulates the expression of the matrix metalloproteinase-7 in human colorectal cancer. Am. J. Pathol. 1999, 155, 1033–1038. [Google Scholar] [CrossRef]
- Crawford, H.C.; Fingleton, B.M.; Rudolph-Owen, L.A.; Goss, K.J.H.; Rubinfeld, B.; Polakis, P.; Matrisian, L.M. The metalloproteinase matrilysin is a target of β-catenin transactivation in intestinal tumors. Oncogene 1999, 18, 2883–2891. [Google Scholar] [CrossRef]
- Spears, E.; Neufeld, K.L. Novel double-negative feedback loop between adenomatous polyposis coli and Musashi1 in colon epithelia. J. Biol. Chem. 2011, 286, 4946–4950. [Google Scholar] [CrossRef]
- Yang, A.D.; Fan, F.; Camp, E.R.; van Buren, G.; Liu, W.; Somcio, R.; Gray, M.J.; Cheng, H.; Hoff, P.M.; Ellis, L.M. Chronic oxaliplatin resistance induces epithelial-to-mesenchymal transition in colorectal cancer cell lines. Clin. Cancer Res. 2006, 12, 4147–4153. [Google Scholar] [CrossRef]
- Zhao, X.; Ma, Y.; Luo, J.; Xu, K.; Tian, P.; Lu, C.; Song, J. Blocking the WNT/β-catenin pathway in cancer treatment: Pharmacological targets and drug therapeutic potential. Heliyon 2024, 10, e35989. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, M.; Deng, K. Blocking the Wnt/β-catenin signaling pathway to treat colorectal cancer: Strategies to improve current therapies (Review). Int. J. Oncol. 2023, 62, 24. [Google Scholar] [CrossRef]
- Artavanis-Tsakonas, S.; Rand, M.D.; Lake, R.J. Notch signaling: Cell fate control and signal integration in development. Science 1999, 284, 770–776. [Google Scholar] [CrossRef]
- Katoh, M.; Katoh, M. Notch signaling in gastrointestinal tract (review). Int. J. Oncol. 2007, 30, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Li, J.L.; Harris, A.L. Notch signaling from tumor cells: A new mechanism of angiogenesis. Cancer Cell 2005, 8, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Meng, R.D.; Shelton, C.C.; Li, Y.-M.; Qin, L.-X.; Notterman, D.; Paty, P.B.; Schwartz, G.K. γ-Secretase inhibitors abrogate oxaliplatin-induced activation of the Notch-1 signaling pathway in colon cancer cells resulting in enhanced chemosensitivity. Cancer Res. 2009, 69, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, L.s.; Santos, S.; Inglés-Esteve, J.; Muñoz-Canoves, P.; Bigas, A. p65-NFκB synergizes with Notch to activate transcription by triggering cytoplasmic translocation of the nuclear receptor corepressor N-CoR. J. Cell Sci. 2002, 115, 1295–1303. [Google Scholar] [CrossRef]
- Liu, Z.-J.; Xiao, M.; Balint, K.; Soma, A.; Pinnix, C.C.; Capobianco, A.J.; Velazquez, O.C.; Herlyn, M. Inhibition of endothelial cell proliferation by Notch1 signaling is mediated by repressing MAPK and PI3K/Akt pathways and requires MAML1. FASEB J. 2006, 20, 1009–1011. [Google Scholar] [CrossRef]
- Wang, Z.; Banerjee, S.; Li, Y.; Rahman, K.W.; Zhang, Y.; Sarkar, F.H. Down-regulation of Notch-1 inhibits invasion by inactivation of nuclear factor-κB, vascular endothelial growth factor, and matrix metalloproteinase-9 in pancreatic cancer cells. Cancer Res. 2006, 66, 2778–2784. [Google Scholar] [CrossRef]
- Koduru, S.; Kumar, R.; Srinivasan, S.; Evers, M.B.; Damodaran, C. Notch-1 inhibition by Withaferin-A: A therapeutic target against colon carcinogenesis. Mol. Cancer Ther. 2010, 9, 202–210. [Google Scholar] [CrossRef]
- Leow, C.C.; Romero, M.S.; Ross, S.; Polakis, P.; Gao, W.Q. Hath1, down-regulated in colon adenocarcinomas, inhibits proliferation and tumorigenesis of colon cancer cells. Cancer Res. 2004, 64, 6050–6057. [Google Scholar] [CrossRef]
