Exploring the Role of Inflammation and Metabolites in Bell’s Palsy and Potential Treatment Strategies
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Design
2.2. Sources of Data
2.2.1. Plasma Protein Screening
2.2.2. Merging with the GWAS Catalogue
2.2.3. Selection of Immune Cells
2.3. Genetic Instrumental Variable (IV) Selection
2.3.1. Identification of SNPs Significantly Associated with the Phenotype
2.3.2. Detection of SNPs Strongly Linked to the Phenotype
2.3.3. Integration, Concordance, and Correction of Palindromic SNPs
2.3.4. Evaluation of IV Strength
2.4. MR Analysis and Sensitivity Analysis
2.5. Bioinformatics Analysis
2.5.1. Protein–Protein Interaction (PPI) Online Network
2.5.2. Gene Ontology (GO) Enrichment Analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG) Analysis
2.5.3. Core Protein Screening
2.5.4. Colocalization Analysis
3. Results
3.1. MR Analysis Results and Sensitivity Analysis
3.1.1. Screening for Inflammation-Related Proteins
3.1.2. Immune Cells
3.1.3. Results of Metabolites
3.2. Results of Bioinformatics Analysis
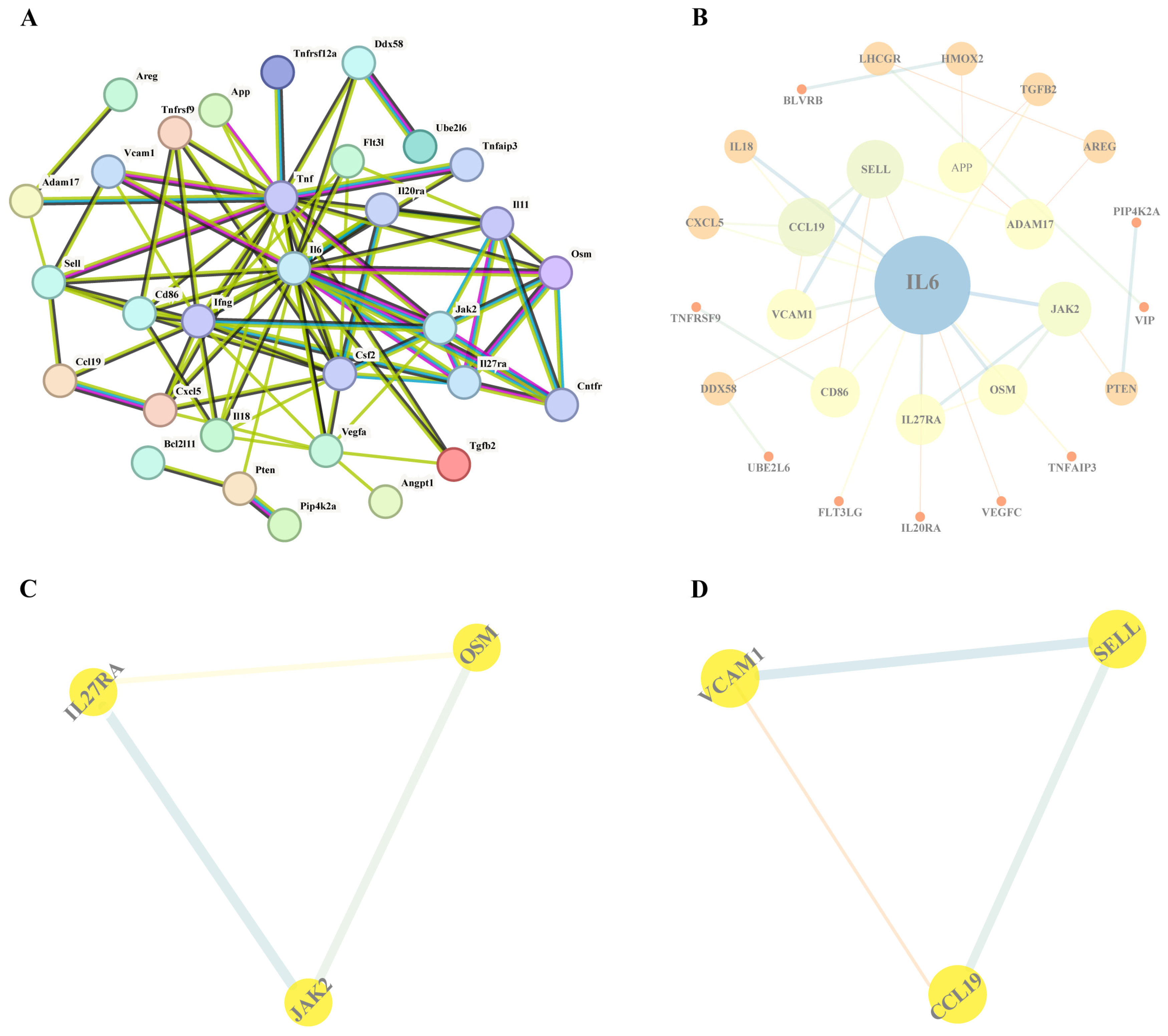
3.2.1. PPI Network Analysis
3.2.2. GO and KEGG Analysis
3.2.3. Results of Core Protein Screening
3.2.4. Results of Colocalization Analysis
3.2.5. Genetic Correlation Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eviston, T.J.; Croxson, G.R.; Kennedy, P.G.; Hadlock, T.; Krishnan, A.V. Bell’s palsy: Aetiology, clinical features and multidisciplinary care. J. Neurol. Neurosurg. Psychiatry 2015, 86, 1356–1361. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xu, L.; Luo, T.; Wu, F.; Zhao, B.; Li, X. The etiology of Bell’s palsy: A review. J. Neurol. 2020, 267, 1896–1905. [Google Scholar] [CrossRef]
- Guez-Barber, D.; Swami, S.K.; Harrison, J.B.; McGuire, J.L. Differentiating Bell’s Palsy from Lyme-Related Facial Palsy. Pediatrics 2022, 149, e2021053992. [Google Scholar] [CrossRef]
- Gagyor, I.; Madhok, V.B.; Daly, F.; Sullivan, F. Antiviral treatment for Bell’s palsy (idiopathic facial paralysis). Cochrane Database Syst. Rev. 2019, 9, CD001869. [Google Scholar] [CrossRef] [PubMed]
- Greco, A.; Gallo, A.; Fusconi, M.; Marinelli, C.; Macri, G.F.; de Vincentiis, M. Bell’s palsy and autoimmunity. Autoimmun. Rev. 2012, 12, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Jawanda, M.K. Surge of Bell’s Palsy in the era of COVID-19: Systematic review. Eur. J. Neurol. 2022, 29, 2526–2543. [Google Scholar] [CrossRef]
- Marra, C.M. Bell’s palsy and HSV-1 infection. Muscle Nerve 1999, 22, 1476–1478. [Google Scholar] [CrossRef]
