Anticancer Potential of Prebiotics: Targeting Estrogen Receptors and PI3K/AKT/mTOR in Breast Cancer
Abstract
1. Introduction
2. Estrogen Receptor Signaling in Breast Cancer
2.1. Role of Estrogen Receptors in BC Development and Progression
2.2. Mechanisms of Endocrine Resistance and Targeted Therapies
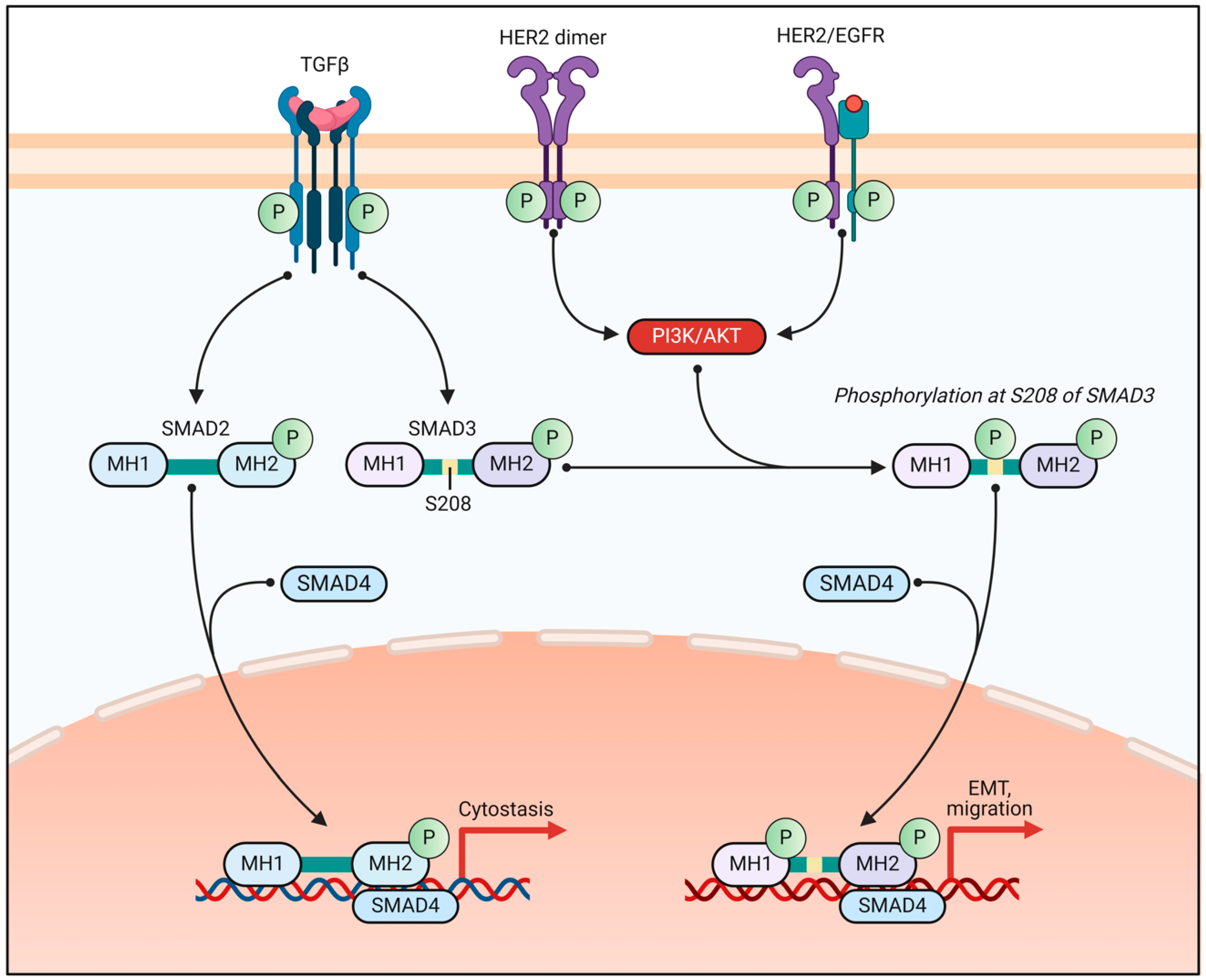
2.3. Crosstalk Between ER Signaling and Other Pathways in BC
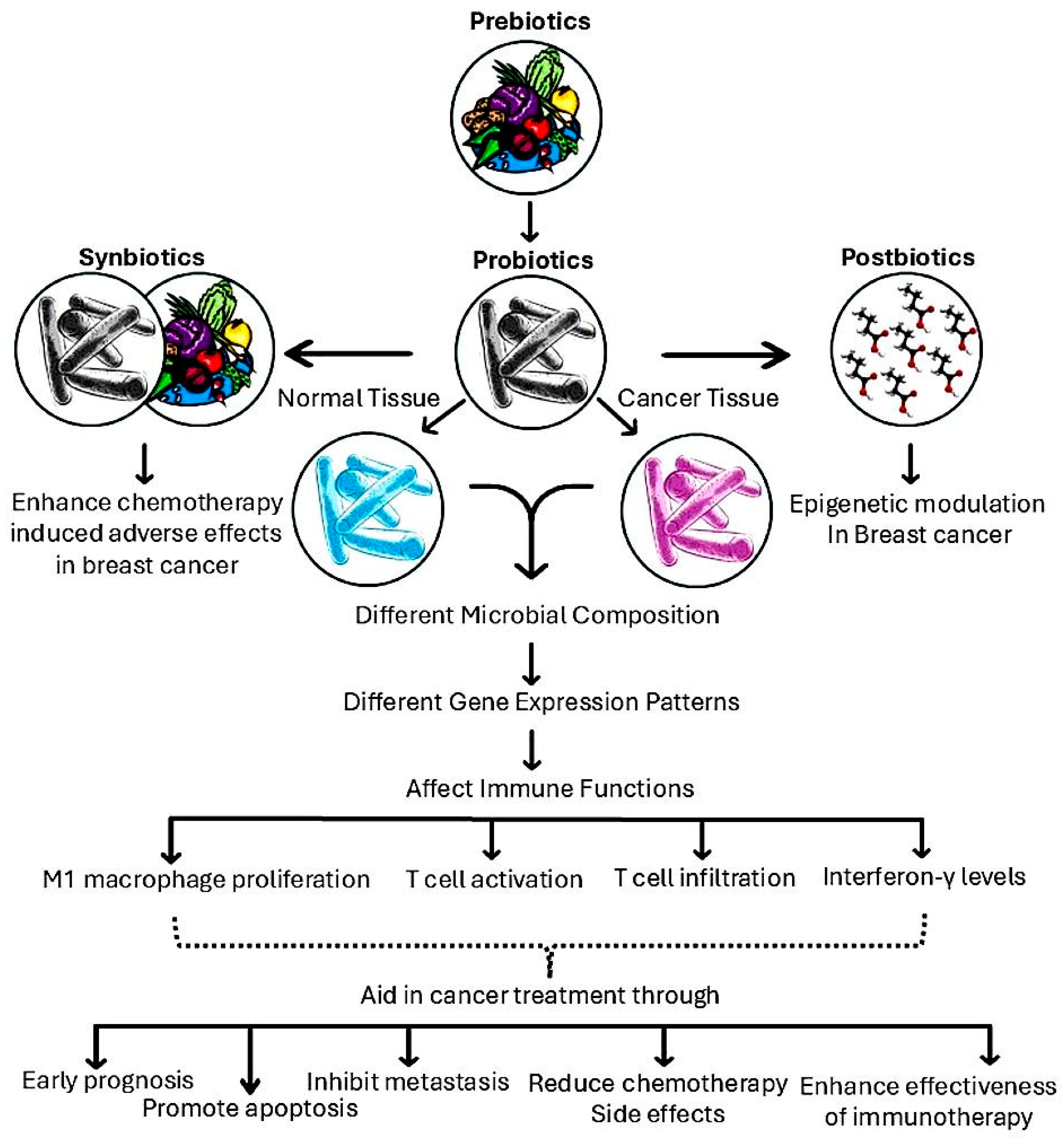
3. The Anticancer Potential of Prebiotics
3.1. The Microbiota–Estrogen Axis
3.2. SCFAs and Epigenetic Reprogramming
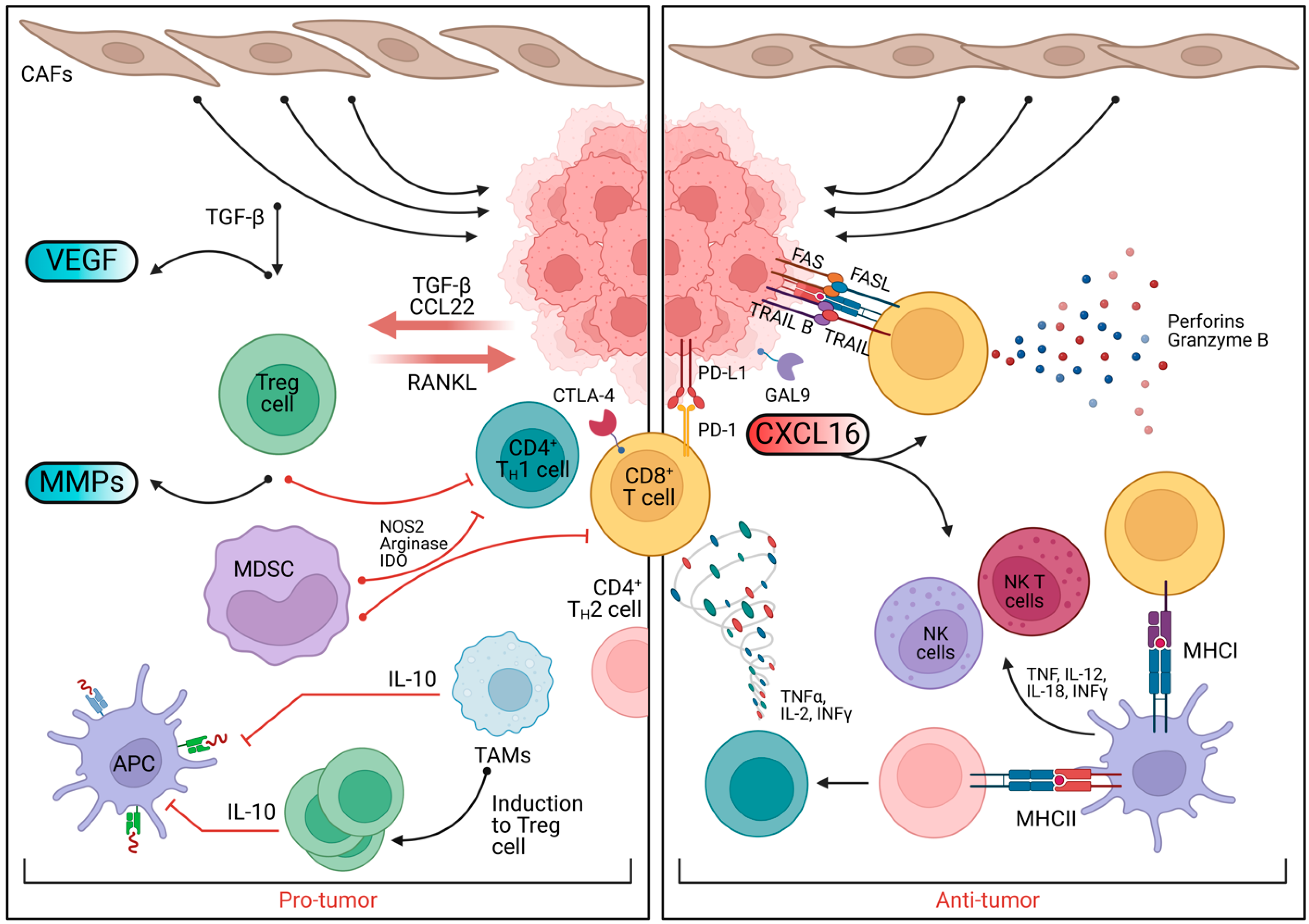
3.3. Anti-Inflammatory Roles of Prebiotics in BC
3.4. Dietary Interventions Targeting mTOR in ER+ BC
4. Evidence from Preclinical and Clinical Studies
4.1. Prebiotics: Systemic Health
4.2. Preclinical Studies
4.2.1. Breast Microbiota and Tumor Biology
4.2.2. Gut Microbiota and Breast Cancer Prognosis
4.2.3. Intratumoral Microbiota and Metastasis
4.2.4. Targeting Microbiota to Improve Therapeutic Outcomes
5. Translational and Clinical Applications of Prebiotics in BC
5.1. The Role of Prebiotics in BC Treatment
5.2. Synergistic Effects of Prebiotics and Exercise
5.3. Prebiotics and Immune Checkpoint Therapy
5.4. Gut Microbiota Modulation by Phytochemicals
5.5. The Role of SCFAs in BC Treatment
5.6. Combination Therapies with SCFAs and Phytochemicals
5.7. Synbiotics as Supportive Care in BC Treatment
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Giaquinto, A.N.; Sung, H.; Newman, L.A.; Freedman, R.A.; Smith, R.A.; Star, J.; Jemal, A.; Siegel, R.L. Breast cancer statistics 2024. CA Cancer J. Clin. 2024, 74, 477–495. [Google Scholar] [CrossRef]
- Menon, G.; Alkabban, F.M.; Ferguson, T. Breast Cancer. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Ye, J.; Baer, J.M.; Faget, D.V.; Morikis, V.A.; Ren, Q.; Melam, A.; Delgado, A.P.; Luo, X.; Bagchi, S.M.; Belle, J.I.; et al. Senescent CAFs Mediate Immunosuppression and Drive Breast Cancer Progression. Cancer Discov. 2024, 14, 1302–1323. [Google Scholar] [CrossRef]
- Slamon, D.J.; Diéras, V.; Rugo, H.S.; Harbeck, N.; Im, S.A.; Gelmon, K.A.; Lipatov, O.N.; Walshe, J.M.; Martin, M.; Chavez-MacGregor, M.; et al. Overall Survival With Palbociclib Plus Letrozole in Advanced Breast Cancer. J. Clin. Oncol. 2024, 42, 994–1000. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Ai, M.; Liu, C. The impact of lipidome on breast cancer: A Mendelian randomization study. Lipids Health Dis. 2024, 23, 109. [Google Scholar] [CrossRef]
- Wang, D.; Liu, X.; Hong, W.; Xiao, T.; Xu, Y.; Fang, X.; Tang, H.; Zheng, Q.; Meng, X. Muscone abrogates breast cancer progression through tumor angiogenic suppression via VEGF/PI3K/Akt/MAPK signaling pathways. Cancer Cell Int. 2024, 24, 214. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, J.; Zhao, Y.; Fan, Z.; Yang, Y.; Mao, Y.; Yang, J.; Ma, S. CD93 regulates breast cancer growth and vasculogenic mimicry through the PI3K/AKT/SP2 signaling pathway activated by integrin β1. J. Biochem. Mol. Toxicol. 2024, 38, e23688. [Google Scholar] [CrossRef] [PubMed]
- Di Pace, B.; Padley, R.H. Survivorship and breast cancer: Navigating the continuum of care. J. Surg. Oncol. 2024, 130, 36–37. [Google Scholar] [CrossRef]
- Montazeri Aliabadi, H. Molecular Targets for Breast Cancer Therapy. Biomolecules 2024, 14, 1219. [Google Scholar] [CrossRef]
- Jia, W.; Lin, X.; Chen, X.; Li, H.; Zhang, X.; Zhang, Y.; Chen, Y.; Wang, B.; Chen, X.; Chen, J.; et al. Rujifang inhibits triple-negative breast cancer growth via the PI3K/AKT pathway. J. Ethnopharmacol. 2024, 327, 118011. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, L.; He, Y.; Jiang, D.; Sun, J.; Luo, Q.; Liang, H.; Wang, T.; Li, F.; Tang, Y.; et al. PI3K PROTAC overcomes the lapatinib resistance in PIK3CA-mutant HER2 positive breast cancer. Cancer Lett. 2024, 598, 217112. [Google Scholar] [CrossRef]
- Gao, F.; Liu, S.; Wang, J.; Wei, G.; Yu, C.; Zheng, L.; Sun, L.; Wang, G.; Sun, Y.; Bao, Y.; et al. TSP50 facilitates breast cancer stem cell-like properties maintenance and epithelial-mesenchymal transition via PI3K p110α mediated activation of AKT signaling pathway. J. Exp. Clin. Cancer Res. 2024, 43, 201. [Google Scholar] [CrossRef] [PubMed]
- Rossetti, S.; Broege, A.; Sen, A.; Khan, S.; MacNeil, I.; Molden, J.; Kopher, R.; Schulz, S.; Laing, L. Gedatolisib shows superior potency and efficacy versus single-node PI3K/AKT/mTOR inhibitors in breast cancer models. NPJ Breast Cancer 2024, 10, 40. [Google Scholar] [CrossRef] [PubMed]
- Smit, D.J.; Brauer, H.; Horn, S.; Yigit, G.; Haider, M.T.; Pogenberg, V.; Schumacher, U.; Pantel, K.; Jücker, M. Functional characterization of PI3K C2 domain mutations detected in breast cancer circulating tumor cells and metastatic cells. Cell. Signal. 2024, 121, 111270. [Google Scholar] [CrossRef]
- Guarner, F.; Sanders, M.E.; Szajewska, H.; Cohen, H.; Eliakim, R.; Herrera-deGuise, C.; Karakan, T.; Merenstein, D.; Piscoya, A.; Ramakrishna, B.; et al. World Gastroenterology Organisation Global Guidelines: Probiotics and Prebiotics. J. Clin. Gastroenterol. 2024, 58, 533–553. [Google Scholar] [CrossRef] [PubMed]
- Leach, S.T. Role of Probiotics and Prebiotics in Gut Symbiosis. Nutrients 2024, 16, 238. [Google Scholar] [CrossRef]
- Aguilera, M.; Daddaoua, A. Prebiotics and Probiotics: Healthy Biotools for Molecular Integrative and Modulation Approaches 2.0. Int. J. Mol. Sci. 2024, 25, 4872. [Google Scholar] [CrossRef]
- Floch, M.H. Probiotics and Prebiotics. Gastroenterol. Hepatol. 2014, 10, 680–681. [Google Scholar]
- Buhaș, M.C.; Candrea, R.; Gavrilaș, L.I.; Miere, D.; Tătaru, A.; Boca, A.; Cătinean, A. Transforming Psoriasis Care: Probiotics and Prebiotics as Novel Therapeutic Approaches. Int. J. Mol. Sci. 2023, 24, 1225. [Google Scholar] [CrossRef]
- Khosravi, S.; Tabatabaei-Malazy, O.; Emamgholipour, S.; Sojoodi, M.; Shabani, P. Editorial: Prebiotics in the management of obesity and associated metabolic disorders. Front. Endocrinol. 2024, 15, 1432530. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, B.; Zhang, J.; Dong, J.; Ma, J.; Zhang, Y.; Jin, K.; Lu, J. Effect of prebiotics, probiotics, synbiotics on depression: Results from a meta-analysis. BMC Psychiatry 2023, 23, 477. [Google Scholar] [CrossRef]
