Exploring Saliva as a Sample for Non-Invasive Glycemic Monitoring in Diabetes: A Scoping Review
Abstract
1. Introduction
2. Results and Discussion
2.1. General Findings and Descriptive Statistics
2.2. Salivary Glucose Monitoring in T1D: Evidence and Perspectives
2.3. Type 2 Diabetes (T2D) Monitoring Through Salivary Biomarkers
2.4. Saliva-Based Monitoring in GD
2.5. Salivary Glucose Monitoring in Mixed Diabetes Populations: Challenges and Insights
2.6. Expert Opinion: Research Limitations and Future Prospects
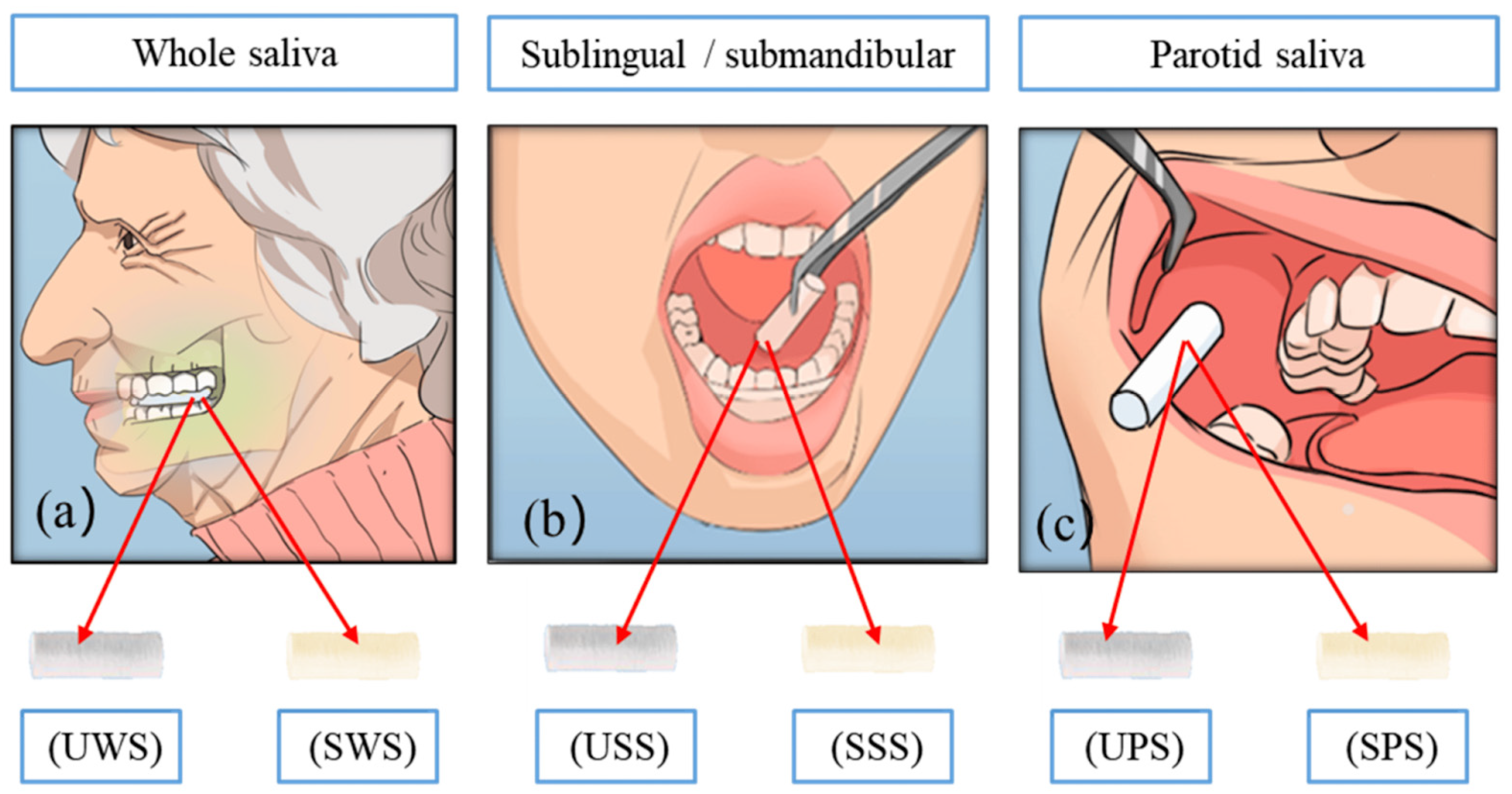
- (A)
- Type of collection:
- -
- Stimulated: Saliva is collected by chewing paraffin or sugar-free gum, enhancing flow.
- -
- (B)
- (C)
- Pre-collection care: Rigorous oral hygiene protocols are essential. Patients should refrain from brushing their teeth, using mouthwashes, smoking, or consuming any substances that may affect their salivary glucose levels for 30 min before collection. It is also advisable for patients to rinse their mouths with water to eliminate food debris before sample collection [4].
- (D)
- Collection method: The patient should slightly tilt their head forward to promote the accumulation of saliva in the oral cavity before expectorating into a sterile tube [15,74]. To mitigate the degradation of sensitive peptides, samples should be collected into pre-chilled polypropylene tubes maintained on ice [17]. Following their collection, samples should be centrifuged at 3000 RPM for 20 min to yield a clear supernatant for glucose analysis [17,55,58,74].
- (E)
- Storage and processing: Saliva must be stored at specified controlled temperatures. Salivary glucose can be preserved for at least one month at −20 °C; however, its levels begin to decline after two freeze/thaw cycles. For optimal long-term stability, it is recommended to freeze saliva samples in aliquots immediately post-collection at −20 °C [17,58].
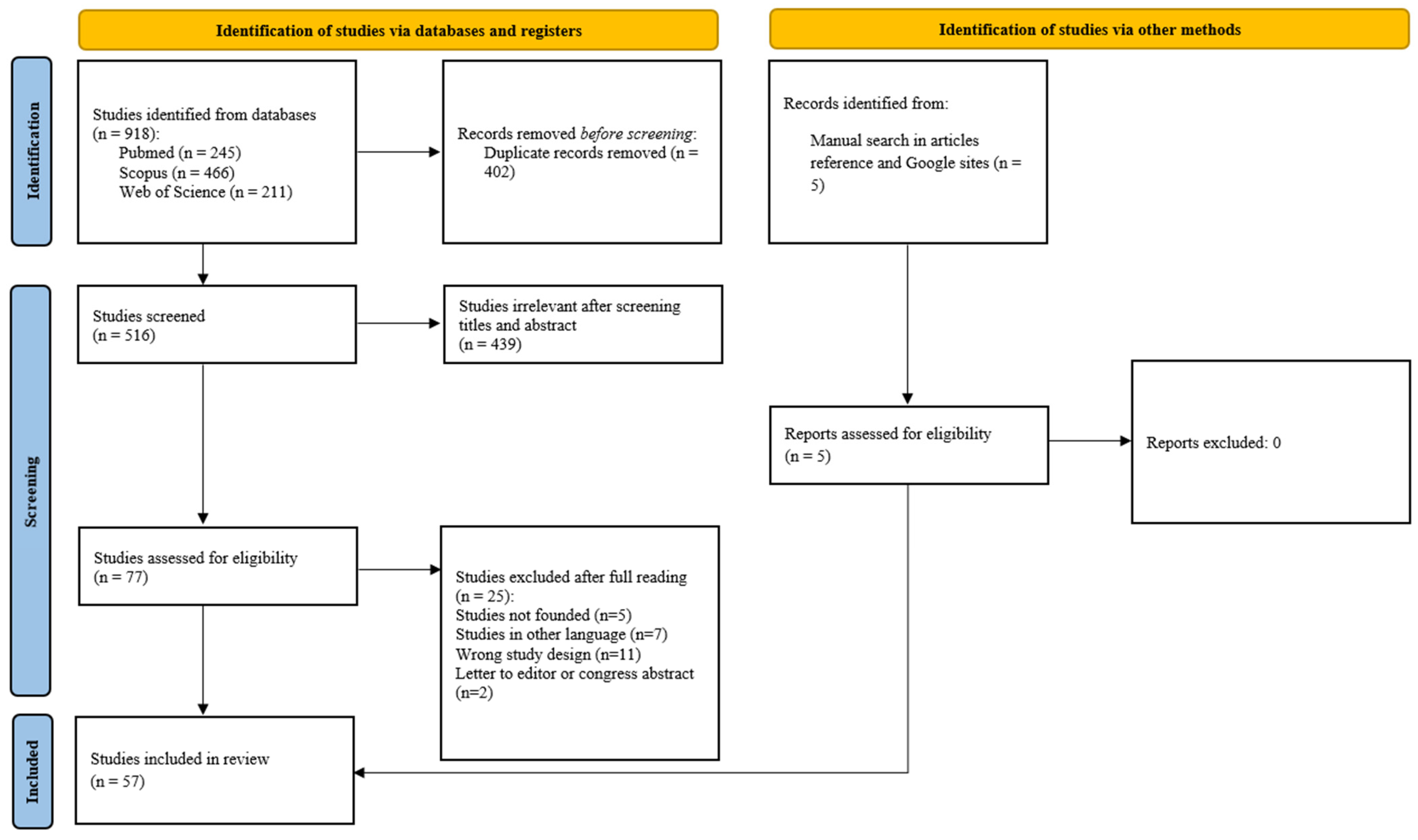
3. Materials and Methods
3.1. The Research Strategy
3.2. Eligibility Criteria
3.3. Study Selection
3.4. Data Extraction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DM | Diabetes mellitus |
| T1D | Type 1 diabetes |
| T2D | Type 2 diabetes |
| ADA | American Diabetes Association |
| GD | Gestational diabetes |
| HbA1c | Glycated hemoglobin |
| CRP | C-reactive protein |
| IL-6 | Interleukin-6 |
| IgA | Immunoglobulin A |
| TNF-α | Tumor necrosis factor-alpha |
| OGTT | Oral Glucose Tolerance Test |
| AGEs | Advanced glycation end products |
| AUC | Area under the curve |
| LLMs | Large language models |
| CFU | Candida colony-forming units |
| LADA | Latent Autoimmune Diabetes in Adults |
| UWS | Unstimulated whole saliva |
| SWS | Stimulated whole saliva |
| USS | Unstimulated sublingual saliva |
| SSS | Stimulated sublingual saliva |
| UPS | Unstimulated parotid saliva |
| SPS | Stimulated parotid saliva |
References
- American Diabetes Association Professional Practice Committee. Introduction and Methodology: Standards of Care in Diabetes—2025. Diabetes Care 2024, 48, S1–S5. [Google Scholar] [CrossRef]
- Harreiter, J.; Roden, M. Diabetes mellitus: Definition, classification, diagnosis, screening and prevention (Update 2023). Wien. Klin. Wochenschr. 2023, 135, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Darenskaya, M.A.; Kolesnikova, L.I.; Kolesnikov, S.I. Oxidative Stress: Pathogenetic Role in Diabetes Mellitus and Its Complications and Therapeutic Approaches to Correction. Bull. Exp. Biol. Med. 2021, 171, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Zhang, H.; Zhu, J.; Liao, Z.; Wang, S.; Liu, W. Correlations of Salivary and Blood Glucose Levels among Six Saliva Collection Methods. Int. J. Environ. Res. Public Health 2022, 19, 4122. [Google Scholar] [CrossRef]
- Sacks, D.B.; Arnold, M.; Bakris, G.L.; Bruns, D.E.; Horvath, A.R.; Lernmark, Å.; Metzger, B.E.; Nathan, D.M.; Kirkman, M.S. Guidelines and Recommendations for Laboratory Analysis in the Diagnosis and Management of Diabetes Mellitus. Diabetes Care 2023, 46, e151–e199. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. 2. Diagnosis and Classification of Diabetes: Standards of Care in Diabetes–2024. Diabetes Care 2024, 47, S20–S42. [Google Scholar] [CrossRef]
- International Expert Committee. International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care 2009, 32, 1327–1334. [Google Scholar] [CrossRef]
- Hirst, J.A.; McLellan, J.H.; Price, C.P.; English, E.; Feakins, B.G.; Stevens, R.J.; Farmer, A.J. Performance of point-of-care HbA1c test devices: Implications for use in clinical practice—A systematic review and meta-analysis. Clin. Chem. Lab. Med. 2017, 55, 167–180. [Google Scholar] [CrossRef]
- Diabetes Prevention Program Research Group. HbA1c as a predictor of diabetes and as an outcome in the diabetes prevention program: A randomized clinical trial. Diabetes Care 2015, 38, 51–58. [Google Scholar] [CrossRef]
- Ko, A.; Liao, C. Salivary glucose measurement: A holy ground for next generation of non-invasive diabetic monitoring. Hybrid Adv. 2023, 3, 13. [Google Scholar] [CrossRef]
- Nathan, D.M.; Genuth, S.; Lachin, J.; Cleary, P.; Crofford, O.; Davis, M.; Rand, L.; Siebert, C. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N. Engl. J. Med. 1993, 329, 977–986. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, R.P.; Sharma, N.; Gupta, V.B.; Jain, S.; Agarwal, V.; Goyal, S. Noninvasive Method for Glucose Level Estimation by Saliva. J. Diabetes Metab. 2013, 4, 266. [Google Scholar]
- Liao, C.; Xiao, S.; Wang, X. Bench-to-bedside: Translational development landscape of biotechnology in healthcare. Health Sci. Rev. 2023, 7, 100097. [Google Scholar] [CrossRef]
- Lenters-Westra, E.; Slingerland, R.J. Six of eight hemoglobin A1c point-of-care instruments do not meet the general accepted analytical performance criteria. Clin. Chem. 2010, 56, 44–52. [Google Scholar] [CrossRef]
