Associations of Skin Autofluorescence with Diabetic Kidney Disease in Type 2 Diabetes
Abstract
:1. Introduction
2. Methods
2.1. Study Population
2.2. Definition and GA-Classification of DKD
2.3. Assessment of Clinical Parameters
2.4. Assessment of SAF Levels
2.5. Statistical Analysis
3. Results
3.1. Logistic Regression Analysis Between SAF and DKD Incidence
3.2. Linear Regression Analysis Between SAF and Renal Function Parameters
3.3. Distribution of Renal Function Stages in T2DM Patients with Different SAF Levels
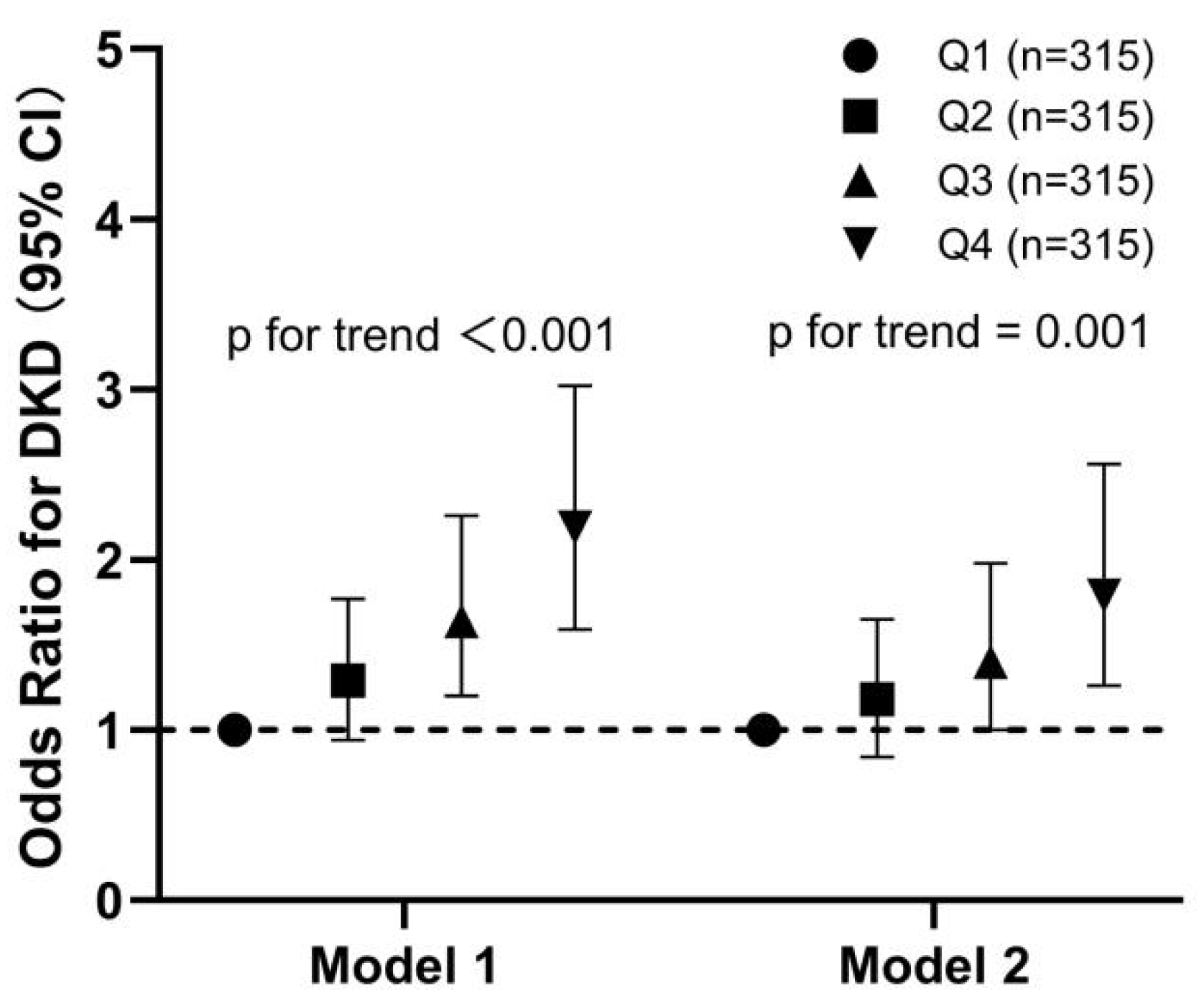
3.4. Relationship of the Quartiles of SAF Levels with the OR of DKD in All Participants
3.5. RCS Regression Analysis Examining the Association Between SAF Levels and the Occurrence of DKD
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L.; et al. 2. Classification and Diagnosis of Diabetes: Standards of Care in Diabetes-2023. Diabetes Care 2023, 46, S19–S40. [Google Scholar] [CrossRef] [PubMed]
- Kahn, S.E.; Cooper, M.E.; Del Prato, S. Pathophysiology and treatment of type 2 diabetes: Perspectives on the past, present, and future. Lancet 2014, 383, 1068–1083. [Google Scholar] [CrossRef]
- Alicic, R.Z.; Rooney, M.T.; Tuttle, K.R. Diabetic Kidney Disease: Challenges, Progress, and Possibilities. Clin. J. Am. Soc. Nephrol. 2017, 12, 2032–2045. [Google Scholar] [CrossRef] [PubMed]
- Zimmet, P.; Alberti, K.G.; Shaw, J. Global and societal implications of the diabetes epidemic. Nature 2001, 414, 782–787. [Google Scholar] [CrossRef]
- van der Velde, M.; Matsushita, K.; Coresh, J.; Astor, B.C.; Woodward, M.; Levey, A.; de Jong, P.; Gansevoort, R.T.; van der Velde, M.; Matsushita, K.; et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative meta-analysis of high-risk population cohorts. Kidney Int. 2011, 79, 1341–1352. [Google Scholar] [CrossRef]
- Piperi, C.; Adamopoulos, C.; Dalagiorgou, G.; Diamanti-Kandarakis, E.; Papavassiliou, A.G. Crosstalk between advanced glycation and endoplasmic reticulum stress: Emerging therapeutic targeting for metabolic diseases. J. Clin. Endocrinol. Metab. 2012, 97, 2231–2242. [Google Scholar] [CrossRef] [PubMed]
- Peppa, M.; Vlassara, H. Advanced glycation end products and diabetic complications: A general overview. Hormones 2005, 4, 28–37. [Google Scholar] [CrossRef]