- Laudani, S.; Grosso, G. Gut-liver axis: May prebiotics play a role? Int. J. Food Sci. Nutr. 2023, 74, 719–720. [Google Scholar] [CrossRef]
- Nicolucci, A.C.; Hume, M.P.; Martínez, I.; Mayengbam, S.; Walter, J.; Reimer, R.A. Prebiotics Reduce Body Fat and Alter Intestinal Microbiota in Children Who Are Overweight or With Obesity. Gastroenterology 2017, 153, 711–722. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.W.; Li, R.Q.; An, J.X.; Xie, T.Q.; Han, Z.Y.; Xu, R.; Fang, Y.; Zhang, X.Z. Prebiotics-Encapsulated Probiotic Spores Regulate Gut Microbiota and Suppress Colon Cancer. Adv. Mater. 2020, 32, e2004529. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.; Xu, M.; Li, Y.; Chen, L.; Li, H. Prebiotics, Probiotics and Nutrients in Cardiovascular and Kidney Disease. Nutrients 2023, 15, 4284. [Google Scholar] [CrossRef]
- Whisner, C.M.; Weaver, C.M. Prebiotics and Bone. Adv. Exp. Med. Biol. 2017, 1033, 201–224. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Wu, G.; Wang, Y.; Wang, J.; Li, H.; Yang, K.; Gu, H.; Sun, W.; Qian, C.; Ren, T.; et al. USP35, regulated by estrogen and AKT, promotes breast tumorigenesis by stabilizing and enhancing transcriptional activity of estrogen receptor α. Cell Death Dis. 2021, 12, 619. [Google Scholar] [CrossRef]
- Miziak, P.; Baran, M.; Błaszczak, E.; Przybyszewska-Podstawka, A.; Kałafut, J.; Smok-Kalwat, J.; Dmoszyńska-Graniczka, M.; Kiełbus, M.; Stepulak, A. Estrogen Receptor Signaling in Breast Cancer. Cancers 2023, 15, 4689. [Google Scholar] [CrossRef] [PubMed]
- Sellitto, A.; Alexandrova, E.; Rocco, D.; Weisz, A.; Lamberti, J.; Coviello, E.; Pecoraro, G.; Memoli, D.; Tarallo, R.; Rizzo, F.; et al. Insights into the Role of Estrogen Receptor β in Triple-Negative Breast Cancer. Cancers 2020, 12, 1477. [Google Scholar] [CrossRef]
- Porras, L.; Mader, S.; Ismail, H. Positive Regulation of Estrogen Receptor Alpha in Breast Tumorigenesis. Cells 2021, 10, 2966. [Google Scholar] [CrossRef]
- Clusan, L.; Percevault, F.; Jullion, E.; Le Goff, P.; Tiffoche, C.; Fernandez-Calero, T.; Métivier, R.; Marin, M.; Pakdel, F.; Michel, D.; et al. Codon adaptation by synonymous mutations impacts the functional properties of the estrogen receptor-alpha protein in breast cancer cells. Mol. Oncol. 2023, 17, 1302–1323. [Google Scholar] [CrossRef]
- Munne, P.; Klefström, J.; Heikkilä, P.; Väänänen, J.; Räty, I.; Hukkinen, K.; Kivento, M.; Ruuska, J.; Monni, O.; Pouwels, J.; et al. Compressive stress-mediated p38 activation required for ER\u03b1\u2009+\u2009phenotype in breast cancer. Nat. Commun. 2021, 12, 6967. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, M.R.; Wander, S.A.; Hamilton, E.; Razavi, P.; Bardia, A. Next-generation selective estrogen receptor degraders and other novel endocrine therapies for management of metastatic hormone receptor-positive breast cancer: Current and emerging role. Ther. Adv. Med. Oncol. 2022, 14, 175883592211136. [Google Scholar] [CrossRef]
- Rodriguez, A.C.; Vahrenkamp, J.M.; Berrett, K.C.; Clark, K.A.; Guillen, K.P.; Scherer, S.D.; Yang, C.-H.; Welm, B.E.; Janát-Amsbury, M.M.; Graves, B.J.; et al. ETV4 Is Necessary for Estrogen Signaling and Growth in Endometrial Cancer Cells. Cancer Res. 2020, 80, 1234–1245. [Google Scholar] [CrossRef]
- Zhou, Y.; Chu, P.; Wang, Y.; Li, N.; Gao, Q.; Wang, S.; Wei, J.; Xue, G.; Zhao, Y.; Jia, H.; et al. Epinephrine promotes breast cancer metastasis through a ubiquitin-specific peptidase 22-mediated lipolysis circuit. Sci. Adv. 2024, 10, eado1533. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Yang, A.; Chen, B.; Deng, X.; Xie, J.; Dai, D.; Zhang, J.; Tang, H.; Wu, T.; Zhou, Z.; et al. crVDAC3 alleviates ferroptosis by impeding HSPB1 ubiquitination and confers trastuzumab deruxtecan resistance in HER2-low breast cancer. Drug Resist. Updates 2024, 77, 101126. [Google Scholar] [CrossRef] [PubMed]
- Boscolo Bielo, L.; Trapani, D.; Nicolò, E.; Valenza, C.; Guidi, L.; Belli, C.; Kotteas, E.; Marra, A.; Prat, A.; Fusco, N.; et al. The evolving landscape of metastatic HER2-positive, hormone receptor-positive Breast Cancer. Cancer Treat. Rev. 2024, 128, 102761. [Google Scholar] [CrossRef]
- Oliveira, M.; Pominchuk, D.; Nowecki, Z.; Hamilton, E.; Kulyaba, Y.; Andabekov, T.; Hotko, Y.; Melkadze, T.; Nemsadze, G.; Neven, P.; et al. Camizestrant, a next-generation oral SERD, versus fulvestrant in post-menopausal women with oestrogen receptor-positive, HER2-negative advanced breast cancer (SERENA-2): A multi-dose, open-label, randomised, phase 2 trial. Lancet Oncol. 2024, 25, 1424–1439. [Google Scholar] [CrossRef]
- Jeffreys, S.A.; Mok, K.; Becker, T.M.; Balakrishnar, B.; Neubauer, H.; Powter, B.; De Souza, P.; Soon, P.; Franken, A. Endocrine Resistance in Breast Cancer: The Role of Estrogen Receptor Stability. Cells 2020, 9, 2077. [Google Scholar] [CrossRef]
- Clusan, L.; Pakdel, F.; Le Goff, P.; Flouriot, G. A Closer Look at Estrogen Receptor Mutations in Breast Cancer and Their Implications for Estrogen and Antiestrogen Responses. Int. J. Mol. Sci. 2021, 22, 756. [Google Scholar] [CrossRef]
- Lloyd, M.R.; Brett, J.O.; Carmeli, A.; Weipert, C.M.; Zhang, N.; Yu, J.; Bucheit, L.; Medford, A.J.; Wagle, N.; Bardia, A.; et al. CDK4/6 Inhibitor Efficacy in ESR1-Mutant Metastatic Breast Cancer. NEJM Evid. 2024, 3, EVIDoa2300231. [Google Scholar] [CrossRef]
- Zhuang, T.; Zhang, S.; Liu, D.; Li, Z.; Li, X.; Li, J.; Yang, P.; Zhang, C.; Cui, J.; Fu, M.; et al. USP36 promotes tumorigenesis and tamoxifen resistance in breast cancer by deubiquitinating and stabilizing ERα. J. Exp. Clin. Cancer Res. 2024, 43, 249. [Google Scholar] [CrossRef]
- Neupane, N.; Thapa, S.; Falkson, C.; Gurusinghe, S.; Bawek, S.; Dhakal, A.; O’Regan, R.; Ghaffary, E.M.; Mirmosayyeb, O. Oral SERD, a Novel Endocrine Therapy for Estrogen Receptor-Positive Breast Cancer. Cancers 2024, 16, 619. [Google Scholar] [CrossRef]
- Parisian, A.D.; Barratt, S.A.; Hodges-Gallagher, L.; Ortega, F.E.; Peña, G.; Sapugay, J.; Robello, B.; Sun, R.; Kulp, D.; Palanisamy, G.S.; et al. Palazestrant (OP-1250), A Complete Estrogen Receptor Antagonist, Inhibits Wild-type and Mutant ER-positive Breast Cancer Models as Monotherapy and in Combination. Mol. Cancer Ther. 2024, 23, 285–300. [Google Scholar] [CrossRef] [PubMed]