- Kennedy, P.G. Herpes simplex virus type 1 and Bell’s palsy-a current assessment of the controversy. J. Neurovirol. 2010, 16, 1–5. [Google Scholar] [CrossRef]
- Yilmaz, M.; Tarakcioglu, M.; Bayazit, N.; Bayazit, Y.A.; Namiduru, M.; Kanlikama, M. Serum cytokine levels in Bell’s palsy. J. Neurol. Sci. 2002, 197, 69–72. [Google Scholar] [CrossRef]
- Gorodezky, C.; Carranza, J.M.; Bustamante, A.; Yescas, P.; Martinez, A.; Alonso Vilatela, M.E. The HLA system and T-cell subsets in Bell’s palsy. Acta Otolaryngol. 1991, 111, 1070–1074. [Google Scholar] [CrossRef]
- Aviel, A.; Ostfeld, E.; Burstein, R.; Marshak, G.; Bentwich, Z. Peripheral blood T and B lymphocyte subpopulations in Bell’s palsy. Ann. Otol. Rhinol. Laryngol. 1983, 92, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Tang, L.; Zhou, Y.; Zhao, S.; Zhu, H. Causal relationship between gut microbiota and cancers: A two-sample Mendelian randomisation study. BMC Med. 2023, 21, 66. [Google Scholar] [CrossRef]
- Higbee, D.H.; Granell, R.; Sanderson, E.; Davey Smith, G.; Dodd, J.W. Lung function and cardiovascular disease: A two-sample Mendelian randomisation study. Eur. Respir. J. 2021, 58, 2003196. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Xiao, J.; Ji, J.; Chen, L. Association of lipid-lowering drugs with COVID-19 outcomes from a Mendelian randomization study. eLife 2021, 10, e73873. [Google Scholar] [CrossRef]
- Williams, J.A.; Burgess, S.; Suckling, J.; Lalousis, P.A.; Batool, F.; Griffiths, S.L.; Palmer, E.; Karwath, A.; Barsky, A.; Gkoutos, G.V.; et al. Inflammation and Brain Structure in Schizophrenia and Other Neuropsychiatric Disorders: A Mendelian Randomization Study. JAMA Psychiatry 2022, 79, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Carter, A.R.; Sanderson, E.; Hammerton, G.; Richmond, R.C.; Davey Smith, G.; Heron, J.; Taylor, A.E.; Davies, N.M.; Howe, L.D. Mendelian randomisation for mediation analysis: Current methods and challenges for implementation. Eur. J. Epidemiol. 2021, 36, 465–478. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, Z.; Pärna, K.; van Zon, S.K.R.; Snieder, H.; Thio, C.H.L. Mediators of the association between educational attainment and type 2 diabetes mellitus: A two-step multivariable Mendelian randomisation study. Diabetologia 2022, 65, 1364–1374. [Google Scholar] [CrossRef]
- Bulik-Sullivan, B.K.; Loh, P.R.; Finucane, H.K.; Ripke, S.; Yang, J.; Patterson, N.; Daly, M.J.; Price, A.L.; Neale, B.M. LD Score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat. Genet. 2015, 47, 291–295. [Google Scholar] [CrossRef]
