Does Left Ventricular Rotational Mechanics Depend on Aortic Valve Annular Dimensions in Healthy Adults?—A Three-Dimensional Speckle-Tracking Echocardiography-Derived Analysis from the MAGYAR-Healthy Study
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
- 1.
- Only a small number of healthy subjects have been involved in the present retrospective study in which complete 2D Doppler echocardiography extended with 3DSTE has been performed. Further studies with a larger number of healthy people are warranted following appropriate power analysis to make the whole analysis statistically stronger. The retrospective design of the present study may lead to selection bias, which should be considered when interpreting results as well;
- 2.
- Females have smaller, whole males have larger AVA dimensions as demonstrated above, which suggest effects of gender distribution on findings suggesting further investigations in this topic [26];
- 3.
- There are significant qualitative differences between the images taken during 2D echocardiography and 3DSTE. The routine 2D echocardiography still comes with significantly better image quality and spatial and temporal resolution, which partially limits the use of 3DSTE in daily clinical practice. The average frame rate achievable during 3DSTE is low (32 ± 3 fps). Another important factor is the size of the transducer, which is for 3DSTE larger than the one used in 2D echocardiography, limiting the efficiency of data acquisitions. Finally, during the processing of the data, digital acquisition of six subvolumes during six cardiac cycles occur, which may increase the chance of creating a stitching or a movement artifact. All these technical problems could have effects on the measured data [10,11,12,13,16,27];
- 4.
- Although a 3DSTE offers simultaneous assessment of the number of LV functional features like strains, the present study aimed to assess only LV rotational parameters [28];
- 5.
- Moreover, 3DSTE is also capable of creating 3D virtual models of other cardiac chambers, but the present study did not aim for such analyses;
- 6.
