Aberrant Expression and Oncogenic Activity of SPP1 in Hodgkin Lymphoma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bioinformatic Analyses
2.2. Cell Lines and Treatments
2.3. Polymerase Chain Reaction (PCR) Analyses
2.4. Protein Analysis
2.5. Genomic Profiling Analysis
3. Results
3.1. SPP1 Expression in HL Patients and Cell Lines
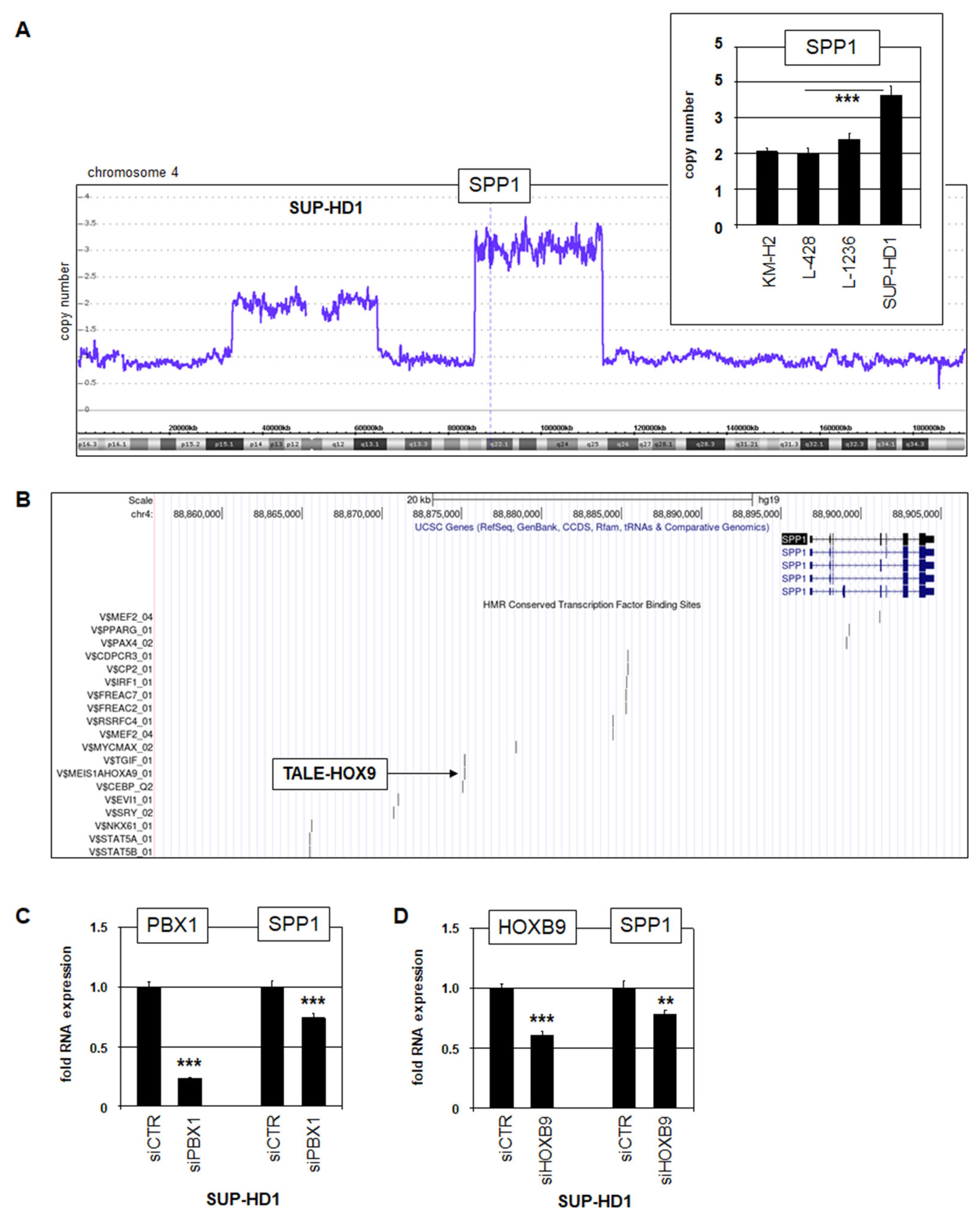
3.2. Aberrant Activation of SPP1 in HL
3.3. SPP1-Signalling in HL
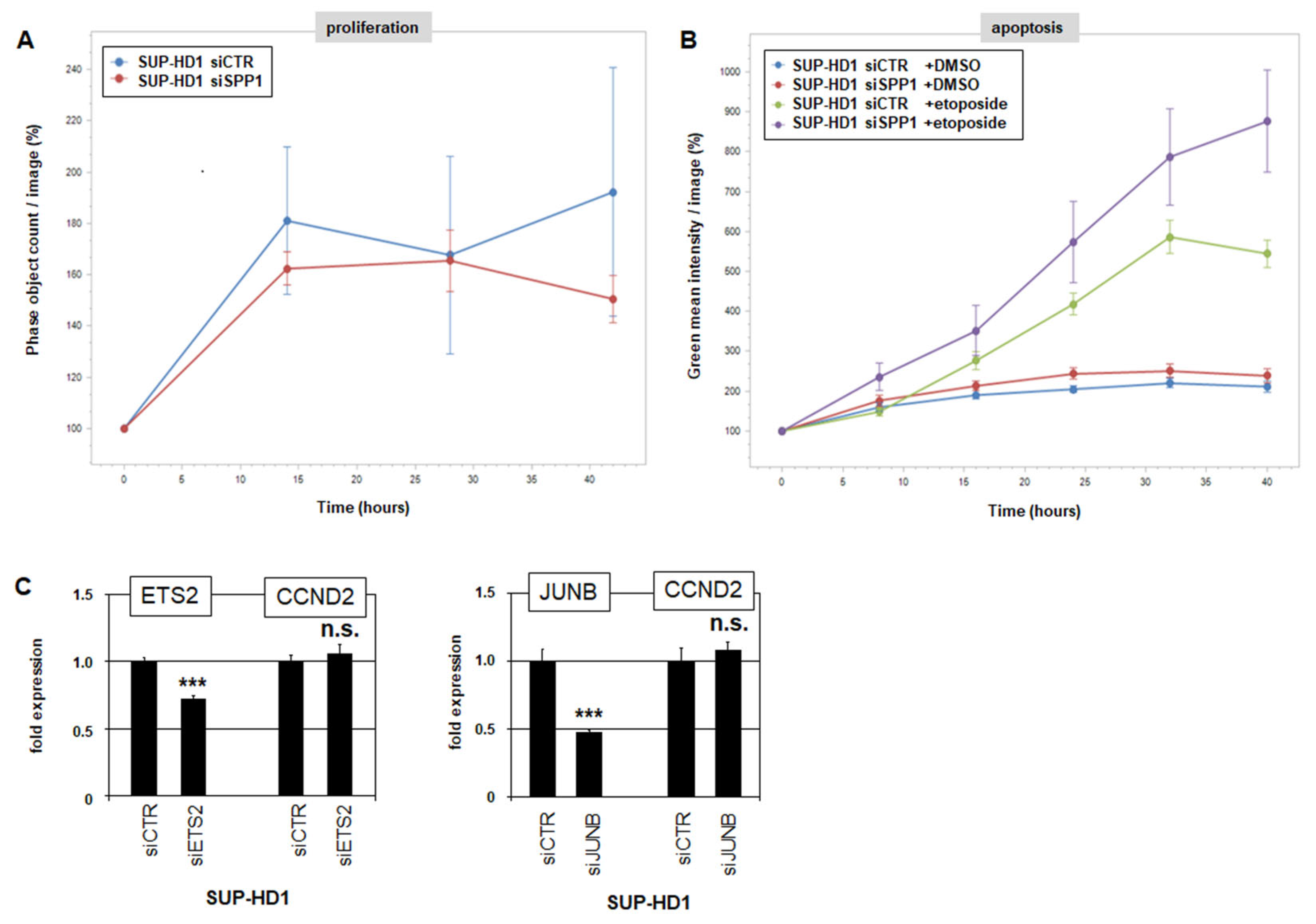
3.4. ETS2 Activation via SPP1-Signalling
3.5. SPP1 Inhibits Apoptosis in HL
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weniger, M.A.; Küppers, R. Molecular biology of Hodgkin lymphoma. Leukemia 2021, 35, 968–981. [Google Scholar] [CrossRef] [PubMed]
- Alaggio, R.; Amador, C.; Anagnostopoulos, I.; Attygalle, A.D.; Araujo, I.B.O.; Berti, E.; Bhagat, G.; Borges, A.M.; Boyer, D.; Calaminici, M.; et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Lymphoid Neoplasms. Leukemia 2022, 36, 1720–1748. [Google Scholar] [CrossRef] [PubMed]
- De Re, V.; Lopci, E.; Brisotto, G.; Elia, C.; Mussolin, L.; Mascarin, M.; d’Amore, E.S.G.; AIEOP The Hodgkin’s Lymphoma Research Network. Preliminary study of the relationship between osteopontin and relapsed Hodgkin’s lymphoma. Biomedicines 2023, 12, 31. [Google Scholar] [CrossRef]
- Kosydar, S.; Ansell, S.M. The biology of classical Hodgkin lymphoma. Semin. Hematol. 2024, 61, 212–220. [Google Scholar] [CrossRef]
- Mathas, S.; Hinz, M.; Anagnostopoulos, I.; Krappmann, D.; Lietz, A.; Jundt, F.; Bommert, K.; Mechta-Grigoriou, F.; Stein, H.; Dörken, B.; et al. Aberrantly expressed c-Jun and JunB are a hallmark of Hodgkin lymphoma cells, stimulate proliferation and synergize with NF-kappa B. EMBO J. 2002, 21, 4104–4113. [Google Scholar] [CrossRef] [PubMed]
- Abate-Shen, C. Deregulated homeobox gene expression in cancer: Cause or consequence? Nat. Rev. Cancer 2002, 2, 777–785. [Google Scholar] [CrossRef]