- Glaviano, A.; Wander, S.A.; Baird, R.D.; Yap, K.C.H.; Lam, H.Y.; Toi, M.; Carbone, D.; Geoerger, B.; Serra, V.; Jones, R.H.; et al. Mechanisms of sensitivity and resistance to CDK4/CDK6 inhibitors in hormone receptor-positive breast cancer treatment. Drug Resist. Updates 2024, 76, 101103. [Google Scholar] [CrossRef] [PubMed]
- Pedroza, D.A.; Subramani, R.; Tiula, K.; Do, A.; Rashiraj, N.; Galvez, A.; Chatterjee, A.; Bencomo, A.; Rivera, S.; Lakshmanaswamy, R. Crosstalk between progesterone receptor membrane component 1 and estrogen receptor α promotes breast cancer cell proliferation. Lab. Investig. 2021, 101, 733–744. [Google Scholar] [CrossRef] [PubMed]
- Lü, J.; Zhao, Q.; Guo, Y.; Li, D.; Xie, H.; Liu, C.; Hu, X.; Liu, S.; Hou, Z.; Wei, X.; et al. Regulation of ERα-dependent breast cancer metastasis by a miR-29a signaling. J. Exp. Clin. Cancer Res. 2023, 42, 93. [Google Scholar] [CrossRef]
- Hao, C.; Wei, Y.; Meng, W.; Zhang, J.; Yang, X. PI3K/AKT/mTOR inhibitors for hormone receptor-positive advanced breast cancer. Cancer Treat. Rev. 2025, 132, 102861. [Google Scholar] [CrossRef]
- Khorasani, A.B.S.; Hafezi, N.; Sanaei, M.J.; Jafari-Raddani, F.; Pourbagheri-Sigaroodi, A.; Bashash, D. The PI3K/AKT/mTOR signaling pathway in breast cancer: Review of clinical trials and latest advances. Cell Biochem. Funct. 2024, 42, e3998. [Google Scholar] [CrossRef]
- Zhang, H.P.; Jiang, R.Y.; Zhu, J.Y.; Sun, K.N.; Huang, Y.; Zhou, H.H.; Zheng, Y.B.; Wang, X.J. PI3K/AKT/mTOR signaling pathway: An important driver and therapeutic target in triple-negative breast cancer. Breast Cancer 2024, 31, 539–551. [Google Scholar] [CrossRef]
- Mery, B.; Trédan, O.; Le Romancer, M.; Poulard, C. Targeting AKT in ER-Positive HER2-Negative Metastatic Breast Cancer: From Molecular Promises to Real Life Pitfalls? Int. J. Mol. Sci. 2021, 22, 13512. [Google Scholar] [CrossRef]
- Lee, B.J.; Boyer, J.A.; Burnett, G.L.; Thottumkara, A.P.; Tibrewal, N.; Wilson, S.L.; Hsieh, T.; Marquez, A.; Lorenzana, E.G.; Evans, J.W.; et al. Selective inhibitors of mTORC1 activate 4EBP1 and suppress tumor growth. Nat. Chem. Biol. 2021, 17, 1065–1074. [Google Scholar] [CrossRef] [PubMed]
- Meng, D.; Zhao, X.; Yang, Y.C.; Navickas, A.; Helland, C.; Goodarzi, H.; Singh, M.; Bandyopadhyay, S. A bi-steric mTORC1-selective inhibitor overcomes drug resistance in breast cancer. Oncogene 2023, 42, 2207–2217. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Qi, L.; Wu, B.; Long, M.; Li, Z.; Yang, M.; Jin, T.; Tu, C. Oncogene PRR14 promotes breast cancer through activation of PI3K signal pathway and inhibition of CHEK2 pathway. Cell Death Dis. 2020, 11, 464. [Google Scholar] [CrossRef]
- Nunnery, S.E.; Mayer, I.A. Targeting the PI3K/AKT/mTOR Pathway in Hormone-Positive Breast Cancer. Drugs 2020, 80, 1685–1697. [Google Scholar] [CrossRef]
- Jiang, W.; Zhong, S.; Chen, Z.; Qian, J.; Huang, X.; Zhang, H.; Wen, L.; Zhang, Y.; Yao, G. 2D-CuPd nanozyme overcome tamoxifen resistance in breast cancer by regulating the PI3K/AKT/mTOR pathway. Biomaterials 2022, 294, 121986. [Google Scholar] [CrossRef]
- Minini, M.; Senni, A.; He, X.; Proietti, S.; Liguoro, D.; Catizone, A.; Giuliani, A.; Mancini, R.; Fuso, A.; Cucina, A.; et al. miR-125a-5p impairs the metastatic potential in breast cancer via IP6K1 targeting. Cancer Lett. 2021, 520, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Ezine, E.; Dumaz, N.; Lebbe, C. Unmasking the tumourigenic role of SIN1/MAPKAP1 in the mTOR complex 2. Clin. Transl. Med. 2023, 13, e1464. [Google Scholar] [CrossRef]
- Arnold, M.; Morgan, E.; Rumgay, H.; Mafra, A.; Singh, D.; Laversanne, M.; Vignat, J.; Gralow, J.R.; Cardoso, F.; Siesling, S. Current and future burden of breast cancer: Global statistics for 2020 and 2040. Breast 2022, 66, 15–23. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Sánchez-Alcoholado, L.; Ramos-Molina, B.; Otero, A.; Laborda-Illanes, A.; Ordóñez, R.; Medina, J.A.; Gómez-Millán, J.; Queipo-Ortuño, M.I. The role of the gut microbiome in colorectal cancer development and therapy response. Cancers 2020, 12, 1406. [Google Scholar] [CrossRef]
- Mir, R.; Albarqi, S.A.; Albalawi, W.; Alatwi, H.E.; Alatawy, M.; Bedaiwi, R.I.; Almotairi, R.; Husain, E.; Zubair, M.; Alanazi, G. Emerging role of gut microbiota in breast cancer development and its implications in treatment. Metabolites 2024, 14, 683. [Google Scholar] [CrossRef] [PubMed]
- Sunkata, R.; Herring, J.; Walker, L.T.; Verghese, M. Chemopreventive potential of probiotics and prebiotics. Food Nutr. Sci. 2014, 5, 1800–1809. [Google Scholar] [CrossRef][Green Version]
- Ruo, S.W.; Alkayyali, T.; Win, M.; Tara, A.; Joseph, C.; Kannan, A.; Srivastava, K.; Ochuba, O.; Sandhu, J.K.; Went, T.R. Role of gut microbiota dysbiosis in breast cancer and novel approaches in prevention, diagnosis, and treatment. Cureus 2021, 13, e17472. [Google Scholar] [CrossRef] [PubMed]
- Arnone, A.A.; Cook, K.L. Gut and breast microbiota as endocrine regulators of hormone receptor-positive breast cancer risk and therapy response. Endocrinology 2023, 164, bqac177. [Google Scholar] [CrossRef]
- Sharma, M.; Arora, I.; Stoll, M.L.; Li, Y.; Morrow, C.D.; Barnes, S.; Berryhill, T.F.; Li, S.; Tollefsbol, T.O. Nutritional combinatorial impact on the gut microbiota and plasma short-chain fatty acids levels in the prevention of mammary cancer in Her2/neu estrogen receptor-negative transgenic mice. PLoS ONE 2020, 15, e0234893. [Google Scholar] [CrossRef]
- Hilakivi-Clarke, L.; Verma, V.; McDermott, M.; Koak, P.; Andrade, F.d.O. Foods may modify responsiveness to cancer immune checkpoint blockers by altering both the gut microbiota and activation of estrogen receptors in immune cells. Front. Microbiomes 2022, 1, 1049688. [Google Scholar] [CrossRef]
- Chen, S.; Chen, Y.; Ma, S.; Zheng, R.; Zhao, P.; Zhang, L.; Liu, Y.; Yu, Q.; Deng, Q.; Zhang, K. Dietary fibre intake and risk of breast cancer: A systematic review and meta-analysis of epidemiological studies. Oncotarget 2016, 7, 80980. [Google Scholar] [CrossRef]
- Kumari, N.; Kumari, R.; Dua, A.; Singh, M.; Kumar, R.; Singh, P.; Duyar-Ayerdi, S.; Pradeep, S.; Ojesina, A.I.; Kumar, R. From gut to hormones: Unraveling the role of gut microbiota in (phyto) estrogen modulation in health and disease. Mol. Nutr. Food Res. 2024, 68, 2300688. [Google Scholar] [CrossRef]
- Parida, S.; Sharma, D. The microbiome–estrogen connection and breast cancer risk. Cells 2019, 8, 1642. [Google Scholar] [CrossRef]