- 3DSTE-based measurement of parameters featuring LV rotational mechanics are validated; therefore, further validation in the present study was not aimed to be performed again.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guzzetti, E.; Côté, N.; Clavel, M.A.; Pibarot, P. Chapter 37—Ventricular–arterial coupling and arterial load in aortic valve disease. In Textbook of Arterial Stiffness and Pulsatile Hemodynamics in Health and Disease; Chirinos, J.A., Ed.; Academic Press: Cambridge, MA, USA, 2022; pp. 591–607. [Google Scholar]
- Nemes, A.; Kalapos, A.; Domsik, P.; Lengyel, C.; Orosz, A.; Forster, T. Correlations between echocardiographic aortic elastic properties and left ventricular rotation and twist—Insights from the three-dimensional speckle-tracking echocardiographic MAGYAR-Healthy Study. Clin. Physiol. Funct. Imaging 2013, 33, 381–385. [Google Scholar] [PubMed]
- Sunagawa, K.; Maughan, W.L.; Sagawa, K. Optimal arterial resistance for the maximal stroke work studied in isolated canine left ventricle. Circ. Res. 1985, 56, 586–595. [Google Scholar] [PubMed]
- Anderson, R.H. Clinical anatomy of the aortic root. Heart 2000, 84, 670–673. [Google Scholar] [PubMed]
- Nakatani, S. Left ventricular rotation and twist: Why should we learn? J. Cardiovasc. Ultrasound 2011, 19, 1–6. [Google Scholar] [CrossRef]
- Bloechlinger, S.; Grander, W.; Bryner, J.; Dünser, M.W. Left ventricular rotation: A neglected aspect of the cardiac cycle. Intensive Care Med. 2011, 37, 156–163. [Google Scholar] [CrossRef]
- Omar, A.M.S.; Vallabhajosyula, S.; Sengupta, P.P. Left Ventricular Twist and Torsion. Research Observations and Clinical Applications. Circ. Cardiovasc. Imaging 2015, 8, e003029. [Google Scholar]
- Sengupta, P.P.; Tajik, A.J.; Chandrasekaran, K.; Khandheria, B.K. Twist mechanics of the left ventricle: Principles and application. JACC Cardiovasc. Imaging 2008, 1, 366–376. [Google Scholar] [CrossRef]
- Stöhr, E.J.; Shave, R.E.; Baggish, A.L.; Weiner, R.B. Left ventricular twist mechanics in the context of normal physiology and cardiovascular disease: A review of studies using speckle tracking echocardiography. Am. J. Physiol. Heart Circ. Physiol. 2016, 311, H633–H644. [Google Scholar]
- Ammar, K.A.; Paterick, T.E.; Khandheria, B.K.; Jan, M.F.; Kramer, C.; Umland, M.M.; Tercius, A.J.; Baratta, L.; Tajik, A.J. Myocardial mechanics: Understanding and applying three-dimensional speckle tracking echocardiography in clinical practice. Echocardiography 2012, 29, 861–872. [Google Scholar]