- Monnier, V.M.; Vishwanath, V.; Frank, K.E.; Elmets, C.A.; Dauchot, P.; Kohn, R.R. Relation between complications of type I diabetes mellitus and collagen-linked fluorescence. N. Engl. J. Med. 1986, 314, 403–408. [Google Scholar] [CrossRef]
- Makita, Z.; Radoff, S.; Rayfield, E.J.; Yang, Z.; Skolnik, E.; Delaney, V.; Friedman, E.A.; Cerami, A.; Vlassara, H. Advanced glycosylation end products in patients with diabetic nephropathy. N. Engl. J. Med. 1991, 325, 836–842. [Google Scholar] [CrossRef]
- Chen, Y.; Lee, K.; Ni, Z.; He, J.C. Diabetic Kidney Disease: Challenges, Advances, and Opportunities. Kidney Dis. 2020, 6, 215–225. [Google Scholar] [CrossRef]
- Andrassy, K.M. Comments on ‘KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease’. Kidney Int. 2013, 84, 622–623. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Yin, J.; Ma, X.; Mo, Y.; Li, C.; Lu, W.; Bao, Y.; Zhou, J.; Jia, W. Low-carbohydrate diets lead to greater weight loss and better glucose homeostasis than exercise: A randomized clinical trial. Front. Med. 2021, 15, 460–471. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, L.; Zhang, L.; Zhang, G.; Liu, Y.; Wang, A. A portable system for noninvasive assessment of advanced glycation end-products using skin fluorescence and reflectance spectrum. J. Appl. Spectrosc. 2012, 79, 431–436. [Google Scholar] [CrossRef]
- Zhang, Y.; Hou, H.; Zhang, Y.; Wang, Y.; Zhu, L.; Dong, M.; Liu, Y. Tissue intrinsic fluorescence recovering by an empirical approach based on the PSO algorithm and its application in type 2 diabetes screening. Biomed. Opt. Express 2018, 9, 1795–1808. [Google Scholar] [CrossRef]
- Luevano-Contreras, C.; Chapman-Novakofski, K. Dietary advanced glycation end products and aging. Nutrients 2010, 2, 1247–1265. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Chen, X.; Luo, T.; Ran, X.; Hu, J.; Cheng, Q.; Yang, S.; Wu, J.; Li, Q.; Wang, Z. Early-Onset Type 2 Diabetes as a Risk Factor for End-Stage Renal Disease in Patients With Diabetic Kidney Disease. Prev. Chronic Dis. 2020, 17, E50. [Google Scholar] [CrossRef]
- Altemtam, N.; Russell, J.; El Nahas, M. A study of the natural history of diabetic kidney disease (DKD). Nephrol. Dial. Transplant. 2012, 27, 1847–1854. [Google Scholar] [CrossRef]