- Kwa, M.; Plottel, C.S.; Blaser, M.J.; Adams, S. The intestinal microbiome and estrogen receptor–positive female breast cancer. J. Natl. Cancer Inst. 2016, 108, djw029. [Google Scholar]
- Fernández-Murga, M.L.; Gil-Ortiz, F.; Serrano-García, L.; Llombart-Cussac, A. A New Paradigm in the Relationship between Gut Microbiota and Breast Cancer: β-glucuronidase Enzyme Identified as Potential Therapeutic Target. Pathogens 2023, 12, 1086. [Google Scholar] [CrossRef]
- Basnet, J.; Eissa, M.A.; Yanes Cardozo, L.L.; Romero, D.G.; Rezq, S. Impact of Probiotics and Prebiotics on Gut Microbiome and Hormonal Regulation. Gastrointest. Disord. 2024, 6, 801–815. [Google Scholar] [CrossRef] [PubMed]
- Bindels, L.B.; Delzenne, N.M.; Cani, P.D.; Walter, J. Towards a more comprehensive concept for prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, types, sources, mechanisms, and clinical applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef] [PubMed]
- Singarayar, M.S.; Chandrasekaran, A.; Balasundaram, D.; Veerasamy, V.; Neethirajan, V.; Thilagar, S. Prebiotics: Comprehensive Analysis of Sources, Structural Characteristics and Mechanistic Roles in Disease Regulation. Microb. Pathog. 2024, 197, 107071. [Google Scholar] [CrossRef]
- Hu, J.; Wang, J.; Li, Y.; Xue, K.; Kan, J. Use of dietary fibers in reducing the risk of several cancer types: An umbrella review. Nutrients 2023, 15, 2545. [Google Scholar] [CrossRef]
- Kalyanaraman, B.; Cheng, G.; Hardy, M. The role of short-chain fatty acids in cancer prevention and cancer treatment. Arch. Biochem. Biophys. 2024, 761, 110172. [Google Scholar] [CrossRef]
- Feitelson, M.A.; Arzumanyan, A.; Medhat, A.; Spector, I. Short-chain fatty acids in cancer pathogenesis. Cancer Metastasis Rev. 2023, 42, 677–698. [Google Scholar] [CrossRef]
- Schoeller, A.; Karki, K.; Jayaraman, A.; Chapkin, R.S.; Safe, S. Short chain fatty acids exhibit selective estrogen receptor downregulator (SERD) activity in breast cancer. Am. J. Cancer Res. 2022, 12, 3422. [Google Scholar]
- Wu, H.; Van Der Pol, W.J.; Dubois, L.G.; Morrow, C.D.; Tollefsbol, T.O. Dietary Supplementation of Inulin Contributes to the Prevention of Estrogen Receptor-Negative Mammary Cancer by Alteration of Gut Microbial Communities and Epigenetic Regulations. Int. J. Mol. Sci. 2023, 24, 9015. [Google Scholar] [CrossRef]
- Kim, S.; Kim, J. The effect of combined therapy with 5-aza-2′-deoxycytidine, sodium butyrate, and tamoxifen on apoptosis of breast cancer cell lines. J. Clin. Oncol. 2011, 29 (Suppl. 27), 197. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, X.; Wang, W.; Li, X.; Sun, X.; Zhao, Y.; Wang, Q.; Li, Y.; Hu, F.; Ren, H. Metabolic reprogramming and therapeutic resistance in primary and metastatic breast cancer. Mol. Cancer 2024, 23, 261. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Ba-Alawi, W.; Deblois, G.; Cruickshank, J.; Duan, S.; Lima-Fernandes, E.; Haight, J.; Tonekaboni, S.A.M.; Fortier, A.-M.; Kuasne, H. GLUT1 inhibition blocks growth of RB1-positive triple negative breast cancer. Nat. Commun. 2020, 11, 4205. [Google Scholar] [CrossRef]
- Serhan, H.A.; Bao, L.; Cheng, X.; Qin, Z.; Liu, C.-J.; Heth, J.A.; Udager, A.M.; Soellner, M.B.; Merajver, S.D.; Morikawa, A. Targeting fatty acid synthase in preclinical models of TNBC brain metastases synergizes with SN-38 and impairs invasion. NPJ Breast Cancer 2024, 10, 43. [Google Scholar] [CrossRef]
- Tao, Z.; Wang, Y. The health benefits of dietary short-chain fatty acids in metabolic diseases. Crit. Rev. Food Sci. Nutr. 2023, 65, 1579–1592. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Du, W.; Ni, Y.; Lan, G.; Shi, G. The effect of short-chain fatty acids on M2 macrophages polarization in vitro and in vivo. Clin. Exp. Immunol. 2022, 207, 53–64. [Google Scholar] [CrossRef]
- Tian, P.; Yang, W.; Guo, X.; Wang, T.; Tan, S.; Sun, R.; Xiao, R.; Wang, Y.; Jiao, D.; Xu, Y. Early life gut microbiota sustains liver-resident natural killer cells maturation via the butyrate-IL-18 axis. Nat. Commun. 2023, 14, 1710. [Google Scholar] [CrossRef]
- Ali Khan, W.; Khan, W.A. Cytochrome P450-mediated estrogen metabolites and autoimmunity: Relationship and link to free radicals. Curr. Drug Metab. 2016, 17, 65–74. [Google Scholar] [CrossRef]
- Al-Qubati, A.A.; Rahmadi, M.; Widiandani, T.; Al-Maamari, J.N.; Khotib, J. The role of IL-1, IL-6 and TNF-α in breast cancer development and progression. Pharm. Educ. 2024, 24, 32–38. [Google Scholar] [CrossRef]
- Zhang, Y.; Xi, Y.; Yang, C.; Gong, W.; Wang, C.; Wu, L.; Wang, D. Short-chain fatty acids attenuate 5-fluorouracil-induced thp-1 cell inflammation through inhibiting nf-κb/nlrp3 signaling via glycerolphospholipid and sphingolipid metabolism. Molecules 2023, 28, 494. [Google Scholar] [CrossRef]
- Malczewski, A.B.; Navarro, S.; Coward, J.I.; Ketheesan, N. Microbiome-derived metabolome as a potential predictor of response to cancer immunotherapy. J. Immunother. Cancer 2020, 8, e001383. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly-y, M.; Glickman, J.N.; Garrett, W.S. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Boucher, E.; Plazy, C.; Richard, M.L.; Suau, A.; Mangin, I.; Cornet, M.; Aldebert, D.; Toussaint, B.; Hannani, D. Inulin prebiotic reinforces host cancer immunosurveillance via ɣδ T cell activation. Front. Immunol. 2023, 14, 1104224. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, X.; Zou, Y.; Gong, J.; Ge, Z.; Lin, X.; Zhang, W.; Huang, H.; Zhao, J.; Saw, P.E.; et al. A high-fat diet promotes cancer progression by inducing gut microbiota–mediated leucine production and PMN-MDSC differentiation. Proc. Natl. Acad. Sci. USA 2024, 121, e2306776121. [Google Scholar] [CrossRef]
- Wang, J.; Guo, Y.; Fang, X.; Zhang, Y.; Nie, D. Short-Chain Fatty Acids Suppress mTOR Signaling in Colon Cancer Cells via Long Non-Coding RNA RMST. Kinases Phosphatases 2024, 2, 136–150. [Google Scholar] [CrossRef]
- To, N.B.; Truong, V.N.; Ediriweera, M.K.; Cho, S.K. Effects of Combined Pentadecanoic Acid and Tamoxifen Treatment on Tamoxifen Resistance in MCF-7/SC Breast Cancer Cells. Int. J. Mol. Sci. 2022, 23, 1340. [Google Scholar] [CrossRef]