- Urbano-Moral, J.A.; Patel, A.R.; Maron, M.S.; Arias-Godinez, J.A.; Pandian, N.G. Three-dimensional speckle-tracking echocardiography: Methodological aspects and clinical potential. Echocardiography 2012, 29, 997–1010. [Google Scholar]
- Muraru, D.; Niero, A.; Rodriguez-Zanella, H.; Cherata, D.; Badano, L. Three-dimensional speckle-tracking echocardiography: Benefits and limitations of integrating myocardial mechanics with three-dimensional imaging. Cardiovasc. Diagn. Ther. 2018, 8, 101–117. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Lin, Y.; Ji, M.; Wu, W.; Li, H.; Qian, M.; Zhang, L.; Xie, M.; Li, Y. Clinical Utility of Three-Dimensional Speckle-Tracking Echocardiography in Heart Failure. J. Clin. Med. 2022, 11, 6307. [Google Scholar] [CrossRef] [PubMed]
- Tamborini, G.; Fusini, L.; Muratori, M.; Cefalù, C.; Gripari, P.; Ali, S.G.; Pontone, G.; Andreini, D.; Bartorelli, A.L.; Alamanni, F.; et al. Feasibility and accuracy of three-dimensional transthoracic echocardiography vs. multidetector computed tomography in the evaluation of aortic valve annulus in patient candidates to transcatheter aortic valve implantation. Eur. Heart J. Cardiovasc. Imaging 2014, 15, 1316–1323. [Google Scholar] [CrossRef] [PubMed]
- Naguib, K.I.; Attia, M.A.; Bashandy, M.S.; Reihan, M.S.; Dabash, T.A.; El-Salam, A.B.A.; Helal, H.H.; Bahbah, E.I. The role of trans-thoracic echocardiography in the assessment of aortic annular diameter. Medicine 2021, 100, e24682. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef]
- Nemes, A.; Kormányos, Á.; Ruzsa, Z.; Achim, A.; Ambrus, N.; Lengyel, C. Left ventricular rotational mechanics and left ventricular volumes: Is there a relationship in healthy adults?—Three-dimensional speckle-tracking echocardiography-derived insights from the MAGYAR-Healthy Study. Quant. Imaging Med. Surg. 2023, 13, 6583–6589. [Google Scholar] [CrossRef]
- Nemes, A.; Kormányos, Á.; Ambrus, N.; Lengyel, C. Complex Relationship of Left Ventricular Rotational Mechanics and Deformation Represented by Strain Parameters in Healthy Adults-Detailed Analysis from the Three-Dimensional Speckle-Tracking Echocardiographic MAGYAR-Healthy Study. J. Clin. Med. 2023, 12, 7389. [Google Scholar] [CrossRef]