- Blasi, F.; Bruckmann, C.; Penkov, D.; Dardaei, L. A tale of TALE, PREP1, PBX1, and MEIS1: Interconnections and competition in cancer. Bioessays 2017, 39, 1600245. [Google Scholar] [CrossRef]
- Holland, P.W.; Booth, H.A.; Bruford, E.A. Classification and nomenclature of all human homeobox genes. BMC Biol. 2007, 5, 47. [Google Scholar] [CrossRef]
- Nagel, S.; Pommerenke, C.; Meyer, C.; MacLeod, R.A.F.; Drexler, H.G. Establishment of the TALE-code reveals aberrantly activated homeobox gene PBX1 in Hodgkin lymphoma. PLoS ONE 2021, 16, e0246603. [Google Scholar] [CrossRef]
- Nagel, S.; Meyer, C.; Pommerenke, C. Establishment of the lymphoid ETS-code reveals deregulated ETS genes in Hodgkin lymphoma. PLoS ONE 2023, 18, e0288031. [Google Scholar] [CrossRef]
- Kureshi, C.T.; Dougan, S.K. Cytokines in cancer. Cancer Cell. 2025, 43, 15–35. [Google Scholar] [CrossRef] [PubMed]
- Rangaswami, H.; Bulbule, A.; Kundu, G.C. Osteopontin: Role in cell signaling and cancer progression. Trends Cell Biol. 2006, 16, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Kashyap, D.; Garg, V.K. Osteopontin in cancer. Adv. Clin. Chem. 2024, 118, 87–110. [Google Scholar] [PubMed]
- Mirabelli, P.; Coppola, L.; Salvatore, M. Cancer Cell Lines Are Useful Model Systems for Medical Research. Cancers 2019, 11, 1098. [Google Scholar] [CrossRef]
- Weniger, M.A.; Melzner, I.; Menz, C.K.; Wegener, S.; Bucur, A.J.; Dorsch, K.; Mattfeldt, T.; Barth, T.F.; Möller, P. Mutations of the tumor suppressor gene SOCS-1 in classical Hodgkin lymphoma are frequent and associated with nuclear phospho-STAT5 accumulation. Oncogene 2006, 25, 2679–2684. [Google Scholar] [CrossRef]
- Davis, S.; Meltzer, P.S. GEOquery: A bridge between the Gene Expression Omnibus (GEO) and BioConductor. Bioinformatics 2007, 23, 1846–1847. [Google Scholar] [CrossRef]
- Brune, V.; Tiacci, E.; Pfeil, I.; Döring, C.; Eckerle, S.; van Noesel, C.J.; Klapper, W.; Falini, B.; von Heydebreck, A.; Metzler, D.; et al. Origin and pathogenesis of nodular lymphocyte-predominant Hodgkin lymphoma as revealed by global gene expression analysis. J. Exp. Med. 2008, 205, 2251–2268. [Google Scholar] [CrossRef]
