Serum Biomarkers in Patent Ductus Arteriosus in Preterm Infants: A Narrative Review
Abstract
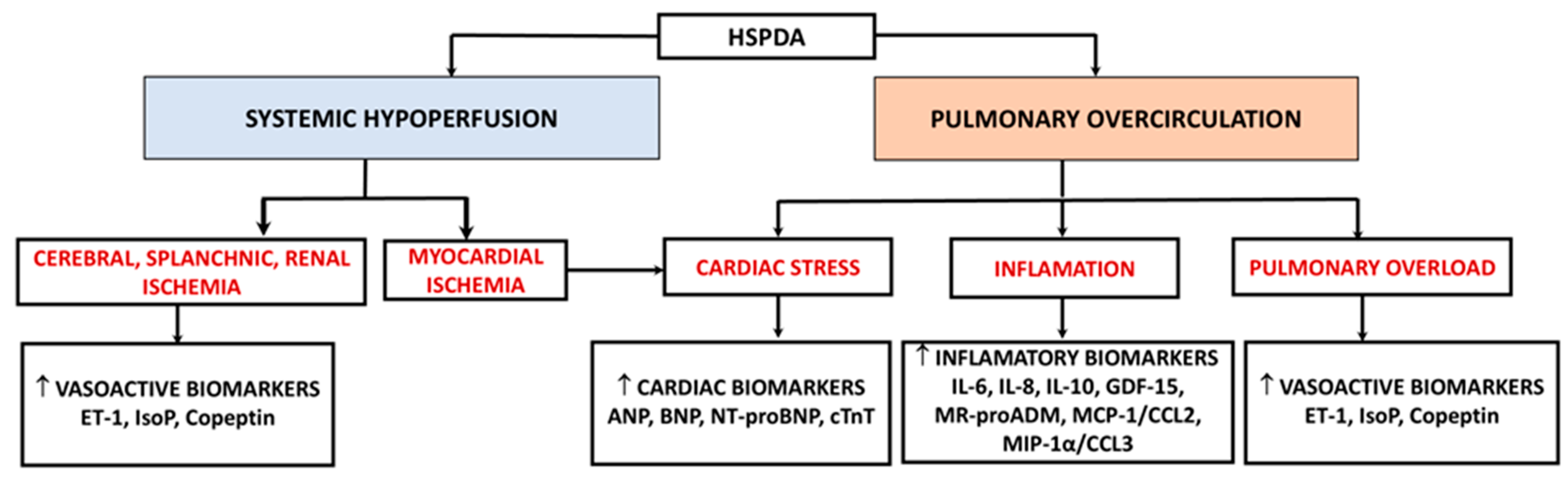
1. Introduction
2. Materials and Methods
3. Results
3.1. Cardiovascular Markers
3.1.1. Natriuretic Peptides (NPs)
- Atrial natriuretic peptide (ANP)
- Brain natriuretic peptide (BNP)
3.1.2. Cardiac Troponin T (cT)
3.2. Vasoactive Biomarkers
3.2.1. Mid-Regional Pro-Adrenomedullin (MR-proADM)
3.2.2. Endothelin-1 (ET-1)
3.2.3. Copeptin
3.2.4. Isoprostanes (IPs)
3.3. Inflammatory Biomarkers
3.3.1. Interleukin-6 (IL-6)
3.3.2. Interleukin-8 (IL-8)
3.3.3. Interleukin-10 (IL-10)
3.3.4. Growth Differentiation Factor 15 (GDF-15)
3.3.5. Monocyte Chemoattractant Protein-1 (MCP-1/CCL2)
3.3.6. Macrophage Inflammatory Protein-1α (MIP-1α/CCL3)
3.4. Importance and Limitation of Biomarkers Use
Limitation of This Review
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Kikuchi, N.; Goto, T.; Katsumata, N.; Murakami, Y.; Shinohara, T.; Maebayashi, Y.; Sakakibara, A.; Saito, C.; Hasebe, Y.; Hoshiai, M.; et al. Correlation between the Closure Time of Patent Ductus Arteriosus in Preterm Infants and Long-Term Neurodevelopmental Outcome. J. Cardiovasc. Dev. Dis. 2024, 11, 26. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, S.G.V.; McBrien, A. Ductus arteriosus and fetal echocardiography: Implications for practice. Semin. Fetal Neonatal Med. 2018, 23, 285–291. [Google Scholar] [CrossRef]
- Gournay, V. The ductus arteriosus: Physiology, regulation, and functional and congenital anomalies. Arch. Cardiovasc. Dis. 2011, 104, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Pugnaloni, F.; Doni, D.; Lucente, M.; Fiocchi, S.; Capolupo, I. Ductus Arteriosus in Fetal and Perinatal Life. J. Cardiovasc. Dev. Dis. 2024, 11, 113. [Google Scholar] [CrossRef] [PubMed]
- Hundscheid, T.; van den Broek, M.; van der Lee, R.; de Boode, W.P. Understanding the pathobiology in patent ductus arteriosus in prematurity-beyond prostaglandins and oxygen. Pediatr. Res. 2019, 86, 28–38. [Google Scholar] [CrossRef]
- Sarzani, R.; Allevi, M.; Di Pentima, C.; Schiavi, P.; Spannella, F.; Giulietti, F. Role of Cardiac Natriuretic Peptides in Heart Structure and Function. Int. J. Mol. Sci. 2022, 23, 14415. [Google Scholar] [CrossRef]
- Crockett, S.L.; Berger, C.D.; Shelton, E.L.; Reese, J. Molecular and mechanical factors contributing to ductus arteriosus patency and closure. Congenit. Heart Dis. 2019, 14, 15–20. [Google Scholar] [CrossRef]
- Ovalı, F. Molecular and Mechanical Mechanisms Regulating Ductus Arteriosus Closure in Preterm Infants. Front. Pediatr. 2020, 8, 516. [Google Scholar] [CrossRef]
- Thébaud, B.; Wu, X.C.; Kajimoto, H.; Bonnet, S.; Hashimoto, K.; Michelakis, E.D.; Archer, S.L. Developmental absence of the O2 sensitivity of L-type calcium channels in preterm ductus arteriosus smooth muscle cells impairs O2 constriction contributing to patent ductus arteriosus. Pediatr. Res. 2008, 63, 176–181. [Google Scholar] [CrossRef]
- Hung, Y.C.; Yeh, J.L.; Hsu, J.H. Molecular Mechanisms for Regulating Postnatal Ductus Arteriosus Closure. Int. J. Mol. Sci. 2018, 19, 1861. [Google Scholar] [CrossRef]
- Chatziantoniou, A.; Rorris, F.-P.; Samanidis, G.; Kanakis, M. Keeping the Ductus Arteriosus Patent: Current Strategy and Perspectives. Diagnostics 2025, 15, 241. [Google Scholar] [CrossRef] [PubMed]
- Hamrick, S.E.G.; Sallmon, H.; Rose, A.T.; Porras, D.; Shelton, E.L.; Reese, J.; Hansmann, G. Patent Ductus Arteriosus of the Preterm Infant. Pediatrics 2020, 146, e20201209. [Google Scholar] [CrossRef]
- Singh, Y.; Lakshminrusimha, S. Pathophysiology and Management of Persistent Pulmonary Hypertension of the Newborn. Clin. Perinatol. 2021, 48, 595–618. [Google Scholar] [CrossRef] [PubMed]
- Bakas, A.M.; Healy, H.M.; Bell, K.A.; Brown, D.W.; Mullen, M.; Scheid, A. Prenatal duct closure leading to severe pulmonary hypertension in a preterm neonate-a case report. Cardiovasc. Diagn. Ther. 2020, 10, 1691–1695. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, C.S.; Silva, P.V.; Castelo, R.; Tiago, J. Premature closure of ductus arteriosus after a single dose of diclofenac during pregnancy. BMJ Case Rep. 2021, 14, e243485. [Google Scholar] [CrossRef]
- Allegaert, K.; Mian, P.; Lapillonne, A.; van den Anker, J.N. Maternal paracetamol intake and fetal ductus arteriosus constriction or closure: A case series analysis. Br. J. Clin. Pharmacol. 2019, 85, 245–251. [Google Scholar] [CrossRef]
- Hauben, M.; Bai, S.; Hung, E.; Lobello, K.; Tressler, C.; Zucal, V.P. Maternal paracetamol intake and fetal ductus arteriosus constriction/closure: Comprehensive signal evaluation using the Austin Bradford Hill criteria. Eur. J. Clin. Pharmacol. 2021, 77, 1019–1028. [Google Scholar] [CrossRef]