- Nemes, A.; Kormányos, Á.; Ambrus, N.; Lengyel, C. Insights into the Associations Between Systolic Left Ventricular Rotational Mechanics and Left Atrial Peak Reservoir Strains in Healthy Adults from the MAGYAR-Healthy Study. Biomedicines 2024, 12, 2515. [Google Scholar] [CrossRef]
- Nemes, A.; Kormányos, Á.; Ruzsa, Z.; Achim, A.; Ambrus, N.; Lengyel, C. Dependence of Left Ventricular Rotational Mechanics on Left Atrial Volumes in Non-Smoker Healthy Adults: Analysis Based on the Three-Dimensional Speckle-Tracking Echocardiographic MAGYAR-Healthy Study. J. Clin. Med. 2023, 12, 1235. [Google Scholar] [CrossRef]
- Nemes, A.; Kovács, Z.; Kormányos, Á.; Domsik, P.; Kalapos, A.; Ajtay, Z.; Lengyel, C. Left ventricular apical rotation is associated with mitral annular function in healthy subjects. Results from the three-dimensional speckle-tracking echocardiographic MAGYAR-Healthy Study. Physiol. Int. 2020, 107, 145–154. [Google Scholar] [CrossRef]
- Zhang, J.; Chowienczyk, P.J.; Spector, T.D.; Jiang, B. Relation of arterial stiffness to left ventricular structure and function in healthy women. Cardiovasc. Ultrasound 2018, 16, 21. [Google Scholar]
- Ashraf, M.; Myronenko, A.; Nguyen, T.; Inage, A.; Smith, W.; Lowe, R.I.; Thiele, K.; Gibbons Kroeker, C.A.; Tyberg, J.V.; Smallhorn, J.F.; et al. Defining left ventricular apex-to-base twist mechanics computed from high-resolution 3D echocardiography: Validation against sonomicrometry. JACC Cardiovasc. Imaging 2010, 3, 227–234. [Google Scholar]
- Zhou, Z.; Ashraf, M.; Hu, D.; Dai, X.; Xu, Y.; Kenny, B.; Cameron, B.; Nguyen, T.; Xiong, L.; Sahn, D.J. Three-dimensional speckle-tracking imaging for left ventricular rotation measurement: An in vitro validation study. J. Ultrasound. Med. 2010, 29, 903–909. [Google Scholar] [CrossRef]
- Andrade, J.; Cortez, L.D.; Campos, O.; Arruda, A.L.; Pinheiro, J.; Vulcanis, L.; Shiratsuchi, T.S.; Kalil-Filho, R.; Cerri, G.G. Left ventricular twist: Comparison between two- and three-dimensional speckle-tracking echocardiography in healthy volunteers. Eur. J. Echocardiogr. 2011, 12, 76–79. [Google Scholar]
- Agahi, S.; Yaseri, M.; Eftekhari, M.R.; Geraiely, B.; Sardari, A.; Sattarzadeh Badkoubeh, R.; Larti, F. Revisiting the Normal Ranges of Aortic Valve Area in 2D Echocardiography and Its Association With Age, Sex, and Anthropometric Characteristics. Echocardiography 2024, 41, e70029. [Google Scholar]
