The Modified Canine Groove Model of Osteoarthritis †
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.1.1. Original Groove Model
2.1.2. Modified Groove Model
2.1.3. Housing
2.2. Induction of Joint Degeneration
2.2.1. Original Groove Procedure
2.2.2. Modified Groove Procedure
2.2.3. Perioperative Management
2.3. Outcome Measures
2.3.1. General
2.3.2. Force Plate Analysis
2.3.3. Cartilage Integrity
2.3.4. Chondrocyte Activity
2.3.5. Synovial Tissue Inflammation
2.3.6. Calculations
2.4. Statistical Analysis
3. Results
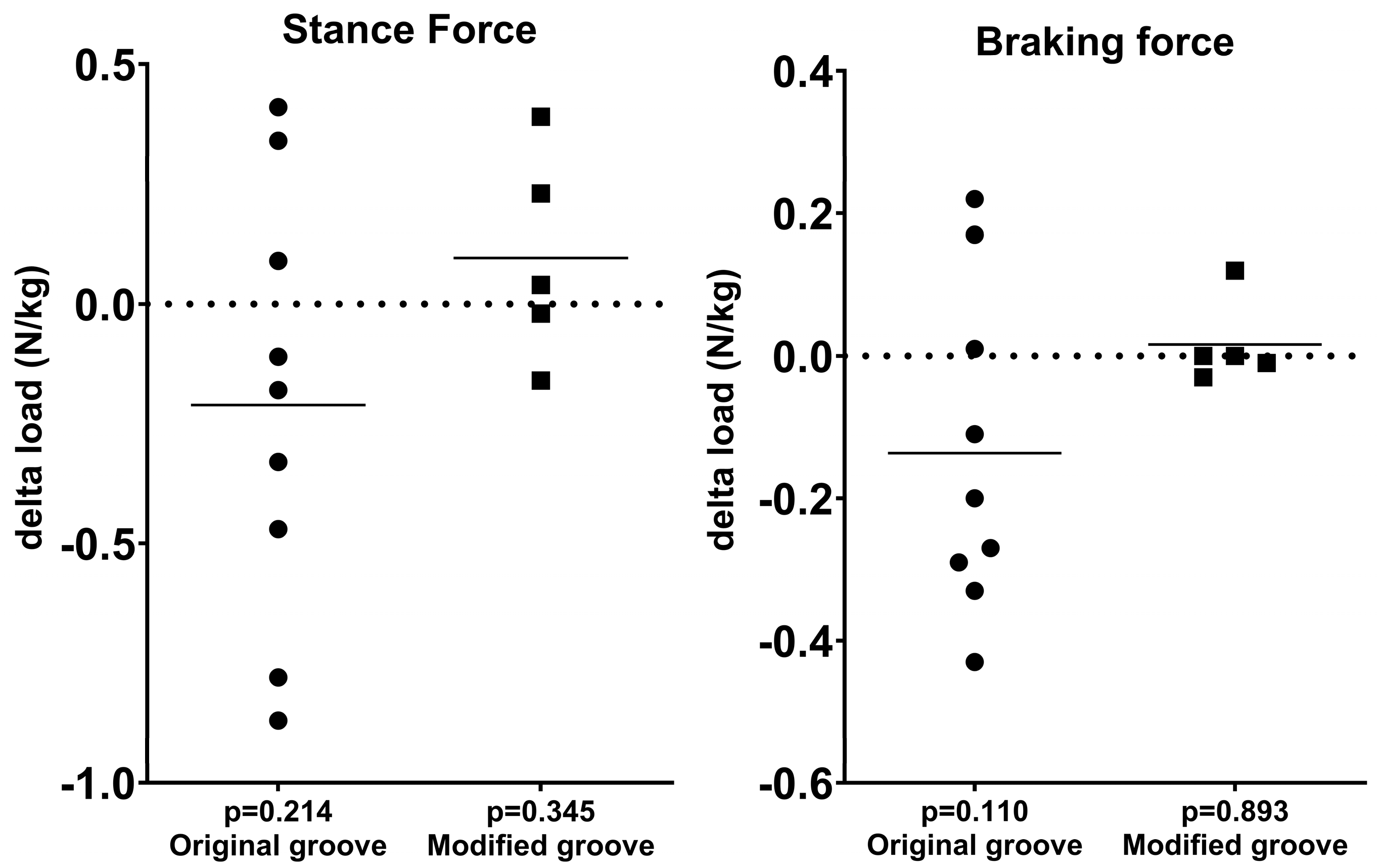
3.1. Force Plate Analysis
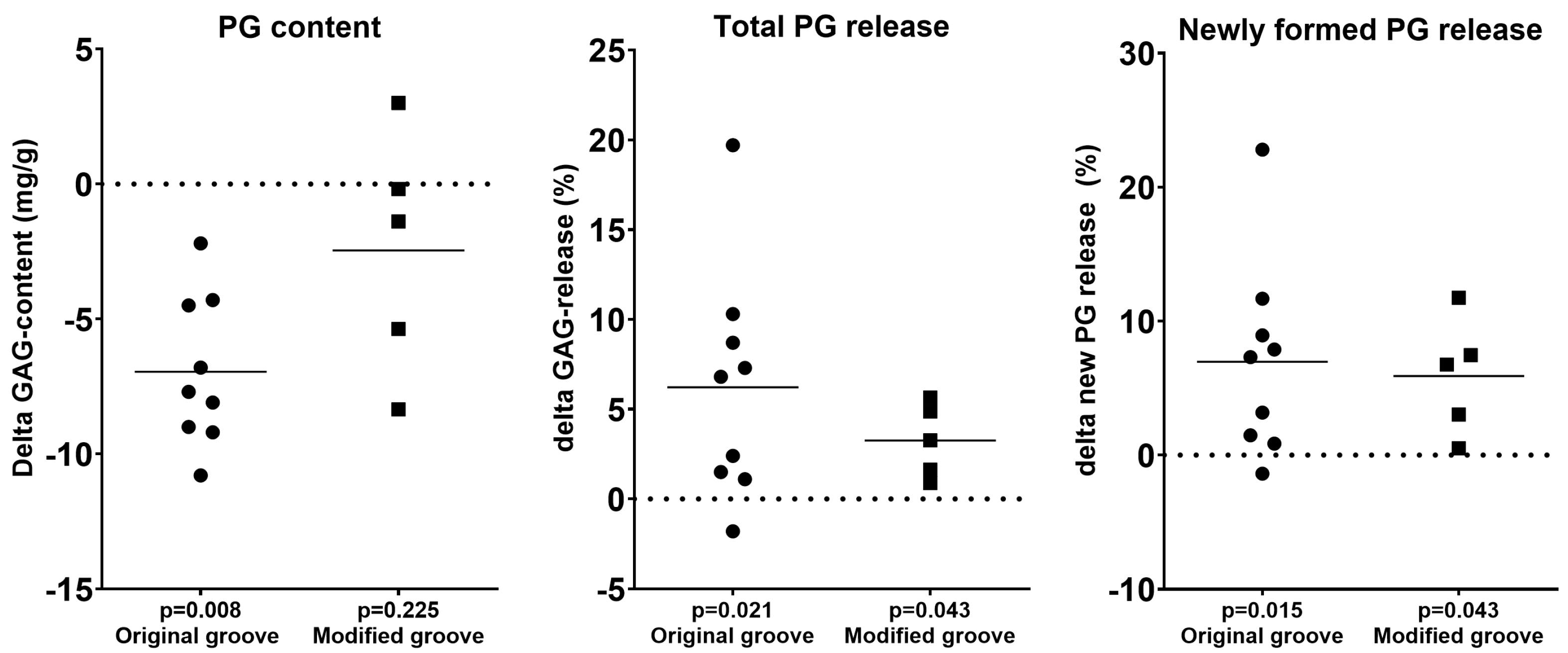
3.2. Cartilage Integrity
3.3. Synovial Inflammation
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| OA | Osteoarthritis |
| ACLT | Anterior cruciate ligament transection |
| DMOADs | Disease-modifying osteoarthritis drugs |
| Fz | Maximum stance force |
| Fymax | Braking force |
| OARSI | Osteoarthritis Research Society International |
| GAG | Glycosaminoglycan |
| PG | Proteoglycan |
| NSAID | Non-steroidal anti-inflammatory drugs |
| MRI | Magnetic resonance imaging |
References
- Loeser, R.F.; Goldring, S.R.; Scanzello, C.R.; Goldring, M.B. Osteoarthritis A Disease of the Joint as an Organ. Arthritis Rheum. 2012, 64, 1697–1707. [Google Scholar] [CrossRef] [PubMed]
- Steinmetz, J.D.; Culbreth, G.T.; Haile, L.M.; Rafferty, Q.; Lo, J.; Fukutaki, K.G.; Cruz, J.A.; Smith, A.E.; Vollset, S.E.; Brooks, P.M.; et al. Global, regional, and national burden of osteoarthritis, 1990–2020 and projections to 2050: A systematic analysis for the Global Burden of Disease Study 2021. Lancet Rheumatol. 2023, 5, e508–e522. [Google Scholar] [CrossRef]
- Antony, B.; Jones, G.; Jin, X.; Ding, C. Do early life factors affect the development of knee osteoarthritis in later life: A narrative review. Arthritis Res. Ther. 2016, 18, 202. [Google Scholar] [CrossRef] [PubMed]
- Kuyinu, E.L.; Narayanan, G.; Nair, L.S.; Laurencin, C.T. Animal models of osteoarthritis: Classification, update, and measurement of outcomes. J. Orthop. Surg. Res. 2016, 11, 19. [Google Scholar] [CrossRef]
- Gregory, M.H.; Capito, N.; Kuroki, K.; Stoker, A.M.; Cook, J.L.; Sherman, S.L. A Review of Translational Animal Models for Knee Osteoarthritis. Arthritis 2012, 2012, 764621. [Google Scholar] [CrossRef]