- Operle, M.; Anderson, S. Premature Closure of the Ductus Arteriosus in an Otherwise Healthy Fetus. J. Diagn. Med. Sonogr. 2019, 35, 235–239. [Google Scholar] [CrossRef]
- Zielinsky, P.; Piccoli ALJr Manica, J.L.; Nicoloso, L.H. New insights on fetal ductal constriction: Role of maternal ingestion of polyphenol-rich foods. Expert. Rev. Cardiovasc. Ther. 2010, 8, 291–298. [Google Scholar] [CrossRef]
- Sung, S.I.; Chang, Y.S.; Kim, J.; Choi, J.H.; Ahn, S.Y.; Park, W.S. Natural evolution of ductus arteriosus with noninterventional conservative management in extremely preterm infants born at 23–28 weeks of gestation. PLoS ONE 2019, 14, e0212256. [Google Scholar] [CrossRef]
- Chesi, E.; Rossi, K.; Ancora, G.; Baraldi, C.; Corradi, M.; Di Dio, F.; Di Fazzio, G.; Galletti, S.; Mescoli, G.; Papa, I.; et al. Patent ductus arteriosus (also non-hemodynamically significant) correlates with poor outcomes in very low birth weight infants. A multicenter cohort study. PLoS ONE 2024, 19, e0306769. [Google Scholar] [CrossRef] [PubMed]
- Cucerea, M.; Moscalu, M.; Ognean, M.L.; Fagarasan, A.; Toma, D.; Marian, R.; Anciuc-Crauciuc, M.; Racean, A.; Gall, Z.; Simon, M. Impact of Early Surfactant Administration on Ductus Arteriosus Assessed at 24 h in Preterm Neonates Less than 32 Weeks of Gestational Age. Biomedicines 2024, 12, 1136. [Google Scholar] [CrossRef]
- Kotidis, C.; Wertheim, D.; Weindling, M.; Rabe, H.; Turner, M.A. Assessing patent ductus arteriosus in preterm infants from standard neonatal intensive care monitoring. Eur. J. Pediatr. 2022, 181, 1117–1124. [Google Scholar] [CrossRef]
- Benitz, W.E. Committee on Fetus and Newborn, American Academy of Pediatrics. Patent Ductus Arteriosus in Preterm Infants. Pediatrics 2016, 137, e20153730. [Google Scholar] [CrossRef]
- Singh, Y.; Fraisse, A.; Erdeve, O.; Atasay, B. Echocardiographic Diagnosis and Hemodynamic Evaluation of Patent Ductus Arteriosus in Extremely Low Gestational Age Newborn (ELGAN) Infants. Front. Pediatr. 2020, 8, 573627. [Google Scholar] [CrossRef]
- Kluckow, M.; Evans, N. Early echocardiographic prediction of symptomatic patent ductus arteriosus in preterm infants undergoing mechanical ventilation. J. Pediatr. 1995, 127, 774–779. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, M.; Gokulakrishnan, G.; Price, J.; Fernandes, C.J.; Leeflang, M.; Pammi, M. Diagnosing significant PDA using natriuretic peptides in preterm neonates: A systematic review. Pediatrics 2015, 135, e510–e525. [Google Scholar] [CrossRef] [PubMed]
- Surak, A.; Sidhu, A.; Ting, J.Y. Should we “eliminate” PDA shunt in preterm infants? A narrative review. Front. Pediatr. 2024, 12, 1257694. [Google Scholar] [CrossRef]
- Rubattu, S.; Volpe, M. Natriuretic Peptides in the Cardiovascular System: Multifaceted Roles in Physiology, Pathology and Therapeutics. Int. J. Mol. Sci. 2019, 20, 3991. [Google Scholar] [CrossRef]
- Weil, J.; Bidlingmaier, F.; Döhlemann, C.; Kuhnle, U.; Strom, T.; Lang, R.E. Comparison of plasma atrial natriuretic peptide levels in healthy children from birth to adolescence and in children with cardiac diseases. Pediatr. Res. 1986, 20, 1328–1331. [Google Scholar] [CrossRef]
- Weir, F.J.; Smith, A.; Littleton, P.; Carter, N.; Hamilton, P.A. Atrial natriuretic peptide in the diagnosis of patent ductus arteriosus. Acta Paediatr. 1992, 81, 672–675. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Huo, Y.; Chen, Q.; Hou, X. Application of B-Type Natriuretic Peptide in Neonatal Diseases. Front. Pediatr. 2021, 9, 767173. [Google Scholar] [CrossRef] [PubMed]
- Fritz, A.S.; Keller, T.; Kribs, A.; Hünseler, C. Reference values for N-terminal Pro-brain natriuretic peptide in premature infants during their first weeks of life. Eur. J. Pediatr. 2021, 180, 1193–1201. [Google Scholar] [CrossRef]
- Weber, M.; Hamm, C. Role of B-type natriuretic peptide (BNP) and NT-proBNP in clinical routine. Heart 2006, 92, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Koch, A.; Singer, H. Normal values of B type natriuretic peptide in infants, children, and adolescents. Heart 2003, 89, 875–878. [Google Scholar] [CrossRef]
- Cantinotti, M.; Storti, S.; Parri, M.S.; Murzi, M.; Clerico, A. Reference values for plasma B-type natriuretic peptide in the first days of life. Clin. Chem. 2009, 55, 1438–1440. [Google Scholar] [CrossRef]
- de Lemos, J.A.; McGuire, D.K.; Drazner, M.H. B-type natriuretic peptide in cardiovascular disease. Lancet 2003, 362, 316–322. [Google Scholar] [CrossRef]