- Sampsell, K.; Wang, W.; Ohland, C.; Mager, L.F.; Pett, N.; Lowry, D.E.; Sales, K.M.; McNeely, M.L.; McCoy, K.D.; Culos-Reed, S.N. Exercise and prebiotic fiber provide gut microbiota-driven benefit in a survivor to germ-free mouse translational model of breast cancer. Cancers 2022, 14, 2722. [Google Scholar] [CrossRef]
- Sun, J.; Chen, S.; Zang, D.; Sun, H.; Sun, Y.; Chen, J. Butyrate as a promising therapeutic target in cancer: From pathogenesis to clinic. Int. J. Oncol. 2024, 64, 44. [Google Scholar] [CrossRef]
- Dong, Y.; Zhang, K.; Wei, J.; Ding, Y.; Wang, X.; Hou, H.; Wu, J.; Liu, T.; Wang, B.; Cao, H. Gut microbiota-derived short-chain fatty acids regulate gastrointestinal tumor immunity: A novel therapeutic strategy? Front. Immunol. 2023, 14, 1158200. [Google Scholar] [CrossRef]
- Laborda-Illanes, A.; Sanchez-Alcoholado, L.; Dominguez-Recio, M.E.; Jimenez-Rodriguez, B.; Lavado, R.; Comino-Méndez, I.; Alba, E.; Queipo-Ortuño, M.I. Breast and gut microbiota action mechanisms in breast cancer pathogenesis and treatment. Cancers 2020, 12, 2465. [Google Scholar] [CrossRef]
- Plovier, H.; Everard, A.; Druart, C.; Depommier, C.; Van Hul, M.; Geurts, L.; Matamoros, S.; Delzenne, N.M.; de Vos, W.M.; Cani, P.D. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat. Med. 2017, 23, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Miquel, S.; Martín, R.; Rossi, O.; Bermúdez-Humarán, L.G.; Chatel, J.M.; Sokol, H.; Thomas, M.; Wells, J.M.; Langella, P. Faecalibacterium prausnitzii and human intestinal health. Curr. Opin. Microbiol. 2013, 16, 255–261. [Google Scholar] [CrossRef]
- Round, J.L.; Mazmanian, S.K. The gut microbiota shapes intestinal immune responses during health and disease. Nat. Rev. Immunol. 2009, 9, 313–323. [Google Scholar] [CrossRef]
- Tamanai-Shacoori, Z.; Smida, I.; Bousarghin, L.; Rodriguez, C.; Fong, S.B.; Grandjean, T.; Del Carmen, S.; Bonnaure-Mallet, M. Roseburia spp.: A marker of health? Future Microbiol. 2017, 12, 157–170. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, X.; Sun, Y.; Hu, B.; Xiao, Y.; Chen, B.; Wang, M.; Li, L.; Wang, H. Probiotic Clostridium butyricum alleviates intestinal inflammation in mice through crosstalk between TLR2 and TLR9 signaling pathways. Front. Microbiol. 2019, 10, 964. [Google Scholar] [CrossRef]
- Pederzoli, F.; Riba, M.; Venegoni, C.; Marandino, L.; Bandini, M.; Alchera, E.; Locatelli, I.; Raggi, D.; Giannatempo, P.; Provero, P.; et al. Stool Microbiome Signature Associated with Response to Neoadjuvant Pembrolizumab in Patients with Muscle-invasive Bladder Cancer. Eur. Urol. 2024, 85, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Roelands, J.; Kuppen, P.J.K.; Ahmed, E.I.; Mall, R.; Masoodi, T.; Singh, P.; Monaco, G.; Raynaud, C.; de Miranda, N.; Ferraro, L.; et al. An integrated tumor, immune and microbiome atlas of colon cancer. Nat. Med. 2023, 29, 1273–1286. [Google Scholar] [CrossRef]
- Ryu, S.W.; Kim, J.S.; Oh, B.S.; Choi, W.J.; Yu, S.Y.; Bak, J.E.; Park, S.H.; Kang, S.W.; Lee, J.; Jung, W.Y.; et al. Gut Microbiota Eubacterium callanderi Exerts Anti-Colorectal Cancer Activity. Microbiol. Spectr. 2022, 10, e0253122. [Google Scholar] [CrossRef]
- Sungkanuparph, S.; Chansirikarnjana, S.; Vorachit, M. Eubacterium bacteremia and colon cancer. Scand. J. Infect. Dis. 2002, 34, 941–943. [Google Scholar] [CrossRef]
- Akbaba, M.; Gökmen, G.G.; Kışla, D.; Nalbantsoy, A. In Vivo Investigation of Supportive Immunotherapeutic Combination of Bifidobacterium infantis 35624 and Doxorubicin in Murine Breast Cancer. Probiotics Antimicrob. Proteins 2023, 15, 880–888. [Google Scholar] [CrossRef]
- Kitagawa, K.; Oda, T.; Saito, H.; Araki, A.; Gonoi, R.; Shigemura, K.; Hashii, Y.; Katayama, T.; Fujisawa, M.; Shirakawa, T. Development of oral cancer vaccine using recombinant Bifidobacterium displaying Wilms’ tumor 1 protein. Cancer Immunol. Immunother. 2017, 66, 787–798. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Hu, W.; Liu, W.X.; Zhao, L.Y.; Huang, D.; Liu, X.D.; Chan, H.; Zhang, Y.; Zeng, J.D.; Coker, O.O.; et al. Streptococcus thermophilus Inhibits Colorectal Tumorigenesis Through Secreting β-Galactosidase. Gastroenterology 2021, 160, 1179–1193.e14. [Google Scholar] [CrossRef]
- Qian, Y.; Kang, Z.; Zhao, L.; Chen, H.; Zhou, C.; Gao, Q.; Wang, Z.; Liu, Q.; Cui, Y.; Li, X.; et al. Berberine might block colorectal carcinogenesis by inhibiting the regulation of B-cell function by Veillonella parvula. Chin. Med. J. (Engl.) 2023, 136, 2722–2731. [Google Scholar] [CrossRef]
- Sędzikowska, A.; Szablewski, L. Human Gut Microbiota in Health and Selected Cancers. Int. J. Mol. Sci. 2021, 22, 13440. [Google Scholar] [CrossRef] [PubMed]
- Notting, F.; Pirovano, W.; Sybesma, W.; Kort, R. The butyrate-producing and spore-forming bacterial genus Coprococcus as a potential biomarker for neurological disorders. Gut Microbiome 2023, 4, e16. [Google Scholar] [CrossRef]
- Wegmann, U.; Nueno Palop, C.; Mayer, M.J.; Crost, E.; Narbad, A. Complete Genome Sequence of Desulfovibrio piger FI11049. Genome Announc. 2017, 5, e01528-16. [Google Scholar] [CrossRef]
- Ma, Y.; Hébert, J.R.; Li, W.; Bertone-Johnson, E.R.; Olendzki, B.; Pagoto, S.L.; Tinker, L.; Rosal, M.C.; Ockene, I.S.; Ockene, J.K. Association between dietary fiber and markers of systemic inflammation in the Women’s Health Initiative Observational Study. Nutrition 2008, 24, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Shukla, S.; Sinha, S.; Meeran, S.M. Role of adipokines and cytokines in obesity-associated breast cancer: Therapeutic targets. Cytokine Growth Factor Rev. 2013, 24, 503–513. [Google Scholar] [CrossRef]
- Tajik, N.; Keshavarz, S.; Masoudkabir, F.; Djalali, M.; Sadrzadeh-Yeganeh, H.H.; Eshraghian, M.; Chamary, M.; Ahmadivand, Z.; Yazdani, T.; Javanbakht, M. Effect of diet-induced weight loss on inflammatory cytokines in obese women. J. Endocrinol. Investig. 2013, 36, 211–215. [Google Scholar]
- Wang, H.-X.; Wang, Y.-P. Gut microbiota-brain axis. Chin. Med. J. 2016, 129, 2373–2380. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, Y.F.; Miao, J.; Zheng, R.F.; Li, J.Y. Short-chain fatty acids: Important components of the gut-brain axis against AD. Biomed. Pharmacother. 2024, 175, 116601. [Google Scholar] [CrossRef]
- Hays, K.E.; Pfaffinger, J.M.; Ryznar, R. The interplay between gut microbiota, short-chain fatty acids, and implications for host health and disease. Gut Microbes 2024, 16, 2393270. [Google Scholar] [CrossRef]