- Fernàndez, V.; Salamero, O.; Espinet, B.; Solé, F.; Royo, C.; Navarro, A.; Camacho, F.; Beà, S.; Hartmann, E.; Amador, V.; et al. Genomic and gene expression profiling defines indolent forms of mantle cell lymphoma. Cancer Res. 2010, 70, 1408–1418. [Google Scholar] [CrossRef]
- Quentmeier, H.; Pommerenke, C.; Dirks, W.G.; Eberth, S.; Koeppel, M.; MacLeod, R.A.F.; Nagel, S.; Steube, K.; Uphoff, C.C.; Drexler, H.G. The LL-100 panel: 100 cell lines for blood cancer studies. Sci. Rep. 2019, 9, 8218. [Google Scholar] [CrossRef]
- Koblitz, J.; Dirks, W.G.; Eberth, S.; Nagel, S.; Steenpass, L.; Pommerenke, C. DSMZCellDive: Diving into high-throughput cell line data. F1000Research 2022, 11, 420. [Google Scholar] [CrossRef]
- Uhlen, M.; Karlsson, M.J.; Zhong, W.; Tebani, A.; Pou, C.; Mikes, J.; Lakshmikanth, T.; Forsström, B.; Edfors, F.; Odeberg, J.; et al. A genome-wide transcriptomic analysis of protein-coding genes in human blood cells. Science 2019, 366, eaax9198. [Google Scholar] [CrossRef] [PubMed]
- Schlegelberger, B.; Weber-Matthiesen, K.; Himmler, A.; Bartels, H.; Sonnen, R.; Kuse, R.; Feller, A.C.; Grote, W. Cytogenetic findings and results of combined immunophenotyping and karyotyping in Hodgkin’s disease. Leukemia 1994, 8, 72–80. [Google Scholar] [PubMed]
- MacLeod, R.A.; Spitzer, D.; Bar-Am, I.; Sylvester, J.E.; Kaufmann, M.; Wernich, A.; Drexler, H.G. Karyotypic dissection of Hodgkin’s disease cell lines reveals ectopic subtelomeres and ribosomal DNA at sites of multiple jumping translocations and genomic amplification. Leukemia 2000, 14, 1803–1814. [Google Scholar] [CrossRef]
- Steidl, C.; Telenius, A.; Shah, S.P.; Farinha, P.; Barclay, L.; Boyle, M.; Connors, J.M.; Horsman, D.E.; Gascoyne, R.D. Genome-wide copy number analysis of Hodgkin Reed-Sternberg cells identifies recurrent imbalances with correlations to treatment outcome. Blood 2010, 116, 418–427. [Google Scholar] [CrossRef]
