Nomogram Predicting In-Hospital Mortality in Patients with Myocardial Infarction Treated with Primary Coronary Interventions Based on Logistic and Angiographic Predictors
Abstract
:1. Introduction
2. Materials and Methods
2.1. ACS GRU Registry
2.2. Study Population
2.3. Selection of Variables
- A panel of general variables: sex, patient age, and STEMI/NSTEMI/UA;
- A panel of angiographic variables: vascular access, extent of coronary atherosclerotic lesions—result of coronary angiography (CAG), PCI on the left main or proximal left anterior descending artery (PCI LM/prox LAD), restenosis in a DES in the infarct-related artery (IRA), Thrombolysis in Myocardial Infarction (TIMI) flow grade before and after PCI, pre-dilatation with balloon (semi-compliant, SC; or non-compliant, NC), post-dilatation with NC balloon after stent implantation, bifurcation PCI, coronary artery calcification in IRA, cardiopulmonary resuscitation (CPR) during PCI, and unsuccessful PCI;
- A panel of variables related to the logistical aspects of the MI treatment process: type of presentation, time of hospital admission, and time of PCI.
2.4. Endpoint and Definitions
- Admitted directly from home/a public place (patient was transferred by the EMS team or arrived at the Emergency Department on their own);
- Transferred from another hospital;
- Transferred from another department of the parent hospital to which they were previously admitted for another condition.
2.5. Statistical Analyses
3. Results
4. Discussion
- Angiographic variables, such as CAG results and suboptimal flow after PCI, were important predictors of in-hospital mortality;
- Logistical aspects of the MI treatment process, such as the time of PCI and the mode of presentation of patients with MI, contributed to in-hospital mortality;
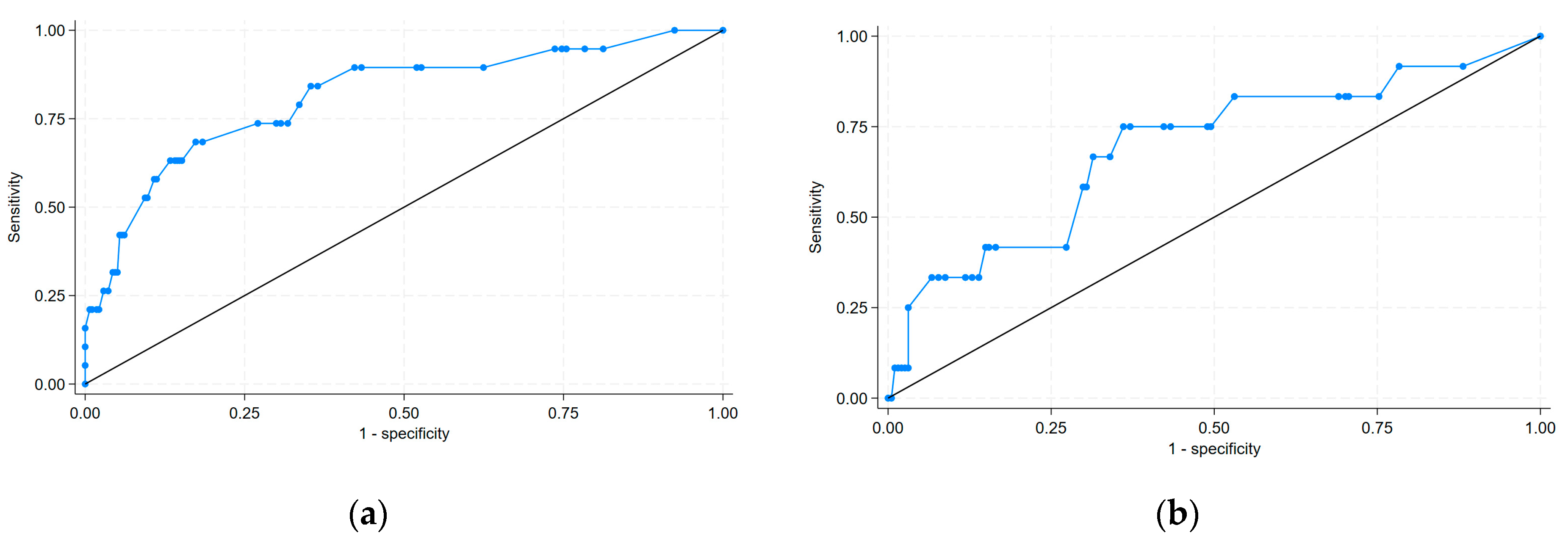
- Discrimination was good in both the derivation and validation sets.