- Tiongco, R.E.; Bituin, A.; Arceo, E.; Rivera, N.; Singian, E. Salivary glucose as a non-invasive biomarker of type 2 diabetes mellitus. J. Clin. Exp. Dent. 2018, 10, e902–e907. [Google Scholar] [CrossRef]
- Bordbar, M.M.; Hosseini, M.S.; Sheini, A.; Safaei, E.; Halabian, R.; Daryanavard, S.M.; Samadinia, H.; Bagheri, H. Monitoring saliva compositions for non-invasive detection of diabetes using a colorimetric-based multiple sensor. Sci. Rep. 2023, 13, 16174. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, H.; Wang, S.; Lu, J.; He, J.; Liu, L.; Liu, W. Obtaining a Reliable Diagnostic Biomarker for Diabetes Mellitus by Standardizing Salivary Glucose Measurements. Biomolecules 2022, 12, 1335. [Google Scholar] [CrossRef]
- Bellagambi, F.G.; Lomonaco, T.; Salvo, P.; Vivaldi, F.; Hangouët, M.; Ghimenti, S.; Biagini, D.; Di Francesco, F.; Fuoco, R.; Errachid, A. Saliva sampling: Methods and devices. An overview. TrAC Trends Anal. Chem. 2020, 124, 115781. [Google Scholar] [CrossRef]
- Liao, C.; Chen, X.; Fu, Y. Salivary analysis: An emerging paradigm for non-invasive healthcare diagnosis and monitoring. Interdiscip. Med. 2023, 1, e20230009. [Google Scholar] [CrossRef]
- Naing, C.; Mak, J.W. Salivary glucose in monitoring glycaemia in patients with type 1 diabetes mellitus: A systematic review. J. Diabetes Metab. Disord. 2017, 16, 2. [Google Scholar] [CrossRef]
- Kumari, S.; Samara, M.; Ampadi Ramachandran, R.; Gosh, S.; George, H.; Wang, R.; Pesavento, R.P.; Mathew, M.T. A Review on Saliva-Based Health Diagnostics: Biomarker Selection and Future Directions. Biomed. Mater. Devices 2023, 2, 121–138. [Google Scholar] [CrossRef] [PubMed]
- Satish, B.N.; Srikala, P.; Maharudrappa, B.; Awanti, S.M.; Kumar, P.; Hugar, D. Saliva: A tool in assessing glucose levels in Diabetes Mellitus. J. Int. Oral Health 2014, 6, 114–117. [Google Scholar] [PubMed]
- Englander, H.R.; Jeffay, A.I.; Fuller, J.B.; Chauncey, H.H. Glucose Concentrations in Blood Plasma and Parotid Saliva of Individuals with and Without Diabetes Mellitus. J. Dent. Res. 1963, 42, 1246. [Google Scholar] [CrossRef]
- Campbell, M.J.A. Glucose in the saliva of the non-diabetic and the diabetic patient. Arch. Oral Biol. 1965, 10, 197–205. [Google Scholar] [CrossRef]
- Ben-Aryeh, H.; Cohen, M.; Kanter, Y.; Szargel, R.; Laufer, D. Salivary composition in diabetic patients. J. Diabet. Complicat. 1988, 2, 96–99. [Google Scholar] [CrossRef]
- Darwazeh, A.M.G.; MacFarlane, T.W.; McCuish, A.; Lamey, P.-J. Mixed salivary glucose levels and candidal carriage in patients with diabetes mellitus. J. Oral Pathol. Med. 1991, 20, 280–283. [Google Scholar] [CrossRef]
- Belazi, M.A.; Galli-Tsinopoulou, A.; Drakoulakos, D.; Fleva, A.; Papanayiotou, P.H. Salivary alterations in insulin-dependent diabetes mellitus. Int. J. Paediatr. Dent. 1998, 8, 29–33. [Google Scholar] [CrossRef]
- Sashikumar, R.; Kannan, R. Salivary glucose levels and oral candidal carriage in type II diabetics. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2010, 109, 706–711. [Google Scholar] [CrossRef]
- Jurysta, C.; Bulur, N.; Oguzhan, B.; Satman, I.; Yilmaz, T.M.; Malaisse, W.J.; Sener, A. Salivary glucose concentration and excretion in normal and diabetic subjects. J. Biomed. Biotechnol. 2009, 2009, 430426. [Google Scholar] [CrossRef]
- Hegde, A.; Shenoy, R.; D’Mello, P.; Smitha, A.; Tintu, A.; Manjrekar, P. Alternative markers of glycemic status in diabetes mellitus. Biomed. Res. 2010, 21, 4. [Google Scholar]
- Vasconcelos, A.C.; Soares, M.S.; Almeida, P.C.; Soares, T.C. Comparative study of the concentration of salivary and blood glucose in type 2 diabetic patients. J. Oral Sci. 2010, 52, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Panchbhai, A.S.; Degwekar, S.S.; Bhowte, R.R. Estimation of salivary glucose, salivary amylase, salivary total protein and salivary flow rate in diabetics in India. J. Oral Sci. 2010, 52, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Nagalaxmi, V.; Priyanka, V. Can saliva be a marker for predicting Type 1 diabetes mellitus?—A pilot study. J. Indian Acad. Oral Med. Radiol. 2011, 23, 579–582. [Google Scholar] [CrossRef]
- Gheena, S.; Chandrasekhar, T.; Pratibhax, R. Salivary characteristics of diabetic children. Braz. J. Oral Sci. 2011, 10, 93–97. [Google Scholar]
- Abikshyeet, P.; Ramesh, V.; Oza, N. Glucose estimation in the salivary secretion of diabetes mellitus patients. Diabetes Metab. Syndr. Obes. Targets Ther. 2012, 5, 149–154. [Google Scholar] [CrossRef]
- Balan, P.; Babu, S.G.; Sucheta, K.N.; Shetty, S.R.; Rangare, A.L.; Castelino, R.L.; Fazil, A.K. Can saliva offer an advantage in monitoring of diabetes mellitus?—A case control study. J. Clin. Exp. Dent. 2014, 6, e335–e338. [Google Scholar] [CrossRef]
- Kumar, S.; Padmashree, S.; Jayalekshmi, R. Correlation of salivary glucose, blood glucose and oral candidal carriage in the saliva of type 2 diabetics: A case-control study. Contemp. Clin. Dent. 2014, 5, 312–317. [Google Scholar] [CrossRef]
- Shahbaz, S.; Katti, G.; Ghali, S.R.; Katti, C.; Diwakar, D.D.; Guduba, V. Salivary alterations in type 1 diabetes mellitus patients: Salivary glucose could be noninvasive tool for monitoring diabetes mellitus. Indian J. Dent. Res. 2014, 25, 420–424. [Google Scholar] [CrossRef]
- Patel, B.J.; Dave, B.; Dave, D.; Karmakar, P.; Shah, M.; Sarvaiya, B. Comparison and Correlation of Glucose Levels in Serum and Saliva of Both Diabetic and Non-diabetic Patients. J. Int. Oral Health 2015, 7, 70–76. [Google Scholar]
- Gupta, S.; Sandhu, S.V.; Bansal, H.; Sharma, D. Comparison of salivary and serum glucose levels in diabetic patients. J. Diabetes Sci. Technol. 2015, 9, 91–96. [Google Scholar] [CrossRef]