- Senarath, R.M.U.S.; Burton, M.; Fernando, W.M.K.T.; Jayasena, V.; Brennan, C.; Fernando, W.M.A.D.B. Role of short chain fatty acids on astrocytes and microglia in Alzheimer’s disease brain. Int. J. Food Sci. Technol. 2024, 59, 5902–5911. [Google Scholar] [CrossRef]
- Ortlek, B.E.; Akan, O.B. Modeling and Analysis of SCFA-Driven Vagus Nerve Signaling in the Gut-Brain Axis via Molecular Communication. arXiv 2024, arXiv:2412.19945. [Google Scholar]
- Hsu, C.-Y.; Khachatryan, L.G.; Younis, N.K.; Mustafa, M.A.; Ahmad, N.; Athab, Z.H.; Polyanskaya, A.V.; Kasanave, E.V.; Mirzaei, R.; Karampoor, S. Microbiota-derived short chain fatty acids in pediatric health and diseases: From gut development to neuroprotection. Front. Microbiol. 2024, 15, 1456793. [Google Scholar] [CrossRef]
- Gu, X.; Fan, M.; Zhou, Y.; Zhang, Y.; Wang, L.; Gao, W.; Li, T.; Wang, H.; Si, N.; Wei, X. Intestinal endogenous metabolites affect neuroinflammation in 5× FAD mice by mediating “gut-brain” axis and the intervention with Chinese Medicine. Alzheimer’s Res. Ther. 2024, 16, 222. [Google Scholar] [CrossRef] [PubMed]
- Siva Venkatesh, I.P.; Majumdar, A.; Basu, A. Prophylactic administration of gut microbiome metabolites abrogated microglial activation and subsequent neuroinflammation in an experimental model of Japanese encephalitis. ACS Chem. Neurosci. 2024, 15, 1712–1727. [Google Scholar] [CrossRef]
- Kishimoto, Y.; Zhu, W.; Hosoda, W.; Sen, J.M.; Mattson, M.P. Chronic mild gut inflammation accelerates brain neuropathology and motor dysfunction in α-synuclein mutant mice. Neuromolecular Med. 2019, 21, 239–249. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Wang, Z.-J.; Moscatello, A.; Kingsbury, C.; Cozene, B.; Farooq, J.; Saft, M.; Sadanandan, N.; Gonzales-Portillo, B.; Zhang, H. Inflammatory gut as a pathologic and therapeutic target in Parkinson’s disease. Cell Death Discov. 2022, 8, 396. [Google Scholar] [CrossRef]
- Abdel-Haq, R.; Schlachetzki, J.C.; Boktor, J.C.; Cantu-Jungles, T.M.; Thron, T.; Zhang, M.; Bostick, J.W.; Khazaei, T.; Chilakala, S.; Morais, L.H. A prebiotic diet modulates microglial states and motor deficits in α-synuclein overexpressing mice. Elife 2022, 11, e81453. [Google Scholar] [CrossRef]
- Stephenson, E.; Mclaughlin, M.; Bray, J.W.; Saxton, J.M.; Vince, R.V. Nutrition Modulation of Cardiotoxicity in Breast Cancer: A Scoping Review. Nutrients 2024, 16, 3777. [Google Scholar] [CrossRef] [PubMed]
- Becerril-Alarcón, Y.; Campos-Gómez, S.; Valdez-Andrade, J.J.; Campos-Gómez, K.A.; Reyes-Barretero, D.Y.; Benitez-Arciniega, A.D.; Valdés-Ramos, R.; Soto-Piña, A.E. Inulin supplementation reduces systolic blood pressure in women with breast cancer undergoing neoadjuvant chemotherapy. Cardiovasc. Ther. 2019, 2019, 5707150. [Google Scholar] [CrossRef]
- Onyszkiewicz, M.; Gawrys-Kopczynska, M.; Konopelski, P.; Aleksandrowicz, M.; Sawicka, A.; Koźniewska, E.; Samborowska, E.; Ufnal, M. Butyric acid, a gut bacteria metabolite, lowers arterial blood pressure via colon-vagus nerve signaling and GPR41/43 receptors. Pflügers Arch.-Eur. J. Physiol. 2019, 471, 1441–1453. [Google Scholar] [CrossRef] [PubMed]
- Muralitharan, R.R.; Zheng, T.; Dinakis, E.; Xie, L.; Barbaro-Wahl, A.; Jama, H.A.; Nakai, M.; Patterson, M.; Johnson, C.; Salimova, E. GPR41/43 regulates blood pressure by improving gut epithelial barrier integrity to prevent TLR4 activation and renal inflammation. bioRxiv 2023. [Google Scholar] [CrossRef]
- Hsu, C.-N.; Yu, H.-R.; Lin, I.-C.; Tiao, M.-M.; Huang, L.-T.; Hou, C.-Y.; Chang-Chien, G.-P.; Lin, S.; Tain, Y.-L. Sodium butyrate modulates blood pressure and gut microbiota in maternal tryptophan-free diet-induced hypertension rat offspring. J. Nutr. Biochem. 2022, 108, 109090. [Google Scholar] [CrossRef]
- Robles-Vera, I.; Toral, M.; de la Visitación, N.; Aguilera-Sánchez, N.; Redondo, J.M.; Duarte, J. Protective effects of short-chain fatty acids on endothelial dysfunction induced by angiotensin II. Front. Physiol. 2020, 11, 277. [Google Scholar] [CrossRef]
- German, R.; Marino, N.; Hemmerich, C.; Podicheti, R.; Rusch, D.B.; Stiemsma, L.T.; Gao, H.; Xuei, X.; Rockey, P.; Storniolo, A.M. Exploring breast tissue microbial composition and the association with breast cancer risk factors. Breast Cancer Res. 2023, 25, 82. [Google Scholar] [CrossRef] [PubMed]
- Tzeng, A.; Sangwan, N.; Jia, M.; Liu, C.-C.; Keslar, K.S.; Downs-Kelly, E.; Fairchild, R.L.; Al-Hilli, Z.; Grobmyer, S.R.; Eng, C. Human breast microbiome correlates with prognostic features and immunological signatures in breast cancer. Genome Med. 2021, 13, 60. [Google Scholar] [CrossRef]
- Terrisse, S.; Derosa, L.; Iebba, V.; Ghiringhelli, F.; Vaz-Luis, I.; Kroemer, G.; Fidelle, M.; Christodoulidis, S.; Segata, N.; Thomas, A.M. Intestinal microbiota influences clinical outcome and side effects of early breast cancer treatment. Cell Death Differ. 2021, 28, 2778–2796. [Google Scholar] [CrossRef]
- Aarnoutse, R.; Ziemons, J.; Hillege, L.E.; de Vos-Geelen, J.; de Boer, M.; Bisschop, S.M.; Vriens, B.E.; Vincent, J.; van de Wouw, A.J.; Le, G.N. Changes in intestinal microbiota in postmenopausal oestrogen receptor-positive breast cancer patients treated with (neo) adjuvant chemotherapy. NPJ Breast Cancer 2022, 8, 89. [Google Scholar] [CrossRef]
- Calderón-Pérez, L.; Gosalbes, M.J.; Yuste, S.; Valls, R.M.; Pedret, A.; Llauradó, E.; Jimenez-Hernandez, N.; Artacho, A.; Pla-Pagà, L.; Companys, J. Gut metagenomic and short chain fatty acids signature in hypertension: A cross-sectional study. Sci. Rep. 2020, 10, 6436. [Google Scholar] [CrossRef] [PubMed]
- Fu, A.; Yao, B.; Dong, T.; Chen, Y.; Yao, J.; Liu, Y.; Li, H.; Bai, H.; Liu, X.; Zhang, Y. Tumor-resident intracellular microbiota promotes metastatic colonization in breast cancer. Cell 2022, 185, 1356–1372.e26. [Google Scholar] [CrossRef]
- Soto-Pantoja, D.R.; Gaber, M.; Arnone, A.A.; Bronson, S.M.; Cruz-Diaz, N.; Wilson, A.S.; Clear, K.Y.; Ramirez, M.U.; Kucera, G.L.; Levine, E.A. Diet alters entero-mammary signaling to regulate the breast microbiome and tumorigenesis. Cancer Res. 2021, 81, 3890–3904. [Google Scholar] [CrossRef] [PubMed]
- Park, E.M.; Chelvanambi, M.; Bhutiani, N.; Kroemer, G.; Zitvogel, L.; Wargo, J.A. Targeting the gut and tumor microbiota in cancer. Nat. Med. 2022, 28, 690–703. [Google Scholar] [CrossRef]