4.1. Angiographic Variables
4.2. Logistic Variables
4.3. Limitations
- Considering the retrospective nature of this study, some unknown factors were prone to data deviation and led to inevitable bias;
- The present investigation was a single-centre study with a small sample size. The risk factors included in this study were not comprehensive, and bias could not be avoided;
- In terms of model validation, only internal validation was performed;
- Due to the small size of the population (and the random allocation of the patients to the derivation and validation sets), there were no patients in the derivation set who underwent PCI on the weekend between 22:00 and 8:00.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MDPI | Multidisciplinary Digital Publishing Institute |
| CA | Cardiac arrest |
| CL | Catheterisation laboratory |
| CPR | Cardiopulmonary resuscitation |
| DES | Drug eluting stent |
| ESC | European Society of Cardiology |
| EMS | Emergency Medical Services |
| IRA | Infarction-related artery |
| LAD | Left anterior descending artery |
| LM | Left main |
| NSTEMI | Non-ST-elevation myocardial infarction |
| NC | Non-compliant |
| PCI | Primary coronary intervention |
| SC | Semi-compliant |
| STEMI | ST-elevation myocardial infarction |
| TIMI | Thrombolysis in Myocardial Infarction flow grade |
| UA | Unstable angina |
References
- Timmis, A.; Vardas, P.; Townsend, N.; Torbica, A.; Katus, H.; De Smedt, D.; Gale, C.P.; Maggioni, A.P.; Petersen, S.E.; Huculeci, R.; et al. European Society of Cardiology: Cardiovascular Disease Statistics 2021. Eur. Heart J. 2022, 43, 716–799. [Google Scholar] [CrossRef] [PubMed]
- Kalra, A.; Rehman, H.; Khera, S.; Thyagarajan, B.; Bhatt, D.L.; Kleiman, N.S.; Yeh, R.W. New-Generation Coronary Stents: Current Data and Future Directions. Curr. Atheroscler. Rep. 2017, 19, 14. [Google Scholar] [CrossRef]
- Wallentin, L.; Becker, R.C.; Budaj, A.; Cannon, C.P.; Emanuelsson, H.; Held, C.; Horrow, J.; Husted, S.; James, S.; Katus, H.; et al. Ticagrelor versus Clopidogrel in Patients with Acute Coronary Syndromes. N. Engl. J. Med. 2009, 361, 1045–1057. [Google Scholar] [CrossRef]
- Gawinski, L.; Burzynska, M.; Kamecka, K.; Kozlowski, R. Practical Aspects of the Use of Telematic Systems in the Diagnosis of Acute Coronary Syndrome in Poland. Medicina 2022, 58, 554. [Google Scholar] [CrossRef]
- Multicenter Postinfarction Research Group. Risk Stratification and Survival after Myocardial Infarction. N. Engl. J. Med. 1983, 309, 331–336. [Google Scholar] [CrossRef]