- Bulik-Sullivan, B.; Finucane, H.K.; Anttila, V.; Gusev, A.; Day, F.R.; Loh, P.R.; Duncan, L.; Perry, J.R.; Patterson, N.; Robinson, E.B.; et al. An atlas of genetic correlations across human diseases and traits. Nat. Genet. 2015, 47, 1236–1241. [Google Scholar] [CrossRef]
- Heckmann, J.G.; Urban, P.P.; Pitz, S.; Guntinas-Lichius, O.; Gágyor, I. The Diagnosis and Treatment of Idiopathic Facial Paresis (Bell’s Palsy). Dtsch. Arztebl. Int. 2019, 116, 692–702. [Google Scholar] [CrossRef]
- Patel, D.K.; Levin, K.H. Bell palsy: Clinical examination and management. Cleve Clin. J. Med. 2015, 82, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Turriziani, O.; Falasca, F.; Maida, P.; Gaeta, A.; De Vito, C.; Mancini, P.; De Seta, D.; Covelli, E.; Attanasio, G.; Antonelli, G. Early collection of saliva specimens from Bell’s palsy patients: Quantitative analysis of HHV-6, HSV-1, and VZV. J. Med. Virol. 2014, 86, 1752–1758. [Google Scholar] [CrossRef] [PubMed]
- Kikuoka, Y.; Haginomori, S.I.; Ayani, Y.; Jin-Nin, T.; Ichihara, T.; Inaka, Y.; Ozaki, A.; Inui, T.; Kawata, R. Recurrent facial palsy: Characteristics of ipsilateral and alternative palsies of 104 cases. Auris Nasus Larynx 2023, 50, 507–512. [Google Scholar] [CrossRef]
- Freire de Castro, R.; Crema, D.; Neiva, F.C.; Pinto, R.; Suzuki, F.A. Prevalence of herpes zoster virus reactivation in patients diagnosed with Bell’s palsy. J. Laryngol. Otol. 2022, 136, 975–978. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Zhao, W.; Liu, W.; Li, Y.; Wang, X.; Xun, J.; Davgadorj, C. CCL19 enhances CD8+ T-cell responses and accelerates HBV clearance. J. Gastroenterol. 2021, 56, 769–785. [Google Scholar] [CrossRef]
- Yusuf-Makagiansar, H.; Anderson, M.E.; Yakovleva, T.V.; Murray, J.S.; Siahaan, T.J. Inhibition of LFA-1/ICAM-1 and VLA-4/VCAM-1 as a therapeutic approach to inflammation and autoimmune diseases. Med. Res. Rev. 2002, 22, 146–167. [Google Scholar] [CrossRef]
- Singh, V.; Kaur, R.; Kumari, P.; Pasricha, C.; Singh, R. ICAM-1 and VCAM-1: Gatekeepers in various inflammatory and cardiovascular disorders. Clin. Chim. Acta 2023, 548, 117487. [Google Scholar] [CrossRef]
- Yan, Y.; Chen, R.; Wang, X.; Hu, K.; Huang, L.; Lu, M.; Hu, Q. CCL19 and CCR7 Expression, Signaling Pathways, and Adjuvant Functions in Viral Infection and Prevention. Front. Cell Dev. Biol. 2019, 7, 212. [Google Scholar] [CrossRef]