- Bernardo, G.; Le Noci, V.; Di Modica, M.; Montanari, E.; Triulzi, T.; Pupa, S.M.; Tagliabue, E.; Sommariva, M.; Sfondrini, L. The emerging role of the microbiota in breast cancer progression. Cells 2023, 12, 1945. [Google Scholar] [CrossRef] [PubMed]
- Karami, P.; Goli, H.R.; Abediankenari, S.; Chandani, S.R.; Jafari, N.; Ghasemi, M.; Ahanjan, M. Anti-tumor effects of Bacteroides fragilis and Bifidobacterium bifidum culture supernatants on mouse breast cancer. Gene Rep. 2023, 33, 101815. [Google Scholar] [CrossRef]
- Adumuah, N.N.; Quarshie, J.T.; Danwonno, H.; Aikins, A.R.; Ametefe, E.N. Exploring Anti-Breast Cancer Effects of Live Pediococcus acidilactici and Its Cell-Free Supernatant Isolated from Human Breast Milk. Int. J. Breast Cancer 2024, 2024, 1841909. [Google Scholar] [CrossRef]
- Kaluza, J.; Komatsu, S.; Lauriola, M.; Harris, H.R.; Bergkvist, L.; Michaëlsson, K.; Wolk, A. Long-term consumption of non-fermented and fermented dairy products and risk of breast cancer by estrogen receptor status–Population-based prospective cohort study. Clin. Nutr. 2021, 40, 1966–1973. [Google Scholar] [CrossRef]
- Wang, M.Y.; Sang, L.X.; Sun, S.Y. Gut microbiota and female health. World J. Gastroenterol. 2024, 30, 1655–1662. [Google Scholar] [CrossRef]
- An, J.; Moon, B. 85P Microbiome analysis in patients with breast cancer via an oral prebiotics therapy. ESMO Open 2023, 8, 101308. [Google Scholar] [CrossRef]
- Smith, J.; Johnson, R. The effects of exercise on gut microbiota in breast cancer survivors and its impact on tumor progression. J. Cancer Immunol. 2023, 45, 122–130. [Google Scholar]
- Johnson, R.; Martinez, P. Prebiotic supplementation and exercise in breast cancer survivors: A synergistic approach. Front. Nutr. 2021, 7, 1085–1095. [Google Scholar]
- Taylor, D.; Lee, M. Exercise modulates gut microbiota and immune responses in breast cancer survivors: A 12-week intervention study. J. Cancer Res. 2022, 34, 345–355. [Google Scholar]
- Martinez, P.; Lee, M. Synergistic effects of prebiotics and exercise on improving immune responses and gut microbiota in breast cancer patients. Oncol. Rep. 2023, 42, 230–240. [Google Scholar]
- Lee, M.; Taylor, D. Integrating exercise and prebiotics in breast cancer care: Potential for improving clinical outcomes. Clin. Cancer Res. 2024, 30, 510–518. [Google Scholar]
- Li, H.; Dong, T.; Tao, M.; Zhao, H.; Lan, T.; Yan, S.; Gong, X.; Hou, Q.; Ma, X.; Song, Y. Fucoidan enhances the anti-tumor effect of anti-PD-1 immunotherapy by regulating gut microbiota. Food Funct. 2024, 15, 3463–3478. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, S.; Liu, Y. Fucoidan enhances anti-PD-1 immunotherapy efficacy through modulation of gut microbiota and immune responses. J. Cancer Immunol. 2024, 45, 221–230. [Google Scholar]
- Zhao, X.; Wang, J.; Liu, X. Immune-modulatory effects of fucoidan: Enhancing dendritic cell function and macrophage activation in cancer immunotherapy. Int. J. Immunol. 2023, 38, 800–810. [Google Scholar]
- Xu, F.; Li, Q.; Zhang, T. Fucoidan as an immune booster in cancer therapy: Modulation of cytokine production and immune cell proliferation. Cell. Immunol. Rep. 2022, 29, 350–360. [Google Scholar]
- Wang, Y.; Zhang, W.; Xu, Z. Combination therapy of fucoidan and anti-PD-1 monoclonal antibodies enhances immune responses against cancer. Cancer Immunother. Rev. 2023, 58, 111–120. [Google Scholar]
- Zhang, M.; Zhang, H.; Xu, Y. Fucoidan-induced modulation of the gut microbiome enhances the efficacy of anti-PD-1 immunotherapy in murine models of cancer. J. Cancer Res. Ther. 2021, 40, 845–856. [Google Scholar]
- Kumar, A.; Singh, M.; Reddy, S. The role of short-chain fatty acids in immunotherapy resistance and tumor progression. Oncol. Rep. 2022, 33, 201–210. [Google Scholar]
- Yang, J.; Li, Y.; Zhou, Y. Fucoidan modulates tumor metabolism and microbiome diversity to enhance anti-tumor immunity. Front. Immunol. 2023, 14, 1023–1034. [Google Scholar]
- Han, B.; Jiang, P.; Jiang, L.; Li, X.; Ye, X. Three phytosterols from sweet potato inhibit MCF7-xenograft-tumor growth through modulating gut microbiota homeostasis and SCFAs secretion. Food Res. Int. 2021, 141, 110147. [Google Scholar] [CrossRef]
- Su, J.; Chen, X.; Xiao, Y.; Li, D.; Li, M.; Li, H.; Huang, J.; Lai, Z.; Su, Z.; Xie, Y. Bruceae fructus oil inhibits triple-negative breast cancer by restraining autophagy: Dependence on the gut microbiota-mediated amino acid regulation. Front. Pharmacol. 2021, 12, 727082. [Google Scholar] [CrossRef]
- Kim, J.-K.; Choi, M.S.; Kim, J.-Y.; Yu, J.S.; Seo, J.I.; Yoo, H.H.; Kim, D.-H. Ginkgo biloba leaf extract suppresses intestinal human breast cancer resistance protein expression in mice: Correlation with gut microbiota. Biomed. Pharmacother. 2021, 140, 111712. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Fan, L. The effect of Poria cocos ethanol extract on the intestinal barrier function and intestinal microbiota in mice with breast cancer. J. Ethnopharmacol. 2021, 266, 113456. [Google Scholar] [CrossRef]
- Chen, Q.; Li, Q.; Liang, Y.; Zu, M.; Chen, N.; Canup, B.S.; Luo, L.; Wang, C.; Zeng, L.; Xiao, B. Natural exosome-like nanovesicles from edible tea flowers suppress metastatic breast cancer via ROS generation and microbiota modulation. Acta Pharm. Sin. B 2022, 12, 907–923. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, X. Butyrate and propionate downregulate estrogen receptor alpha expression and mimic histone deacetylase inhibitor effects in breast cancer cells. J. Nutr. Biochem. 2023, 98, 108789. [Google Scholar]
- Li, X.; Zhang, Y. Sodium butyrate inhibits MCF-7 breast cancer cell proliferation and induces apoptosis more effectively than sodium propionate. Breast Cancer Res. Treat. 2022, 191, 345–357. [Google Scholar]
- Smith, R.; Patel, S. Epigenetic modulation by short-chain fatty acids: Implications for cancer therapy. Front. Oncol. 2021, 11, 654321. [Google Scholar]
- Jones, D.; Wang, L. Free fatty acid receptors as targets for SCFA-mediated regulation of breast cancer cell behavior. J. Lipid Res. 2020, 61, 1234–1245. [Google Scholar]
- Wang, L.; Zhang, H. Short-chain fatty acids enhance anti-tumor immunity by modulating immune cell function in breast cancer. Front. Immunol. 2021, 12, 654321. [Google Scholar]