- Granger, C.B.; Goldberg, R.J.; Dabbous, O.; Pieper, K.S.; Eagle, K.A.; Cannon, C.P.; Van De Werf, F.; Avezum, A.; Goodman, S.G.; Flather, M.D.; et al. Predictors of Hospital Mortality in the Global Registry of Acute Coronary Events. Arch. Intern. Med. 2003, 163, 2345–2353. [Google Scholar] [CrossRef] [PubMed]
- Timóteo, A.T.; Aguiar Rosa, S.; Afonso Nogueira, M.; Belo, A.; Cruz Ferreira, R. ProACS Risk Score: An Early and Simple Score for Risk Stratification of Patients with Acute Coronary Syndromes. Rev. Port. Cardiol. 2017, 36, 77–83. [Google Scholar] [CrossRef] [PubMed]
- McNamara, R.L.; Kennedy, K.F.; Cohen, D.J.; Diercks, D.B.; Moscucci, M.; Ramee, S.; Wang, T.Y.; Connolly, T.; Spertus, J.A. Predicting In-Hospital Mortality in Patients with Acute Myocardial Infarction. J. Am. Coll. Cardiol. 2016, 68, 626–635. [Google Scholar] [CrossRef]
- Chin, C.T.; Chen, A.Y.; Wang, T.Y.; Alexander, K.P.; Mathews, R.; Rumsfeld, J.S.; Cannon, C.P.; Fonarow, G.C.; Peterson, E.D.; Roe, M.T. Risk Adjustment for In-Hospital Mortality of Contemporary Patients with Acute Myocardial Infarction: The Acute Coronary Treatment and Intervention Outcomes Network (ACTION) Registry-Get with the Guidelines (GWTG) Acute Myocardial Infarction Mortality Model and Risk Score. Am. Heart J. 2011, 161, 113–122.e2. [Google Scholar] [CrossRef]
- de Mulder, M.; Gitt, A.; van Domburg, R.; Hochadel, M.; Seabra-Gomes, R.; Serruys, P.W.; Silber, S.; Weidinger, F.; Wijns, W.; Zeymer, U.; et al. EuroHeart Score for the Evaluation of In-Hospital Mortality in Patients Undergoing Percutaneous Coronary Intervention. Eur. Heart J. 2011, 32, 1398–1408. [Google Scholar] [CrossRef]
- Peterson, E.D.; Dai, D.; DeLong, E.R.; Brennan, J.M.; Singh, M.; Rao, S.V.; Shaw, R.E.; Roe, M.T.; Ho, K.K.L.; Klein, L.W.; et al. Contemporary Mortality Risk Prediction for Percutaneous Coronary Intervention: Results from 588,398 Procedures in the National Cardiovascular Data Registry. J. Am. Coll. Cardiol. 2010, 55, 1923–1932. [Google Scholar] [CrossRef] [PubMed]
- Gao, N.; Qi, X.; Dang, Y.; Li, Y.; Wang, G.; Liu, X.; Zhu, N.; Fu, J. Establishment and Validation of a Risk Model for Prediction of In-Hospital Mortality in Patients with Acute ST-Elevation Myocardial Infarction after Primary PCI. BMC Cardiovasc. Disord. 2020, 20, 513. [Google Scholar] [CrossRef] [PubMed]
- de Castro, P.P.N.; Castro, M.A.N.; Nascimento, G.A.; Moura, I.; Pena, J.L.B. Predictors of Hospital Mortality Based on Primary Angioplasty Treatment: A Multicenter Case-Control Study. Arq. Bras. Cardiol. 2022, 119, 448–457. [Google Scholar] [CrossRef]
