Immunogenic Cell Death and Metabolic Reprogramming in Cancer: Mechanisms, Synergies, and Innovative Therapeutic Strategies
Abstract
1. Introduction
2. Molecular Mechanisms and Features of ICD
2.1. Mechanisms of ICD Activation
2.2. Core Features of ICD
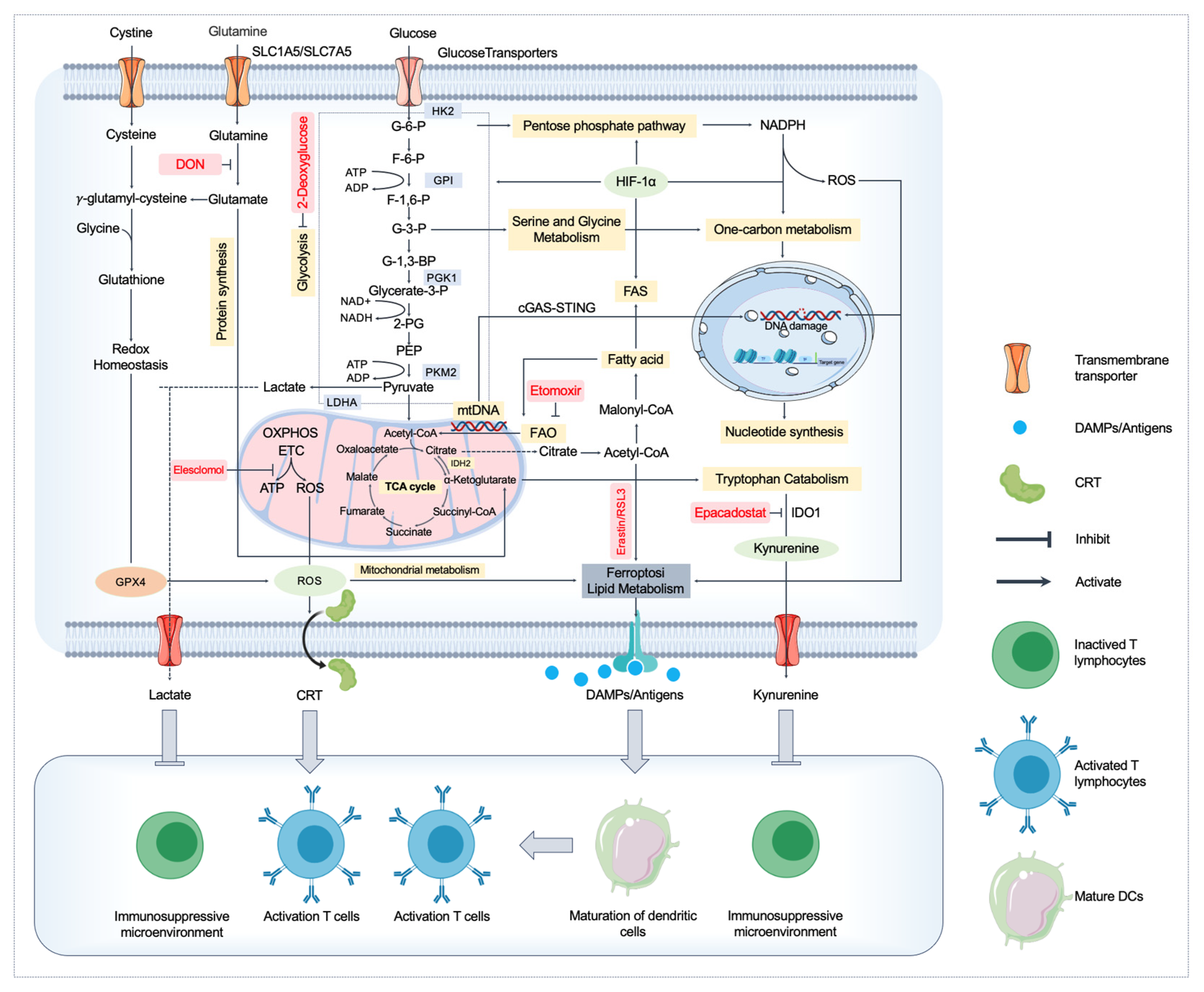
3. Metabolic Regulation of ICD
3.1. Disruption of Mitochondrial Function and ICD
3.1.1. Mitochondrial Dysfunction and ICD Induction
3.1.2. Mitochondrial ROS and ICD
3.1.3. mtDNA and the cGAS-STING Pathway in ICD
3.2. Lipid Metabolism, Ferroptosis, and ICD
3.2.1. Lipid Metabolism and Its Role in ICD
3.2.2. Ferroptosis and Its Interplay with ICD
3.2.3. Lipid Peroxidation and Immune Activation
4. Amino Acid Metabolism and ICD
4.1. Glutamine Metabolism
4.2. Tryptophan Catabolism and Immune Suppression
4.3. Serine and Glycine Metabolism
5. Immune Microenvironment Regulation by Metabolites
6. Targeting Metabolism to Enhance ICD and Cancer Immunotherapy
6.1. Metabolic Reprogramming to Enhance ICD Immunogenicity
6.2. Targeted Delivery Systems for ICD and Metabolic Regulation
6.3. Combination Therapies: Synergizing ICD with Metabolic Inhibitors
6.4. Personalized Treatment Approaches
6.5. Omics Approaches for Metabolic Reprogramming in Clinical Application
6.6. Clinical Applications and Success Stories of Combining ICD and Metabolic Inhibitors
6.7. Genetic Strategies to Enhance ICD: CRISPR/Cas9
7. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ICD | Immunogenic cell death |
| DAMPs | Damage-associated molecular patterns |
| HMGB1 | High-mobility group box 1 |
| CRT | Calreticulin |
| TME | Tumor microenvironment |
| DCs | Dendritic cells |
| CTLs | Cytotoxic T lymphocytes |
| TAAs | Tumor-associated antigens |
| ROS | Reactive oxygen species |
| ER | Endoplasmic reticulum |
| ATP | Adenosine triphosphate |
| P2X7 | P2X Purinoceptor 7 |
| NLRP3 | NLR family pyrin domain-containing protein 3 |
| IL-1β | Interleukin-1β |
| TLR4 | Toll-like receptor 4 |
| RAGE | Receptor of advanced glycation endproduct |
| OXPHOS | Oxidative phosphorylation |
| mtDNA | Mitochondrial DNA |
| cGAS | cyclic GMP-AMP synthase |
| STING | Stimulator of interferon genes |
| PRRs | Pattern recognition receptors |
| FASN | Fatty acid synthase |
| ACSL4 | acyl-CoA synthetase long-chain family member 4 |
| GPX4 | Glutathione peroxidase 4 |
| PDT | Photodynamic therapy |
| 4-HNE | 4-hydroxy-2-nonenal |
| UPR | Unfolded protein response |
| DON | 6-diazo-5-oxo-L-norleucine |
| IDO1 | 2,3-dioxygenase 1 |
| SAMe | S-adenosylmethionine |
| PD-1 | Programmed death-1 |
| PD-L1 | Programmed cell death ligand 1 |
| AMPK | Adenosine 5′-monophosphate-activated protein kinase |
| CRISPR/Cas9 | Clustered regularly interspaced short palindromic repeats—CRISPR-associated protein 9 |
| RIPK1 | Receptor-interacting protein kinase 1 |
| RIPK3 | Receptor-interacting protein kinase 3 |
| MLKL | Mixed lineage kinase domain-like protein |
| LDHA | Lactate dehydrogenase A |
| HK2 | Hexokinase 2 |
References
- Meier, P.; Legrand, A.J.; Adam, D.; Silke, J. Immunogenic cell death in cancer: Targeting necroptosis to induce antitumour immunity. Nat. Rev. Cancer 2024, 24, 299–315. [Google Scholar] [CrossRef] [PubMed]
- Gielecińska, A.; Kciuk, M.; Yahya, E.B.; Ainane, T.; Mujwar, S.; Kontek, R. Apoptosis, necroptosis, and pyroptosis as alternative cell death pathways induced by chemotherapeutic agents? Biochim. Biophys. Acta Rev. Cancer 2023, 1878, 189024. [Google Scholar] [CrossRef] [PubMed]
- Hänggi, K.; Ruffell, B. Cell death, therapeutics, and the immune response in cancer. Trends Cancer 2023, 9, 381–396. [Google Scholar] [CrossRef]
- Kroemer, G.; Galluzzi, L.; Kepp, O.; Zitvogel, L. Immunogenic cell death in cancer therapy. Annu. Rev. Immunol. 2013, 31, 51–72. [Google Scholar] [CrossRef]
- Krysko, D.V.; Garg, A.D.; Kaczmarek, A.; Krysko, O.; Agostinis, P.; Vandenabeele, P. Immunogenic cell death and DAMPs in cancer therapy. Nat. Rev. Cancer 2012, 12, 860–875. [Google Scholar] [CrossRef]
- Fan, J.; Gillespie, K.P.; Mesaros, C.; Blair, I.A. HMGB2-induced calreticulin translocation required for immunogenic cell death and ferroptosis of cancer cells are controlled by the nuclear exporter XPO1. Commun. Biol. 2024, 7, 1234. [Google Scholar] [CrossRef]
- Luu, M.; Riester, Z.; Baldrich, A.; Reichardt, N.; Yuille, S.; Busetti, A.; Klein, M.; Wempe, A.; Leister, H.; Raifer, H.; et al. Microbial short-chain fatty acids modulate CD8+ T cell responses and improve adoptive immunotherapy for cancer. Nat. Commun. 2021, 12, 4077. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Rong, Y.; Tang, X.; Yi, K.; Qi, P.; Hou, J.; Liu, W.; He, Y.; Gao, X.; Yuan, C.; et al. Engineered exosomes as an in situ DC-primed vaccine to boost antitumor immunity in breast cancer. Mol. Cancer 2022, 21, 45. [Google Scholar] [CrossRef]