- Kara, K.; Lehmann, N.; Neumann, T.; Kälsch, H.; Möhlenkamp, S.; Dykun, I.; Broecker-Preuss, M.; Pundt, N.; Moebus, S.; Jöckel, K.H.; et al. NT-proBNP is superior to BNP for predicting first cardiovascular events in the general population: The Heinz Nixdorf Recall Study. Int. J. Cardiol. 2015, 183, 155–161. [Google Scholar] [CrossRef]
- Dasgupta, S.; Aly, A.M.; Malloy, M.H.; Okorodudu, A.O.; Jain, S.K. NTproBNP as a surrogate biomarker for early screening of pulmonary hypertension in preterm infants with bronchopulmonary dysplasia. J. Perinatol. 2018, 38, 1252–1257. [Google Scholar] [CrossRef]
- Fritz, A.S.; Keller, T.; Kribs, A.; Hünseler, C. Diseases associated with prematurity in correlation with N-terminal pro-brain natriuretic peptide levels during the early postnatal life. Eur. J. Pediatr. 2023, 182, 3075–3082. [Google Scholar] [CrossRef]
- Schroeder, L.; Ebach, F.; Melaku, T.; Strizek, B.; Jimenez-Cruz, J.; Dolscheid-Pommerich, R.; Mueller, A.; Kipfmueller, F. Longitudinal evaluation of hemodynamic blood and echocardiographic biomarkers for the prediction of BPD and BPD-related pulmonary hypertension in very-low-birth-weight preterm infants. Eur. J. Pediatr. 2024, 184, 15. [Google Scholar] [CrossRef] [PubMed]
- Neumann, R.P.; Gerull, R.; Hasler, P.W.; Wellmann, S.; Schulzke, S.M. Vasoactive peptides as biomarkers for the prediction of retinopathy of prematurity. Pediatr. Res. 2024, 95, 1868–1874. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Li, B.; Wang, Z.; Zou, J.; Jia, Y.; Yoshida, S.; Zhou, Y. Novel Potential Biomarkers for Retinopathy of Prematurity. Front. Med. 2022, 9, 840030. [Google Scholar] [CrossRef]
- Yang, C.; Ma, J.; Guo, L.; Li, B.; Wang, L.; Li, M.; Wang, T.; Xu, P.; Zhao, C. NT-Pro-BNP and echocardiography for the early assessment of cardiovascular dysfunction in neonates with sepsis. Medicine 2022, 101, e30439. [Google Scholar] [CrossRef]
- Czernik, C.; Lemmer, J.; Metze, B.; Koehne, P.S.; Mueller, C.; Obladen, M. B-type natriuretic peptide to predict ductus intervention in infants <28 weeks. Pediatr. Res. 2008, 64, 286–290. [Google Scholar] [CrossRef]
- Cui, Q.; Liu, X.; Su, G.; Zhou, C.; Wang, J. Change and clinical significance of serum cortisol, BNP, and PGE-2 levels in premature infants with patent ductus arteriosus. Transl. Pediatr. 2021, 10, 2573–2578. [Google Scholar] [CrossRef]
- König, K.; Guy, K.J.; Drew, S.M.; Barfield, C.P. B-type and N-terminal pro-B-type natriuretic peptides are equally useful in assessing patent ductus arteriosus in very preterm infants. Acta Paediatr. 2015, 104, e139–e142. [Google Scholar] [CrossRef] [PubMed]
- Parra-Bravo, J.R.; Valdovinos-Ponce, M.T.; García, H.; Núñez-Enríquez, J.C.; Jiménez-Cárdenas, M.L.; Avilés-Monjaraz, R.; Lavana-Hernández, W. B-type brain natriuretic peptide as marker of hemodynamic overload of the patent ductus arteriosus in the preterm infant]. Arch. Cardiol. Mex. 2020, 91, 17–24. [Google Scholar] [CrossRef]
- Kim, J.S.; Shim, E.J. B-type natriuretic Peptide assay for the diagnosis and prognosis of patent ductus arteriosus in preterm infants. Korean Circ. J. 2012, 42, 192–196. [Google Scholar] [CrossRef][Green Version]
- Choi, B.M.; Lee, K.H.; Eun, B.L.; Yoo, K.H.; Hong, Y.S.; Son, C.S.; Lee, J.W. Utility of rapid B-type natriuretic peptide assay for diagnosis of symptomatic patent ductus arteriosus in preterm infants. Pediatrics 2005, 115, e255–e261. [Google Scholar] [CrossRef]
- Mine, K.; Ohashi, A.; Tsuji, S.; Nakashima, J.; Hirabayashi, M.; Kaneko, K. B-type natriuretic peptide for assessment of haemodynamically significant patent ductus arteriosus in premature infants. Acta Paediatr. 2013, 102, e347–e352. [Google Scholar] [CrossRef] [PubMed]
- Sanjeev, S.; Pettersen, M.; Lua, J.; Thomas, R.; Shankaran, S.; L’Ecuyer, T. Role of plasma B-type natriuretic peptide in screening for hemodynamically significant patent ductus arteriosus in preterm neonates. J. Perinatol. 2005, 25, 709–713. [Google Scholar] [CrossRef] [PubMed]
- Kalra, V.K.; DeBari, V.A.; Zauk, A.; Kataria, P.; Myridakis, D.; Kiblawi, F. Point-of-care testing for B-type natriuretic peptide in premature neonates with patent ductus arteriosus. Ann. Clin. Lab. Sci. 2011, 41, 131–137. [Google Scholar] [PubMed]
- Zekri, H.S.; Said, R.N.; Hegazy, R.A.; Darwish, R.K.; Kamel, A.; Abd El Hakim, N.G. B-type Natriuretic Peptide: A Diagnostic Biomarker for a Hemodynamically Significant PDA. Open Access Maced. J. Med. Sci. 2022, 10, 877–883. [Google Scholar] [CrossRef]
- Lee, J.H.; Shin, J.H.; Park, K.H.; Rhie, Y.J.; Park, M.S.; Choi, B.M. Can early B-type natriuretic peptide assays predict symptomatic patent ductus arteriosus in extremely low birth weight infants? Neonatol. 2013, 103, 118–122. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, Z.-L.; Gong, L.; Zhang, Z.; Zhang, S.-C.; Zhou, Y.-X. N-terminal pro-brain natriuretic peptide used for screening hemodynamically significant patent ductus arteriosus in very low birth weight infants: How and when? Clin. Hemorheol. Microcirc. 2020, 75, 335–347. [Google Scholar] [CrossRef]