- Gawinski, L.; Burzynska, M.; Marczak, M.; Kozlowski, R. Assessment of In-Hospital Mortality and Its Risk Factors in Patients with Myocardial Infarction Considering the Logistical Aspects of the Treatment Process-A Single-Center, Retrospective, Observational Study. Int. J. Environ. Res. Public. Health 2023, 20, 3603. [Google Scholar] [CrossRef]
- Gawinski, L.; Engelseth, P.; Kozlowski, R. Application of Modern Clinical Risk Scores in the Global Assessment of Risks Related to the Diagnosis and Treatment of Acute Coronary Syndromes in Everyday Medical Practice. Int. J. Environ. Res. Public. Health 2021, 18, 9103. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D. Fourth Universal Definition of Myocardial Infarction (2018). J. Am. Coll. Cardiol. 2018, 72, 2231–2264. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- TIMI Study Group. The Thrombolysis in Myocardial Infarction (TIMI) Trial. Phase I Findings. N. Engl. J. Med. 1985, 312, 932–936. [Google Scholar] [CrossRef]
- Madhavan, M.V.; Tarigopula, M.; Mintz, G.S.; Maehara, A.; Stone, G.W.; Généreux, P. Coronary Artery Calcification: Pathogenesis and Prognostic Implications. J. Am. Coll. Cardiol. 2014, 63, 1703–1714. [Google Scholar] [CrossRef]
- Swets, J.A. Measuring the Accuracy of Diagnostic Systems. Science 1988, 240, 1285–1293. [Google Scholar] [CrossRef]
- Lemeshow, S.; Hosmer, D.W. A Review of Goodness of Fit Statistics for Use in the Development of Logistic Regression Models. Am. J. Epidemiol. 1982, 115, 92–106. [Google Scholar] [CrossRef]
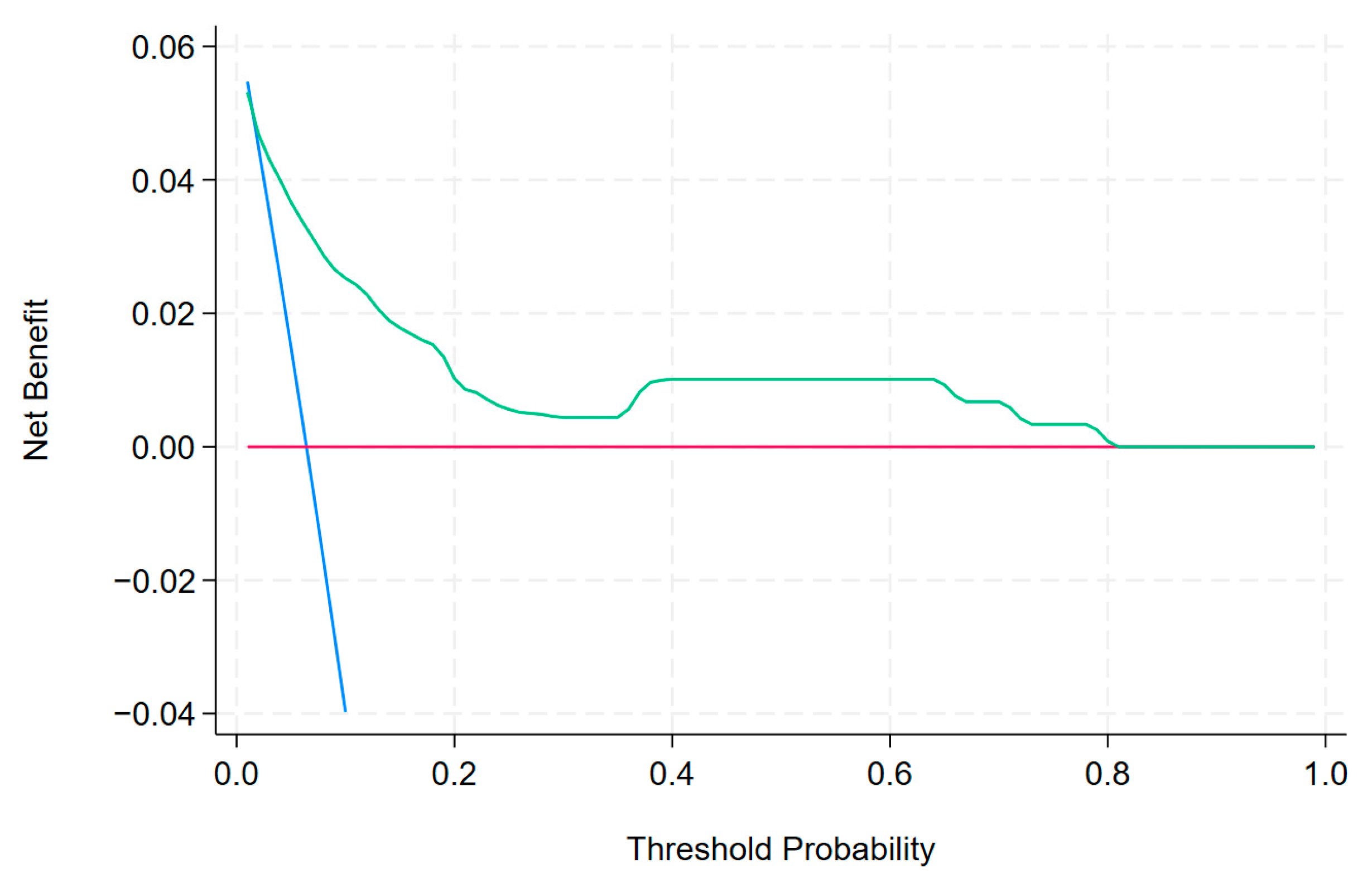
- Vickers, A.J.; Elkin, E.B. Decision Curve Analysis: A Novel Method for Evaluating Prediction Models. Med. Decis. Mak. 2006, 26, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Puymirat, E.; Simon, T.; Cayla, G.; Cottin, Y.; Elbaz, M.; Coste, P.; Lemesle, G.; Motreff, P.; Popovic, B.; Khalife, K.; et al. Acute Myocardial Infarction: Changes in Patient Characteristics, Management, and 6-Month Outcomes Over a Period of 20 Years in the FAST-MI Program (French Registry of Acute ST-Elevation or Non-ST-Elevation Myocardial Infarction) 1995 to 2015. Circulation 2017, 136, 1908–1919. [Google Scholar] [CrossRef]
- Tasar, O.; Karabay, A.K.; Oduncu, V.; Kirma, C. Predictors and Outcomes of No-Reflow Phenomenon in Patients with Acute ST-Segment Elevation Myocardial Infarction Undergoing Primary Percutaneous Coronary Intervention. Coron. Artery Dis. 2019, 30, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Liu, Y.; Wang, W.; Chen, J.; Yang, J.; Wen, J.; Gao, J.; Shao, C.; Tang, Y.-D. A Nomogram Predicting 30-Day Mortality in Patients Undergoing Percutaneous Coronary Intervention. Front. Cardiovasc. Med. 2022, 9, 897020. [Google Scholar] [CrossRef]