- Zhou, Z.; Liu, Y.; Song, W.; Jiang, X.; Deng, Z.; Xiong, W.; Shen, J. Metabolic reprogramming mediated PD-L1 depression and hypoxia reversion to reactivate tumor therapy. J. Control. Release 2022, 352, 793–812. [Google Scholar] [CrossRef]
- Zhao, P.; Wang, S.; Jiang, J.; Gao, Y.; Wang, Y.; Zhao, Y.; Zhang, J.; Zhang, M.; Huang, Y. Targeting lactate metabolism and immune interaction in breast tumor via protease-triggered delivery. J. Control. Release 2023, 358, 706–717. [Google Scholar] [CrossRef]
- Yuan, C.S.; Deng, Z.W.; Qin, D.; Mu, Y.Z.; Chen, X.G.; Liu, Y. Hypoxia-modulatory nanomaterials to relieve tumor hypoxic microenvironment and enhance immunotherapy: Where do we stand? Acta Biomater. 2021, 125, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Yang, J.; Yu, S.; Xiang, Z.; Zeng, Y.; Shen, M.; Kou, X.; Wu, Q.; Gong, C. Self-sufficient nanoparticles with dual-enzyme activity trigger radical storms and activate cascade-amplified antitumor immunologic responses. Acta. Pharm. Sin. B 2024, 14, 821–835. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wu, M.; Liu, X.; Shao, S.; Huang, J.; Liu, B.; Liang, T. Pyroptosis Remodeling Tumor Microenvironment to Enhance Pancreatic Cancer Immunotherapy Driven by Membrane Anchoring Photosensitizer. Adv. Sci. 2022, 9, e2202914. [Google Scholar] [CrossRef]
- Xia, L.; Oyang, L.; Lin, J.; Tan, S.; Han, Y.; Wu, N.; Yi, P.; Tang, L.; Pan, Q.; Rao, S.; et al. The cancer metabolic reprogramming and immune response. Mol. Cancer 2021, 20, 28. [Google Scholar] [CrossRef] [PubMed]
- Leone, R.D.; Zhao, L.; Englert, J.M.; Sun, I.M.; Oh, M.H.; Sun, I.H.; Arwood, M.L.; Bettencourt, I.A.; Patel, C.H.; Wen, J.; et al. Glutamine blockade induces divergent metabolic programs to overcome tumor immune evasion. Science 2019, 366, 1013–1021. [Google Scholar] [CrossRef]
- Nie, T.; Fang, Y.; Zhang, R.; Cai, Y.; Wang, X.; Jiao, Y.; Wu, J. Self-healable and pH-responsive spermidine/ferrous ion complexed hydrogel Co-loaded with CA inhibitor and glucose oxidase for combined cancer immunotherapy through triple ferroptosis mechanism. Bioact. Mater. 2025, 47, 51–63. [Google Scholar] [CrossRef]
- Avolio, R.; Matassa, D.S.; Criscuolo, D.; Landriscina, M.; Esposito, F. Modulation of Mitochondrial Metabolic Reprogramming and Oxidative Stress to Overcome Chemoresistance in Cancer. Biomolecules 2020, 10, 135. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Xin, Z.; Sun, X.; Hu, Y.; Zhang, C.; Yan, R.; Wang, Y.; Lu, M.; Huang, J.; Du, X.; et al. Activation of ACLY by SEC63 deploys metabolic reprogramming to facilitate hepatocellular carcinoma metastasis upon endoplasmic reticulum stress. J. Exp. Clin. Cancer Res. 2023, 42, 108. [Google Scholar] [CrossRef]
- Chen, X.; Cubillos-Ruiz, J.R. Endoplasmic reticulum stress signals in the tumour and its microenvironment. Nat. Rev. Cancer 2021, 21, 71–88. [Google Scholar] [CrossRef]
- Tharp, K.M.; Kersten, K.; Maller, O.; Timblin, G.A.; Stashko, C.; Canale, F.P.; Menjivar, R.E.; Hayward, M.K.; Berestjuk, I.; Ten Hoeve, J.; et al. Tumor-associated macrophages restrict CD8+ T cell function through collagen deposition and metabolic reprogramming of the breast cancer microenvironment. Nat. Cancer 2024, 5, 1045–1062. [Google Scholar] [CrossRef]
- Xiao, J.; Wang, S.; Chen, L.; Ding, X.; Dang, Y.; Han, M.; Zheng, Y.; Shen, H.; Wu, S.; Wang, M.; et al. 25-Hydroxycholesterol regulates lysosome AMP kinase activation and metabolic reprogramming to educate immunosuppressive macrophages. Immunity 2024, 57, 1087–1104.e7. [Google Scholar] [CrossRef] [PubMed]
- Morrissey, S.M.; Zhang, F.; Ding, C.; Montoya-Durango, D.E.; Hu, X.; Yang, C.; Wang, Z.; Yuan, F.; Fox, M.; Zhang, H.G.; et al. Tumor-derived exosomes drive immunosuppressive macrophages in a pre-metastatic niche through glycolytic dominant metabolic reprogramming. Cell. Metab. 2021, 33, 2040–2058.e10. [Google Scholar] [CrossRef]
- Chen, L.; Huang, L.; Gu, Y.; Cang, W.; Sun, P.; Xiang, Y. Lactate-Lactylation Hands between Metabolic Reprogramming and Immunosuppression. Int. J. Mol. Sci. 2022, 23, 11943. [Google Scholar] [CrossRef]
- Morad, G.; Helmink, B.A.; Sharma, P.; Wargo, J.A. Hallmarks of response, resistance, and toxicity to immune checkpoint blockade. Cell 2021, 184, 5309–5337. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Chen, L.; Li, Y.; Hu, Z.; He, F. Ferroptosis, necroptosis, and pyroptosis in the tumor microenvironment: Perspectives for immunotherapy of SCLC. Semin. Cancer Biol. 2022, 86, 273–285. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, W.; Xie, Z.; Wang, X.; Sun, J.; Ran, F.; Jiang, W.; Liu, Y.; Wang, Z.; Ran, H.; et al. NIR-responsive copper nanoliposome composites for cascaded ferrotherapy via ferroptosis actived ICD and IFN-γ released. Biomaterials 2024, 308, 122570. [Google Scholar] [CrossRef] [PubMed]
- Orehek, S.; Ramuta, T.Ž.; Lainšček, D.; Malenšek, Š.; Šala, M.; Benčina, M.; Jerala, R.; Hafner-Bratkovič, I. Cytokine-armed pyroptosis induces antitumor immunity against diverse types of tumors. Nat. Commun. 2024, 15, 10801. [Google Scholar] [CrossRef]