- VanHeyst, K.A.; Choi, S.H.; Kingsley, D.T.; Huang, A.Y. Ectopic Tumor VCAM-1 Expression in Cancer Metastasis and Therapy Resistance. Cells 2022, 11, 3922. [Google Scholar] [CrossRef]
- Madhok, V.B.; Gagyor, I.; Daly, F.; Somasundara, D.; Sullivan, M.; Gammie, F.; Sullivan, F. Corticosteroids for Bell’s palsy (idiopathic facial paralysis). Cochrane Database Syst. Rev. 2016, 7, CD001942. [Google Scholar] [CrossRef]
- Xin, P.; Xu, X.; Deng, C.; Liu, S.; Wang, Y.; Zhou, X.; Ma, H.; Wei, D.; Sun, S. The role of JAK/STAT signaling pathway and its inhibitors in diseases. Int. Immunopharmacol. 2020, 80, 106210. [Google Scholar] [CrossRef] [PubMed]
- Trammell, C.E.; Ramirez, G.; Sanchez-Vargas, I.; St Clair, L.A.; Ratnayake, O.C.; Luckhart, S.; Perera, R.; Goodman, A.G. Coupled small molecules target RNA interference and JAK/STAT signaling to reduce Zika virus infection in Aedes aegypti. PLoS Pathog. 2022, 18, e1010411. [Google Scholar] [CrossRef]
- Reece, M.D.; Song, C.; Hancock, S.C.; Pereira Ribeiro, S.; Kulpa, D.A.; Gavegnano, C. Repurposing BCL-2 and Jak 1/2 inhibitors: Cure and treatment of HIV-1 and other viral infections. Front. Immunol. 2022, 13, 1033672. [Google Scholar] [CrossRef]
- Li, X.; Yan, Z.; Ma, J.; Li, G.; Liu, X.; Peng, Z.; Zhang, Y.; Huang, S.; Luo, J.; Guo, X. TRIM28 promotes porcine epidemic diarrhea virus replication by mitophagy-mediated inhibition of the JAK-STAT1 pathway. Int. J. Biol. Macromol. 2024, 254, 127722. [Google Scholar] [CrossRef]
- Pratumchai, I.; Zak, J.; Huang, Z.; Min, B.; Oldstone, M.B.A.; Teijaro, J.R. B cell-derived IL-27 promotes control of persistent LCMV infection. Proc. Natl. Acad. Sci. USA 2022, 119, e2116741119. [Google Scholar] [CrossRef]
- Pradhan, A.; Lambert, Q.T.; Reuther, G.W. Transformation of hematopoietic cells and activation of JAK2-V617F by IL-27R, a component of a heterodimeric type I cytokine receptor. Proc. Natl. Acad. Sci. USA 2007, 104, 18502–18507. [Google Scholar] [CrossRef] [PubMed]
- Do, J.; Kim, D.; Kim, S.; Valentin-Torres, A.; Dvorina, N.; Jang, E.; Nagarajavel, V.; DeSilva, T.M.; Li, X.; Ting, A.H.; et al. Treg-specific IL-27Rα deletion uncovers a key role for IL-27 in Treg function to control autoimmunity. Proc. Natl. Acad. Sci. USA 2017, 114, 10190–10195. [Google Scholar] [CrossRef] [PubMed]
- Korobova, Z.R.; Arsentieva, N.A.; Santoni, A.; Totolian, A.A. Role of IL-27 in COVID-19: A Thin Line between Protection and Disease Promotion. Int. J. Mol. Sci. 2024, 25, 7953. [Google Scholar] [CrossRef]