- Bin Rakhis, S.A.; AlDuwayhis, N.M.; Aleid, N.; AlBarrak, A.N.; Aloraini, A.A. Glycemic Control for Type 2 Diabetes Mellitus Patients: A Systematic Review. Cureus 2022, 14, e26180. [Google Scholar] [CrossRef]
- Rigon, F.A.; Ronsoni, M.F.; Vianna, A.G.D.; de Lucca Schiavon, L.; Hohl, A.; van de Sande-Lee, S. Flash glucose monitoring system in special situations. Arch. Endocrinol. Metab. 2022, 66, 883–894. [Google Scholar] [CrossRef]
- Cleary, P.A.; Braffett, B.H.; Orchard, T.; Lyons, T.J.; Maynard, J.; Cowie, C.; Gubitosi-Klug, R.A.; Way, J.; Anderson, K.; Barnie, A.; et al. Clinical and technical factors associated with skin intrinsic fluorescence in subjects with type 1 diabetes from the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study. Diabetes Technol. Ther. 2013, 15, 466–474. [Google Scholar] [CrossRef]
- Monnier, V.M.; Bautista, O.; Kenny, D.; Sell, D.R.; Fogarty, J.; Dahms, W.; Cleary, P.A.; Lachin, J.; Genuth, S. Skin collagen glycation, glycoxidation, and crosslinking are lower in subjects with long-term intensive versus conventional therapy of type 1 diabetes: Relevance of glycated collagen products versus HbA1c as markers of diabetic complications. DCCT Skin Collagen Ancillary Study Group. Diabetes Control Complicat. Trial. Diabetes 1999, 48, 870–880. [Google Scholar]
- Jaisson, S.; Gillery, P. Methods to assess advanced glycation end-products. Curr. Opin. Clin. Nutr. Metab. Care 2021, 24, 411–415. [Google Scholar] [CrossRef] [PubMed]
- den Hollander, N.C.; Mulder, D.J.; Graaff, R.; Thorpe, S.R.; Baynes, J.W.; Smit, G.P.; Smit, A.J. Advanced glycation end products and the absence of premature atherosclerosis in glycogen storage disease Ia. J. Inherit. Metab. Dis. 2007, 30, 916–923. [Google Scholar] [CrossRef]
- Meerwaldt, R.; Hartog, J.W.; Graaff, R.; Huisman, R.J.; Links, T.P.; den Hollander, N.C.; Thorpe, S.R.; Baynes, J.W.; Navis, G.; Gans, R.O.; et al. Skin autofluorescence, a measure of cumulative metabolic stress and advanced glycation end products, predicts mortality in hemodialysis patients. J. Am. Soc. Nephrol. 2005, 16, 3687–3693. [Google Scholar] [CrossRef]
- Meerwaldt, R.; Graaff, R.; Oomen, P.H.N.; Links, T.P.; Jager, J.J.; Alderson, N.L.; Thorpe, S.R.; Baynes, J.W.; Gans, R.O.B.; Smit, A.J. Simple non-invasive assessment of advanced glycation endproduct accumulation. Diabetologia 2004, 47, 1324–1330. [Google Scholar] [CrossRef] [PubMed]