- Arora, K.S.; Binjoo, N.; Reddy, G.V.; Kaur, P.; Modgil, R.; Negi, L.S. Determination of normal range for fasting salivary glucose in Type 1 diabetics. J. Int. Soc. Prev. Community Dent. 2015, 5, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Lakshmi, P.V.; Sridevi, E.; Sai Sankar, A.J.; Manoj Kumar, M.G.; Sridhar, M.; Sujatha, B. Diagnostic perspective of saliva in insulin dependent diabetes mellitus children: An in vivo study. Contemp. Clin. Dent. 2015, 6, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, R.; Gopinathan, D.M.; Sukumaran, S. Estimation of Salivary Glucose and Glycogen Content in Exfoliated Buccal Mucosal Cells of Patients with Type II Diabetes Mellitus. J. Clin. Diagn. Res. 2015, 9, ZC89–ZC93. [Google Scholar] [CrossRef]
- Gupta, A.; Singh, S.K.; Padmavathi, B.N.; Rajan, S.Y.; Mamatha, G.P.; Kumar, S.; Roy, S.; Sareen, M. Evaluation of Correlation of Blood Glucose and Salivary Glucose Level in Known Diabetic Patients. J. Clin. Diagn. Res. 2015, 9, ZC106–ZC109. [Google Scholar] [CrossRef]
- Indira, M.; Chandrashekar, P.; Kattappagari, K.K.; Chandra, L.P.; Chitturi, R.T.; Bv, R.R. Evaluation of salivary glucose, amylase, and total protein in Type 2 diabetes mellitus patients. Indian J. Dent. Res. 2015, 26, 271–275. [Google Scholar] [CrossRef]
- Kadashetti, V.; Baad, R.; Malik, N.; Shivakumar, K.M.; Vibhute, N.; Belgaumi, U.; Gugawad, S.; Pramod, R.C. Glucose Level Estimation in Diabetes Mellitus By Saliva: A Bloodless Revolution. Rom. J. Intern. Med. 2015, 53, 248–252. [Google Scholar] [CrossRef]
- Mussavira, S.; Dharmalingam, M.; Omana Sukumaran, B. Salivary glucose and antioxidant defense markers in type II diabetes mellitus. Turk. J. Med. Sci. 2015, 45, 141–147. [Google Scholar] [CrossRef]
- Smriti, K.; Pai, K.M.; Ravindranath, V.; Gadicherla, S.; Pentapati, K.C. Salivary Glucose as a Diagnostic Marker for Diabetes Mellitus. J. Diabetes Sci. Technol. 2016, 10, 991–992. [Google Scholar] [CrossRef]
- Dhanya, M.; Hegde, S. Salivary glucose as a diagnostic tool in Type II diabetes mellitus: A case-control study. Niger. J. Clin. Pract. 2016, 19, 486–490. [Google Scholar] [CrossRef]
- Puttaswamy, K.A.; Puttabudhi, J.H.; Raju, S. Correlation between Salivary Glucose and Blood Glucose and the Implications of Salivary Factors on the Oral Health Status in Type 2 Diabetes Mellitus Patients. J. Int. Soc. Prev. Community Dent. 2017, 7, 28–33. [Google Scholar] [CrossRef]
- Wang, B.; Du, J.; Zhu, Z.; Ma, Z.; Wang, S.; Shan, Z. Evaluation of Parotid Salivary Glucose Level for Clinical Diagnosis and Monitoring Type 2 Diabetes Mellitus Patients. BioMed Res. Int. 2017, 2017, 2569707. [Google Scholar] [CrossRef] [PubMed]
- Abd-Elraheem, S.E.; El Saeed, A.M.; Mansour, H.H. Salivary changes in type 2 diabetic patients. Diabetes Metab. Syndr. 2017, 11 (Suppl. S2), S637–S641. [Google Scholar] [CrossRef] [PubMed]
- Shaik, S.; Jayam, R.; Bokkasam, V.; Dirasantchu, S.; Venkata, S.S.; Praveen, S. Salivary Glucose and Oral Mucosal Alterations in Type II Diabetic Mellitus Patients. J. Indian Acad. Oral Med. Radiol. 2017, 29, 259–262. [Google Scholar] [CrossRef]
- Carramolino-Cuellar, E.; Lauritano, D.; Silvestre, F.J.; Carinci, F.; Lucchese, A.; Silvestre-Rangil, J. Salivary flow and xerostomia in patients with type 2 diabetes. J. Oral Pathol. Med. 2017, 47, 526–530. [Google Scholar] [CrossRef]
- Gupta, S.; Nayak, M.T.; Sunitha, J.D.; Dawar, G.; Sinha, N.; Rallan, N.S. Correlation of salivary glucose level with blood glucose level in diabetes mellitus. J. Oral Maxillofac. Pathol. 2017, 21, 334–339. [Google Scholar] [CrossRef]
- Ghafouri, F.; Arab, H.; Keshavarzi, F. Evaluation of Fasting Blood Sugar via Salivary Glucose in Type 2 Diabetes Mellitus. Iran. J. Diabetes Obes. 2018, 10, 4. [Google Scholar]
- Bhattacharyya, A.; Chandra, S.; Singh, A.; Raj, V.; Gupta, B. Salivary glucose levels and oral candidal carriage in Type 2 diabetics. J. Oral Biol. Craniofacial Res. 2018, 8, 158–164. [Google Scholar] [CrossRef]
- Harish, S.; Shantaram, M. A Comparative and correlative study between blood and salivary glucose with blood HbA1c in type 2 diabetes. Int. J. Pharm. Sci. Res. 2019, 10, 5. [Google Scholar] [CrossRef]
- Fares, S.; Said, M.S.M.; Ibrahim, W.; Amin, T.T.; Saad, N.E.S. Accuracy of salivary glucose assessment in diagnosis of diabetes and prediabestes. Diabetes Metab. Syndr. 2019, 13, 1543–1547. [Google Scholar] [CrossRef]
- Mishra, N.; Trivedi, A.; Gajdhar, S.K.; Bhagwat, H.; Khutwad, G.K.; Mall, P.E.; Kulkarni, D. Correlation of Blood Glucose Levels, Salivary Glucose Levels and Oral Colony Forming Units of Candida albicans in Type 2 Diabetes Mellitus Patients. J. Contemp. Dent. Pract. 2019, 20, 494–498. [Google Scholar]
- Ephraim, R.K.D.; Anto, E.O.; Acheampong, E.; Fondjo, L.A.; Barnie, R.B.; Sakyi, S.A.; Asare, A. Fasting salivary glucose levels is not a better measure for identifying diabetes mellitus than serum or capillary blood glucose levels: Comparison in a Ghanaian population. Heliyon 2019, 5, e01286. [Google Scholar] [CrossRef] [PubMed]
- Ragunathan, H.; Aswath, N.; Sarumathi, T. Salivary glucose estimation: A noninvasive method. Indian J. Dent. Sci. 2019, 11, 25. [Google Scholar] [CrossRef]
- Hegde, S.S.; Sattur, A.P.; Bargale, A.B.; Rao, G.S.; Shetty, R.S.; Kulkarni, R.D.; Ajantha, G.S. Estimation and correlation of serum and salivary glucose and immunoglobulin A levels and salivary candidal carriage in diabetic and non-diabetic patients. J. Dent. Res. Dent. Clin. Dent. Prospect. 2020, 14, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Mrag, M.; Kassab, A.; Omezzine, A.; Belkacem, C.R.; Ben, F.I.F.; Douki, N.; Laouani, K.C.; Bouslema, A.; Ben, A.F. Saliva diagnostic utility in patients with type 2 diabetes: Future standard method. J. Med. Biochem. 2020, 39, 140–148. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, T.; Bhargava, M.; Raj, R.; Vaibhav, V.; Kishore, J. Salivary and Serum Glucose Levels in Diabetes Mellitus Patients versus Control—A Randomised Control Trial. J. Med. Life 2020, 13, 235–240. [Google Scholar] [CrossRef]