- Ciecko, A.E.; Foda, B.; Barr, J.Y.; Ramanathan, S.; Atkinson, M.A.; Serreze, D.V.; Geurts, A.M.; Lieberman, S.M.; Chen, Y.G. Interleukin-27 Is Essential for Type 1 Diabetes Development and Sjögren Syndrome-like Inflammation. Cell Rep. 2019, 29, 3073–3086.e5. [Google Scholar] [CrossRef]
- Masjedi, A.; Hajizadeh, F.; Beigi Dargani, F.; Beyzai, B.; Aksoun, M.; Hojjat-Farsangi, M.; Zekiy, A.; Jadidi-Niaragh, F. Oncostatin M: A mysterious cytokine in cancers. Int. Immunopharmacol. 2021, 90, 107158. [Google Scholar] [CrossRef]
- Domaniku-Waraich, A.; Agca, S.; Toledo, B.; Sucuoglu, M.; Özen, S.D.; Bilgic, S.N.; Arabaci, D.H.; Kashgari, A.E.; Kir, S. Oncostatin M signaling drives cancer-associated skeletal muscle wasting. Cell Rep. Med. 2024, 5, 101498. [Google Scholar] [CrossRef] [PubMed]
- Hermans, D.; Houben, E.; Baeten, P.; Slaets, H.; Janssens, K.; Hoeks, C.; Hosseinkhani, B.; Duran, G.; Bormans, S.; Gowing, E.; et al. Oncostatin M triggers brain inflammation by compromising blood-brain barrier integrity. Acta Neuropathol. 2022, 144, 259–281. [Google Scholar] [CrossRef] [PubMed]
- McGarry, T.; Orr, C.; Wade, S.; Biniecka, M.; Wade, S.; Gallagher, L.; Low, C.; Veale, D.J.; Fearon, U. JAK/STAT Blockade Alters Synovial Bioenergetics, Mitochondrial Function, and Proinflammatory Mediators in Rheumatoid Arthritis. Arthritis Rheumatol. 2018, 70, 1959–1970. [Google Scholar] [CrossRef]
- Heinrich, P.C.; Behrmann, I.; Haan, S.; Hermanns, H.M.; Müller-Newen, G.; Schaper, F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem. J. 2003, 374, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Ryter, S.W. Heme Oxygenase-1: An Anti-Inflammatory Effector in Cardiovascular, Lung, and Related Metabolic Disorders. Antioxidants 2022, 11, 555. [Google Scholar] [CrossRef]
- Guo, M.; Li, R.; Xiao, Q.; Fan, X.; Li, N.; Shang, Y.; Wei, L.; Chai, T. Protective Role of Rabbit Nucleotide-Binding Oligomerization Domain-2 (NOD2)-Mediated Signaling Pathway in Resistance to Enterohemorrhagic Escherichia coli Infection. Front. Cell Infect. Microbiol. 2018, 8, 220. [Google Scholar] [CrossRef]
- Frietze, K.K.; Brown, A.M.; Das, D.; Franks, R.G.; Cunningham, J.L.; Hayward, M.; Nickels, J.T., Jr. Lipotoxicity reduces DDX58/Rig-1 expression and activity leading to impaired autophagy and cell death. Autophagy 2022, 18, 142–160. [Google Scholar] [CrossRef]
- Cegarra, C.; Chaves, C.; Déon, C.; Do, T.M.; Dumas, B.; Frenzel, A.; Kuhn, P.; Roudieres, V.; Guillemot, J.C.; Lesuisse, D. Exploring ITM2A as a new potential target for brain delivery. Fluids Barriers CNS 2022, 19, 25. [Google Scholar] [CrossRef]