- Nuntnarumit, P.; Khositseth, A.; Thanomsingh, P. N-terminal probrain natriuretic peptide and patent ductus arteriosus in preterm infants. J. Perinatol. 2009, 29, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Harris, S.L.; More, K.; Dixon, B.; Troughton, R.; Pemberton, C.; Horwood, J.; Ellis, N.; Austin, N. Factors affecting N-terminal pro-B-type natriuretic peptide levels in preterm infants and use in determination of haemodynamic significance of patent ductus arteriosus. Eur. J. Pediatr. 2018, 177, 521–532. [Google Scholar] [CrossRef]
- Gudmundsdottir, A.; Bartocci, M.; Picard, O.; Ekström, J.; Chakhunashvili, A.; Bohlin, K.; Attner, C.; Printz, G.; Karlsson, M.; Mohlkert, L.A.; et al. Early N-Terminal Pro B-Type Natriuretic Peptide (NTproBNP) Plasma Values and Associations with Patent Ductus Arteriosus Closure and Treatment-An Echocardiography Study of Extremely Preterm Infants. J. Clin. Med. 2022, 11, 667. [Google Scholar] [CrossRef]
- Ramakrishnan, S.; Heung, Y.M.; Round, J.; Morris, T.P.; Collinson, P.; Williams, A.F. Early N-terminal pro-brain natriuretic peptide measurements predict clinically significant ductus arteriosus in preterm infants. Acta Paediatr. 2009, 98, 1254–1259. [Google Scholar] [CrossRef]
- Asrani, P.; Aly, A.M.; Jiwani, A.K.; Niebuhr, B.R.; Christenson, R.H.; Jain, S.K. High-sensitivity troponin T in preterm infants with a hemodynamically significant patent ductus arteriosus. J. Perinatol. 2018, 38, 1483–1489. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Blanco, S.; Oulego-Erroz, I.; Gautreaux-Minaya, S.; Perez-Muñuzuri, A.; Couce-Pico, M.L. Early NT-proBNP levels as a screening tool for the detection of hemodynamically significant patent ductus arteriosus during the first week of life in very low birth weight infants. J. Perinatol. 2018, 38, 881–888. [Google Scholar] [CrossRef] [PubMed]
- Buddhe, S.; Dhuper, S.; Kim, R.; Weichbrod, L.; Mahdi, E.; Shah, N.; Kona, S.; Sokal, M. NT-proBNP Levels Improve the Ability of Predicting a Hemodynamically Significant Patent Ductus Arteriosus in Very Low-Birth-Weight Infants. J. Clin. Neonatol. 2012, 1, 82–86. [Google Scholar] [CrossRef]
- Lin, Y.-L.; Hung, Y.-L.; Shen, C.-M.; Chen, Y.-C.; Hsieh, W.-S. Can NT-proBNP Levels Be an Early Biomarker of Reduced Left Ventricular Ejection Fraction in Preterm Infants? Children 2022, 9, 1002. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Lee, E.H.; Lee, J.H.; Choi, B.M.; Hong, Y.S. Individualized ibuprofen treatment using serial B-type natriuretic peptide measurement for symptomatic patent ductus arteriosus in very preterm infants. Korean J. Pediatr. 2017, 60, 175–180. [Google Scholar] [CrossRef][Green Version]
- Gokulakrishnan, G.; Kulkarni, M.; He, S.; Leeflang, M.M.; Cabrera, A.G.; Fernandes, C.J.; Pammi, M. Brain natriuretic peptide and N-terminal brain natriuretic peptide for the diagnosis of haemodynamically significant patent ductus arteriosus in preterm neonates. Cochrane Database Syst. Rev. 2022, 12, CD013129. [Google Scholar] [CrossRef]
- Köstekci, Y.E.; Erdeve, Ö. Patent ductus arteriosus (PDA): Recent recommendations for to close or not to close. Glob. Pediatr. Dec. 2023, 2, 100128. [Google Scholar] [CrossRef]
- Tarkowska, A.; Furmaga-Jabłońska, W. The Evaluation of Cardiac Troponin T in Newborns. Biomed. Hub. 2017, 2, 1–7. [Google Scholar] [CrossRef]
- Karlén, J.; Karlsson, M.; Eliasson, H.; Bonamy, A.E.; Halvorsen, C.P. Cardiac Troponin T in Healthy Full-Term Infants. Pediatr. Cardiol. 2019, 40, 1645–1654. [Google Scholar] [CrossRef]
- Osredkar, J.; Bajrić, A.; Možina, H.; Lipar, L.; Jerin, A. Cardiac Troponins I and T as Biomarkers of Cardiomyocyte Injury—Advantages and Disadvantages of Each. Appl. Sci. 2024, 14, 6007. [Google Scholar] [CrossRef]
- Kontos, M.C.; Turlington, J.S. High-Sensitivity Troponins in Cardiovascular Disease. Curr. Cardiol. Rep. 2020, 22, 30. [Google Scholar] [CrossRef] [PubMed]
- Trevisanuto, D.; Pitton, M.; Altinier, S.; Zaninotto, M.; Plebani, M.; Zanardo, V. Cardiac troponin I, cardiac troponin T and creatine kinase MB concentrations in umbilical cord blood of healthy term neonates. Acta Paediatr. 2003, 92, 1463–1467. [Google Scholar] [CrossRef] [PubMed]
- Clark, S.J.; Newland, P.; Yoxall, C.W.; Subhedar, N.V. Concentrations of cardiac troponin T in neonates with and without respiratory distress. Arch. Dis. Child. Fetal Neonatal Ed. 2004, 89, F348–F352. [Google Scholar] [CrossRef]