- Moore, G.; Annett, S.; McClements, L.; Robson, T. Top Notch Targeting Strategies in Cancer: A Detailed Overview of Recent Insights and Current Perspectives. Cells 2020, 9, 1503. [Google Scholar] [CrossRef]
- Li, X.; Yan, X.; Wang, Y.; Kaur, B.; Han, H.; Yu, J. The Notch signaling pathway: A potential target for cancer immunotherapy. J. Hematol. Oncol. 2023, 16, 45. [Google Scholar] [CrossRef]
- Jung, B.; Staudacher, J.J.; Beauchamp, D. Transforming Growth Factor β Superfamily Signaling in Development of Colorectal Cancer. Gastroenterology 2017, 152, 36–52. [Google Scholar] [CrossRef] [PubMed]
- Itatani, Y.; Kawada, K.; Sakai, Y. Transforming Growth Factor-β Signaling Pathway in Colorectal Cancer and Its Tumor Microenvironment. Int. J. Mol. Sci. 2019, 20, 5822. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wu, Y.; Tian, T. TGF-β Signaling in Metastatic Colorectal Cancer (mCRC): From Underlying Mechanism to Potential Applications in Clinical Development. Int. J. Mol. Sci. 2022, 23, 14436. [Google Scholar] [CrossRef] [PubMed]
- Tauriello, D.V.F.; Sancho, E.; Batlle, E. Overcoming TGFβ-mediated immune evasion in cancer. Nat. Rev. Cancer 2022, 22, 25–44. [Google Scholar] [CrossRef]
- Barcellos-Hoff, M.H.; Gulley, J.L. Molecular Pathways and Mechanisms of TGFβ in Cancer Therapy. Clin. Cancer Res. 2023, 29, 2025–2033. [Google Scholar] [CrossRef]
- Sadeghi Rad, H.; Monkman, J.; Warkiani, M.E.; Ladwa, R.; O’Byrne, K.; Rezaei, N.; Kulasinghe, A. Understanding the tumor microenvironment for effective immunotherapy. Med. Res. Rev. 2021, 41, 1474–1498. [Google Scholar] [CrossRef]
- Shiravand, Y.; Khodadadi, F.; Kashani, S.M.A.; Hosseini-Fard, S.R.; Hosseini, S.; Sadeghirad, H.; Ladwa, R.; O’Byrne, K.; Kulasinghe, A. Immune Checkpoint Inhibitors in Cancer Therapy. Curr. Oncol. 2022, 29, 3044–3060. [Google Scholar] [CrossRef]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef]
- Borelli, B.; Antoniotti, C.; Carullo, M.; Germani, M.M.; Conca, V.; Masi, G. Immune-Checkpoint Inhibitors (ICIs) in Metastatic Colorectal Cancer (mCRC) Patients beyond Microsatellite Instability. Cancers 2022, 14, 4974. [Google Scholar] [CrossRef]
- Liebl, M.C.; Hofmann, T.G. Identification of responders to immune checkpoint therapy: Which biomarkers have the highest value? J. Eur. Acad. Dermatol. Venereol. 2019, 33 (Suppl. S8), 52–56. [Google Scholar] [CrossRef] [PubMed]
- Moon, B.; Go, A.; Park, S.; Kim, H.J.; An, D.; Kim, J.; Lee, J.Y.; Kim, J.H.; Hwang, J.Y.; Kim, J.A. Discovery of novel WRN inhibitors for treating MSI-H colorectal cancers. Bioorg. Med. Chem. Lett. 2025, 120, 130141. [Google Scholar] [CrossRef] [PubMed]
- Picco, G.; Rao, Y.; Al Saedi, A.; Lee, Y.; Vieira, S.F.; Bhosle, S.; May, K.; Herranz-Ors, C.; Walker, S.J.; Shenje, R.; et al. Novel WRN Helicase Inhibitors Selectively Target Microsatellite-Unstable Cancer Cells. Cancer Discov. 2024, 14, 1457–1475. [Google Scholar] [CrossRef] [PubMed]
- Bhamidipati, D.; Subbiah, V. Tumor-agnostic drug development in dMMR/MSI-H solid tumors. Trends Cancer 2023, 9, 828–839. [Google Scholar] [CrossRef]
- Liebs, S.; Keilholz, U.; Kehler, I.; Schweiger, C.; Haybäck, J.; Nonnenmacher, A. Detection of mutations in circulating cell-free DNA in relation to disease stage in colorectal cancer. Cancer Med. 2019, 8, 3761–3769. [Google Scholar] [CrossRef]
- Ponomaryova, A.A.; Rykova, E.Y.; Solovyova, A.I.; Tarasova, A.S.; Kostromitsky, D.N.; Dobrodeev, A.Y.; Afanasiev, S.A.; Cherdyntseva, N.V. Genomic and Transcriptomic Research in the Discovery and Application of Colorectal Cancer Circulating Markers. Int. J. Mol. Sci. 2023, 24, 12407. [Google Scholar] [CrossRef]