- Cook, J.; Kuroki, K.; Visco, D.; Pelletier, J.-P.; Schulz, L.; Lafeber, F. The OARSI histopathology initiative e recommendations for histological assessments of osteoarthritis in the dog. Osteoarthr. Cartil. 2010, 18, 66–79. [Google Scholar] [CrossRef]
- Proffen, B.L.; McElfresh, M.; Fleming, B.C.; Murray, M.M. A comparative anatomical study of the human knee and six animal species. Knee 2012, 19, 493–499. [Google Scholar] [CrossRef]
- Meeson, R.L.; Todhunter, R.J.; Blunn, G.; Nuki, G.; Pitsillides, A.A. Spontaneous dog osteoarthritis—A One Medicine vision. Nat. Rev. Rheumatol. 2019, 15, 273–287. [Google Scholar] [CrossRef] [PubMed]
- Coxib and traditional NSAID Trialists’ (CNT) Collaboration; Bhala, N.; Emberson, J.; Merhi, A.; Abramson, S.; Arber, N.; Baron, J.A.; Bombardier, C.; Cannon, C.; Farkouh, M.E.; et al. Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: Meta-analyses of individual participant data from randomised trials. Lancet 2013, 382, 769–779. [Google Scholar] [CrossRef]
- Burnett, R.A., 3rd; Khalid, S.; DeBenedetti, A.; Terhune, E.B.; Angotti, M.L.; Della Valle, C.J. Intra-articular corticosteroid injections are associated with a dose-dependent risk of total knee arthroplasty at 5 years. Knee Surg. Sports Traumatol. Arthrosc. 2022, 31, 426–431. [Google Scholar] [CrossRef]
- Gao, J.; Xia, Z.; Mary, H.B.; Joseph, J.; Luo, J.N.; Joshi, N. Overcoming barriers for intra-articular delivery of disease-modifying osteoarthritis drugs. Trends Pharmacol. Sci. 2022, 43, 171–187. [Google Scholar] [CrossRef] [PubMed]
- Marijnissen, A.; van Roermund, P.; TeKoppele, J.; Bijlsma, J.; Lafeber, F. The canine ‘groove’ model, compared with the ACLT model of osteoarthritis. Osteoarthr. Cartil. 2002, 10, 145–155. [Google Scholar] [CrossRef]
- Mastbergen, S.C.; Marijnissen, A.C.; Vianen, M.E.; van Roermund, P.M.; Bijlsma, J.W.; Lafeber, F.P. The canine ‘groove’ model of osteoarthritis is more than simply the expression of surgically applied damage. Osteoarthr. Cartil. 2005, 14, 39–46. [Google Scholar] [CrossRef]