- Montone, R.A.; Camilli, M.; Buono, M.G.D.; Meucci, M.C.; Gurgoglione, F.; Russo, M.; Crea, F.; Niccoli, G. “No-reflow”: Update su diagnosi, fisiopatologia e strategie terapeutiche. G. Ital. Cardiol. 2020, 21, 4–14. [Google Scholar]
- Yu, Y.-Y.; Zhao, B.-W.; Ma, L.; Dai, X.-C. Association Between Out-of-Hour Admission and Short- and Long-Term Mortality in Acute Myocardial Infarction: A Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 2021, 8, 752675. [Google Scholar] [CrossRef]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.-A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the Management of Acute Coronary Syndromes. Eur. Heart J. Acute Cardiovasc. Care 2024, 13, 55–161. [Google Scholar] [CrossRef]
- Tokarek, T.; Dziewierz, A.; Plens, K.; Rakowski, T.; Jaroszyńska, A.; Bartuś, S.; Siudak, Z. Percutaneous Coronary Intervention during On- and off-Hours in Patients with ST-Segment Elevation Myocardial Infarction. Hell. J. Cardiol. 2021, 62, 212–218. [Google Scholar] [CrossRef]
- Eggers, K.M.; James, S.K.; Jernberg, T.; Lindahl, B. Timing of Coronary Angiography in Patients with Non-ST-Elevation Acute Coronary Syndrome: Long-Term Clinical Outcomes from the Nationwide SWEDEHEART Registry. EuroIntervention 2022, 18, 582–589. [Google Scholar] [CrossRef]
- Jollis, J.G.; Granger, C.B.; Zègre-Hemsey, J.K.; Henry, T.D.; Goyal, A.; Tamis-Holland, J.E.; Roettig, M.L.; Ali, M.J.; French, W.J.; Poudel, R.; et al. Treatment Time and In-Hospital Mortality Among Patients with ST-Segment Elevation Myocardial Infarction, 2018–2021. JAMA 2022, 328, 2033–2040. [Google Scholar] [CrossRef] [PubMed]
- Smilowitz, N.R.; Gupta, N.; Guo, Y.; Bangalore, S. Comparison of Outcomes of Patients with Sepsis With Versus Without Acute Myocardial Infarction and Comparison of Invasive Versus Noninvasive Management of the Patients With Infarction. Am. J. Cardiol. 2016, 117, 1065–1071. [Google Scholar] [CrossRef] [PubMed]


| Variables | Derivation Set (n = 359) | Validation Set (n = 244) | ||
|---|---|---|---|---|
| Dead (n = 20) | Alive (n = 339) | Dead (n = 15) | Alive (n = 229) | |
| General factors: | ||||
| Age (per 10-year increase) | ||||
| <40 years old | 20.00% (1) | 80.00% (4) | 0% (0) | 100% (3) |
| 40–49 years old | 9.09% (1) | 90.91% (10) | 0% (0) | 100% (9) |
| 50–59 years old | 0% (0) | 100% (72) | 8.70% (4) | 91.30% (42) |
| 60–69 years old | 4.62% (6) | 95.38% (124) | 3.30% (3) | 96.70% (88) |