- Rigalleau, V.; Pucheux, Y.; Couffinhal, T.; Tessier, F.J.; Howsam, M.; Rubin, S.; Helmer, C.; Alkhami, F.; Larroumet, A.; Blanco, L.; et al. Skin autofluorescence of advanced glycation end-products, glycemic memory, and diabetes complications. Diabetes Metab. 2025, 51, 101600. [Google Scholar] [CrossRef] [PubMed]
- Stróżecki, P.; Kurowski, R.; Flisiński, M.; Stefańska, A.; Odrowąż-Sypniewska, G.; Manitius, J. Advanced glycation end products and arterial stiffness in patients with diabetic nephropathy and patients with chronic kidney disease without diabetes. Pol. Arch. Med. Wewn. 2013, 123, 609–616. [Google Scholar] [CrossRef]
- Thieme, K.; Da Silva, K.S.; Fabre, N.T.; Catanozi, S.; Monteiro, M.B.; Santos-Bezerra, D.P.; Costa-Pessoa, J.M.; Oliveira-Souza, M.; Machado, U.F.; Passarelli, M.; et al. N-Acetyl Cysteine Attenuated the Deleterious Effects of Advanced Glycation End-Products on the Kidney of Non-Diabetic Rats. Cell Physiol. Biochem. 2016, 40, 608–620. [Google Scholar] [CrossRef]
- Jin, Q.; Lau, E.S.; Luk, A.O.; Ozaki, R.; Chow, E.Y.; So, T.; Yeung, T.; Loo, K.-M.; Lim, C.K.; Kong, A.P.; et al. Skin autofluorescence is associated with progression of kidney disease in type 2 diabetes: A prospective cohort study from the Hong Kong diabetes biobank. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 436–446. [Google Scholar] [CrossRef]
- Gerrits, E.G.; Lutgers, H.L.; Kleefstra, N.; Graaff, R.; Groenier, K.H.; Smit, A.J.; Gans, R.O.; Bilo, H.J. Skin autofluorescence: A tool to identify type 2 diabetic patients at risk for developing microvascular complications. Diabetes Care 2008, 31, 517–521. [Google Scholar] [CrossRef]
- Ahdi, M.; Gerdes, V.E.A.; Graaff, R.; Kuipers, S.; Smit, A.J.; Meesters, E.W. Skin autofluorescence and complications of diabetes: Does ethnic background or skin color matter? Diabetes Technol. Ther. 2015, 17, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Krhač, M.; Lovrenčić, M.V. Update on biomarkers of glycemic control. World J. Diabetes 2019, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]



| Variable | Without DKD | With DKD | p Value a | A1 | A2 | A3 | p Value b | G1 and G2 | G3 | G4 and G5 | p Value b |
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Participants | 682 | 577 | 726 | 360 | 173 | 1119 | 114 | 26 | |||