- Marijnissen, A.; van Roermund, P.; Verzijl, N.; Tekoppele, J.; Bijlsma, J.; Lafeber, F. Steady progression of osteoarthritic features in the canine groove model. Osteoarthr. Cartil. 2002, 10, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Intema, F.; Mastbergen, S.; Van Rinsum, C.; Castelein, R.; Marijnissen, A.K.; Bijlsma, J.; Lafeber, F. Cartilage integrity and proteoglycan turnover are comparable in canine experimentally induced and human joint degeneration. Rheumatol. Rep. 2010, 2, e7. [Google Scholar] [CrossRef]
- Frost-Christensen, L.N.; Mastbergen, S.C.; Vianen, M.E.; Hartog, A.; DeGroot, J.; Voorhout, G.; Van Wees, A.M.C.; Lafeber, F.P.J.G.; Hazewinkel, H.A.W. Degeneration, inflammation, regeneration, and pain/disability in dogs following destabilization or articular cartilage grooving of the stifle joint. Osteoarthr. Cartil. 2008, 16, 1327–1335. [Google Scholar] [CrossRef]
- Wiegant, K.; Intema, F.; van Roermund, P.M.; Rijbroek, A.D.B.; Doornebal, A.; Hazewinkel, H.A.W.; Lafeber, F.P.J.G.; Mastbergen, S.C. Mastbergen. Evidence of Cartilage Repair by Joint Distraction in a Canine Model of Osteoarthritis. Arthritis Rheumatol. 2015, 67, 465–474. [Google Scholar] [CrossRef]
- Wohl, G.R.; Shymkiw, R.C.; Matyas, J.R.; Kloiber, R.; Zernicke, R.F. Periarticular cancellous bone changes following anterior cruciate ligament injury. J. Appl. Physiol. 2001, 91, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Budsberg, S.C.; Verstraete, M.C.; Soutas-Little, R.W. Force plate analysis of the walking gait in healthy dogs. Am. J. Vet. Res. 1987, 48, 915–918. [Google Scholar] [CrossRef]
- Moreau, M.; Lussier, B.; Ballaz, L.; Troncy, E. Kinetic measurements of gait for osteoarthritis research in dogs and cats. Can. Vet. J. 2014, 55, 1057–1065. [Google Scholar]
- Hyllested, J.; Veje, K.; Ostergaard, K. Histochemical studies of the extracellular matrix of human articular cartilage—A review. Osteoarthr. Cartil. 2002, 10, 333–343. [Google Scholar] [CrossRef]
- An, Y.H.; Martin, K.L. Handbook of Histology Methods for Bone and Cartilage; Springer: New York, NY, USA, 2003. [Google Scholar]
- Whiteman, P. The quantitative measurement of Alcian Blue–glycosaminoglycan complexes. Biochem. J. 1973, 131, 343–350. [Google Scholar] [CrossRef]
- Lafeber, F.P.; van Roy, H.; Wilbrink, B.; Huber-Bruning, O.; Bijlsma, J.W. Human osteoarthritic cartilage is synthetically more active but in culture less vital than normal cartilage. J. Rheumatol. 1992, 19, 123–129. [Google Scholar]