| 70–79 years old | 6.74% (6) | 93.26% (83) | 8.00% (4) | 92.00% (46) |
| 89–89 years old | 10.64% (5) | 89.36% (42) | 6.98% (3) | 93.02% (40) |
| ≥90 years old | 20.00% (1) | 80.00% (4) | 50.00% (1) | 50.00% (1) |
| Women | 3.42% (4) | 96.58% (113) | 9.41% (8) | 90.59% (77) |
| Men | 6.61% (16) | 93.39% (226) | 4.40% (7) | 95.60% (152) |
| STEMI | 8.06% (10) | 91.94% (114) | 8.60% (8) | 91.40% (85) |
| NSTEMI/UA | 4.26% (10) | 95.74% (225) | 4.64% (7) | 95.36% (144) |
| Angiographic factors: | ||||
| Vascular access: | ||||
| Right radial artery | 4.03% (12) | 95.97% (286) | 4.31% (9) | 95.69% (200) |
| Left radial artery | 13.79% (8) | 86.21% (50) | 18.18% (6) | 81.82% (27) |
| Right/left femoral artery | 0% (0) | 100% (3) | 0% (0) | 100% (2) |
| Result of coronarography study: | ||||
| One-vessel disease | 2.33% (2) | 97.67% (84) | 6.90% (4) | 93.10% (54) |
| Double-vessel disease | 3.26% (3) | 96.74% (89) | 1.92% (1) | 98.08% (51) |
| Multi-vessel disease with affected LM | 6.56% (8) | 93.44% (114) | 3.41% (3) | 96.59% (85) |
| Multi-vessel disease without affected LM | 13.95% (6) | 86.05% (37) | 16.13% (5) | 83.87% (26) |
| PCI on LM/proximal LAD | 11.90% (10) | 86.10% (74) | 9.1% (4) | 90.9% (40) |
| Restenosis in a DES in IRA | 6.25% (1) | 93.75% (15) | 16.67% (1) | 83.33% (5) |
| Post-PCI TIMI flow grades 0–1 | 44.44% (4) | 55.56% (5) | 50.00% (2) | 50.00% (2) |
| Post-PCI TIMI flow grades 2–3 | 4.95% (15) | 95.05% (288) | 4.95% (10) | 95.05% (192) |
| Pre-dilatation with balloon (SC or NC) | 6.15% (16) | 93.85% (244) | 5.75% (10) | 94.25% (164) |
| Post-dilatation with NC balloon | 3.69% (8) | 96.31% (209) | 3.95% (6) | 96.05% (146) |
| PCI in bifurcation | 2.38% (1) | 97.62% (41) | 4.00% (1) | 96.00% (24) |
| Coronary artery calcifications in IRA | 12.5% (6) | 87.5% (42) | 6.67% (2) | 93.33% (28) |
| CA with subsequent CPR (CL stage) | 50.00% (4) | 50.00% (4) | 50.00% (2) | 50.00% (2) |
| Unsuccessful PCI | 50.00% (4) | 50.00% (4) | 50.00% (2) | 50.00% (2) |
| Logistical factors: | ||||
| Mode of presentation | ||||
| Admission from home/public place | 4.07% (12) | 95.93% (283) | 6.73% (14) | 93.27% (194) |
| Admission from another hospital | 8.33% (4) | 91.67% (44) | 0.00% (0) | 100% (28) |
| Admission from another department | 25.00% (4) | 75.00% (12) | 12.50% (1) | 87.50% (7) |
| Time of hospital admission: | ||||
| Weekday from 8:00 to 14:00 | 2.21% (3) | 97.79% (133) | 3.49% (3) | 96.51% (83) |
| Weekday from 14:00 to 22:00 | 11.96% (11) | 88.04% (81) | 5.56% (3) | 94.44% (51) |
| Weekday from 22:00 to 8:00 | 6.82% (3) | 93.18% (41) | 7.69% (3) | 92.31% (36) |