- Gupta, V.; Kaur, A. Salivary glucose levels in diabetes mellitus patients: A case-control study. J. Oral Maxillofac. Pathol. 2020, 24, 187. [Google Scholar] [CrossRef]
- Dharmakeerthi, K.I.; Ponweera, M.P.; Moragoda, E.H.; Galgamuwa, L.S.; Jayasekara, K.; Kaluarachchi, V.; Bulugahapitiya, U. Correlation Between Blood Glucose and Salivary Glucose in Type 2 Diabetes Mellitus Patients. Malays. J. Med. Health Sci. 2021, 17, 5. [Google Scholar]
- Ganesan, A.; Muthukrishnan, A.; Veeraraghavan, V. Effectiveness of Salivary Glucose in Diagnosing Gestational Diabetes Mellitus. Contemp. Clin. Dent. 2021, 12, 294–300. [Google Scholar] [CrossRef]
- Egboh, V.O.; Ohwin, P.E.; Daubry, T.M.E.; Ofulue, O.O.; Nwogueze, B.C.; Ojugbeli, E.T.; Osuagwu, U.L.; Nwangwa, E.K. Comparative Analysis of Fasting Blood Glucose and Salivary Electrolytes Concentrations among Individuals with Type II Diabetes: A Randomized Controlled Hospital Based Study. Toxicol. Rep. 2022, 9, 1268–1272. [Google Scholar] [CrossRef]
- Ganesan, A.; Muthukrishnan, A.; Veeraraghavan, V.P.; Kumar, N.G. Effectiveness of Salivary Glucose as a Reliable Alternative in Diagnosis of Type 1 Diabetes Mellitus: A Cross-Sectional Study. J. Pharm. Bioallied Sci. 2022, 14, S557–S562. [Google Scholar] [CrossRef]
- Cheprasova, A.; Mittova, V.O.; Kryl’skii, E.D.; Verevkin, A.N.; Pashkov, A.N.; Popov, S.S. Oxidative status, carbohydrate, and lipid metabolism indicators in saliva and blood serum of type 1 diabetes mellitus patients. Biomed. Res. Ther. 2022, 9, 5233–5240. [Google Scholar] [CrossRef]
- Choudhry, A.A.; Kumar, P.; Prasad, M.; Mohapatra, T.; Sharma, P. Validation of salivary glucose as a screening tool of diabetes mellitus. Rom. J. Intern. Med. = Rev. Roum. Med. Interne 2022, 60, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Pandey, D.; Chandra, P.; Somanna, P.; Sampath, R.; Pachipulusu, B. Saliva as a diagnostic tool for glucose estimation in diabetic patients. J. Indian Acad. Oral Med. Radiol. 2023, 35, 527. [Google Scholar] [CrossRef]
- Shettigar, L.; Sivaraman, S.; Rao, R.; Arun, S.A.; Chopra, A.; Kamath, S.U.; Rana, R. Correlational analysis between salivary and blood glucose levels in individuals with and without diabetes mellitus: A cross-sectional study. Acta Odontol. Scand. 2024, 83, 101–111. [Google Scholar] [CrossRef]
- IDF. IDF Diabetes Atlas; IDF: Brussels, Belgium, 2021. [Google Scholar]
- Subramanian, S.; Khan, F.; Hirsch, I.B. New advances in type 1 diabetes. BMJ 2024, 384, e075681. [Google Scholar] [CrossRef]
- Dall, T.M.; Yang, W.; Gillespie, K.; Mocarski, M.; Byrne, E.; Cintina, I.; Beronja, K.; Semilla, A.P.; Iacobucci, W.; Hogan, P.F. The Economic Burden of Elevated Blood Glucose Levels in 2017: Diagnosed and Undiagnosed Diabetes, Gestational Diabetes Mellitus, and Prediabetes. Diabetes Care 2019, 42, 1661–1668. [Google Scholar] [CrossRef]
- Lee, H.; Hong, Y.J.; Baik, S.; Hyeon, T.; Kim, D.-H. Enzyme-Based Glucose Sensor: From Invasive to Wearable Device. Adv. Healthc. Mater. 2018, 7, 1701150. [Google Scholar] [CrossRef]
- ADA. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2013, 37, S81–S90. [Google Scholar] [CrossRef]
- Gale, E.A.M.; Gillespie, K.M. Diabetes and gender. Diabetologia 2001, 44, 3–15. [Google Scholar] [CrossRef]
- Fox, D.A.; Islam, N.; Sutherland, J.; Reimer, K.; Amed, S. Type 1 diabetes incidence and prevalence trends in a cohort of Canadian children and youth. Pediatr. Diabetes 2018, 19, 501–505. [Google Scholar] [CrossRef]
- Samuelsson, U.; Lindblad, B.; Carlsson, A.; Forsander, G.; Ivarsson, S.; Kockum, I.; Lernmark, Å.; Marcus, C.; Ludvigsson, J. Residual beta cell function at diagnosis of type 1 diabetes in children and adolescents varies with gender and season. Diabetes/Metab. Res. Rev. 2013, 29, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Blohmé, G.; Nyström, L.; Arnqvist, H.J.; Lithner, F.; Littorin, B.; Olsson, P.O.; Scherstén, B.; Wibell, L.; Ostman, J. Male predominance of type 1 (insulin-dependent) diabetes mellitus in young adults: Results from a 5-year prospective nationwide study of the 15-34-year age group in Sweden. Diabetologia 1992, 35, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Holman, N.; Young, B.; Gadsby, R. Current prevalence of Type 1 and Type 2 diabetes in adults and children in the UK. Diabet. Med. 2015, 32, 1119–1120. [Google Scholar] [CrossRef]
- IDF. IDF Diabetes Atlas; IDF: Brussels, Belgium, 2015. [Google Scholar]
- Ng, M.; Fleming, T.; Robinson, M.; Thomson, B.; Graetz, N.; Margono, C.; Mullany, E.C.; Biryukov, S.; Abbafati, C.; Abera, S.F.; et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014, 384, 766–781. [Google Scholar] [CrossRef]
- Bruno, G.; Runzo, C.; Cavallo-Perin, P.; Merletti, F.; Rivetti, M.; Pinach, S.; Novelli, G.; Trovati, M.; Cerutti, F.; Pagano, G. Incidence of type 1 and type 2 diabetes in adults aged 30-49 years: The population-based registry in the province of Turin, Italy. Diabetes Care 2005, 28, 2613–2619. [Google Scholar] [CrossRef]
- Xu, S.T.; Sun, M.; Xiang, Y. Global, regional, and national trends in type 2 diabetes mellitus burden among adolescents and young adults aged 10–24 years from 1990 to 2021: A trend analysis from the Global Burden of Disease Study 2021. World J. Pediatr. 2025, 21, 73–89. [Google Scholar] [CrossRef]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef]
- Bommer, C.; Heesemann, E.; Sagalova, V.; Manne-Goehler, J.; Atun, R.; Bärnighausen, T.; Vollmer, S. The global economic burden of diabetes in adults aged 20-79 years: A cost-of-illness study. Lancet Diabetes Endocrinol. 2017, 5, 423–430. [Google Scholar] [CrossRef]
- Harrison, R.; Bowen, W.H. Flow rate and organic constituents of whole saliva in insulin-dependent diabetic children and adolescents. Pediatr. Dent. 1987, 9, 287–291. [Google Scholar]