- Jansen, M.P.; Boymans, T.A.; Custers, R.J.; Van Geenen, R.C.; Van Heerwaarden, R.J.; Huizinga, M.R.; Nellensteijn, J.M.; Sollie, R.; Spruijt, S.; Mastbergen, S.C. Knee Joint Distraction as Treatment for Osteoarthritis Results in Clinical and Structural Benefit: A Systematic Review and Meta-Analysis of the Limited Number of Studies and Patients Available. Cartilage 2021, 13, 1113S–1123S. [Google Scholar] [CrossRef]
- Goldring, M.B.; Goldring, S.R. Articular cartilage and subchondral bone in the pathogenesis of osteoarthritis. Ann. N. Y. Acad. Sci. 2010, 1192, 230–237. [Google Scholar] [CrossRef]
- Andrade, R.; Vasta, S.; Pereira, R.; Pereira, H.; Papalia, R.; Karahan, M.; Oliveira, J.M.; Reis, R.L.; Espregueira-Mendes, J. Knee donor-site morbidity after mosaicplasty—A systematic review. J. Exp. Orthop. 2016, 3, 31. [Google Scholar] [CrossRef]
- Englund, M.; Roemer, F.W.; Hayashi, D.; Crema, M.D.; Guermazi, A. Meniscus pathology, osteoarthritis and the treatment controversy. Nat. Rev. Rheumatol. 2012, 8, 412–419. [Google Scholar] [CrossRef]
- Silverstein, A.; Stefani, R.; Sobczak, E.; Tong, E.; Attur, M.; Shah, R.; Bulinski, J.; Ateshian, G.; Hung, C. Toward understanding the role of cartilage particulates in synovial inflammation. Osteoarthr. Cartil. 2017, 25, 1353–1361. [Google Scholar] [CrossRef]
- Waxman, A.S.; Robinson, D.A.; Evans, R.B.; Hulse, D.A.; Innes, J.F.; Conzemius, M.G. Relationship between objective and subjective assessment of limb function in normal dogs with an experimentally induced lameness. Vet. Surg. 2008, 37, 241–246. [Google Scholar] [CrossRef]
- Voss, K.; Imhof, J.; Kaestner, S.; Montavon, P.M. Force plate gait analysis at the walk and trot in dogs with low-grade hindlimb lameness. Vet. Comp. Orthop. Traumatol. 2007, 20, 299–304. [Google Scholar] [CrossRef]
- Becerra, J.; Andrades, J.A.; Guerado, E.; Zamora-Navas, P.; López-Puertas, J.M.; Reddi, A.H. Articular cartilage: Structure and regeneration. Tissue Eng. Part B Rev. 2010, 16, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Little, C.B.; Hunter, D.J. Post-traumatic osteoarthritis: From mouse models to clinical trials. Nat. Rev. Rheumatol. 2013, 9, 485–497. [Google Scholar] [CrossRef] [PubMed]