- Yildirim, A.; Ozgen, F.; Ucar, B.; Alatas, O.; Tekin, N.; Kilic, Z. The diagnostic value of troponin T level in the determination of cardiac damage in perinatal asphyxia newborns. Fetal Pediatr. Pathol. 2016, 35, 29–36. [Google Scholar] [CrossRef]
- Jones, R.; Heep, A.; Odd, D. Biochemical and clinical predictors of hypoxic-ischemic encephalopathy after perinatal asphyxia. J. Matern. Fetal Neonatal Med. 2018, 31, 791–796. [Google Scholar] [CrossRef]
- El-Khuffash, A.F.; Molloy, E.J. Influence of a patent ductus arteriosus on cardiac troponin T levels in preterm infants. J. Pediatr. 2008, 153, 350–353. [Google Scholar] [CrossRef]
- Mohamed, M.H.; Aboraya, H.M.; Makawy, M.A.; Elgebaly, H.H. Cardiac Troponin T in very low birthweight preterm infants with patent ductus arteriosus. QJM Int. J. Med. 2020, 113 (Suppl. S1), i219–i220. [Google Scholar] [CrossRef]
- Omar, H.R.; Abed, N.T.; El-Falah, A.A.; Elsayes, M.E. High-sensitivity troponin T in preterm infants with a hemodynamically significant patent ductus arteriosus. Int. J. Health Sci. 2022, 6, 8220–8230. [Google Scholar] [CrossRef]
- Vaisbourd, Y.; Sharif, D.; Riskin, A.; Yaniv, L.; Dinur, G.; Amen, K.; Bader, D.; Kugelman, A. The effect of patent ductus arteriosus on coronary artery blood flow in premature infants: A prospective observational pilot study. J. Perinatol. 2020, 40, 1366–1374. [Google Scholar] [CrossRef]
- Veysizadeh, M.; Khodadadi, M.; Zarkesh, M.R.; Kamrani, K.; Kaveh, M.; Shariat, M. Finding a biomarker to predict patent ductus arteriosus in preterm babies. World J. Adv. Res. Rev. Oct. 2022, 16, 259–265. [Google Scholar] [CrossRef]
- Tavakoli, A.; Rezaei, M.; Moghtaderi, M. Reduction of Serum Troponin I in Premature Neonates After Closure of Patent Ductus Arteriosus. Shiraz E-Med. J. 2024, 25, e152701. [Google Scholar] [CrossRef]
- Koyama, T.; Kuriyama, N.; Suzuki, Y.; Saito, S.; Tanaka, R.; Iwao, M.; Tanaka, M.; Maki, T.; Itoh, H.; Ihara, M.; et al. Mid-regional pro-adrenomedullin is a novel biomarker for arterial stiffness as the criterion for vascular failure in a cross-sectional study. Sci. Rep. 2021, 11, 305, Erratum in Sci. Rep. 2021, 11, 17638. https://doi.org/10.1038/s41598-021-96984-3. [Google Scholar] [CrossRef]
- Krintus, M.; Kozinski, M.; Braga, F.; Kubica, J.; Sypniewska, G.; Panteghini, M. Plasma midregional proadrenomedullin (MR-proADM) concentrations and their biological determinants in a reference population. Clin. Chem. Lab. Med. 2018, 56, 1161–1168. [Google Scholar] [CrossRef]
- Valenzuela-Sánchez, F.; Valenzuela-Méndez, B.; Rodríguez-Gutiérrez, J.F.; Estella-García, Á.; González-García, M.Á. New role of biomarkers: Mid-regional pro-adrenomedullin, the biomarker of organ failure. Ann. Transl. Med. 2016, 4, 329. [Google Scholar] [CrossRef]
- Oncel, M.Y.; Dilmen, U.; Erdeve, O.; Ozdemir, R.; Calisici, E.; Yurttutan, S.; Canpolat, F.E.; Oguz, S.S.; Uras, N. Proadrenomedullin as a prognostic marker in neonatal sepsis. Pediatr. Res. 2012, 72, 507–512. [Google Scholar] [CrossRef][Green Version]
- Fahmey, S.S.; Mostafa, H.; Elhafeez, N.A.; Hussain, H. Diagnostic and prognostic value of proadrenomedullin in neonatal sepsis. Korean J. Pediatr. 2018, 61, 156–159. [Google Scholar] [CrossRef] [PubMed]
- Admaty, D.; Benzing, J.; Burkhardt, T.; Lapaire, O.; Hegi, L.; Szinnai, G.; Morgenthaler, N.G.; Bucher, H.U.; Bührer, C.; Wellmann, S. Plasma midregional proadrenomedullin in newborn infants: Impact of prematurity and perinatal infection. Pediatr. Res. 2012, 72, 70–76. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wu, R.Z.; Rong, X.; Ren, Y.; He, X.X.; Xiang, R.L. Heart rate variability, adrenomedullin and B-type natriuretic peptide before and after transcatheter closure in children with patent ductus arteriosus. Zhonghua Xin Xue Guan Bing Za Zhi 2010, 38, 334–336. [Google Scholar]
- Kowalczyk, A.; Kleniewska, P.; Kolodziejczyk, M.; Skibska, B.; Goraca, A. The role of endothelin-1 and endothelin receptor antagonists in inflammatory response and sepsis. Arch. Immunol. Ther. Exp. 2015, 63, 41–52. [Google Scholar] [CrossRef]
- Buendgens, L.; Yagmur, E.; Bruensing, J.; Herbers, U.; Baeck, C.; Trautwein, C.; Koch, A.; Tacke, F. C-terminal proendothelin-1 (CT-proET-1) is associated with organ failure and predicts mortality in critically ill patients. J. Intensive Care 2017, 5, 25. [Google Scholar] [CrossRef]
- Houde, M.; Desbiens, L.; D’Orléans-Juste, P. Endothelin-1, Biosynthesis, Signaling and Vasoreactivity. Adv. Pharmacol. 2016, 77, 143–175. [Google Scholar] [CrossRef]
- Niu, J.O.; Munshi, U.K.; Siddiq, M.M.; Parton, L.A. Early increase in endothelin-1 in tracheal aspirates of preterm infants: Correlation with bronchopulmonary dysplasia. J. Pediatr. 1998, 132, 965–970. [Google Scholar] [CrossRef] [PubMed]