- Miller, C.S.; Foley, J.D.; Bailey, A.L.; Campell, C.L.; Humphries, R.L.; Christodoulides, N.; Floriano, P.N.; Simmons, G.; Bhagwandin, B.; Jacobson, J.W.; et al. Current developments in salivary diagnostics. Biomark. Med. 2010, 4, 171–189. [Google Scholar] [CrossRef]
- Mirzaii-Dizgah, I.; Mirzaii-Dizgah, M.; Mirzaii-Dizgah, M. Stimulated Saliva Glucose as a Diagnostic Specimen for Detection of Diabetes Mellitus. J. Arch. Mil. Med. 2013, 1, 24–27. [Google Scholar] [CrossRef]
- Ladgotra, A.; Verma, P.; Raj, S.S. Estimation of Salivary and Serum Biomarkers in Diabetic and Non Diabetic Patients—A Comparative Study. J. Clin. Diagn. Res. 2016, 10, ZC56–ZC61. [Google Scholar] [CrossRef] [PubMed]
- Negrato, C.A.; Tarzia, O. Buccal alterations in diabetes mellitus. Diabetol. Metab. Syndr. 2010, 2, 3. [Google Scholar] [CrossRef]
- Buchanan, T.A.; Xiang, A.; Kjos, S.L.; Watanabe, R. What is gestational diabetes? Diabetes Care 2007, 30 (Suppl. S2), S105–S111. [Google Scholar] [CrossRef]
- Noctor, E.; Crowe, C.; Carmody, L.A.; Saunders, J.A.; Kirwan, B.; O’Dea, A.; Gillespie, P.; Glynn, L.G.; McGuire, B.E.; O’Neill, C.; et al. Abnormal glucose tolerance post-gestational diabetes mellitus as defined by the International Association of Diabetes and Pregnancy Study Groups criteria. Eur. J. Endocrinol. 2016, 175, 287–297. [Google Scholar] [CrossRef]
- Kim, C.; Newton, K.M.; Knopp, R.H. Gestational diabetes and the incidence of type 2 diabetes: A systematic review. Diabetes Care 2002, 25, 1862–1868. [Google Scholar] [CrossRef]
- Setji, T.L.; Brown, A.J.; Feinglos, M.N. Gestational Diabetes Mellitus. Clin. Diabetes 2005, 23, 17–24. [Google Scholar] [CrossRef]
- Choudhury, A.A.; Devi Rajeswari, V. Gestational diabetes mellitus—A metabolic and reproductive disorder. Biomed. Pharmacother. 2021, 143, 112183. [Google Scholar] [CrossRef]
- Dong, Y.; Zhai, Y.; Wang, J.; Chen, Y.; Xie, X.; Zhang, C.; Liu, J.; Lu, Y.; Tang, G.; Han, L.; et al. Glycated albumin in pregnancy: Reference intervals establishment and its predictive value in adverse pregnancy outcomes. BMC Pregnancy Childbirth 2020, 20, 12. [Google Scholar] [CrossRef]
- Rodrigo, N.; Glastras, S.J. The Emerging Role of Biomarkers in the Diagnosis of Gestational Diabetes Mellitus. J. Clin. Med. 2018, 7, 120. [Google Scholar] [CrossRef]
- Bogdanet, D.; O’Shea, P.; Lyons, C.; Shafat, A.; Dunne, F. The Oral Glucose Tolerance Test-Is It Time for a Change?—A Literature Review with an Emphasis on Pregnancy. J. Clin. Med. 2020, 9, 3451. [Google Scholar] [CrossRef] [PubMed]
- Lages, M.; Barros, R.; Moreira, P.; Guarino, M.P. Metabolic Effects of an Oral Glucose Tolerance Test Compared to the Mixed Meal Tolerance Tests: A Narrative Review. Nutrients 2022, 14, 2032. [Google Scholar] [CrossRef] [PubMed]
- Nunes, L.A.S.; de Macedo, D.V. Saliva as a diagnostic fluid in sports medicine: Potential and limitations. J. Bras. Patol. Med. Lab. 2013, 49, 247–255. [Google Scholar] [CrossRef]
- Alarcón-Sánchez, M.A.; Becerra-Ruiz, J.S.; Avetisyan, A.; Heboyan, A. Activity and levels of TNF-α, IL-6 and IL-8 in saliva of children and young adults with dental caries: A systematic review and meta-analysis. BMC Oral Health 2024, 24, 816. [Google Scholar] [CrossRef]
- Lima-Aragão, M.V.; de Oliveira-Junior Jde, J.; Maciel, M.C.; Silva, L.A.; do Nascimento, F.R.; Guerra, R.N. Salivary profile in diabetic patients: Biochemical and immunological evaluation. BMC Res. Notes 2016, 9, 103. [Google Scholar] [CrossRef]
- Marques, R.C.R.; da Silva, J.R.; Vieira Lima, C.P.; Stefani, C.M.; Damé-Teixeira, N. Salivary parameters of adults with diabetes mellitus: A systematic review and meta-analysis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2022, 134, 176–189. [Google Scholar] [CrossRef]
- Ferizi, L.; Dragidella, F.; Spahiu, L.; Begzati, A.; Kotori, V. The Influence of Type 1 Diabetes Mellitus on Dental Caries and Salivary Composition. Int. J. Dent. 2018, 2018, 5780916. [Google Scholar] [CrossRef]
- Akkemik, Ö.; Kesim, S.; Çabuk Renklibay, E.; Ökdemir, D.; Saraymen, R.; Kurtoğlu, S. Saliva and GCF cytokine levels in insulin-dependent diabetic teens and their relationship with metabolic status and disease duration. Pediatr. Dent. J. 2023, 33, 192–198. [Google Scholar] [CrossRef]
- Moore, P.A.; Guggenheimer, J.; Etzel, K.R.; Weyant, R.J.; Orchard, T. Type 1 diabetes mellitus, xerostomia, and salivary flow rates. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2001, 92, 281–291. [Google Scholar] [CrossRef]
- Desai, P.; Donovan, L.; Janowitz, E.; Kim, J.Y. The Clinical Utility of Salivary Biomarkers in the Identification of Type 2 Diabetes Risk and Metabolic Syndrome. Diabetes Metab. Syndr. Obes. Targets Ther. 2020, 13, 3587–3599. [Google Scholar] [CrossRef]
- Agho, E.T.; Owotade, F.J.; Kolawole, B.A.; Oyetola, E.O.; Adedeji, T.A. Salivary inflammatory biomarkers and glycated haemoglobin among patients with type 2 diabetic mellitus. BMC Oral Health 2021, 21, 101. [Google Scholar] [CrossRef] [PubMed]
- Aisha, H.; Nashiya, F.; Shyma, Z.; Farooqui, S.; Zulekha, S.; Francis, P.S. Salivary Glucose as a Potential Biomarker for Monitoring Blood Glucose Levels in Type 2 Diabetes Mellitus: Current Insights and Future Prospects. Indian J. Pharm. Pract. 2024, 17, 109–118. [Google Scholar] [CrossRef]
- Aitken-Saavedra, J.; Rojas-Alcayaga, G.; Maturana-Ramírez, A.; Escobar-Álvarez, A.; Cortes-Coloma, A.; Reyes-Rojas, M.; Viera-Sapiain, V.; Villablanca-Martínez, C.; Morales-Bozo, I. Salivary gland dysfunction markers in type 2 diabetes mellitus patients. J. Clin. Exp. Dent. 2015, 7, e501–e505. [Google Scholar] [CrossRef][Green Version]
- Srinivasan, M.; Blackburn, C.; Mohamed, M.; Sivagami, A.V.; Blum, J. Literature–Based Discovery of Salivary Biomarkers for Type 2 Diabetes Mellitus. Biomark. Insights 2015, 10, BMI.S22177. [Google Scholar] [CrossRef]