- van der Weiden, G.S.; Meij, B.P.; van de Belt, A.; Custers, R.J.; Karperien, M.; Lafeber, F.; Mastbergen, S. The Modified Canine Groove Model of Osteoarthritis. In Proceedings of the 2024 OARSI World Congress on Osteoarthritis, Vienna, Austria, 18–21 April 2024. [Google Scholar]



| Original Groove Model | Modified Groove Model | |||||
|---|---|---|---|---|---|---|
| Outcomes | Experimental | Control | p | Experimental | Control | p |
| Stance force | 4.0 ± 0.5 | 4.2 ± 0.3 | 0.214 | 3.9 ± 0.3 | 3.8 ± 0.2 | 0.345 |
| Braking force | 0.6 ± 0.1 | 0.8 ± 0.2 | 0.110 | 0.6 ± 0.2 | 0.6 ± 0.1 | 0.893 |
| Macroscopy of cartilage | 1.5 ± 0.5 | 0.1 ± 0.2 | 0.007 | 0.9 ± 0.2 | 0.0 ± 0.0 | 0.034 |
| Histology of cartilage | 9.4 ± 2.3 | 4.4 ± 1.7 | 0.008 | 6.1 ± 2.2 | 4.3 ± 1.3 | 0.043 |
| Macroscopy of synovial tissue | 2.1 ± 0.7 | 1.0 ± 0.4 | 0.007 | 1.0 ± 0.4 | 0.0 ± 0.0 | 0.039 |
| Histology of synovial tissue | 4.1 ± 1.5 | 1.1 ± 0.6 | 0.008 | 9.6 ± 3.0 | 4.7 ± 2.9 | 0.043 |
| PG content | 27.8 ± 3.3 | 34.8 ± 2.9 | 0.008 | 24.7 ± 6.9 | 27.1 ± 8.6 | 0.225 |
| Newly formed PG released | 50.2 ± 10.4 | 43.2 ± 4.9 | 0.015 | 39.8 ± 14.1 | 33.9 ± 11.0 | 0.043 |
| Total PG released | 27.7 ± 7.6 | 21.5 ± 2.9 | 0.021 | 19.0 ± 4.6 | 15.8 ± 4.1 | 0.043 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van der Weiden, G.S.; Meij, B.P.; van de Belt, A.; Custers, R.J.H.; Both, S.K.; Karperien, M.; Mastbergen, S.C. The Modified Canine Groove Model of Osteoarthritis. Biomedicines 2025, 13, 913. https://doi.org/10.3390/biomedicines13040913
van der Weiden GS, Meij BP, van de Belt A, Custers RJH, Both SK, Karperien M, Mastbergen SC. The Modified Canine Groove Model of Osteoarthritis. Biomedicines. 2025; 13(4):913. https://doi.org/10.3390/biomedicines13040913
Chicago/Turabian Stylevan der Weiden, Goran S., Björn P. Meij, Amy van de Belt, Roel J. H. Custers, Sanne K. Both, Marcel Karperien, and Simon C. Mastbergen. 2025. "The Modified Canine Groove Model of Osteoarthritis" Biomedicines 13, no. 4: 913. https://doi.org/10.3390/biomedicines13040913
APA Stylevan der Weiden, G. S., Meij, B. P., van de Belt, A., Custers, R. J. H., Both, S. K., Karperien, M., & Mastbergen, S. C. (2025). The Modified Canine Groove Model of Osteoarthritis. Biomedicines, 13(4), 913. https://doi.org/10.3390/biomedicines13040913