| Age, years | 68.00 [58.25~74.00] | 68.00 [60.00~76.00] | 0.025 | 68.00 [59.00~75.00] | 68.00 [59.00~76.00] | 68.00 [60.00~75.00] | 0.538 | 68.00 [59.00~75.00] | 71.50 [65.00~79.00] ### | 72.50 [61.25~78.25] | <0.001 |
| Male gender, n (%) | 370 (54.25%) | 316 (54.77%) | 0.900 | 391 (53.86%) | 193 (53.61%) | 102 (58.96%) | 0.444 | 506 (45.22%) | 54 (47.37%) | 13 (50%) | 0.815 |
| BMI, kg/m2 | 25.16 ± 11.17 | 25.17 ± 4.27 | 0.979 | 25.2 ± 10.86 | 24.86 ± 4.42 | 25.63 ± 4.09 | 0.621 | 25.05 ± 9.14 | 26.14 ± 3.78 | 25.78 ± 3.89 | 0.417 |
| SBP, mmHg | 134.47 ± 20.54 | 132.40 ± 20.28 | 0.150 0.074 | 134.05 ± 20.29 | 132.24 ± 20.99 | 134 ± 19.86 | 0.442 0.369 | 133.84 ± 20.38 | 130.48 ± 20.6 | 133.19 ± 21.66 | 0.165 0.246 |
| DBP, mmHg | 76.38 ± 11.12 | 75.48 ± 10.82 | 76.18 ± 11.04 | 76.01 ± 11.23 | 74.99 ± 10.22 | 76.17 ± 11 | 74.44 ± 10.97 | 73.81 ± 10.2 | |||
| Duration, years | 8.00 [1.00~15.00] | 10.00 [4.00~18.00] | <0.001 | 8.00 [1.00~15.00] | 10.00 [3.00~16.00] * | 11.00 [8.00~20.00] *** | <0.001 | 10.00 [2.00~15.00] | 10.00 [5.50~20.00] # | 20.00 [10.00~20.00] ### | <0.001 |
| HbA1c, % | 10.24 ± 2.71 | 10.36 ± 2.86 | 0.414 | 10.28 ± 2.74 | 10.37 ± 2.81 | 10.22 ± 2.88 | 0.823 | 10.33 ± 2.75 | 10.24 ± 3.06 | 8.82 ± 2.41 * | 0.023 |
| FPG, mmol/L | 7.12 [5.96~9.15] | 7.36 [5.97~9.68] | 0.121 | 7.12 [5.96~9.17] | 7.35 [6.02~9.62] | 7.57 [5.88~9.87] | 0.356 | 7.27 [6.00~9.34] | 7.12 [5.96~9.65] | 6.90 [5.08~9.71] | 0.244 |
| FCP, ng/mL | 1.42 [0.89~2.08] | 1.64 [0.96~2.64] | <0.001 | 1.48 [0.92~2.15] | 1.48 [0.86~2.47] | 1.93 [1.14~2.91] *** | <0.001 | 1.42 [0.90~2.17] | 2.45 [1.68~3.39] ### | 2.73 [1.32~4.94] ### | <0.001 |
| GA, % | 30.86 ± 12.39 | 31.30 ± 13.66 | 0.552 | 31.04 ± 12.68 | 31.28 ± 13.01 | 30.69 ± 14.21 | 0.885 | 31.14 ± 12.8 | 31.6 ± 14.73 | 25.1 ± 11.72 # | 0.040 |
| TC, mmol/L | 4.04 ± 0.87 | 4.08 ± 1.03 | 0.444 | 4.03 ± 0.88 | 4.07 ± 0.93 | 4.13 ± 1.21 | 0.491 | 4.06 ± 0.92 | 3.98 ± 1.15 | 4.19 ± 1.18 | 0.685 |
| TG, mmol/L | 1.21 [0.87 ~ 1.74] | 1.33 [0.93~1.86] | 0.004 | 1.22 [0.87~1.76] | 1.28 [0.91~1.87] | 1.35 [0.95~1.78] | 0.065 | 1.22 [0.88~1.75] | 1.57 [1.15~2.00] ### | 1.40 [1.11~2.02] | <0.001 |
| HDL-C, mmol/L | 1.11 [0.91~1.32] | 1.02 [0.87~1.27] | 0.001 | 1.10 [0.90~1.31] | 1.05 [0.89~1.29] | 0.99 [0.84~1.27] * | 0.019 | 1.09 [0.90~1.31] | 0.94 [0.80~1.18] ### | 0.87 [0.78~1.32] | <0.001 |