- Crusell, M.K.W.; Brink, L.R.; Nielsen, T.; Allin, K.H.; Hansen, T.; Damm, P.; Lauenborg, J.; Hansen, T.H.; Pedersen, O. Gestational diabetes and the human salivary microbiota: A longitudinal study during pregnancy and postpartum. BMC Pregnancy Childbirth 2020, 20, 69. [Google Scholar] [CrossRef]
- Bulut, A.; Akca, G.; Keskin Aktan, A.; Akbulut, K.G.; Babül, A. The significance of blood and salivary oxidative stress markers and chemerin in gestational diabetes mellitus. Taiwan. J. Obstet. Gynecol. 2021, 60, 695–699. [Google Scholar] [CrossRef]
- Li, Y.; Feng, Y.; Yang, Z.; Zhou, Z.; Jiang, D.; Luo, J. Untargeted metabolomics of saliva in pregnant women with and without gestational diabetes mellitus and healthy non-pregnant women. Front. Cell. Infect. Microbiol. 2023, 13, 1206462. [Google Scholar] [CrossRef]
- Zygula, A.; Kosinski, P.; Zwierzchowska, A.; Sochacka, M.; Wroczynski, P.; Makarewicz-Wujec, M.; Pietrzak, B.; Wielgos, M.; Rzentala, M.; Giebultowicz, J. Oxidative stress markers in saliva and plasma differ between diet-controlled and insulin-controlled gestational diabetes mellitus. Diabetes Res. Clin. Pract. 2019, 148, 72–80. [Google Scholar] [CrossRef]
- Nam, Y.; Kim, Y.Y.; Chang, J.Y.; Kho, H.S. Salivary biomarkers of inflammation and oxidative stress in healthy adults. Arch. Oral Biol. 2019, 97, 215–222. [Google Scholar] [CrossRef]
- Shah, V.S.; Pareikh, D.; Manjunatha, B.S. Salivary alpha-amylase-biomarker for monitoring type II diabetes. J. Oral Maxillofac. Pathol. 2021, 25, 441–445. [Google Scholar] [CrossRef]
- Vuletić, L.; Špalj, S.; Rogić, D.; Peroš, K. The rise in glucose concentration in saliva samples mixed with test foods monitored using a glucometer: An observational pilot study. J. Oral Biosci. 2019, 61, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Kubala, E.; Strzelecka, P.; Grzegocka, M.; Lietz-Kijak, D.; Gronwald, H.; Skomro, P.; Kijak, E. A Review of Selected Studies That Determine the Physical and Chemical Properties of Saliva in the Field of Dental Treatment. BioMed Res. Int. 2018, 2018, 6572381. [Google Scholar] [CrossRef] [PubMed]
- López, M.E.; Colloca, M.E.; Páez, R.G.; Schallmach, J.N.; Koss, M.A.; Chervonagura, A. Salivary characteristics of diabetic children. Braz. Dent. J. 2003, 14, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Lamey, P.-J.; Fisher, B.M.; Frier, B.M. The Effects of Diabetes and Autonomic Neuropathy on Parotid Salivary Flow in Man. Diabet. Med. 1986, 3, 537–540. [Google Scholar] [CrossRef]
- Zhu, P.; Hu, J.; Li, X.; Zhu, Q. Using Blockchain Technology to Enhance the Traceability of Original Achievements. IEEE Trans. Eng. Manag. 2023, 70, 1693–1707. [Google Scholar] [CrossRef]
- Hu, J.; Zhu, P.; Li, J.; Qi, Y.; Xia, Y.; Wang, F.Y. A Secure Medical Information Storage and Sharing Method Based on Multiblockchain Architecture. IEEE Trans. Comput. Soc. Syst. 2024, 11, 6392–6406. [Google Scholar] [CrossRef]
- Sun, L.; Liu, D.; Wang, M.; Han, Y.; Zhang, Y.; Zhou, B.; Ren, Y.; Zhu, P. Taming Unleashed Large Language Models with Blockchain for Massive Personalized Reliable Healthcare. IEEE J. Biomed. Health Inform. 2025, 1–20. [Google Scholar] [CrossRef]
- Peters, M.D.; Godfrey, C.M.; Khalil, H.; McInerney, P.; Parker, D.; Soares, C.B. Guidance for conducting systematic scoping reviews. Int. J. Evid.-Based Healthc. 2015, 13, 141–146. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]


| Author and Year | Type of Diabetes | Number of Diabetic and Non-Diabetic (♂/♀) | Saliva Sample | Fasting (Yes/No) | Biomarkers |
|---|---|---|---|---|---|
| Englander et al., 1963 [23] | Not specified | 26 and 26 | Stimulated (lemon juice) | Yes | Plasma glucose |
| Campbell et al., 1965 [24] | Not specified | 60 and 60 | Spontaneous | No, 2 h after last meal | Plasma glucose, galacturonic acid, glucuronic acid, lactose, and others |
| Ben-Aryeh et al., 1988 [25] | Not specified | 31 (17/14) and 35 (20/15) | Spontaneous and stimulated (citric acid) | No, 1 h after last meal | Plasma glucose, sodium, potassium, protein, and amylase |
| Darwazeh et al., 1991 [26] | Not specified | 41 and 34 | Spontaneous | No, 2 h after last meal | Plasma glucose, HbA1C, and candida count |
| Belazi et al., 1998 [27] | T1D | 10 (5/5) and 10 (6/5) | Spontaneous | No, 2 h after last meal | Plasma glucose |
| Sashikumar et al., 2009 [28] | T2D | 100 and 50 | Spontaneous and stimulated (citric acid) | No, 2 h after last meal | Plasma glucose, HbA1C, and candida count |
| Jurysta et al., 2009 [29] | T1D and T2D | 84 (36/48) and 38 (16/22) | Spontaneous and stimulated (mastication) | Yes | Plasma glucose |
| Hegde et al., 2010 [30] | T2D | 26 and 21 | Spontaneous | Not informed | Plasma glucose, pH, and oxidative stress markers |
| Vasconcelos et al., 2010 [31] | T2D | 40 and 40 | Spontaneous | No, 90 min after last meal | Plasma glucose |
| Panchbhai et al., 2010 [32] | T1D and T2D | 40 (16/24) and 80 (25/15 and 22/18) | Spontaneous | No, 2 h after last meal | Plasma glucose |
| Nagalaxmi et al., 2011 [33] | T1D | 50 (28/22) and 50 (28/22) | Spontaneous and draining | Yes | Plasma glucose |
| Gheena et al., 2011 [34] | T1D | 32 and 32 | Spontaneous | No, 1 h after last meal | Plasma glucose, cholesterol, albumin, and total protein |
| Abikshyeet et al., 2012 [35] | T2D | 106 (52/54) and 15 (9/6) | Spontaneous | Yes | Plasma glucose and HbA1C |
| Agrawal et al., 2013 [12] | Not specified | 40 and 40 | Spontaneous | No, 30 min after last meal | Plasma glucose |
| Balan et al., 2014 [36] | T2D | 30 and 60 | Spontaneous | No, 2 h after last meal | Plasma glucose |
| Kumar et al., 2014 [37] | T2D | 60 and 30 | Spontaneous | No, 2 h after last meal | Plasma glucose, HbA1C, and salivary candida count |
| Satish et al., 2014 [22] | T2D | 20 and 10 | Spontaneous | Yes | Plasma glucose |
| Shahbaz et al., 2014 [38] | T1D | 30 and 30 (30/30) | Spontaneous | Yes | Plasma glucose, total protein, and albumin |
| Patel et al., 2015 [39] | T1D and T2D | 50 and 50 | Spontaneous | Not informed | Plasma glucose |