- Nagel, S.; Meyer, C.; Quentmeier, H.; Kaufmann, M.; Drexler, H.G.; MacLeod, R.A. Chromothripsis in Hodgkin lymphoma. Genes Chromosomes Cancer 2013, 52, 741–747. [Google Scholar] [CrossRef]
- Nagel, S.; Burek, C.; Venturini, L.; Scherr, M.; Quentmeier, H.; Meyer, C.; Rosenwald, A.; Drexler, H.G.; MacLeod, R.A. Comprehensive analysis of homeobox genes in Hodgkin lymphoma cell lines identifies dysregulated expression of HOXB9 mediated via ERK5 signaling and BMI1. Blood 2007, 109, 3015–3023. [Google Scholar] [CrossRef]
- Hodge, C.; Liao, J.; Stofega, M.; Guan, K.; Carter-Su, C.; Schwartz, J. Growth hormone stimulates phosphorylation and activation of elk-1 and expression of c-fos, egr-1, and junB through activation of extracellular signal-regulated kinases 1 and 2. J. Biol. Chem. 1998, 273, 31327–31336. [Google Scholar] [CrossRef]
- Coffer, P.; de Jonge, M.; Mettouchi, A.; Binetruy, B.; Ghysdael, J.; Kruijer, W. junB promoter regulation: Ras mediated transactivation by c-Ets-1 and c-Ets-2. Oncogene 1994, 9, 911–921. [Google Scholar]
- Yao, F.; Wang, X.; Cui, Z.K.; Lan, H.; Ai, X.; Song, Q.; Chen, Z.; Yang, J.; Wu, B.; Bai, X. ETS2 promotes epithelial-to-mesenchymal transition in renal fibrosis by targeting JUNB transcription. Lab. Investig. 2020, 100, 438–453. [Google Scholar] [CrossRef]
- Tun, H.W.; Personett, D.; Baskerville, K.A.; Menke, D.M.; Jaeckle, K.A.; Kreinest, P.; Edenfield, B.; Zubair, A.C.; O’Neill, B.P.; Lai, W.R.; et al. Pathway analysis of primary central nervous system lymphoma. Blood 2008, 111, 3200–3210. [Google Scholar] [CrossRef]
- Sud, A.; Cooke, R.; Swerdlow, A.J.; Houlston, R.S. Genome-wide homozygosity signature and risk of Hodgkin lymphoma. Sci. Rep. 2015, 5, 14315. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Ji, R.; Zheng, S.; Xia, X.; Du, W.; He, H.; Han, C.; Zhao, W.; Li, X.; Wang, Y.; et al. HOXB9 promotes osteosarcoma cell survival and malignancy under glucose starvation via upregulating SPP1 expression. Biochem. Pharmacol. 2024, 224, 116208. [Google Scholar] [CrossRef] [PubMed]