- Huang, F.Y.; Dai, S.Z.; Xu, W.T.; Xiong, W.; Sun, Y.; Huang, Y.H.; Wang, J.Y.; Lin, Y.Y.; Chen, H.; Tan, G.H.; et al. 3′-epi-12β-hydroxyfroside-mediated autophagy degradation of RIPK1/RIPK3 necrosomes leads to anergy of immunogenic cell death in triple-negative breast cancer cells. Pharmacol. Res. 2023, 187, 106613. [Google Scholar] [CrossRef]
- Ding, Q.; Tang, W.; Li, X.; Ding, Y.; Chen, X.; Cao, W.; Wang, X.; Mo, W.; Su, Z.; Zhang, Q.; et al. Mitochondrial-targeted brequinar liposome boosted mitochondrial-related ferroptosis for promoting checkpoint blockade immunotherapy in bladder cancer. J. Control. Release 2023, 363, 221–234. [Google Scholar] [CrossRef]
- Chen, F.; Tang, H.; Cai, X.; Lin, J.; Kang, R.; Tang, D.; Liu, J. DAMPs in immunosenescence and cancer. Semin. Cancer Biol. 2024, 106–107, 123–142. [Google Scholar] [CrossRef]
- Fucikova, J.; Spisek, R.; Kroemer, G.; Galluzzi, L. Calreticulin and cancer. Cell Res. 2021, 31, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Obeid, M.; Tesniere, A.; Ghiringhelli, F.; Fimia, G.M.; Apetoh, L.; Perfettini, J.L.; Castedo, M.; Mignot, G.; Panaretakis, T.; Casares, N.; et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat. Med. 2007, 13, 54–61. [Google Scholar] [CrossRef]
- Kepp, O.; Bezu, L.; Yamazaki, T.; Di Virgilio, F.; Smyth, M.J.; Kroemer, G.; Galluzzi, L. ATP and cancer immunosurveillance. EMBO J. 2021, 40, e108130. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Hu, D.; Feng, Y.; Wu, C.; Song, Y.; Liu, W.; Li, A.; Wang, Y.; Chen, K.; Tian, M.; et al. Paxillin mediates ATP-induced activation of P2X7 receptor and NLRP3 inflammasome. BMC Biol. 2020, 18, 182. [Google Scholar] [CrossRef] [PubMed]
- Sims, G.P.; Rowe, D.C.; Rietdijk, S.T.; Herbst, R.; Coyle, A.J. HMGB1 and RAGE in inflammation and cancer. Annu. Rev. Immunol. 2010, 28, 367–388. [Google Scholar] [CrossRef]
- Galluzzi, L.; Guilbaud, E.; Schmidt, D.; Kroemer, G.; Marincola, F.M. Targeting immunogenic cell stress and death for cancer therapy. Nat. Rev. Drug. Discov. 2024, 23, 445–460. [Google Scholar] [CrossRef]
- Kroemer, G.; Galassi, C.; Zitvogel, L.; Galluzzi, L. Immunogenic cell stress and death. Nat. Immunol. 2022, 23, 487–500. [Google Scholar] [CrossRef]
- Alzeibak, R.; Mishchenko, T.A.; Shilyagina, N.Y.; Balalaeva, I.V.; Vedunova, M.V.; Krysko, D.V. Targeting immunogenic cancer cell death by photodynamic therapy: Past, present and future. J. Immunother. Cancer 2021, 9, e001926. [Google Scholar] [CrossRef]
- Dai, E.; Zhu, Z.; Wahed, S.; Qu, Z.; Storkus, W.J.; Guo, Z.S. Epigenetic modulation of antitumor immunity for improved cancer immunotherapy. Mol. Cancer 2021, 20, 171. [Google Scholar] [CrossRef]
- Li, Y.; Sun, Q.; Yang, Z.; Luo, M.; Yang, W.; Song, Z.; Liu, K.; Li, H.; Gao, W.; Wu, Q.; et al. Transforming tumors into ‘high-risk bombs’ triggers a neoantigen storm and amplifies immune responses. J. Control. Release 2025, 380, 1080–1094. [Google Scholar] [CrossRef]
- Gordon, K.S.; Kyung, T.; Perez, C.R.; Holec, P.V.; Ramos, A.; Zhang, A.Q.; Agarwal, Y.; Liu, Y.; Koch, C.E.; Starchenko, A.; et al. Screening for CD19-specific chimaeric antigen receptors with enhanced signalling via a barcoded library of intracellular domains. Nat. Biomed. Eng. 2022, 6, 855–866. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Lin, B.; Chen, C.; Wang, H.; Lin, X.; Liu, J.; Ren, Q.; Tao, J.; Zhao, P.; Xu, Y. Synergistic Reinforcing of Immunogenic Cell Death and Transforming Tumor-Associated Macrophages Via a Multifunctional Cascade Bioreactor for Optimizing Cancer Immunotherapy. Adv. Mater. 2022, 34, e2207593. [Google Scholar] [CrossRef]
- Zhang, L.L.; Zhang, D.J.; Shi, J.X.; Huang, M.Y.; Yu, J.M.; Chen, X.J.; Wei, X.; Zou, L.; Lu, J.J. Immunogenic cell death inducers for cancer therapy: An emerging focus on natural products. Phytomedicine 2024, 132, 155828. [Google Scholar] [CrossRef]
- Chen, S.; Sun, Y.; Xie, Y.; Liu, Y.; Hu, H.; Xie, C.; Xu, S.; Zhang, Z.; Zhang, J.; Shen, Y.; et al. Mitochondria-Targeted Icaritin Nanoparticles Induce Immunogenic Cell Death in Hepatocellular Carcinoma. ACS Appl. Mater. Interfaces 2025, 17, 2899–2910. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Zhang, X.; Li, Z.; Mo, F.; Tang, D.; Xiao, H.; Wang, J.; Rong, G.; Liu, T. Oxidative Stress Amplifiers as Immunogenic Cell Death Nanoinducers Disrupting Mitochondrial Redox Homeostasis for Cancer Immunotherapy. Adv. Healthc. Mater. 2023, 12, e2202710. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Dong, J.; Sun, C.; Liu, X.; Liu, Z.; Liu, L.; Kuang, Z.; Zhang, N.; Xiao, D.; Zhou, X.; et al. Raddeanin A Enhances Mitochondrial DNA-cGAS/STING Axis-Mediated Antitumor Immunity by Targeting Transactive Responsive DNA-Binding Protein 43. Adv. Sci. 2023, 10, e2206737. [Google Scholar] [CrossRef]