| Gupta et al., 2015 [40] | T2D | 100 (46/54) and 100 (54/46) | Spontaneous | No | Plasma glucose and HbA1C |
| Arora et al., 2015 [41] | T1D | 100 (64/36) and 100 (47/53) | Spontaneous | Yes | Plasma glucose |
| Lakshmi et al., 2015 [42] | T1D | 30 and 30 | Spontaneous | Yes | Plasma glucose |
| Ravindran et al., 2015 [43] | T2D | 30 and 30 | Spontaneous | Yes | Plasma glucose and HbA1C |
| Gupta et al., 2015 [44] | T1D and T2D | 212 and 38 (106/144) | Spontaneous and aspiration | Yes | Plasma glucose |
| Indira et al., 2015 [45] | T2D | 20 (10/10) and 20 (10/10) | Spontaneous | No, 2 h after last meal | Plasma glucose, salivary amylase, and total protein |
| Kadashetti et al., 2015 [46] | Not specified | 53 (26/27) and 37 (22/15) | Spontaneous | Yes | Plasma glucose |
| Mussavira et al., 2015 [47] | T2D | 53 and 40 | Spontaneous | Yes | Plasma glucose, total protein, uric acid, and antioxidant markers |
| Smriti et al., 2016 [48] | Not specified | 120 and 60 | Spontaneous | Yes | Plasma glucose |
| Dhanya et al., 2016 [49] | T2D | 100 and 100 | Spontaneous | Yes | Plasma glucose |
| Puttaswamy et al., 2017 [50] | T2D | 40 and 60 | Spontaneous | Not informed | Plasma glucose |
| Wang et al., 2017 [51] | T2D | 30 (17/13) and 30 (15/15) | Spontaneous | Yes | Plasma glucose |
| Abd-Elraheem et al., 2017 [52] | T2D | 20 and 20 (20/20) | Spontaneous | Yes | Plasma glucose, HbA1C, IgA, and salivary amylase |
| Shaik et al., 2017 [53] | T2D | 70 and 70 | Spontaneous | Yes and 2 h postprandial | Plasma glucose |
| Carramolino-Cuéllar et al., 2017 [54] | T2D | 46 and 47 | Spontaneous and stimulated (paraffin tablet) | Yes and 2 h postprandial | Plasma glucose |
| Gupta et al., 2017 [55] | Not specified | 80 and 40 | Spontaneous | Yes and postprandial | Plasma glucose |
| Ghafouri et al., 2018 [56] | T2D | 50 and 50 | Spontaneous | Yes | Plasma glucose |
| Tiongco et al., 2018 [15] | T2D | 97 and 34 | Spontaneous | Yes | Plasma glucose |
| Bhattacharyya et al., 2018 [57] | T2D | 97 and 34 | Spontaneous | Yes and 2 h postprandial | Plasma glucose |
| Harish et al., 2019 [58] | T2D | 50 and 50 | Spontaneous | Yes | Plasma glucose, HbA1C |
| Fares et al., 2019 [59] | T2D and pre-diabetes | 154 and 50 | Spontaneous | Not informed | Plasma glucose |
| Mishra et al., 2019 [60] | T2D | 100 and 100 | Spontaneous | No, 2 h after last meal | Plasma glucose and candida count |
| Ephraim et al., 2019 [61] | Not specified | 79 and 59 | Spontaneous | Yes | Plasma glucose |
| Ragunathan et al., 2019 [62] | Not specified | 40 and 40 | Spontaneous | No, 90 min after last meal | Plasma glucose |
| Hegde et al., 2020 [63] | Not specified | 59 and 29 (45/43) | Spontaneous | Not informed | Plasma glucose, IgA, and candida count |
| Mrag et al., 2020 [64] | T2D | 300 and 300 | Spontaneous | Yes | Plasma glucose, urea, amylase, total protein, albumin, electrolytes, C-reactive protein (CRP), and immunoglobulin A (IgA) |
| Kumar et al., 2020 [65] | T1D and T2D | 150 and 50 | Spontaneous | Not informed | Plasma glucose |
| Gupta et al., 2020 [66] | Not specified | 45 and 45 | Spontaneous | Yes and 2 h postprandial | Plasma glucose |
| Dharmakeerthi et al., 2021 [67] | T2D | 120 and 31 | Spontaneous | Yes | Plasma glucose |
| Ganesan et al., 2021 [68] | GD | 100 (0/100) and 99 (0/99) | Spontaneous and stimulated (citric acid) | Yes and 2 h postprandial | Plasma glucose |
| Egboh et al., 2022 [69] | T2D | 45 (23/22) and 40 (20/20) | Spontaneous | Yes | Plasma glucose, sodium, potassium, bicarbonate, and chlorine |
| Cui et al., 2022a [17] | T1D and T2D | 40 and 40 | Spontaneous and stimulated (citric acid) | No, 30 min after last meal | Plasma glucose |
| Cui et al., 2022b [4] | Not specified | 40 and 40 | Spontaneous | No, 30 min after last meal | Plasma glucose |
| Ganesan et al., 2022 [70] | T1D | 79 (38/41) and 100 (58/42) | Spontaneous and stimulated (citric acid) | Yes and 2 h postprandial | Plasma glucose |
| Cheprasova et al., 2022 [71] | T1D | 40 (20/20) and 40 (20/20) | Spontaneous | Not informed | Plasma glucose, salivary protein, salivary cholesterol and triglycerides, and chaperone-like activity |
| Choudhry et al., 2022 [72] | T2D | 100 (67/33) and 100 (65/35) | Spontaneous | Yes | Plasma glucose |
| Pandey et al., 2023 [73] | T1D, T2D and GD | 200 (105/95) and 200 (102/98) | Spontaneous | Not informed | Plasma glucose |
| Shettigar et al., 2024 [74] | T2D | 83 (31/52) and 83 (36/47) | Spontaneous | Not informed | Plasma glucose |
| Author and Year | Type of Diabetes | Fasting Serum Glucose (mg/dL) (DM/Control) | Fasting Salivary Glucose (mg/dL) (DM/Control) | ||
|---|---|---|---|---|---|
| Englander et al., 1963 [23] | Not specified | 142 | 92 | 1.61 | 0.78 |
| Campbell et al., 1965 [24] | Not specified | - | - | - | - |
| Ben-Aryeh et al., 1988 [25] | Not specified | ||||
| Darwazeh et al., 1991 [26] | Not specified | - | - | - | - |
| Belazi et al., 1998 [27] | T1D | - | - | - | - |
| Sashikumar et al., 2009 [28] | T2D | - | - | - | - |
| Jurysta et al., 2009 [29] | T1D and T2D | - | - | Stimulated: ♂ 3.67/♀ 3.15 Not Stimulated: ♂ 3.64/♀ 3.15 | Stimulated: ♂ 0.53/♀ 0.62 Not Stimulated: ♂ 1.42/♀ 1.45 |
| Hegde et al., 2010 [30] | T2D | 144.31 | 99.71 | 10.46 | 7.41 |
| Vasconcelos et al., 2010 [31] | T2D | - | - | - | - |
| Panchbhai et al., 2010 [32] | T1D and T2D | - | - | - | - |
| Nagalaxmi et al., 2011 [33] | T1D | 306.1 | 86.56 | 30.54 | 9.174 |
| Gheena et al., 2011 [34] | T1D | - | - | - | - |
| Abikshyeet et al., 2012 [35] | T2D | 154.70 | 86.82 | 4.22 | 1.23 |
| Agrawal et al., 2013 [12] | Not specified | 171.31 | 92.11 | 10.93 | 6.08 |
| Balan et al., 2014 [36] | T2D | - | - | - | - |
| Kumar et al., 2014 [37] | T2D | - | - | - | - |
| Satish et al., 2014 [22] | T2D | 205.2 | 90.5 | 12.11 | 4.32 |
| Shahbaz et al., 2014 [38] | T1D | 213.8 | 82.96 | 2.1 | 0.813 |