| LDL-C, mmol/L | 2.49 ± 0.84 | 2.51 ± 0.90 | 0.593 | 2.48 ± 0.85 | 2.5 ± 0.8 | 2.57 ± 1.05 | 0.602 | 2.5 ± 0.83 | 2.45 ± 1.09 | 2.55 ± 1.11 | 0.865 |
| eGFR, ml/min/1.73 m2 | 115.65 ± 35.49 | 93.29 ± 45.25 | <0.001 | 111.71 ± 37.81 | 107.61 ± 45.82 | 74.34 ± 34.65 *** | <0.001 | 113.16 ± 37.42 | 48.22 ± 8.29 ### | 22.32 ± 5.39 ### | <0.001 |
| UACR, mg/g | 8.55 [4.90~14.79] | 102.07 [42.45~395.72] | <0.001 | 8.51 [4.90~14.86] | 72.47 [42.04~113.26] *** | 856.32 [430.17~1859.10] *** | <0.001 | 17.10 [7.32~70.22] | 53.69 [17.37~731.89] ### | 1859.10 [977.96~4095.67] ### | <0.001 |
| SAF, AU | 88.65 ± 15.86 | 94.13 ± 17.08 | <0.001 | 89.01 ± 15.85 | 92.85 ± 16.72 *** | 96.7 ± 18.1 *** | <0.001 | 90.31 ± 16.48 | 97.53 ± 16.7 ### | 100.12 ± 15.5 ## | <0.001 |
| SUA, μmol/L | 265.93 ± 78.36 | 300.71 ± 95.57 | <0.001 | 272.16 ± 83.83 | 283.32 ± 88.11 | 319.6 ± 97.14 *** | <0.001 | 270.07 ± 80.42 | 372.65 ± 92.45 ### | 391.92 ± 92.31 ### | <0.001 |
| Diabetes family history, n (%) | 197 (28.89%) | 191 (33.1%) | 0.120 | 205 (28.24%) | 125 (34.72%) | 58 (33.53%) | 0.066 | 768 (68.63%) | 84 (73.68%) | 19 (73.08%) | 0.490 |
| Hypertension history, n (%) | 404 (59.24%) | 379 (65.68%) | 0.022 | 441 (60.74%) | 231 (64.17%) | 111 (64.16%) | 0.465 | 441 (39.41%) | 25 (21.93%) # | 10 (38.46%) | 0.001 |
| Current smoker, n (%) | 184 (26.98%) | 137 (23.74%) | 0.212 | 194 (26.72%) | 91 (25.28%) | 36 (20.81%) | 0.275 | 822 (73.46%) | 93 (81.58%) | 23 (88.46%) | 0.043 |
| Medication, n (%) | |||||||||||
| Lipid-lowering agents | 446 (65.4%) | 396 (68.63%) | 0.248 | 476 (65.56%) | 243 (67.5%) | 123 (71.1%) | 0.364 | 378 (33.78%) | 34 (29.82%) | 5 (19.23%) | 0.218 |
| Aspirin | 190 (27.86%) | 172 (29.81%) | 0.484 | 204 (28.1%) | 108 (30%) | 50 (28.9%) | 0.808 | 803 (71.76%) | 76 (66.67%) | 18 (69.23%) | 0.506 |
| Antihypertensive agents | 329 (48.24%) | 368 (63.78%) | <0.001 | 363 (50%) | 208 (57.78%) | 126 (72.83%) *** | <0.001 | 540 (48.26%) | 21 (18.42%) ### | 1 (3.85%) ### | <0.001 |
| Insulin injection | 201 (29.47%) | 204 (35.36%) | 0.030 | 218 (30.03%) | 123 (34.17%) | 64 (36.99%) | 0.133 | 783 (69.97%) | 62 (54.39%) ## | 9 (34.62%) ### | <0.001 |
| Oral anti-diabetes drugs | 488 (71.55%) | 393 (68.11%) | 0.205 | 515 (70.94%) | 255 (70.83%) | 111 (64.16%) | 0.199 | 327 (29.22%) | 36 (31.58%) | 15 (57.69%) # | 0.007 |