- Kim, J.; Shim, M.K.; Moon, Y.; Kim, J.; Cho, H.; Yun, W.S.; Shim, N.; Seong, J.K.; Lee, Y.; Lim, D.K.; et al. Cancer cell-specific and pro-apoptotic SMAC peptide-doxorubicin conjugated prodrug encapsulated aposomes for synergistic cancer immunotherapy. J. Nanobiotechnology 2024, 22, 109. [Google Scholar] [CrossRef]
- Scharping, N.E.; Rivadeneira, D.B.; Menk, A.V.; Vignali, P.D.A.; Ford, B.R.; Rittenhouse, N.L.; Peralta, R.; Wang, Y.; Wang, Y.; DePeaux, K.; et al. Mitochondrial stress induced by continuous stimulation under hypoxia rapidly drives T cell exhaustion. Nat. Immunol. 2021, 22, 205–215. [Google Scholar] [CrossRef]
- Xu, Z.; Xu, J.; Sun, S.; Lin, W.; Li, Y.; Lu, Q.; Li, F.; Yang, Z.; Lu, Y.; Liu, W. Mecheliolide elicits ROS-mediated ERS driven immunogenic cell death in hepatocellular carcinoma. Redox. Biol. 2022, 54, 102351. [Google Scholar] [CrossRef]
- Yang, J.; Ren, B.; Yin, X.; Xiang, L.; Hua, Y.; Huang, X.; Wang, H.; Mao, Z.; Chen, W.; Deng, J. Expanded ROS Generation and Hypoxia Reversal: Excipient-free Self-assembled Nanotheranostics for Enhanced Cancer Photodynamic Immunotherapy. Adv. Mater. 2024, 36, e2402720. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, M.; Quan, C.; Ren, S.; Chen, W.; Wang, J. ROS-responsive and triple-synergistic mitochondria-targeted polymer micelles for efficient induction of ICD in tumor therapeutics. Bioact. Mater. 2024, 36, 490–507. [Google Scholar] [CrossRef]
- Dai, Y.; Guo, Z.; Leng, D.; Jiao, G.; Chen, K.; Fu, M.; Liu, Y.; Shen, Q.; Wang, Q.; Zhu, L.; et al. Metal-Coordinated NIR-II Nanoadjuvants with Nanobody Conjugation for Potentiating Immunotherapy by Tumor Metabolism Reprogramming. Adv. Sci. 2024, 11, e2404886. [Google Scholar] [CrossRef]
- Wang, L.; He, S.; Liu, R.; Xue, Y.; Quan, Y.; Shi, R.; Yang, X.; Lin, Q.; Sun, X.; Zhang, Z.; et al. A pH/ROS dual-responsive system for effective chemoimmunotherapy against melanoma via remodeling tumor immune microenvironment. Acta. Pharm. Sin. B 2024, 14, 2263–2280. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Pan, Y.F.; Chen, Y.B.; Wan, Q.Q.; Lin, Y.K.; Shang, T.Y.; Xu, M.Y.; Jiang, T.Y.; Pei, M.M.; Tan, Y.X.; et al. Arsenic trioxide augments immunogenic cell death and induces cGAS-STING-IFN pathway activation in hepatocellular carcinoma. Cell Death. Dis. 2024, 15, 300. [Google Scholar] [CrossRef]
- Oresta, B.; Pozzi, C.; Braga, D.; Hurle, R.; Lazzeri, M.; Colombo, P.; Frego, N.; Erreni, M.; Faccani, C.; Elefante, G.; et al. Mitochondrial metabolic reprogramming controls the induction of immunogenic cell death and efficacy of chemotherapy in bladder cancer. Sci. Transl. Med. 2021, 13, eaba6110. [Google Scholar] [CrossRef]
- Tao, J.; Dong, Y.; Wang, B.; Wang, T.; Zhang, A.; Li, S.; Chen, R.; Su, Y.; Jiang, T.; Zhao, X. Dual Metal Nanoflower Oxygen Pump Microneedles Based on Cuproptosis and STING Pathway Activation for Cancer Immunotherapy. Small 2025, 21, e2409187. [Google Scholar] [CrossRef]
- Li, Z.; Chu, Z.; Yang, J.; Qian, H.; Xu, J.; Chen, B.; Tian, T.; Chen, H.; Xu, Y.; Wang, F. Immunogenic Cell Death Augmented by Manganese Zinc Sulfide Nanoparticles for Metastatic Melanoma Immunotherapy. ACS Nano 2022, 16, 15471–15483. [Google Scholar] [CrossRef]
- Pang, X.; Fu, C.; Chen, J.; Su, M.; Wei, R.; Wang, Y.; Lin, W.; Wei, X.; Jiang, X.; Yang, X.; et al. A manganese-phenolic network platform amplifying STING activation to potentiate MRI guided cancer chemo-/chemodynamic/immune therapy. Biomater. Sci. 2023, 11, 3840–3850. [Google Scholar] [CrossRef]
- Yu, S.; Xiao, H.; Ma, L.; Zhang, J.; Zhang, J. Reinforcing the immunogenic cell death to enhance cancer immunotherapy efficacy. Biochim. Biophys. Acta Rev. Cancer 2023, 1878, 188946. [Google Scholar]
- Lim, S.A.; Su, W.; Chapman, N.M.; Chi, H. Lipid metabolism in T cell signaling and function. Nat. Chem. Biol. 2022, 18, 470–481. [Google Scholar] [CrossRef] [PubMed]
- Bian, X.; Liu, R.; Meng, Y.; Xing, D.; Xu, D.; Lu, Z. Lipid metabolism and cancer. J. Exp. Med. 2021, 218, e20201606. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, L.; Wang, Z.; Gu, D.; Zhu, M.; Cai, Y.; Li, L.; Tang, J.; Huang, B.; Bosco, B.; et al. Single-cell transcriptome analysis indicates fatty acid metabolism-mediated metastasis and immunosuppression in male breast cancer. Nat. Commun. 2023, 14, 5590. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhu, T.; Wang, X.; Xiong, F.; Hu, Z.; Qiao, X.; Yuan, X.; Wang, D. ACSL3 and ACSL4, Distinct Roles in Ferroptosis and Cancers. Cancers 2022, 14, 5896. [Google Scholar] [CrossRef] [PubMed]
- Malau, I.A.; Chang, J.P.; Lin, Y.W.; Chang, C.C.; Chiu, W.C.; Su, K.P. Omega-3 Fatty Acids and Neuroinflammation in Depression: Targeting Damage-Associated Molecular Patterns and Neural Biomarkers. Cells 2024, 13, 1791. [Google Scholar] [CrossRef] [PubMed]
- Kanno, T.; Nakajima, T.; Miyako, K.; Endo, Y. Lipid metabolism in Th17 cell function. Pharmacol. Ther. 2023, 245, 108411. [Google Scholar] [CrossRef]