- Baumann, P.; Fouzas, S.; Pramana, I.; Grass, B.; Niesse, O.; Bührer, C.; Wellmann, S. Plasma proendothelin-1 as an early marker of bronchopulmonary dysplasia. Neonatology 2015, 108, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Gerull, R.; Neumann, R.P.; Atkinson, A.; Bernasconi, L.; Schulzke, S.M.; Wellmann, S. Respiratory morbidity in preterm infants predicted by natriuretic peptide (MR-proANP) and endothelin-1 (CT-proET-1). Pediatr. Res. 2022, 91, 1478–1484. [Google Scholar] [CrossRef] [PubMed]
- Letzner, J.; Berger, F.; Schwabe, S.; Benzing, J.; Morgenthaler, N.G.; Bucher, H.U.; Buhrer, C.; Arlettaz, R.; Wellmann, S. Plasma C-terminal pro-endothelin-1 and the natriuretic pro-peptides NT-proBNP and MR-proANP in very preterm infants with patent ductus arteriosus. Neonatology 2012, 101, 116–124. [Google Scholar] [CrossRef]
- Grass, B.; Baumann, P.; Arlettaz, R.; Fouzas, S.; Meyer, P.; Spanaus, K.; Wellmann, S. Cardiovascular biomarkers pro-atrial natriuretic peptide and pro-endothelin-1 to monitor ductus arteriosus evolution in very preterm infants. Early Hum. Dev. 2014, 90, 293–298. [Google Scholar] [CrossRef]
- Sellmer, A.; Hjortdal, V.E.; Bjerre, J.V.; Schmidt, M.R.; Bech, B.H.; Henriksen, T.B. Cardiovascular biomarkers in the evaluation of patent ductus arteriosus in very preterm neonates: A cohort study. Early Hum. Dev. 2020, 149, 105142. [Google Scholar] [CrossRef]
- Yoshimura, M.; Conway-Campbell, B.; Ueta, Y. Arginine vasopressin: Direct and indirect action on metabolism. Peptides 2021, 142, 170555. [Google Scholar] [CrossRef]
- Evers, K.S.; Wellmann, S. Arginine Vasopressin and Copeptin in Perinatology. Front. Pediatr. 2016, 4, 75. [Google Scholar] [CrossRef]
- Jalleh, R.; Torpy, D.J. The Emerging Role of Copeptin. Clin. Biochem. Rev. 2021, 42, 17–25. [Google Scholar] [CrossRef]
- Benzing, J.; Wellmann, S.; Achini, F.; Letzner, J.; Burkhardt, T.; Beinder, E.; Morgenthaler, N.G.; Haagen, U.; Bucher, H.U.; Bührer, C.; et al. Plasma copeptin in preterm infants: A highly sensitive marker of fetal and neonatal stress. J. Clin. Endocrinol. Metab. 2011, 96, E982–E985. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Milne, G.L.; Yin, H.; Brooks, J.D.; Sanchez, S.; Jackson Roberts L 2nd Morrow, J.D. Quantification of F2-isoprostanes in biological fluids and tissues as a measure of oxidant stress. Methods Enzym. 2007, 433, 113–126. [Google Scholar] [CrossRef]
- Dani, C.; Pratesi, S. Patent ductus arteriosus and oxidative stress in preterm infants: A narrative review. Transl. Pediatr. 2020, 9, 835–839. [Google Scholar] [CrossRef]
- Comporti, M.; Signorini, C.; Leoncini, S.; Buonocore, G.; Rossi, V.; Ciccoli, L. Plasma F2-isoprostanes are elevated in newborns and inversely correlated to gestational age. Free Radic. Biol. Med. 2004, 37, 724–732. [Google Scholar] [CrossRef] [PubMed]
- Coviello, C.; Perrone, S.; Buonocore, G.; Negro, S.; Longini, M.; Dani, C.; de Vries, L.S.; Groenendaal, F.; Vijlbrief, D.C.; Benders, M.J.N.L.; et al. Isoprostanes as Biomarker for White Matter Injury in Extremely Preterm Infants. Front. Pediatr. 2021, 8, 618622. [Google Scholar] [CrossRef]
- Lembo, C.; Buonocore, G.; Perrone, S. Oxidative Stress in Preterm Newborns. Antioxidants 2021, 10, 1672. [Google Scholar] [CrossRef]
- Chen, J.X.; O’Mara, P.W.; Poole, S.D.; Brown, N.; Ehinger, N.J.; Slaughter, J.C.; Paria, B.C.; Aschner, J.L.; Reese, J. Isoprostanes as physiological mediators of transition to newborn life: Novel mechanisms regulating patency of the term and preterm ductus arteriosus. Pediatr. Res. 2012, 72, 122–128. [Google Scholar] [CrossRef]
- Küng, E.; Unterasinger, L.; Waldhör, T.; Berger, A.; Wisgrill, L. Cut-off values of serum interleukin-6 for culture-confirmed sepsis in neonates. Pediatr. Res. 2023, 93, 1969–1974. [Google Scholar] [CrossRef] [PubMed]
- Coviello, C.; Tataranno, M.L.; Corsini, I.; Leonardi, V.; Longini, M.; Bazzini, F.; Buonocore, G.; Dani, C. Isoprostanes as Biomarker for Patent Ductus Arteriosus in Preterm Infants. Front. Pediatr. 2020, 8, 555. [Google Scholar] [CrossRef]
- Wei, Y.J.; Hsu, R.; Lin, Y.C.; Wong, T.W.; Kan, C.D.; Wang, J.N. The Association of Patent Ductus Arteriosus with Inflammation: A Narrative Review of the Role of Inflammatory Biomarkers and Treatment Strategy in Premature Infants. Int. J. Mol. Sci. 2022, 23, 13877. [Google Scholar] [CrossRef]
- Cakir, U.; Tayman, C. Systemic Inflammatory Indices as New Biomarkers for Hemodynamically Significant Ductus Arteriosus. Arq. Bras. Cardiol. 2024, 121, e20240211, Portuguese, English. [Google Scholar] [CrossRef] [PubMed]