| Public holiday from 8:00 to 22:00 | 3.57% (2) | 96.43% (54) | 2.33% (1) | 97.67% (42) |
| Public holiday from 22:00 to 8:00 | 3.23% (1) | 96.77% (30) | 22.73% (5) | 77.27% (17) |
| Time of PCI: | ||||
| Weekday from 8:00 to 14:00 | 1.59% (2) | 98.41% (124) | 5.49% (5) | 94.51% (86) |
| Weekday from 14:00 to 22:00 | 8.40% (11) | 91.60% (120) | 4.17% (3) | 95.83% (69) |
| Weekday from 22:00 to 8:00 | 14.29% (4) | 85.71% (24) | 15.00% (3) | 85.00% (17) |
| Public holiday from 8:00 to 22:00 | 5.26% (3) | 94.74% (54) | 4.55% (2) | 95.45% (42) |
| Public holiday from 22:00 to 8:00 | 0% (0) | 100% (17) | 11.76% (2) | 88.24% (15) |
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| Variables | OR | 95% CI | p Value | OR | 95% CI | p Value |
| General factors: | ||||||
| STEMI | 1.519 | 0.60–3.85 | 0.379 | |||
| Women | 0.52 | 0.17–1.62 | 0.260 | |||
| Age (per 10-year increase) | 1.50 | 0.99–2.28 | 0.05 | |||
| Angiographic factors: | ||||||
| Vascular access: | ||||||
| Right radial artery (ref) | ||||||
| Left radial artery | 1.50 | 0.99–2.28 | 0.055 | |||
| Right/left femoral artery | / | / | / | |||
| Result of coronarography study: | ||||||
| One-vessel disease (ref) | ||||||
| Double-vessel disease | 1.37 | 0.22–8.46 | 0.729 | 1.06 | 0.16–7.15 | 0.953 |
| Multi-vessel disease with affected LM | 3.22 | 0.66–15.62 | 0.146 | 2.49 | 0.48–13.08 | 0.279 |
| Multi-vessel disease without affected LM | 7.90 | 1.51–41.32 | 0.014 | 7.68 | 1.34–43.93 | 0.022 |
| PCI on LM/proximal LAD | 3.33 | 1.30–8.52 | 0.012 | |||
| Restenosis in a DES in IRA | 1.04 | 0.13–8.33 | 0.970 | |||
| Post-PCI TIMI flow grades 2–3 (ref) | ||||||
| Post-PCI TIMI flow grades 0–1 | 15.36 | 3.73– 63.13 | 0.000 | 9.84 | 1.93–50.19 | 0.006 |
| Pre-dilatation with balloon (SC or NC) | 3.22 | 0.42–24.90 | 0.261 | |||
| Post-dilatation with NC balloon | 0.36 | 0.13–0.96 | 0.042 | |||
| PCI in bifurcation | 0.34 | 0.04–2.62 | 0.300 | |||
| Coronary artery calcifications in IRA | 2.76 | 0.99–7.66 | 0.051 | |||
| CA with subsequent CPR (CL stage) | 13.55 | 2.79–65.72 | 0.001 | |||
| Unsuccessful PCI | 19.27 | 4.39–84.63 | 0.000 | |||
| Logistical factors: | ||||||
| Mode of presentation: | ||||||
| Admission from home/public place (ref) | ||||||
| Admission from another hospital | 2.15 | 0.66–7.04 | 0.202 | 2.23 | 0.63–7.98 | 0.216 |
| Admission from another department | 6.83 | 1.64–28.54 | 0.008 | 9.13 | 1.75–47.52 | 0.009 |
| Time of hospital admission: | ||||||
| Weekday from 8:00 to 14:00 | ||||||
| Weekday from 14:00 to 22:00 | 5.185 | 1.38–19.48 | 0.015 | |||