- Kelly, B.; O’Neill, L.A. Metabolic reprogramming in macrophages and dendritic cells in innate immunity. Cell. Res. 2015, 25, 771–784. [Google Scholar] [CrossRef]
- Liu, C.; Chikina, M.; Deshpande, R.; Menk, A.V.; Wang, T.; Tabib, T.; Brunazzi, E.A.; Vignali, K.M.; Sun, M.; Stolz, D.B.; et al. Treg Cells Promote the SREBP1-Dependent Metabolic Fitness of Tumor-Promoting Macrophages via Repression of CD8+ T Cell-Derived Interferon-γ. Immunity 2019, 51, 381–397.e6. [Google Scholar] [CrossRef]
- Lin, J.; Lai, Y.; Lu, F.; Wang, W. Targeting ACSLs to modulate ferroptosis and cancer immunity. Trends Endocrinol. Metab. 2024. [CrossRef]
- Li, J.; Liu, J.; Zhou, Z.; Wu, R.; Chen, X.; Yu, C.; Stockwell, B.; Kroemer, G.; Kang, R.; Tang, D. Tumor-specific GPX4 degradation enhances ferroptosis-initiated antitumor immune response in mouse models of pancreatic cancer. Sci. Transl. Med. 2023, 15, eadg3049. [Google Scholar] [CrossRef]
- Wang, W.; Green, M.; Choi, J.E.; Gijón, M.; Kennedy, P.D.; Johnson, J.K.; Liao, P.; Lang, X.; Kryczek, I.; Sell, A.; et al. CD8+ T cells regulate tumour ferroptosis during cancer immunotherapy. Nature 2019, 569, 270–274. [Google Scholar] [CrossRef]
- Liao, P.; Wang, W.; Wang, W.; Kryczek, I.; Li, X.; Bian, Y.; Sell, A.; Wei, S.; Grove, S.; Johnson, J.K.; et al. CD8+ T cells and fatty acids orchestrate tumor ferroptosis and immunity via ACSL4. Cancer Cell 2022, 40, 365–378.e6. [Google Scholar] [CrossRef]
- Li, Y.; Liu, J.; Chen, Y.; Weichselbaum, R.R.; Lin, W. Nanoparticles Synergize Ferroptosis and Cuproptosis to Potentiate Cancer Immunotherapy. Adv. Sci. 2024, 11, e2310309. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zheng, W.; Wang, X.; Li, Z.; Shen, X.; Chen, Q.; Lu, Y.; Chen, K.; Ai, S.; Zhu, Y.; et al. Enhanced Photodynamic Therapy Synergizing with Inhibition of Tumor Neutrophil Ferroptosis Boosts Anti-PD-1 Therapy of Gastric Cancer. Adv. Sci. 2024, 11, e2307870. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Q.; Yang, X.; Zhang, Z.; Yang, J.; Li, Y.; Du, J.; Wang, J.; Fan, K.; Yuan, J.; Zhang, J.; et al. Fe/Mo-Based Lipid Peroxidation Nanoamplifier Combined with Adenosine Immunometabolism Regulation to Augment Anti-Breast Cancer Immunity. Adv. Mater. 2025, 37, e2419120. [Google Scholar] [CrossRef]
- Perkovic, M.N.; Jaganjac, M.; Milkovic, L.; Horvat, T.; Rojo, D.; Zarkovic, K.; Ćorić, M.; Hudolin, T.; Waeg, G.; Orehovec, B.; et al. Relationship between 4-Hydroxynonenal (4-HNE) as Systemic Biomarker of Lipid Peroxidation and Metabolomic Profiling of Patients with Prostate Cancer. Biomolecules 2023, 13, 145. [Google Scholar] [CrossRef]
- Yang, L.; Chu, Z.; Liu, M.; Zou, Q.; Li, J.; Liu, Q.; Wang, Y.; Wang, T.; Xiang, J.; Wang, B. Amino acid metabolism in immune cells: Essential regulators of the effector functions, and promising opportunities to enhance cancer immunotherapy. J. Hematol. Oncol. 2023, 16, 59. [Google Scholar] [CrossRef]
- Zheng, Y.; Yao, Y.; Ge, T.; Ge, S.; Jia, R.; Song, X.; Zhuang, A. Amino acid metabolism reprogramming: Shedding new light on T cell anti-tumor immunity. J. Exp. Clin. Cancer. Res. 2023, 42, 291. [Google Scholar] [CrossRef]
- Cluntun, A.A.; Lukey, M.J.; Cerione, R.A.; Locasale, J.W. Glutamine Metabolism in Cancer: Understanding the Heterogeneity. Trends Cancer 2017, 3, 169–180. [Google Scholar] [CrossRef]
- Nan, D.; Yao, W.; Huang, L.; Liu, R.; Chen, X.; Xia, W.; Sheng, H.; Zhang, H.; Liang, X.; Lu, Y. Glutamine and cancer: Metabolism, immune microenvironment, and therapeutic targets. Cell Commun. Signal. 2025, 23, 45. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Hung, Y.W.; Wang, Y.C.; Chung, Y.; Qi, Y.; Ouyang, C.; Zhong, X.; Hu, W.; Coblentz, A.; Maghami, E.; et al. Glutamine is essential for overcoming the immunosuppressive microenvironment in malignant salivary gland tumors. Theranostics 2022, 12, 6038–6056. [Google Scholar] [CrossRef]
- Metzler, B.; Gfeller, P.; Guinet, E. Restricting Glutamine or Glutamine-Dependent Purine and Pyrimidine Syntheses Promotes Human T Cells with High FOXP3 Expression and Regulatory Properties. J. Immunol. 2016, 196, 3618–3630. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.S.; Gupta, V.K.; Garrido, V.T.; Hadad, R.; Durden, B.C.; Kesh, K.; Giri, B.; Ferrantella, A.; Dudeja, V.; Saluja, A.; et al. Targeting tumor-intrinsic hexosamine biosynthesis sensitizes pancreatic cancer to anti-PD1 therapy. J. Clin. Investig. 2020, 130, 451–465. [Google Scholar] [CrossRef]
- Xue, C.; Li, G.; Zheng, Q.; Gu, X.; Shi, Q.; Su, Y.; Chu, Q.; Yuan, X.; Bao, Z.; Lu, J.; et al. Tryptophan metabolism in health and disease. Cell Metab. 2023, 35, 1304–1326. [Google Scholar] [CrossRef]
- Shi, D.; Wu, X.; Jian, Y.; Wang, J.; Huang, C.; Mo, S.; Li, Y.; Li, F.; Zhang, C.; Zhang, D.; et al. USP14 promotes tryptophan metabolism and immune suppression by stabilizing IDO1 in colorectal cancer. Nat. Commun. 2022, 13, 5644. [Google Scholar] [CrossRef]