- Chapman, M.S.; Wu, L.; Amatucci, A.; Ho, S.N.; Michaelson, J.S. TWEAK signals through JAK-STAT to induce tumor cell apoptosis. Cytokine 2013, 61, 210–217. [Google Scholar] [CrossRef]
- Xiao, G.; Lyu, M.; Wang, Y.; He, S.; Liu, X.; Ni, J.; Li, L.; Fan, G.; Han, J.; Gao, X.; et al. Ginkgo Flavonol Glycosides or Ginkgolides Tend to Differentially Protect Myocardial or Cerebral Ischemia-Reperfusion Injury via Regulation of TWEAK-Fn14 Signaling in Heart and Brain. Front. Pharmacol. 2019, 10, 735. [Google Scholar] [CrossRef]
- White, A.L.; Bix, G.J. VEGFA Isoforms as Pro-Angiogenic Therapeutics for Cerebrovascular Diseases. Biomolecules 2023, 13, 702. [Google Scholar] [CrossRef]
- Wiszniak, S.; Schwarz, Q. Exploring the Intracrine Functions of VEGF-A. Biomolecules 2021, 11, 128. [Google Scholar] [CrossRef]
- Li, J.; Xiang, X.; Xu, H.; Shi, Y. Cilostazol Promotes Angiogenesis and Increases Cell Proliferation After Myocardial Ischemia-Reperfusion Injury Through a cAMP-Dependent Mechanism. Cardiovasc. Eng. Technol. 2019, 10, 638–647. [Google Scholar] [CrossRef]
- Uwimana, A.; Ma, C.; Chen, S.; Ma, X. Metformin therapy as a strategy to compensate anti-VEGF resistance in patients with diabetic macular edema. Medicine 2022, 101, e31266. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Hong, L.; Yang, Y.; Qiao, X.; Cai, W.; Zhong, M.; Wang, M.; Zheng, Z.; Fu, Y. Metformin reduces proteinuria in spontaneously hypertensive rats by activating the HIF-2α-VEGF-A pathway. Eur. J. Pharmacol. 2021, 891, 173731. [Google Scholar] [CrossRef] [PubMed]
- Nesbitt, N.M.; Malone, L.E.; Liu, Z.; Jares, A.; Gnatenko, D.V.; Ma, Y.; Zhu, W.; Bahou, W.F. Divergent erythroid megakaryocyte fates in Blvrb-deficient mice establish non-overlapping cytoprotective functions during stress hematopoiesis. Free Radic. Biol. Med. 2021, 164, 164–174. [Google Scholar] [CrossRef]
- Wu, S.; Li, Z.; Gnatenko, D.V.; Zhang, B.; Zhao, L.; Malone, L.E.; Markova, N.; Mantle, T.J.; Nesbitt, N.M.; Bahou, W.F. BLVRB redox mutation defines heme degradation in a metabolic pathway of enhanced thrombopoiesis in humans. Blood 2016, 128, 699–709. [Google Scholar] [CrossRef] [PubMed]
- Skrivankova, V.W.; Richmond, R.C.; Woolf, B.A.R.; Yarmolinsky, J.; Davies, N.M.; Swanson, S.A.; VanderWeele, T.J.; Higgins, J.P.T.; Timpson, N.J.; Dimou, N.; et al. Strengthening the Reporting of Observational Studies in Epidemiology using Mendelian Randomization: The STROBE-MR Statement. JAMA 2021, 326, 1614–1621. [Google Scholar] [CrossRef]