- Biswas, M.; Sudhakar, S.; Nanda, N.C.; Buckberg, G.; Pradhan, M.; Roomi, A.U.; Gorissen, W.; Houle, H. Two- and three-dimensional speckle tracking echocardiography: Clinical applications and future directions. Echocardiography 2013, 30, 88–105. [Google Scholar] [CrossRef]
- Narang, A.; Addetia, K. An introduction to left ventricular strain. Curr. Opin. Cardiol. 2018, 33, 455–463. [Google Scholar] [CrossRef]

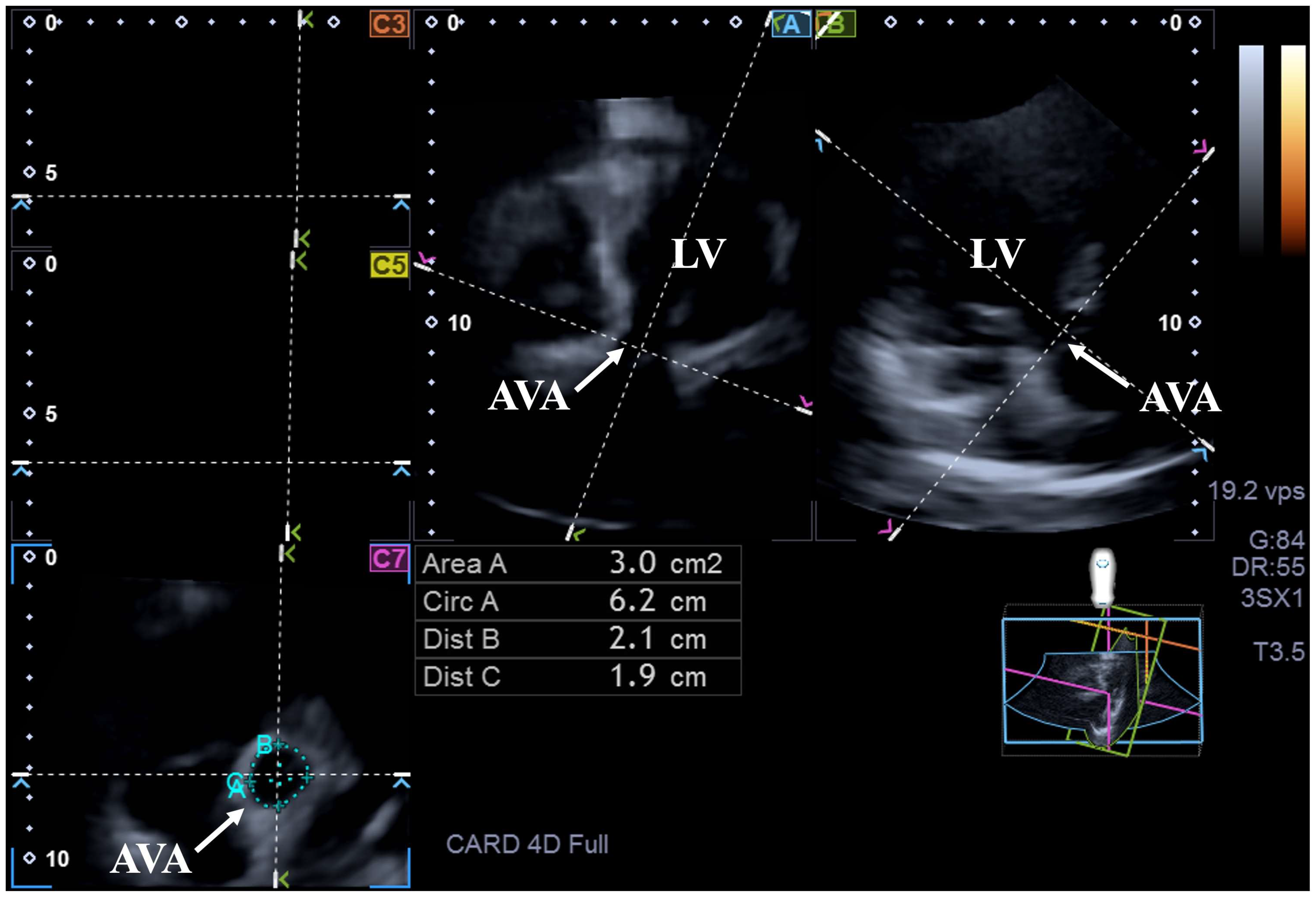
| ED-AVA-A ≤2.28 cm2 (n = 15) | 2.28 cm2 < ED-AVA-A < 4 cm2 (n = 77) | ED-AVA-A ≥4 cm2 (n = 19) | ES-AVA-A ≤2.48 cm2 (n = 11) | 2.48 cm2 < ES-AVA-A <4.22 cm2 (n = 81) | ES-AVA-A ≥4.22 cm2 (n = 19) | |
|---|---|---|---|---|---|---|
| mean age (years) | 33.9 ± 12.2 | 35.7 ± 12.0 | 37.6 ± 2.5 | 36.2 ± 11.2 | 35.5 ± 11.5 | 33.2 ± 10.7 |
| males (%) | 4 (27) | 49 (64) * | 16 (84) * | 1 (9) | 40 (49) ** | 18 (95) **†† |
| LV rotational mechanics | ||||||
| basal LV rotation (°) | −4.60 ± 2.91 | −3.96 ± 2.15 | −4.03 ± 2.26 | −3.90 ± 1.95 | −4.12 ± 2.26 | −3.89 ± 2.59 |
| apical LV rotation (°) | 7.82 ± 4.03 | 9.48 ± 3.69 | 9.93 ± 3.88 | 8.06 ± 4.11 | 9.50 ± 3.84 | 9.38 ± 3.43 |
| LV twist (°) | 12.42 ± 5.32 | 13.44 ± 4.06 | 13.96 ± 4.34 | 11.95 ± 4.62 | 13.61 ± 4.17 | 13.26 ± 4.60 |
| LV twist time (ms) | 384 ± 180 | 342 ± 104 | 301 ± 108 | 380 ± 146 | 345 ± 119 | 300 ± 96 |
| Aortic valve annulus | ||||||
| ED-AVA-Dmax (mm) | 1.56 ± 0.17 ‡ | 2.01 ± 0.20 * | 2.44 ± 0.23 *† | 1.65 ± 0.25 | 1.99 ± 0.25 ** | 2.36 ± 0.29 **††‡ |
| ED-AVA-Dmin (mm) | 1.35 ± 0.10 ‡ | 1.82 ± 0.20 * | 2.19 ± 0.16 *† | 1.41 ± 0.15 | 1.79 ± 0.24 ** | 2.15 ± 0.22 **†† |
| ED-AVA-A (mm) | 1.80 ± 0.27 ‡ | 3.07 ± 0.44 *‡ | 4.46 ± 0.51 *† | 1.98 ± 0.46 | 3.06 ± 0.64 **‡ | 4.14 ± 0.79 **††‡ |
| ED-AVA-P (mm) | 4.84 ± 0.41 ‡ | 6.27 ± 0.45 *‡ | 7.58 ± 0.40 *† | 5.02 ± 0.59 | 6.25 ± 0.66 **‡ | 7.26 ± 0.70 **††‡ |
| ES-AVA-Dmax (mm) | 1.72 ± 0.19 | 2.04 ± 0.24 * | 2.37 ± 0.27 *† | 1.61 ± 0.18 | 2.02 ± 0.20 ** | 2.48 ± 0.19 **†† |
| ES-AVA-Dmin (mm) | 1.57 ± 0.16 | 1.85 ± 0.24 * | 2.17 ± 0.21 *† | 1.43 ± 0.17 | 1.84 ± 0.20 ** | 2.23 ± 0.18 **†† |
| ES-AVA-A (mm) | 2.35 ± 0.40 | 3.28 ± 0.63 * | 4.43 ± 0.82 *† | 1.92 ± 0.28 | 3.23 ± 0.47 ** | 4.73 ± 0.50 **†† |