- Eichberger, J.; Resch, B. Reliability of Interleukin-6 Alone and in Combination for Diagnosis of Early Onset Neonatal Sepsis: Systematic Review. Front. Pediatr. 2022, 10, 840778. [Google Scholar] [CrossRef]
- Gude, S.S.; Peddi, N.C.; Vuppalapati, S.; Venu Gopal, S.; Marasandra Ramesh, H.; Gude, S.S. Biomarkers of Neonatal Sepsis: From Being Mere Numbers to Becoming Guiding Diagnostics. Cureus 2022, 14, e23215. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Cheng, S.; Yu, J.; Lu, Q. Interleukin-8 for diagnosis of neonatal sepsis: A meta-analysis. PLoS ONE 2015, 10, e0127170. [Google Scholar] [CrossRef]
- Dembinski, J.; Behrendt, D.; Heep, A.; Dorn, C.; Reinsberg, J.; Bartmann, P. Cell-associated interleukin-8 in cord blood of term and preterm infants. Clin. Diagn. Lab. Immunol. 2002, 9, 320–323. [Google Scholar] [CrossRef] [PubMed]
- Orlikowsky, T.W.; Neunhoeffer, F.; Goelz, R.; Eichner, M.; Henkel, C.; Zwirner, M.; Poets, C.F. Evaluation of IL-8-concentrations in plasma and lysed EDTA-blood in healthy neonates and those with suspected early onset bacterial infection. Pediatr. Res. 2004, 56, 804–809. [Google Scholar] [CrossRef]
- Olsson, K.W.; Larsson, A.; Jonzon, A.; Sindelar, R. Exploration of potential biochemical markers for persistence of patent ductus arteriosus in preterm infants at 22-27 weeks’ gestation. Pediatr. Res. 2019, 86, 333–338. [Google Scholar] [CrossRef]
- Minshawi, F.; Lanvermann, S.; McKenzie, E.; Jeffery, R.; Couper, K.; Papoutsopoulou, S.; Roers, A.; Muller, W. The Generation of an Engineered Interleukin-10 Protein with Improved Stability and Biological Function. Front. Immunol. 2020, 11, 1794. [Google Scholar] [CrossRef]
- Wollert, K.C.; Kempf, T.; Wallentin, L. Growth Differentiation Factor 15 as a Biomarker in Cardiovascular Disease. Clin. Chem. 2017, 63, 140–151. [Google Scholar] [CrossRef]
- Almudares, F.; Hagan, J.; Chen, X.; Devaraj, S.; Moorthy, B.; Lingappan, K. Growth and differentiation factor 15 (GDF15) levels predict adverse respiratory outcomes in premature neonates. Pediatr. Pulmonol. 2023, 58, 271–278. [Google Scholar] [CrossRef]
- Paneitz, D.C.; Zhou, A.; Yanek, L.; Golla, S.; Avula, S.; Kannankeril, P.J.; Everett, A.D.; Mettler, B.A.; Gottlieb Sen, D. Growth Differentiation Factor 15, A Novel Growth Biomarker for Children With Congenital Heart Disease. World J. Pediatr. Congenit. Heart Surg. 2022, 13, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Deshmane, S.L.; Kremlev, S.; Amini, S.; Sawaya, B.E. Monocyte chemoattractant protein-1 (MCP-1): An overview. J. Interferon Cytokine Res. 2009, 29, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Quesada, C.; Frangogiannis, N.G. Monocyte chemoattractant protein-1/CCL2 as a biomarker in acute coronary syndromes. Curr. Atheroscler. Rep. 2009, 11, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Bhavsar, I.; Miller, C.S.; Al-Sabbagh, M. Macrophage Inflammatory Protein-1 Alpha (MIP-1 alpha)/CCL3, As a Biomarker. Gen. Methods Biomark. Res. Their Appl. 2015, 161, 105464. [Google Scholar] [CrossRef]
- Aikio, O.; Härmä, A.; Härkin, P.; Leskinen, M.; Valkama, M.; Saarela, T.; Salminen, A.; Hallman, M. Inflammatory biomarkers in very preterm infants during early intravenous paracetamol administration. Early Hum. Dev. 2021, 161, 105464. [Google Scholar] [CrossRef]

| GA Weeks | n | Age Days | BNP (pg/mL) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| PDA | No PDA | Cutoff Value | Sensitivity | Specificity | Study Findings | ||||
| Czernik [45] | <28 Median 26 | 67 | 1–2 | 1069 (564–1845) 87 (17–130) # | 247 (121–463) | 550 | 83% | 86% | BNP is correlated with DA size (R = 0.46, p < 0.001) BNP is predictive for PDA treatment |
| Cui Q [46] | 28–32 | 67 | 3 | 95.20 ± 7.42 | 70.15 ± 6.44 | - | 68.9% | 69% | BNP is correlated with early diagnosis and progression of PDA |
| König [47] | <32 | 58 | 1–4 | 486.5 (219–1316) | 190 (95.5–514.5) | - | - | - | BNP is correlated with PDA size (R = 0.35, p = 0.0066) |
| Parra-Bravo [48] | < 32 | 29 | 3–5 | 1061.9 ± 105.7 | 219.9 ± 227.8 | 486.5 | 81% | 92% | BNP is correlated with hsPDA (R = 0.71; p < 0.001) |
| Kim [49] | <37 32.7 (28.4–35.8) | 28 | 4 | 654.68 (428.29–1280) | 124.52 (37.21–290.49) | 412 | 100% | 95% | BNP is correlated with hsPDA |
| Choi [50] | 25–34 | 66 | 3 | 2896 ± 1627 | 208 ± 313 | 1110 | 100% | 95.3% | BNP is correlated with the magnitude of the DA shunt |
| Mine [51] | <33 | 46 | 2–3 | 283.4 (123.1–226.2) | 88.4 (38.6–191.4) | 250 2000 | 80% | 40% | BNP is predictive for PDA treatment (indomethacin) BNP is predictive for PDA surgery |
| Sanjeev [52] | ≤34 | 29 | 2–28 | 508.5 ± 618.2 | 59.5 ± 69.9 | 70 | 92.9% | 73.3% | BNP is correlated with hsPDA |