- Skrivankova, V.W.; Richmond, R.C.; Woolf, B.A.R.; Davies, N.M.; Swanson, S.A.; VanderWeele, T.J.; Timpson, N.J.; Higgins, J.P.T.; Dimou, N.; Langenberg, C.; et al. Strengthening the Reporting of Observational Studies in Epidemiology using Mendelian Randomisation (STROBE-MR): Explanation and Elaboration. BMJ 2021, 375, n2233. [Google Scholar] [CrossRef]


| Exposure | Outcome | nSNP | OR (95%CI) | p-Val | Heterogeneity | Pleiotropy | MR Presso | LDSC | Color | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MR–Egger | Global Test | ||||||||||||
| Q | Q_p-Val | Egger Intercept | p-Val | RSSobs | p-Val | rg | p-rg | PP.H4 | |||||
| CCL19 | Bell palsy | 23 | 1.15 (1.03, 1.29) | 0.013 | 23.10148 | 0.339 | −0.011963 | 0.146 | 39.73911 | 0.331 | 0.2105 | 0.042 | 0.0075119 |
| SELL | Bell palsy | 49 | 1.16 (1.06, 1.27) | <0.001 | 43.825816 | 0.605 | 0.010615 | 0.084 | 49.08359 | 0.548 | 0.0398 | 0.733 | - |
| VCAM1 | Bell palsy | 27 | 1.23 (1.05, 1.43) | 0.009 | 14.857993 | 0.945 | 0.0176444 | 0.162 | 18.20016 | 0.916 | 0.2478 | 0.017 | 0.0224088 |
| IL27RA | Bell palsy | 21 | 1.07 (1.01, 1.13) | 0.031 | 10.554531 | 0.938 | 0.0175772 | 0.259 | 13.12543 | 0.926 | 0.0634 | 0.843 | 0.0213167 |
| OSM | Bell palsy | 15 | 1.17 (1.01, 1.36) | 0.042 | 9.5880892 | 0.727 | −0.008722 | 0.632 | 10.83632 | 0.803 | 0.41934 | 0.021 | 0.0117855 |
| JAK2 | Bell palsy | 17 | 1.37 (1.04, 1.81) | 0.023 | 17.758644 | 0.276 | −0.006678 | 0.716 | 20.32988 | 0.351 | −0.008 | 0.974 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, J.; Yin, Z.; Qiu, Y.; Yang, Y.; Chen, Z.; Wu, J.; Wang, Z. Exploring the Role of Inflammation and Metabolites in Bell’s Palsy and Potential Treatment Strategies. Biomedicines 2025, 13, 957. https://doi.org/10.3390/biomedicines13040957
Lu J, Yin Z, Qiu Y, Yang Y, Chen Z, Wu J, Wang Z. Exploring the Role of Inflammation and Metabolites in Bell’s Palsy and Potential Treatment Strategies. Biomedicines. 2025; 13(4):957. https://doi.org/10.3390/biomedicines13040957
Chicago/Turabian StyleLu, Jiaye, Ziqian Yin, Youjia Qiu, Yayi Yang, Zhouqing Chen, Jiang Wu, and Zhong Wang. 2025. "Exploring the Role of Inflammation and Metabolites in Bell’s Palsy and Potential Treatment Strategies" Biomedicines 13, no. 4: 957. https://doi.org/10.3390/biomedicines13040957
APA StyleLu, J., Yin, Z., Qiu, Y., Yang, Y., Chen, Z., Wu, J., & Wang, Z. (2025). Exploring the Role of Inflammation and Metabolites in Bell’s Palsy and Potential Treatment Strategies. Biomedicines, 13(4), 957. https://doi.org/10.3390/biomedicines13040957