| Patel et al., 2015 [39] | T1D and T2D | 167.06 | 78.94 | 13.96 | 4.61 |
| Gupta et al., 2015 [40] | T2D | - | - | - | - |
| Arora et al., 2015 [41] | T1D | 204.44 | 82.02 | 20.14 | 7.65 |
| Lakshmi et al., 2015 [42] | T1D | - | - | 8.56 | 5.06 |
| Ravindran et al., 2015 [43] | T2D | 230.067 | 92.50 | 6.567 | 1.867 |
| Gupta et al., 2015 [44] | T1D and T2D | T1D = 217.62 T2D = 174.24 Both = 183.86 | 84.18 | T1D = 10.21 T2D = 9.92 Both = 9.98 | 6.8 |
| Indira et al., 2015 [45] | T2D | - | - | - | - |
| Kadashetti et al., 2015 [46] | Not specified | - | - | Group III (<130 mg/dL): 5.78 Group II (130–200 mg/dL): 9.81 Group I (>200 mg/dL): 15.5 | - |
| Mussavira et al., 2015 [47] | T2D | Controlled: 109 Uncontrolled: 211.85 All: 161.07 | 86.30 | Controlled: 8.34 Uncontrolled: 3.41 All: 5.83 | 2.07 |
| Smriti et al., 2016 [48] | Not specified | - | - | Not Medicated = 11.68 Medicated = 9.68 | 6.5 |
| Dhanya et al., 2016 [49] | T2D | 136.30 | 97.78 | 8.47 | 1.20 |
| Puttaswamy et al., 2017 [50] | T2D | - | - | - | - |
| Wang et al., 2017 [51] | T2D | 134.41 | 100.15 | Parotid: 3.24 Mix: 0.57 | Parotid: 1.39 Mix: 0.62 |
| Abd-Elraheem et al., 2017 [52] | T2D | - | - | 10.9 | 4.88 |
| Shaik et al., 2017 [53] | T2D | 201.471 | 101.614 | 7.634 | 5.469 |
| Carramolino-Cuéllar et al., 2017 [54] | T2D | - | - | Not Stimulated: 5.57 Stimulated: 4.31 | Not Stimulated: 3.73 Stimulated: 3.46 |
| Gupta et al., 2017 [55] | Not specified | Controlled: 121.53 Uncontrolled: 283.23 | 78.39 | Controlled: 4.86 Uncontrolled: 11.33 | 0.78 |
| Ghafouri et al., 2018 [56] | T2D | 161.00 | 74.75 | 12.80 | 6.5 |
| Tiongco et al., 2018 [15] | T2D | preDM = 115.8 T2D = 189.1 | 93.7 | preDM = 10.5 T2D = 16.3 | 5.3 |
| Bhattacharyya et al., 2018 [57] | T2D | Controlled 96.62 Uncontrolled 170.76 | 92.51 | Controlled: 9.14 Uncontrolled: 15.21 | 7.18 |
| Harish et al., 2019 [58] | T2D | Controlled 103 Uncontrolled 162 | 91.88 | Controlled: 4.75 Uncontrolled: 6.07 | 4.27 |
| Fares et al., 2019 [59] | T2D and pre-diabetes | DM = 226.89 PreDM = 111.31 | 86.45 | DM = 59.32 PreDM = 42.68 | 23.40 |
| Mishra et al., 2019 [60] | T2D | - | - | - | - |
| Ephraim et al., 2019 [61] | Not specified | 285.22 | 88.28 | 16.58 | 5.76 |
| Ragunathan et al., 2019 [62] | Not specified | - | - | - | - |
| Hegde et al., 2020 [63] | Not specified | - | - | - | - |
| Mrag et al., 2020 [64] | T2D | 180.18 | 78.38 | 7.21 | 3.6 |
| Kumar et al., 2020 [65] | T1D and T2D | - | - | - | - |
| Gupta et al., 2020 [66] | Not specified | 194.53 | 74.71 | 1 | 1.2 |
| Dharmakeerthi et al., 2021 [67] | T2D | 163.03 | 95.24 | 1.38 | 0.36 |
| Ganesan et al., 2021 [68] | GD | - | - | - | - |
| Egboh et al., 2022 [69] | T2D | 180.4 | 19.63 | 11.15 | |
| Cui et al., 2022a [17] | T1D and T2D | - | - | - | - |
| Cui et al., 2022b [4] | Not specified | - | - | - | - |
| Ganesan et al., 2022 [70] | T1D | 142.11 | 87.98 | 6.04 | 1.46 |
| Cheprasova et al., 2022 [71] | T1D | ♂ 183.4 ♀ 169.65 | ♂ 95.04 ♀ 92.7 | ♂ 11.84 ♀ 12.22 | ♂ 3.07 ♀ 3.07 |
| Choudhry et al., 2022 [72] | T2D | 183.36 | 79.6 | 4.37 | 0.92 |
| Pandey et al., 2023 [73] | T1D, T2D, and GD | 228.94 | 96.13 | 13.93 | 5.76 |
| Shettigar et al., 2024 [74] | T2D | - | - | - | - |
| Database | Queries |
|---|---|
| PubMed | (“Diabetes Mellitus”[MeSH Terms] OR “diabetes mellitus, type 1”[MeSH Terms] OR “diabetes mellitus, type 2”[MeSH Terms] OR “diabetes, gestational”[MeSH Terms] OR “Diabetes Mellitus”[Title/Abstract] OR “DM”[Title/Abstract] OR “Type 1 diabetes”[Title/Abstract] OR “T1D”[Title/Abstract] OR “Type 2 diabetes”[Title/Abstract] OR “T2D”[Title/Abstract] OR “Gestational diabetes”[Title/Abstract]) AND (“serum glucose”[Title/Abstract] OR “blood glucose”[Title/Abstract] OR “plasma glucose”[Title/Abstract]) AND (“saliva”[MeSH Terms] OR “saliva”[Title/Abstract] OR (“salivary glucose”[Title/Abstract] OR “salivary”[Title/Abstract])) |
| Scopus | (TITLE-ABS-KEY (“diabetes mellitus” OR “DM” OR “type 1 diabetes” OR “T1D” OR “type 2 diabetes” OR “T2D” OR “gestational diabetes”)) AND (TITLE-ABS-KEY (“serum glucose” OR “blood glucose” OR “plasma glucose”)) AND (TITLE-ABS-KEY (“saliva” OR “salivary glucose” OR “salivary”)) |
| Web of Science | (TS=(“diabetes mellitus”)) OR TS=(DM)) OR TS=(“Type 1 diabetes”)) OR TS=(“Type 2 diabetes”)) OR TS=(“Gestational diabetes”)) OR TS=(“T1D”)) OR TS=(“T2D”) AND (TS=(“serum glucose”)) OR TS=(“blood glucose”)) OR TS=(“plasma glucose”) AND (TS=(“saliva”)) OR TS=(“salivary glucose”)) OR TS=(“salivary”) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calixto, P.S.; Ferraz, F.C.; Dutra, G.C.; Pelozzo, M.J.B.; Trovão, M.E.; Rego, F.G.d.M.; Picheth, G.; Campelo, P.M.S.; Sari, M.H.M. Exploring Saliva as a Sample for Non-Invasive Glycemic Monitoring in Diabetes: A Scoping Review. Biomedicines 2025, 13, 713. https://doi.org/10.3390/biomedicines13030713
Calixto PS, Ferraz FC, Dutra GC, Pelozzo MJB, Trovão ME, Rego FGdM, Picheth G, Campelo PMS, Sari MHM. Exploring Saliva as a Sample for Non-Invasive Glycemic Monitoring in Diabetes: A Scoping Review. Biomedicines. 2025; 13(3):713. https://doi.org/10.3390/biomedicines13030713
Chicago/Turabian StyleCalixto, Patricia Sthefani, Fernanda Cereda Ferraz, Gabriela Carolina Dutra, Maria Julia Belotto Pelozzo, Mariana Eleni Trovão, Fabiane Gomes de Moraes Rego, Geraldo Picheth, Patrícia Maria Stuelp Campelo, and Marcel Henrique Marcondes Sari. 2025. "Exploring Saliva as a Sample for Non-Invasive Glycemic Monitoring in Diabetes: A Scoping Review" Biomedicines 13, no. 3: 713. https://doi.org/10.3390/biomedicines13030713
APA StyleCalixto, P. S., Ferraz, F. C., Dutra, G. C., Pelozzo, M. J. B., Trovão, M. E., Rego, F. G. d. M., Picheth, G., Campelo, P. M. S., & Sari, M. H. M. (2025). Exploring Saliva as a Sample for Non-Invasive Glycemic Monitoring in Diabetes: A Scoping Review. Biomedicines, 13(3), 713. https://doi.org/10.3390/biomedicines13030713