- Bender, M.J.; McPherson, A.C.; Phelps, C.M.; Pandey, S.P.; Laughlin, C.R.; Shapira, J.H.; Medina Sanchez, L.; Rana, M.; Richie, T.G.; Mims, T.S.; et al. Dietary tryptophan metabolite released by intratumoral Lactobacillus reuteri facilitates immune checkpoint inhibitor treatment. Cell 2023, 186, 1846–1862.e26. [Google Scholar] [CrossRef]
- Maganin, A.G.; Souza, G.R.; Fonseca, M.D.; Lopes, A.H.; Guimarães, R.M.; Dagostin, A.; Cecilio, N.T.; Mendes, A.S.; Gonçalves, W.A.; Silva, C.E.; et al. Meningeal dendritic cells drive neuropathic pain through elevation of the kynurenine metabolic pathway in mice. J. Clin. Investig. 2022, 132, e153805. [Google Scholar] [CrossRef]
- Fong, W.; Li, Q.; Ji, F.; Liang, W.; Lau, H.C.H.; Kang, X.; Liu, W.; To, K.K.; Zuo, Z.; Li, X.; et al. Lactobacillus gallinarum-derived metabolites boost anti-PD1 efficacy in colorectal cancer by inhibiting regulatory T cells through modulating IDO1/Kyn/AHR axis. Gut 2023, 72, 2272–2285. [Google Scholar] [CrossRef]
- Jia, S.; Chen, Y.; Zhuo, C.; Hu, M.; Zhang, C.; Cai, H.; Li, X.; Chen, H.; Yu, X. Aptamer-modified melittin micelles efficiently inhibit osteosarcoma deterioration by inducing immunogenic cell death. Colloids Surf. B Biointerfaces 2025, 249, 114512. [Google Scholar] [CrossRef]
- Geeraerts, S.L.; Heylen, E.; De Keersmaecker, K.; Kampen, K.R. The ins and outs of serine and glycine metabolism in cancer. Nat. Metab. 2021, 3, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Tajan, M.; Hennequart, M.; Cheung, E.C.; Zani, F.; Hock, A.K.; Legrave, N.; Maddocks, O.D.K.; Ridgway, R.A.; Athineos, D.; Suárez-Bonnet, A.; et al. Serine synthesis pathway inhibition cooperates with dietary serine and glycine limitation for cancer therapy. Nat. Commun. 2021, 12, 366. [Google Scholar] [CrossRef] [PubMed]
- Pham, V.N.; Bruemmer, K.J.; Toh, J.D.W.; Ge, E.J.; Tenney, L.; Ward, C.C.; Dingler, F.A.; Millington, C.L.; Garcia-Prieto, C.A.; Pulos-Holmes, M.C.; et al. Formaldehyde regulates S-adenosylmethionine biosynthesis and one-carbon metabolism. Science 2023, 382, eabp9201. [Google Scholar] [CrossRef]
- Tong, H.; Jiang, Z.; Song, L.; Tan, K.; Yin, X.; He, C.; Huang, J.; Li, X.; Jing, X.; Yun, H.; et al. Dual impacts of serine/glycine-free diet in enhancing antitumor immunity and promoting evasion via PD-L1 lactylation. Cell. Metab. 2024, 36, 2493–2510.e9. [Google Scholar] [CrossRef] [PubMed]
- Ma, E.H.; Bantug, G.; Griss, T.; Condotta, S.; Johnson, R.M.; Samborska, B.; Mainolfi, N.; Suri, V.; Guak, H.; Balmer, M.L.; et al. Serine is an essential metabolite for effector T cell expansion. Cell. Metab. 2017, 25, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Sandhoff, R.; Luo, X.; Shang, F.; Shi, Q.; Li, Z.; Wu, J.; Ming, Y.; Schwarz, F.; Madi, A.; et al. Serine enrichment in tumors promotes regulatory T cell accumulation through sphinganine-mediated regulation of c-Fos. Sci. Immunol. 2024, 9, eadg8817. [Google Scholar] [CrossRef]
- Saha, S.; Ghosh, M.; Li, J.; Wen, A.; Galluzzi, L.; Martinez, L.A.; Montrose, D.C. Serine Depletion Promotes Antitumor Immunity by Activating Mitochondrial DNA-Mediated cGAS-STING Signaling. Cancer Res. 2024, 84, 2645–2659. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, J.; Huang, W.; Ma, H.; Zhang, S.; Cai, Z.; Lin, W.; Zheng, J. Tumor Microenvironment Reprogrammed Bimetallic Hybrid Nanostimulator for Triggering Radio-Cuproptosis-Immunotherapy. Adv. Healthc. Mater. 2024, 13, e2401902. [Google Scholar] [CrossRef]
- Huang, K.C.; Chiang, S.F.; Lin, P.C.; Hong, W.Z.; Yang, P.C.; Chang, H.P.; Peng, S.L.; Chen, T.W.; Ke, T.W.; Liang, J.A.; et al. TNFα modulates PANX1 activation to promote ATP release and enhance P2RX7-mediated antitumor immune responses after chemotherapy in colorectal cancer. Cell. Death. Dis. 2024, 15, 24. [Google Scholar] [CrossRef]
- Hofbauer, D.; Mougiakakos, D.; Broggini, L.; Zaiss, M.; Büttner-Herold, M.; Bach, C.; Spriewald, B.; Neumann, F.; Bisht, S.; Nolting, J.; et al. β2-microglobulin triggers NLRP3 inflammasome activation in tumor-associated macrophages to promote multiple myeloma progression. Immunity 2021, 54, 1772–1787.e9. [Google Scholar] [CrossRef]
- Jin, S.; Yin, E.; Feng, C.; Sun, Y.; Yang, T.; Yuan, H.; Guo, Z.; Wang, X. Regulating tumor glycometabolism and the immune microenvironment by inhibiting lactate dehydrogenase with platinum(iv) complexes. Chem. Sci. 2023, 14, 8327–8337. [Google Scholar] [CrossRef]
- Apostolova, P.; Pearce, E.L. Lactic acid and lactate: Revisiting the physiological roles in the tumor microenvironment. Trends Immunol. 2022, 43, 969–977. [Google Scholar] [CrossRef]
- Han, S.; Bao, X.; Zou, Y.; Wang, L.; Li, Y.; Yang, L.; Liao, A.; Zhang, X.; Jiang, X.; Liang, D.; et al. d-Lactate modulates M2 tumor-associated macrophages and remodels immunosuppressive tumor microenvironment for hepatocellular carcinoma. Sci. Adv. 2023, 9, eadg2697. [Google Scholar] [CrossRef]