| Kalra [53] | <34 | 52 | 3–7 | 2410 (420–2770) | 23.6 (13.1–32.8) | 123 | 100% | 100% | BNP is predictive for decision for treatment |
| Zekri [54] | ≤35 | 73 | 1–2 | 536 (36–5665) | 59.25 (11.5–331) | 160.5 | 80.49% | 90.62% | BNP is correlated with PDA size |
| Lee [55] | 27.1 ± 2.2 | 73 | 1 | 921 (318–2133) | 152 (91–450) | >200 >900 | 83.9% 54.8% | 61.9% 95.2% | BNP at 24 h is correlated with the magnitude of the of the DA shunt BNP at 24 h—guide for early targeted treatment of hsPDA |
| GA Weeks | n | Age Days | NT-proBNP (pg/mL) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| PDA | No PDA | Cutoff Value | Sensitivity | Specificity | Study Findings | ||||
| Liu Y [56] | 30.6 ± 1.5 | 120 | 1 2 3 | 2050.0 ± 590.5 5716.8 ± 2267.0 5505.1 ± 2210.2 | 1865.4 ± 436.6 2765.5 ± 793.1 1618.7 ± 782.3 | 3689 2331.5 | 83.7% 97.7% | 93.5% 89.6% | NT-proBNP is predictive for hsPDA NT-proBNP is correlated with the magnitude of DA shunt Day three of life is the optimal testing time |
| Nuntnarumit [57] | <37 | 35 | 2 | 16,353 (10,316–104,998) | 3914 (1535–19,516) | 10,180 | 100% | 91% | NT-proBNP is predictive for HsPDA |
| Fritz [40] | ≤31 | 118 | 1–7 | 7843 (2915–14,116) | 1896 (1277–5200) | - | - | - | NT-proBNP is correlated with the severity of PDA |
| König [47] | <32 | 58 | 1–4 | 10,858.5 (6319–42 108) | 7488 (3363–14 227.5) | - | - | - | NT-proBNP is correlated with PDA size |
| Harris [58] | < 30 | 51 | 3 | 1840 (1058) | 178 (140) | 287 | 92% | 92% | NT-proBNP is predictive for hsPDA |
| Gudmundsdottir [59] | <28 | 98 | 3 | 14,600 (7740–28,100) 32,300 (29,100–35,000) * | 1810 (1760–6000) | 6001–9000 15,001–18,000 | 61% 66% | 20% 66% | NT-proBNP is predictive for spontaneous DA closure Predictive for PDA surgery |
| Ramakrishnan [60] | 29 | 56 | 2 | 6952 | 1206 | 2850 | 90% | 89% | NT-proBNP is predictive for PDA treatment |
| Asrani [61] | <34 | 70 | 1–5 | 18,181.02 | 3149.23 | 3460 | 88% | 72% | NT-proBNP is an excellent diagnostic test for PDA |
| Rodriguez-Blanco [62] | ≤32 | 85 | 2–3 | 33,171 (5337–60,684) | 2065 (1093–4448) | 5099 | 94% | 82% | NT-proBNP at 48–96 h of life can be used to exclude hsPDA |
| Buddhe [63] | 27 ± 2.6 | 69 | 3–5 | 24,420 ± 3190 | 3072 ± 332 | 5900 | 96% | 90% | NT-proBNP helps timing of intervention of a hsPDA |
| Lin [64] | 30.8 ± 3.3 | 36 | 2 | 9233.5 | 4262.5 | - | - | - | NT-proBNP might predict the effectiveness of the treatment |
| GA Weeks | n | Age Days | cTnT (pg/mL) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| PDA | No PDA | Cutoff Value | Sensitivity | Specificity | Study Findings | ||||
| Asrani [61] | <34 | 70 | 2 | 251.5 ± 65.6 | 161 ± 22.4 | 170 | 70% | 55% | cTnT is a fair diagnostic test for PDA |
| EL-Khuffash [76] | 28 (26.1–29.5) | 80 | ½–2 | 430 | 130 | 200 | 70% | 75% | cTnT significantly correlated with echocardiographic markers of DA significance |
| Mohamed [77] | 31.7 ± 61.57 | 77 | 2;5–7 | 310 ± 60 | 160 ± 30 | - | - | - | cTnT is correlated with PDA size |
| Omar [78] | <34 | 60 | 1–4 | 182.7 ± 59.62 | 67.23 ± 25.96 | >100 | 93.33% | 90% | cTnT can detect hsPDA |
| Vaisbourd [79] | <32 | 43 | 1–3 | hsPDA 200 ± 100 nhsPDA 120 ± 100 | 100 ± 100 | - | - | - | cTnT is as sensitive as echocardiographic findings in hsPDA |
| Veysizadeh [80] | 32.658 ± 1.554 | 36 | 1–3 | 124.506 ± 113.138 | 112.275 ± 66.546 | - | - | - | There is no correlation between PDA and cTnT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cucerea, M.; Marian, R.; Simon, M.; Anciuc-Crauciuc, M.; Racean, A.; Toth, A.; Simon-Szabó, Z.; Fadur, M.-G.; Moldovan, V.; Moldovan, E. Serum Biomarkers in Patent Ductus Arteriosus in Preterm Infants: A Narrative Review. Biomedicines 2025, 13, 670. https://doi.org/10.3390/biomedicines13030670
Cucerea M, Marian R, Simon M, Anciuc-Crauciuc M, Racean A, Toth A, Simon-Szabó Z, Fadur M-G, Moldovan V, Moldovan E. Serum Biomarkers in Patent Ductus Arteriosus in Preterm Infants: A Narrative Review. Biomedicines. 2025; 13(3):670. https://doi.org/10.3390/biomedicines13030670
Chicago/Turabian StyleCucerea, Manuela, Raluca Marian, Marta Simon, Madalina Anciuc-Crauciuc, Andreea Racean, Andrea Toth, Zsuzsánna Simon-Szabó, Mihaela-Georgiana Fadur, Valeriu Moldovan, and Elena Moldovan. 2025. "Serum Biomarkers in Patent Ductus Arteriosus in Preterm Infants: A Narrative Review" Biomedicines 13, no. 3: 670. https://doi.org/10.3390/biomedicines13030670
APA StyleCucerea, M., Marian, R., Simon, M., Anciuc-Crauciuc, M., Racean, A., Toth, A., Simon-Szabó, Z., Fadur, M.-G., Moldovan, V., & Moldovan, E. (2025). Serum Biomarkers in Patent Ductus Arteriosus in Preterm Infants: A Narrative Review. Biomedicines, 13(3), 670. https://doi.org/10.3390/biomedicines13030670