| ES-AVA-P (mm) | 5.45 ± 0.50 | 6.46 ± 0.62 * | 7.54 ± 0.71 *† | 4.99 ± 0.40 | 6.42 ± 0.49 ** | 7.75 ± 0.44 **†† |
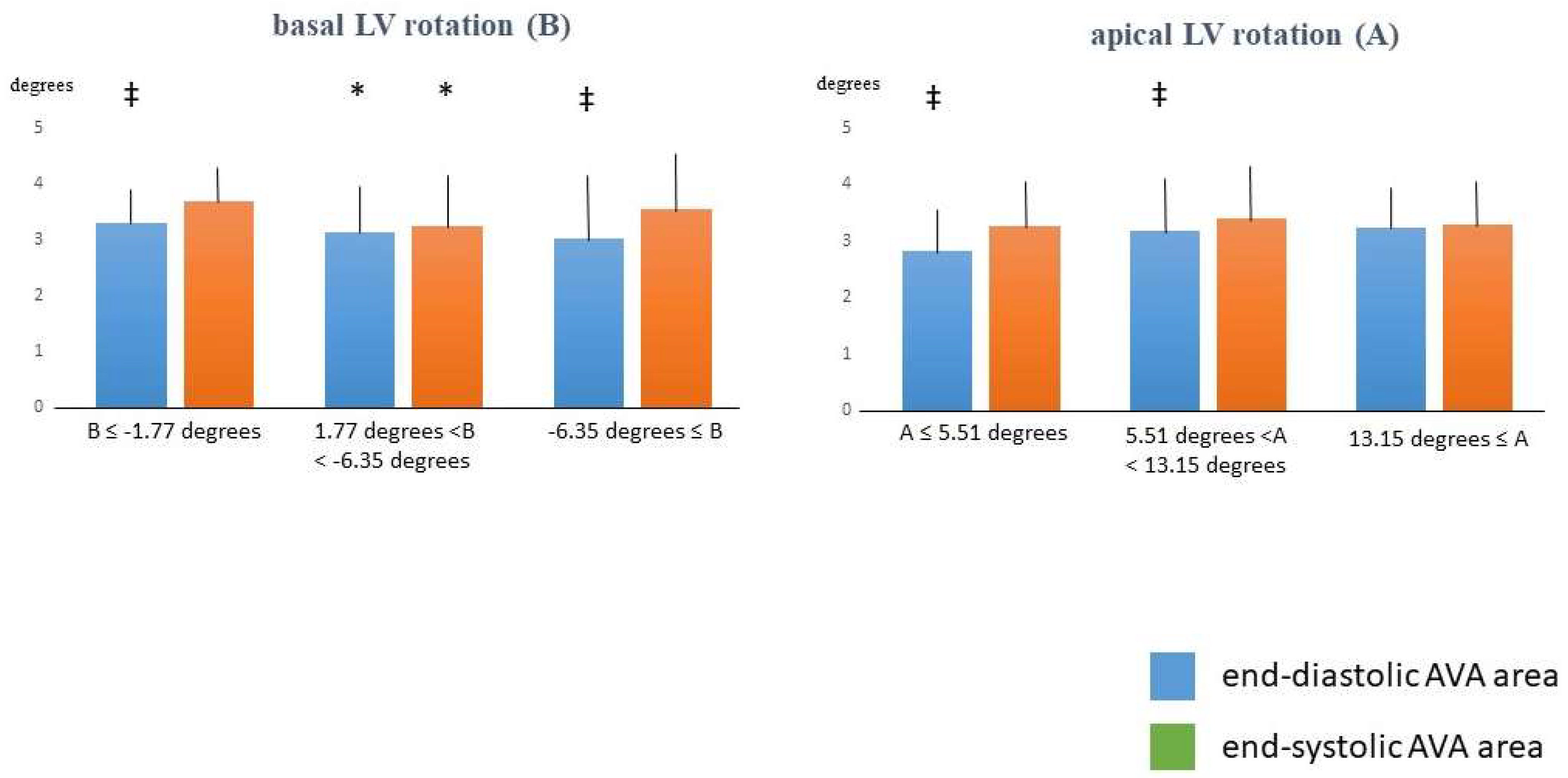
| Basal LV Rotation ≤ −1.77 Degrees (n = 14) | −1.77 Degrees < Basal LV Rotation < −6.35 Degrees (n = 77) | Basal LV Rotation ≥ −6.35 Degrees (n = 20) | Apical LV Rotation ≤ 5.51 Degrees (n = 16) | 5.51 Degrees < Apical LV Rotation < 13.15 Degrees (n = 77) | Apical LV Rotation ≥ 13.15 Degrees (n = 18) | |
|---|---|---|---|---|---|---|
| mean age (years) | 36.1 ± 11.7 | 34.0 ± 11.1 | 39.6 ± 14.4 | 36.8 ± 12.5 | 34.7 ± 12.0 | 36.7 ± 11.6 |
| males (%) | 11 (79) | 44 (57) | 14 (70) | 10 (63) | 46 (60) | 13 (72) |
| LV rotational mechanics | ||||||
| basal LV rotation (°) | −1.14 ± 0.60 | −3.55 ± 1.09 * | −8.06 ± 1.18 *† | −4.03 ± 2.76 | −4.11 ± 2.30 | −3.86 ± 1.75 |
| apical LV rotation (°) | 9.57 ± 3.48 | 9.59 ± 3.92 | 8.67 ± 3.39 | 3.18 ± 1.64 | 9.20 ± 1.91 ** | 15.36 ± 1.38 **†† |
| LV twist (°) | 10.70 ± 3.70 | 13.12 ± 3.92 | 16.72 ± 3.70 *† | 7.20 ± 3.40 | 13.31 ± 2.69 ** | 19.23 ± 2.40 **†† |
| LV twist time (ms) | 289 ± 127 | 349 ± 124 | 343 ± 88 | 311 ± 173 | 340 ± 106 | 353 ± 120 |
| Aortic valve annulus | ||||||
| ED-AVA-Dmax (mm) | 2.08 ± 0.22 | 2.02 ± 0.32 | 1.98 ± 0.34 ‡ | 1.96 ± 0.37 | 2.02 ± 0.31 ‡ | 2.09 ± 0.25 |
| ED-AVA-Dmin (mm) | 1.95 ± 0.16 | 1.81 ± 0.30 * | 1.78 ± 0.32 ‡ | 1.71 ± 0.31 | 1.84 ± 0.30 ‡ | 1.83 ± 0.26 |
| ED-AVA-A (mm) | 3.30 ± 0.53 ‡ | 3.14 ± 0.84 * | 3.02 ± 1.05 ‡ | 2.83 ± 0.78 ‡ | 3.18 ± 0.89 ‡ | 3.24 ± 0.69 |
| ED-AVA-P (mm) | 6.49 ± 0.52 ‡ | 6.31 ± 0.87 * | 6.16 ± 1.03 ‡ | 6.00 ± 0.90 ‡ | 6.33 ± 0.90 ‡ | 6.44 ± 0.67 |
| ES-AVA-Dmax (mm) | 2.19 ± 0.23 | 2.02 ± 0.29 * | 2.10 ± 0.35 | 1.97 ± 0.29 | 2.07 ± 0.31 | 2.06 ± 0.25 |
| ES-AVA-Dmin (mm) | 2.01 ± 0.17 | 1.83 ± 0.29 * | 1.92 ± 0.29 | 1.80 ± 0.30 | 1.90 ± 0.27 | 1.81 ± 0.29 |
| ES-AVA-A (mm) | 3.69 ± 0.65 | 3.25 ± 0.83 * | 3.54 ± 1.03 | 3.26 ± 0.77 | 3.39 ± 0.91 | 3.28 ± 0.74 |
| ES-AVA-P (mm) | 6.86 ± 0.61 | 6.40 ± 0.84 * | 6.65 ± 0.94 | 6.37 ± 0.80 | 6.55 ± 0.89 | 6.43 ± 0.66 |
| Intraobserver Agreement | Interobserver Agreement | |||
|---|---|---|---|---|
| mean ± 2SD Difference in Values Obtained by 2 Measurements of the Same Observer | ICC Between Measurements of the Same Observer | mean ± 2SD Difference in Values Obtained by 2 Observers | ICC Between Independent Measurements of 2 Observers | |