| Variable | Without DKD | With DKD | Statistics Value | p Value a |
|---|---|---|---|---|
| No. of Participants | 563 | 563 | ||
| Age, years | 69.00 [60.00~75.00] | 68.00 [60.00~76.00] | −0.116 | 0.907 |
| Duration, years | 10.00 [3.00~16.00] | 10.00 [4.00~17.00] | −1.311 | 0.190 |
| HbA1c, % | 10.29 ± 2.69 | 10.31 ± 2.84 | −0.141 | 0.888 |
| SBP, mmHg | 133.27 ± 20.52 | 132.59 ± 20.25 | 0.560 | 0.576 |
| DBP, mmHg | 75.85 ± 11.02 | 75.66 ± 10.82 | 0.292 | 0.770 |
| Male gender, n (%) | 293 (52.04%) | 308 (54.71%) | 0.700 | 0.403 |
| BMI, kg/m2 | 25.18 ± 12.17 | 25.17 ± 4.30 | 0.022 | 0.983 |
| FPG, mmol/L | 7.18 [6.00~9.25] | 7.35 [5.96~9.61] | −0.781 | 0.435 |
| C-peptide, ng/mL | 1.41 [0.88~2.06] | 1.65 [0.97~2.64] | −4.289 | <0.001 |
| GA, % | 31.05 ± 12.34 | 31.08 ± 13.65 | −0.044 | 0.965 |
| TC, mmol/L | 4.01 ± 0.90 | 4.08 ± 1.03 | −1.202 | 0.229 |
| TG, mmol/L | 1.20 [0.86~1.74] | 1.33 [0.94~1.86] | −2.982 | 0.003 |
| HDL-C, mmol/L | 1.12 [0.90~1.33] | 1.02 [0.87~1.27] | −3.263 | 0.001 |
| LDL-C, mmol/L | 2.45 ± 0.85 | 2.52 ± 0.90 | −1.347 | 0.178 |
| eGFR, mL/min/1.73 m2 | 114.00 ± 34.17 | 93.35 ± 45.18 | 8.645 | <0.001 |
| UACR, mg/g | 8.83 [5.03~15.52] | 100.77 [42.32~392.49] | −26.925 | <0.001 |
| SAF, AU | 89.58 ± 15.89 | 93.97 ± 17.14 | −4.455 | <0.001 |
| SUA, μmol/L | 263.85 ± 79.67 | 300.63 ± 95.65 | −7.011 | <0.001 |
| Diabetes family history, n (%) | 153 (27.18%) | 187 (33.21%) | 4.590 | 0.032 |
| Hypertension history, n (%) | 338 (60.04%) | 370 (65.72%) | 3.660 | 0.056 |
| Current smoker, n (%) | 145 (25.75%) | 132 (23.45%) | 0.690 | 0.406 |
| Medication, n (%) | ||||
| Lipid-lowering agents | 383 (68.03%) | 387 (68.74%) | 0.040 | 0.848 |
| Aspirin | 169 (30.02%) | 169 (30.02%) | 0.000 | 1.000 |
| Antihypertensive agents | 289 (51.33%) | 357 (63.41%) | 16.300 | <0.001 |
| Insulin injection | 177 (31.44%) | 196 (34.81%) | 1.300 | 0.254 |
| Oral anti-diabetes drugs | 408 (72.47%) | 386 (68.56%) | 1.880 | 0.170 |
| Independent Variables | Simple Regression | Stepwise Multiple Regression | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Estimate | Standard Error | Z Value | p Value | Estimate | Standard Error | Z Value | p Value | OR (95%CI) | |
| Age | 0.010 | 0.004 | 2.318 | 0.020 | |||||