| Colorectal Cancer Stage | Survival Rate After Surgery |
|---|---|
| Stage I | 95–99% |
| Stage II | 68–83% |
| Stage III | 45–65% |
| Drug Used for Colorectal Cancer | Mode of Action | Response Rate | Year of Approval | |
|---|---|---|---|---|
| 1. | 5-Fluorouracil (5-FU) | 5-FU exerts its anticancer effects by inhibiting thymidylate synthase (TS) and incorporating its metabolites into RNA and DNA [17]. | 54% [18] | 1962 |
| 2. | Capecitabine (Xeloda) | Capecitabine was developed as a prodrug of FU, inhibiting thymidylate synthetase [19]. | 51–76% [20] | 1998 |
| 3. | Irinotecan (Camptosar) | Irinotecan is a prodrug that inhibits DNA topoisomerase I [21]. | 19–32% [22] | 1996 |
| 4. | Oxaliplatin (Eloxatin) | DNA replication and transcription disruption through intrastrand links between two adjacent guanine residues or a guanine and an adenine [23]. | 12–24% [24] | 2002 |
| 5. | Trifluridine and tipiracil (Lonsurf) | TFD is a nucleoside analog; it is incorporated into replicating DNA strands, where it inhibits DNA synthesis and further cellular proliferation. TPI is an inhibitor of the enzyme thymidine phosphorylase, which is responsible for the breakdown of the active trifluridine component; thus, TPI boosts the levels of TFD [25]. | 40% [26] | 2015 |
| Drugs Targeted Against HGF/CMet | Mode of Action | Response Rate | Year of Approval | |
|---|---|---|---|---|
| 1. | Cabozantinib | Cabozantinib blocks c-Met, which in turn deactivates the SHH pathway, leading to a reduction in CRC tumor growth and angiogenesis [77]. Cabozantinib is a multi-tyrosine kinase inhibitor, utilized to induce p53 upregulated modulator of apoptosis (PUMA). | 27.6% [78] | 2021 |
| 2. | Crizotinib | Crizotinib targets ALK, ROS1, and the Met/hepatocyte growth-factor receptor (HGFR), and is used as an effective treatment against mCRC [79]. | 2011 |
| Drugs Targeted Against Hedgehog | Mode of Action | Response Rate | Year of Approval | |
|---|---|---|---|---|
| 1. | Vismodegib | Prevents Hh signaling by attaching to Smo and blocking the activation of downstream Hh target genes that inhibit the proliferation of tumors [91]. | 46% [92] | 2012 |
| 2. | Sonidegib | Prevents Hh signaling by attaching to Smo and blocking the activation of downstream Hh target genes that inhibit cancer; also used as an alternative to vismodegib [93,94]. | 47.6% [95] | 2015 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaham, S.H.; Vij, P.; Tripathi, M.K. Advances in Targeted and Chemotherapeutic Strategies for Colorectal Cancer: Current Insights and Future Directions. Biomedicines 2025, 13, 642. https://doi.org/10.3390/biomedicines13030642
Shaham SH, Vij P, Tripathi MK. Advances in Targeted and Chemotherapeutic Strategies for Colorectal Cancer: Current Insights and Future Directions. Biomedicines. 2025; 13(3):642. https://doi.org/10.3390/biomedicines13030642
Chicago/Turabian StyleShaham, Salique H., Puneet Vij, and Manish K. Tripathi. 2025. "Advances in Targeted and Chemotherapeutic Strategies for Colorectal Cancer: Current Insights and Future Directions" Biomedicines 13, no. 3: 642. https://doi.org/10.3390/biomedicines13030642
APA StyleShaham, S. H., Vij, P., & Tripathi, M. K. (2025). Advances in Targeted and Chemotherapeutic Strategies for Colorectal Cancer: Current Insights and Future Directions. Biomedicines, 13(3), 642. https://doi.org/10.3390/biomedicines13030642