| basal LV rotation (°) | 0.3 ± 0.1 | 0.81 (p < 0.01) | 0.3 ± 0.2 | 0.80 (p < 0.01) |
| apical LV rotation (°) | 0.05 ± 0.05 | 0.80 (p < 0.01) | 0.6 ± 0.7 | 0.81 (p < 0.01) |
| ED-AVA-Dmax (mm) | −0.06 ± 0.19 | 0.85 (p < 0.01) | −0.04 ± 0.20 | 0.89 (p < 0.01) |
| ED-AVA-Dmin (mm) | −0.02 ± 0.24 | 0.91 (p < 0.01) | −0.03 ± 0.20 | 0.93 (p < 0.01) |
| ED-AVA-A (mm) | −0.14 ± 0.62 | 0.95 (p < 0.01) | −0.12 ± 0.57 | 0.96 (p < 0.01) |
| ED-AVA-p (mm) | −0.05 ± 0.70 | 0.91 (p < 0.01) | −0.13 ± 0.66 | 0.92 (p < 0.01) |
| ES-AVA-Dmax (mm) | 0.01 ± 0.29 | 0.92 (p < 0.01) | 0.03 ± 0.31 | 0.93 (p < 0.01) |
| ES-AVA-Dmin (mm) | 0.05 ± 0.33 | 0.82 (p < 0.01) | 0.05 ± 0.32 | 0.82 (p < 0.01) |
| ES-AVA-A (mm) | 0.15 ± 0.73 | 0.92 (p < 0.01) | 0.12 ± 0.74 | 0.94 (p < 0.01) |
| ES-AVA-P (mm) | −0.02 ± 0.54 | 0.92 (p < 0.01) | 0.01 ± 0.58 | 0.93 (p < 0.01) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nemes, A.; Ambrus, N.; Lengyel, C. Does Left Ventricular Rotational Mechanics Depend on Aortic Valve Annular Dimensions in Healthy Adults?—A Three-Dimensional Speckle-Tracking Echocardiography-Derived Analysis from the MAGYAR-Healthy Study. Biomedicines 2025, 13, 817. https://doi.org/10.3390/biomedicines13040817
Nemes A, Ambrus N, Lengyel C. Does Left Ventricular Rotational Mechanics Depend on Aortic Valve Annular Dimensions in Healthy Adults?—A Three-Dimensional Speckle-Tracking Echocardiography-Derived Analysis from the MAGYAR-Healthy Study. Biomedicines. 2025; 13(4):817. https://doi.org/10.3390/biomedicines13040817
Chicago/Turabian StyleNemes, Attila, Nóra Ambrus, and Csaba Lengyel. 2025. "Does Left Ventricular Rotational Mechanics Depend on Aortic Valve Annular Dimensions in Healthy Adults?—A Three-Dimensional Speckle-Tracking Echocardiography-Derived Analysis from the MAGYAR-Healthy Study" Biomedicines 13, no. 4: 817. https://doi.org/10.3390/biomedicines13040817
APA StyleNemes, A., Ambrus, N., & Lengyel, C. (2025). Does Left Ventricular Rotational Mechanics Depend on Aortic Valve Annular Dimensions in Healthy Adults?—A Three-Dimensional Speckle-Tracking Echocardiography-Derived Analysis from the MAGYAR-Healthy Study. Biomedicines, 13(4), 817. https://doi.org/10.3390/biomedicines13040817