| Weekday from 22:00 to 8:00 | 3.29 | 0.63–17.08 | 0.156 | |||
| Public holiday from 8:00 to 22:00 | 1.55 | 0.25–9.61 | 0.634 | |||
| Public holiday from 22:00 to 8:00 | 1.38 | 0.14–13.81 | 0.783 | |||
| Time of PCI: | ||||||
| Weekday from 8:00 to 14:00 | ||||||
| Weekday from 14:00 to 22:00 | 4.90 | 1.05–22.93 | 0.043 | 3.16 | 0.63–15.83 | 0.162 |
| Weekday from 22:00 to 8:00 | 9.18 | 1.58–53.31 | 0.013 | 6.67 | 1.04–42.63 | 0.045 |
| Public holiday from 8:00 to 22:00 | 2.97 | 0.48–18.34 | 0.241 | 1.42 | 0.20–10.14 | 0.728 |
| Public holiday from 22:00 to 8:00 | / | / | / | / | / | / |
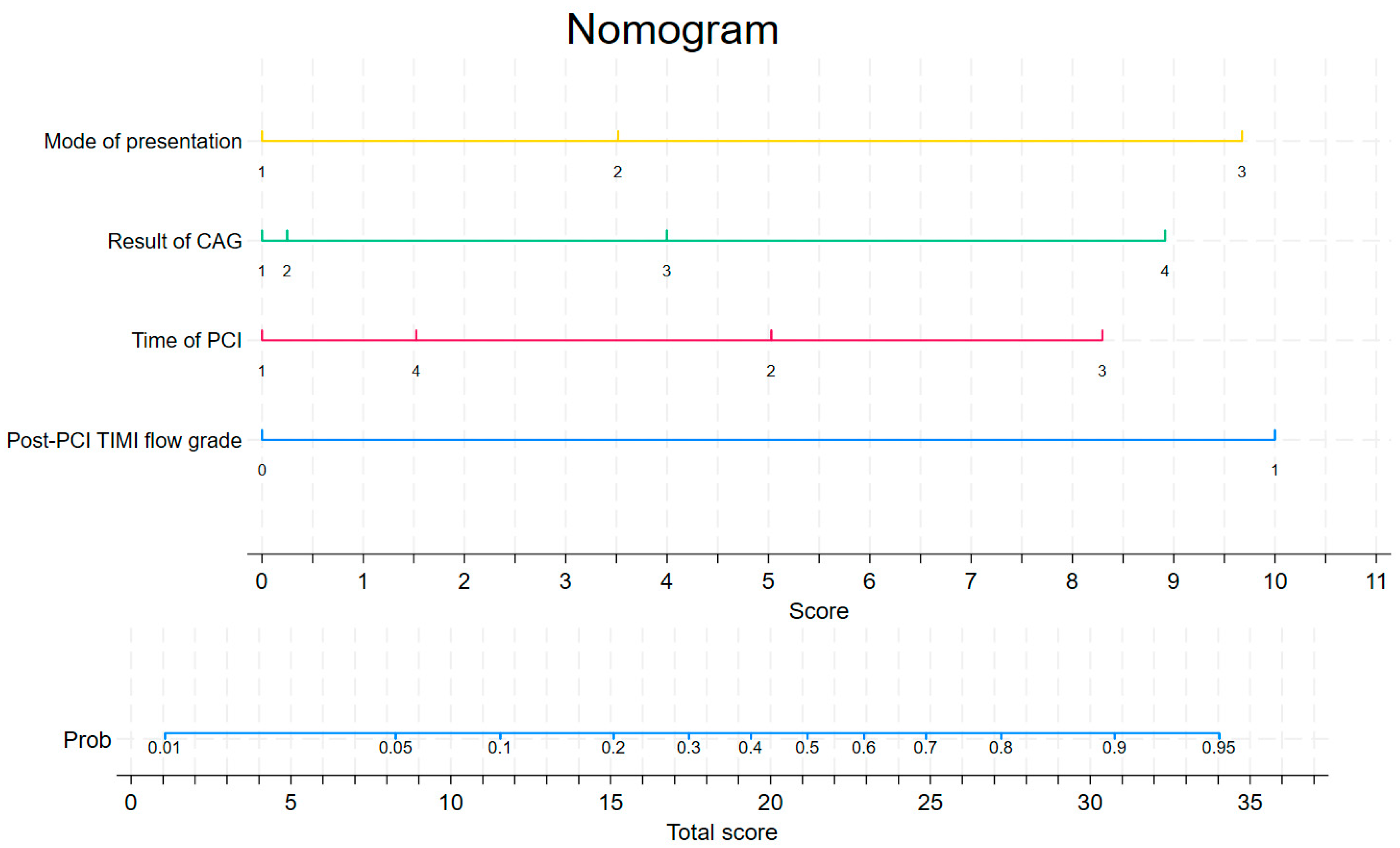
| Mode of Presentation | Score |
|---|---|
| from home/public place | 0 |
| from another hospital | 3.5 |
| from another department | 9.7 |
| Result of coronarography study | Score |
| one-vessel disease | 0 |
| double-vessel disease | 0.2 |
| multi-vessel disease with affected LM | 4.0 |
| multi-vessel disease without affected LM | 8.9 |
| Time of PCI | Score |
| weekday from 8:00 to 14:00 | 0 |
| weekday from 14:00 to 22:00 | 5 |
| weekday from 22:00 to 8:00 | 8.3 |
| public holiday from 8:00 to 22:00 | 1.5 |
| Post-PCI TIMI flow grade | Score |
| TIMI flow grades 0–1 | 10.0 |
| TIMI flow grades 2–3 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gawinski, L.; Milewska, A.; Marczak, M.; Kozlowski, R. Nomogram Predicting In-Hospital Mortality in Patients with Myocardial Infarction Treated with Primary Coronary Interventions Based on Logistic and Angiographic Predictors. Biomedicines 2025, 13, 646. https://doi.org/10.3390/biomedicines13030646
Gawinski L, Milewska A, Marczak M, Kozlowski R. Nomogram Predicting In-Hospital Mortality in Patients with Myocardial Infarction Treated with Primary Coronary Interventions Based on Logistic and Angiographic Predictors. Biomedicines. 2025; 13(3):646. https://doi.org/10.3390/biomedicines13030646
Chicago/Turabian StyleGawinski, Lukasz, Anna Milewska, Michal Marczak, and Remigiusz Kozlowski. 2025. "Nomogram Predicting In-Hospital Mortality in Patients with Myocardial Infarction Treated with Primary Coronary Interventions Based on Logistic and Angiographic Predictors" Biomedicines 13, no. 3: 646. https://doi.org/10.3390/biomedicines13030646
APA StyleGawinski, L., Milewska, A., Marczak, M., & Kozlowski, R. (2025). Nomogram Predicting In-Hospital Mortality in Patients with Myocardial Infarction Treated with Primary Coronary Interventions Based on Logistic and Angiographic Predictors. Biomedicines, 13(3), 646. https://doi.org/10.3390/biomedicines13030646