- Zhang, J.; Muri, J.; Fitzgerald, G.; Gorski, T.; Gianni-Barrera, R.; Masschelein, E.; D’Hulst, G.; Gilardoni, P.; Turiel, G.; Fan, Z.; et al. Endothelial Lactate Controls Muscle Regeneration from Ischemia by Inducing M2-like Macrophage Polarization. Cell Metab. 2020, 31, 1136–1153.e7. [Google Scholar] [CrossRef]
- Cheong, J.E.; Sun, L. Targeting the IDO1/TDO2-KYN-AhR Pathway for Cancer Immunotherapy—Challenges and Opportunities. Trends Pharmacol. Sci. 2018, 39, 307–325. [Google Scholar] [CrossRef]
- Escobar, G.; Tooley, K.; Oliveras, J.P.; Huang, L.; Cheng, H.; Bookstaver, M.L.; Edwards, C.; Froimchuk, E.; Xue, C.; Mangani, D.; et al. Tumor immunogenicity dictates reliance on TCF1 in CD8+ T cells for response to immunotherapy. Cancer Cell 2023, 41, 1662–1679.e7. [Google Scholar] [CrossRef]
- Alvarez-Valadez, K.; Sauvat, A.; Diharce, J.; Leduc, M.; Stoll, G.; Guittat, L.; Lambertucci, F.; Paillet, J.; Motiño, O.; Ferret, L.; et al. Lysosomal damage due to cholesterol accumulation triggers immunogenic cell death. Autophagy 2024, 1–23, online ahead of print. [Google Scholar] [CrossRef]
- O’Rourke, S.A.; Neto, N.G.B.; Devilly, E.; Shanley, L.C.; Fitzgerald, H.K.; Monaghan, M.G.; Dunne, A. Cholesterol crystals drive metabolic reprogramming and M1 macrophage polarization in primary human macrophages. Atherosclerosis 2022, 352, 35–45. [Google Scholar] [CrossRef]
- Li, X.; Lovell, J.F.; Yoon, J.; Chen, X. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat. Rev. Clin. Oncol. 2020, 17, 657–674. [Google Scholar] [CrossRef]
- Liu, X.; Hartman, C.L.; Li, L.; Albert, C.J.; Si, F.; Gao, A.; Huang, L.; Zhao, Y.; Lin, W.; Hsueh, E.C.; et al. Reprogramming lipid metabolism prevents effector T cell senescence and enhances tumor immunotherapy. Sci. Transl. Med. 2021, 13, eaaz6314. [Google Scholar] [CrossRef]
- Bahmani, B.; Gong, H.; Luk, B.T.; Haushalter, K.J.; DeTeresa, E.; Previti, M.; Zhou, J.; Gao, W.; Bui, J.D.; Zhang, L.; et al. Intratumoral immunotherapy using platelet-cloaked nanoparticles enhances antitumor immunity in solid tumors. Nat. Commun. 2021, 12, 1999. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wei, L.; Ma, X.; Wang, J.; Liang, S.; Chen, K.; Wu, M.; Niu, L.; Zhang, Y. pH-sensitive tumor-tropism hybrid membrane-coated nanoparticles for reprogramming the tumor microenvironment and boosting the antitumor immunity. Acta Biomater. 2023, 166, 470–484. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Tian, H.; Fang, M.; Xu, Z.; Tang, D.; Chen, J.; Yin, J.; Xiao, H.; Shang, K.; Han, H.; et al. Activating cGAS-STING pathway with ROS-responsive nanoparticles delivering a hybrid prodrug for enhanced chemo-immunotherapy. Biomaterials 2022, 290, 121856. [Google Scholar] [CrossRef]
- Su, Q.; Wang, Z.; Li, P.; Wei, X.; Xiao, J.; Duan, X. pH and ROS Dual-Responsive Autocatalytic Release System Potentiates Immunotherapy of Colorectal Cancer. Adv. Healthcare Mater. 2024, 13, e2401126. [Google Scholar] [CrossRef]
- Ren, H.; Wu, Z.; Tan, J.; Tao, H.; Zou, W.; Cao, Z.; Wen, B.; Cai, Z.; Du, J.; Deng, Z. Co-delivery Nano System of MS-275 and V-9302 Induces Pyroptosis and Enhances Anti-Tumor Immunity Against Uveal Melanoma. Adv. Sci. 2024, 11, e2404375. [Google Scholar] [CrossRef]
- Ruan, H.; Hu, Q.; Wen, D.; Chen, Q.; Chen, G.; Lu, Y.; Wang, J.; Cheng, H.; Lu, W.; Gu, Z. A Dual-Bioresponsive Drug-Delivery Depot for Combination of Epigenetic Modulation and Immune Checkpoint Blockade. Adv. Mater. 2019, 31, e1806957. [Google Scholar] [CrossRef]
- Lee, Y.; Shinn, J.; Xu, C.; Dobson, H.E.; Neamati, N.; Moon, J.J. Hyaluronic acid-bilirubin nanomedicine-based combination chemoimmunotherapy. Nat. Commun. 2023, 14, 4771. [Google Scholar] [CrossRef]
- Xiao, H.; Li, X.; Liang, S.; Yang, S.; Han, S.; Huang, J.; Shuai, X.; Ren, J. Dual-Responsive Nanomedicine Activates Programmed Antitumor Immunity through Targeting Lymphatic System. ACS Nano 2024, 18, 11070–11083. [Google Scholar] [CrossRef]
- Ciavardelli, D.; Rossi, C.; Barcaroli, D.; Volpe, S.; Consalvo, A.; Zucchelli, M.; De Cola, A.; Scavo, E.; Carollo, R.; D’Agostino, D.; et al. Breast cancer stem cells rely on fermentative glycolysis and are sensitive to 2-deoxyglucose treatment. Cell Death Dis. 2014, 5, e1336. [Google Scholar] [CrossRef]
- Kim, M.; Lee, J.S.; Kim, W.; Lee, J.H.; Jun, B.H.; Kim, K.S.; Kim, D.E. Aptamer-conjugated nano-liposome for immunogenic chemotherapy with reversal of immunosuppression. J. Control. Release 2022, 348, 893–910. [Google Scholar] [CrossRef]
- Jin, S.M.; Lee, S.N.; Kim, J.E.; Yoo, Y.J.; Song, C.; Shin, H.S.; Phuengkham, H.; Lee, C.H.; Um, S.H.; Lim, Y.T. Overcoming Chemoimmunotherapy-Induced Immunosuppression by Assemblable and Depot Forming Immune Modulating Nanosuspension. Adv. Sci. 2021, 8, e2102043. [Google Scholar] [CrossRef]