| Duration | 0.031 | 0.007 | 4.393 | <0.001 | 0.025 | 0.007 | 3.364 | 0.001 | 1.03 (1.01–1.04) |
| C-peptide | 0.248 | 0.049 | 5.024 | <0.001 | 0.171 | 0.054 | 3.185 | 0.001 | 1.19 (1.07–1.32) |
| TG | 0.117 | 0.056 | 2.077 | 0.038 | |||||
| HDL-C | −0.539 | 0.175 | −3.069 | 0.002 | −0.278 | 0.188 | −1.482 | 0.138 | 0.76 (0.52–1.09) |
| SAF | 0.020 | 0.004 | 5.726 | <0.001 | 0.016 | 0.004 | 4.278 | <0.001 | 1.02 (1.01–1.02) |
| SUA | 0.005 | 0.001 | 6.800 | <0.001 | 0.004 | 0.001 | 5.034 | <0.001 | 1 (1–1.01) |
| Hypertension history | 0.275 | 0.117 | 2.348 | 0.019 | |||||
| Independent Variables | Estimate | Standard Error | Statistics | p Value |
|---|---|---|---|---|
| Age | −1.135 | 0.077 | −14.809 | <0.001 |
| C-peptide | −6.351 | 0.788 | −8.061 | <0.001 |
| SAF | −0.140 | 0.060 | −2.324 | 0.020 |
| SUA | −0.157 | 0.011 | −14.157 | <0.001 |
| Hypertension history | −5.227 | 2.002 | −2.611 | 0.009 |
| F value | 148.670 | |||
| F-value-associated p value | <0.001 | |||
| Independent Variables | Estimate | Standard Error | Statistics | p Value |
|---|---|---|---|---|
| Duration | 0.038 | 0.006 | 5.984 | <0.001 |
| C-peptide | 0.167 | 0.042 | 3.990 | <0.001 |
| SAF | 0.012 | 0.003 | 3.952 | <0.001 |
| SUA | 0.003 | 0.001 | 4.265 | <0.001 |
| F value | 30.111 | |||
| F-value-associated p value | <0.001 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Wang, J.; Zhao, Y.; Yuan, Z.; Zhuang, X.; Yin, J. Associations of Skin Autofluorescence with Diabetic Kidney Disease in Type 2 Diabetes. Biomedicines 2025, 13, 764. https://doi.org/10.3390/biomedicines13040764
Liu Z, Wang J, Zhao Y, Yuan Z, Zhuang X, Yin J. Associations of Skin Autofluorescence with Diabetic Kidney Disease in Type 2 Diabetes. Biomedicines. 2025; 13(4):764. https://doi.org/10.3390/biomedicines13040764
Chicago/Turabian StyleLiu, Ziwei, Jingjie Wang, Yuedong Zhao, Zhu Yuan, Xinjuan Zhuang, and Jun Yin. 2025. "Associations of Skin Autofluorescence with Diabetic Kidney Disease in Type 2 Diabetes" Biomedicines 13, no. 4: 764. https://doi.org/10.3390/biomedicines13040764
APA StyleLiu, Z., Wang, J., Zhao, Y., Yuan, Z., Zhuang, X., & Yin, J. (2025). Associations of Skin Autofluorescence with Diabetic Kidney Disease in Type 2 Diabetes. Biomedicines, 13(4), 764. https://doi.org/10.3390/biomedicines13040764