- Conway, C.; Mitra, A.; Jewell, R.; Randerson-Moor, J.; Lobo, S.; Nsengimana, J.; Edward, S.; Sanders, D.S.; Cook, M.; Powell, B.; et al. Gene expression profiling of paraffin-embedded primary melanoma using the DASL assay identifies increased osteopontin expression as predictive of reduced relapse-free survival. Clin. Cancer Res. 2009, 15, 6939–6946. [Google Scholar] [CrossRef] [PubMed]
- Nagel, S. NKL-Code in normal and aberrant hematopoiesis. Cancers 2021, 13, 1961. [Google Scholar] [CrossRef]
- Sanyal, M.; Tung, J.W.; Karsunky, H.; Zeng, H.; Selleri, L.; Weissman, I.L.; Herzenberg, L.A.; Cleary, M.L. B-cell development fails in the absence of the Pbx1 proto-oncogene. Blood 2007, 109, 4191–4199. [Google Scholar] [CrossRef]
- Hartmann, S.; Schuhmacher, B.; Rausch, T.; Fuller, L.; Döring, C.; Weniger, M.; Lollies, A.; Weiser, C.; Thurner, L.; Rengstl, B.; et al. Highly recurrent mutations of SGK1, DUSP2 and JUNB in nodular lymphocyte predominant Hodgkin lymphoma. Leukemia 2016, 30, 844–853. [Google Scholar] [CrossRef]
- Tetsu, O.; McCormick, F. ETS-targeted therapy: Can it substitute for MEK inhibitors? Clin. Transl. Med. 2017, 6, 16. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagel, S.; Meyer, C. Aberrant Expression and Oncogenic Activity of SPP1 in Hodgkin Lymphoma. Biomedicines 2025, 13, 735. https://doi.org/10.3390/biomedicines13030735
Nagel S, Meyer C. Aberrant Expression and Oncogenic Activity of SPP1 in Hodgkin Lymphoma. Biomedicines. 2025; 13(3):735. https://doi.org/10.3390/biomedicines13030735
Chicago/Turabian StyleNagel, Stefan, and Corinna Meyer. 2025. "Aberrant Expression and Oncogenic Activity of SPP1 in Hodgkin Lymphoma" Biomedicines 13, no. 3: 735. https://doi.org/10.3390/biomedicines13030735
APA StyleNagel, S., & Meyer, C. (2025). Aberrant Expression and Oncogenic Activity of SPP1 in Hodgkin Lymphoma. Biomedicines, 13(3), 735. https://doi.org/10.3390/biomedicines13030735