- Sahin, U.; Türeci, Ö. Personalized vaccines for cancer immunotherapy. Science 2018, 359, 1355–1360. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Päivinen, P.; Tripathi, S.; Domènech-Moreno, E.; Wong, I.P.L.; Vaahtomeri, K.; Nagaraj, A.S.; Talwelkar, S.S.; Foretz, M.; Verschuren, E.W.; et al. Inactivation of AMPK Leads to Attenuation of Antigen Presentation and Immune Evasion in Lung Adenocarcinoma. Clin. Cancer Res. 2022, 28, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.J.; Svensson-Arvelund, J.; Lubitz, G.S.; Marabelle, A.; Melero, I.; Brown, B.D.; Brody, J.D. Cancer vaccines: The next immunotherapy frontier. Nat. Cancer 2022, 3, 911–926. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Liu, J.; Ye, Y.; Pan, L.; Deng, H.; Wang, Y.; Yang, Y.; Diao, L.; Lin, S.H.; Mills, G.B.; et al. Multi-omics prediction of immune-related adverse events during checkpoint immunotherapy. Nat. Commun. 2020, 11, 4946. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Qi, Y.; Jin, Z.; Tian, J. Noninvasive imaging in cancer immunotherapy: The way to precision medicine. Cancer Lett. 2019, 466, 13–22. [Google Scholar] [CrossRef]
- Liu, J.; Shi, Y.; Zhang, Y. Multi-omics identification of an immunogenic cell death-related signature for clear cell renal cell carcinoma in the context of 3P medicine and based on a 101-combination machine learning computational framework. EPMA J. 2023, 14, 275–305. [Google Scholar] [CrossRef]
- Vijayan, Y.; James, S.; Viswanathan, A.; Aparna, J.S.; Bindu, A.; Namitha, N.N.; Anantharaman, D.; Babu Lankadasari, M.; Harikumar, K.B. Targeting acid ceramidase enhances antitumor immune response in colorectal cancer. J. Adv. Res. 2024, 65, 73–87. [Google Scholar] [CrossRef]
- Moir, J.; Hyman, M.J.; Gonnah, R.; Flores, A.; Hariprasad, S.M.; Skondra, D. The Association Between Metformin Use and New-Onset ICD Coding of Geographic Atrophy. Investig. Ophthalmol. Vis. Sci. 2024, 65, 23. [Google Scholar] [CrossRef]
- Khoshkhabar, R.; Yazdani, M.; Hoda Alavizadeh, S.; Saberi, Z.; Arabi, L.; Reza Jaafari, M. Chemo-immunotherapy by nanoliposomal epacadostat and docetaxel combination to IDO1 inhibition and tumor microenvironment suppression. Int. Immunopharmacol. 2024, 137, 112437. [Google Scholar] [CrossRef]
- Liang, R.; Ding, D.; Li, Y.; Lan, T.; Ryabtseva, S.; Huang, S.; Ren, J.; Huang, H.; Wei, B. HDACi combination therapy with IDO1i remodels the tumor microenvironment and boosts antitumor efficacy in colorectal cancer with microsatellite stability. J. Nanobiotechnology 2024, 22, 753. [Google Scholar] [CrossRef]
- Kennedy, O.J.; Kicinski, M.; Valpione, S.; Gandini, S.; Suciu, S.; Blank, C.U.; Long, G.V.; Atkinson, V.G.; Dalle, S.; Haydon, A.M.; et al. Prognostic and predictive value of metformin in the European Organisation for Research and Treatment of Cancer 1325/KEYNOTE-054 phase III trial of pembrolizumab versus placebo in resected high-risk stage III melanoma. Eur. J. Cancer 2023, 189, 112900. [Google Scholar] [CrossRef]
- Kelly, C.M.; Qin, L.X.; Whiting, K.A.; Richards, A.L.; Avutu, V.; Chan, J.E.; Chi, P.; Dickson, M.A.; Gounder, M.M.; Keohan, M.L.; et al. A Phase II Study of Epacadostat and Pembrolizumab in Patients with Advanced Sarcoma. Clin. Cancer Res. 2023, 29, 2043–2051. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Li, D.; Zhang, Y.; Huang, Q.; Zhang, Z.; Chen, C.; Xu, C.F.; Chu, X.; Zhang, Y.; Yang, X. HSP70-Promoter-Driven CRISPR/Cas9 System Activated by Reactive Oxygen Species for Multifaceted Anticancer Immune Response and Potentiated Immunotherapy. ACS Nano 2022, 16, 13821–13833. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Song, J.; He, Y.; Liu, Y.; Liu, Z.; Sun, W.; Hu, W.; Lei, Q.Y.; Hu, X.; Chen, Z.; et al. CRISPR/Cas9 Screens Reveal that Hexokinase 2 Enhances Cancer Stemness and Tumorigenicity by Activating the ACSL4-Fatty Acid β-Oxidation Pathway. Adv. Sci. 2022, 9, e2105126. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, J.; Yan, Y.; Yang, C.; Cai, H. Immunogenic Cell Death and Metabolic Reprogramming in Cancer: Mechanisms, Synergies, and Innovative Therapeutic Strategies. Biomedicines 2025, 13, 950. https://doi.org/10.3390/biomedicines13040950
Jiang J, Yan Y, Yang C, Cai H. Immunogenic Cell Death and Metabolic Reprogramming in Cancer: Mechanisms, Synergies, and Innovative Therapeutic Strategies. Biomedicines. 2025; 13(4):950. https://doi.org/10.3390/biomedicines13040950
Chicago/Turabian StyleJiang, Jie, Yan Yan, Chunhui Yang, and Hong Cai. 2025. "Immunogenic Cell Death and Metabolic Reprogramming in Cancer: Mechanisms, Synergies, and Innovative Therapeutic Strategies" Biomedicines 13, no. 4: 950. https://doi.org/10.3390/biomedicines13040950
APA StyleJiang, J., Yan, Y., Yang, C., & Cai, H. (2025). Immunogenic Cell Death and Metabolic Reprogramming in Cancer: Mechanisms, Synergies, and Innovative Therapeutic Strategies. Biomedicines, 13(4), 950. https://doi.org/10.3390/biomedicines13040950