- Li, Y.; Zhang, T. Sodium butyrate in cancer therapy: Epigenetic modulation through HDAC inhibition. Mol. Med. Rep. 2023, 27, 351–358. [Google Scholar]
- Kumar, S.; Sharma, M. Genistein as an epigenetic modulator in breast cancer therapy. Cancer Epigenetics J. 2022, 19, 402–410. [Google Scholar]
- Sharma, M.; Tollefsbol, T.O. Combinatorial epigenetic mechanisms of sulforaphane, genistein, and sodium butyrate in breast cancer inhibition. Exp. Cell Res. 2024, 416, 113160. [Google Scholar] [CrossRef] [PubMed]
- Khazaei, Y.; Basi, A.; Fernandez, M.L.; Foudazi, H.; Bagherzadeh, R.; Shidfar, F. The effects of synbiotics supplementation on reducing chemotherapy-induced side effects in women with breast cancer: A randomized placebo-controlled double-blind clinical trial. BMC Complement. Med. Ther. 2023, 23, 339. [Google Scholar] [CrossRef]
- Thu, M.S.; Ondee, T.; Nopsopon, T.; Farzana, I.A.; Fothergill, J.L.; Hirankarn, N.; Campbell, B.J.; Pongpirul, K. Effect of probiotics in breast cancer: A systematic review and meta-analysis. Biology 2023, 12, 280. [Google Scholar] [CrossRef]
- Vafa, S.; Zarrati, M.; Malakootinejad, M.; Totmaj, A.S.; Zayeri, F.; Salehi, M.; Sanati, V.; Haghighat, S. Calorie restriction and synbiotics effect on quality of life and edema reduction in breast cancer-related lymphedema, a clinical trial. Breast 2020, 54, 37–45. [Google Scholar] [CrossRef]

| Microbial/Metabolite Change | Immune Effects | Relevance to Breast Cancer | Refs. |
|---|---|---|---|
| ↑ Bifidobacterium and Lactobacillus | Strengthen the gut barrier, decrease systemic lipopolysaccharides, increase the IL-10 production, and enhance the dendritic cell function. | Reduced chronic inflammation and improved antitumor T cell priming. | [98,99] |
| ↑ Butyrate-producing bacteria (Faecalibacterium) | ↑ Butyrate levels: HDAC inhibition in T cells and macrophages; promote Treg differentiation and IL-10; suppress M2 macrophages; activate NK cells. | Anti-inflammatory milieu (less NF-κB activity) potentiates innate tumor killing and could limit metastasis. | [98,99] |
| ↑ Propionate and acetate | Via GPR43 on neutrophils and GPR41 on monocytes, modulate chemotaxis and phagocytosis; propionate also inhibits HDAC to reduce Th17 cytokines. | Controlled inflammatory responses (prevent excessive Th17, which can aid tumor angiogenesis); maintain immune surveillance by neutrophils. | [100] |
| ↓ Clostridia and E. coli (high GUS producers) | Lower the microbial antigen load that drives inflammation; possibly reduce TLR activation systemically. | Decreased protumor inflammation (e.g., less IL-6, which can stimulate cancer cell proliferation) may reduce TLR4-mediated tumor growth signals. | [101] |
| ↑ Akkermansia muciniphila | Enhances TLR2 signaling, strengthens the gut barrier, and increases anti-inflammatory cytokines such as IL-10. | Promotes an anti-inflammatory environment and may inhibit tumor progression. | [102] |
| ↑ Faecalibacterium prausnitzii | Produces butyrate, induces Treg differentiation, and suppresses pro-inflammatory cytokines. | Anti-inflammatory milieu; potential reduction in tumor-promoting inflammation. | [103] |
| ↑ Bacteroides fragilis | Polysaccharide A induces IL-10 production and enhances the regulatory T cell function. | Modulates immune response and may reduce inflammation-associated tumorigenesis. | [104] |
| ↑ Roseburia spp. | Butyrate production enhances mucosal immunity and induces Treg cells. | Supports gut integrity; potential protective role against cancer. | [105] |
| ↑ Clostridium butyricum | Butyrate production promotes Treg cells and inhibits pro-inflammatory cytokines. | Anti-inflammatory effects may suppress tumor growth. | [106] |
| ↑ Ruminococcus bromii | Resistant starch degradation; butyrate production; supports the gut barrier. | Enhances gut health; potential indirect effects on tumor suppression. | [107,108] |
| ↑ Eubacterium sp. | Produces butyrate and propionate and modulates immune responses. | SCFA-mediated anti-inflammatory effects; possible tumor suppression. | [109,110] |
| ↑ Bifidobacterium longum | Enhances dendritic cell maturation, increases IFN-γ production, and supports NK cell activity. | Boosts antitumor immunity; potential to enhance immunotherapy efficacy. | [111,112] |
| ↑ Streptococcus thermophilus | Produces lactate and modulates macrophage polarization towards the M1 phenotype. | Promotes antitumor immune responses and may inhibit tumor growth. | [113] |
| ↑ Veillonella parvula | Lactate fermentation to propionate modulates T cell responses. | SCFA-mediated immune modulation; potential anticancer effects. | [114] |
| ↑ Anaerostipes caccae | Butyrate production enhances Treg cell differentiation and suppresses inflammation. | Supports an anti-inflammatory environment and may reduce cancer risk. | [115] |
| ↑ Coprococcus comes | Produces butyrate, modulates the gut barrier function, and influences immune homeostasis. | Maintains gut integrity; potential protective role against tumorigenesis. | [116] |
| ↑ Desulfovibrio piger | Produces hydrogen sulfide, modulates inflammatory responses, and impacts the gut barrier. | Complex role: excessive hydrogen sulfide may promote inflammation, while balanced levels could support gut health. | [117] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabit, H.; Abouelnour, S.; Hassen, B.M.; Magdy, S.; Yasser, A.; Wadan, A.-H.S.; Abdel-Ghany, S.; Radwan, F.; Alqosaibi, A.I.; Hafiz, H.; et al. Anticancer Potential of Prebiotics: Targeting Estrogen Receptors and PI3K/AKT/mTOR in Breast Cancer. Biomedicines 2025, 13, 990. https://doi.org/10.3390/biomedicines13040990
Sabit H, Abouelnour S, Hassen BM, Magdy S, Yasser A, Wadan A-HS, Abdel-Ghany S, Radwan F, Alqosaibi AI, Hafiz H, et al. Anticancer Potential of Prebiotics: Targeting Estrogen Receptors and PI3K/AKT/mTOR in Breast Cancer. Biomedicines. 2025; 13(4):990. https://doi.org/10.3390/biomedicines13040990
Chicago/Turabian StyleSabit, Hussein, Sama Abouelnour, Bassel M. Hassen, Salma Magdy, Ahmed Yasser, Al-Hassan Soliman Wadan, Shaimaa Abdel-Ghany, Faisal Radwan, Amany I. Alqosaibi, Hala Hafiz, and et al. 2025. "Anticancer Potential of Prebiotics: Targeting Estrogen Receptors and PI3K/AKT/mTOR in Breast Cancer" Biomedicines 13, no. 4: 990. https://doi.org/10.3390/biomedicines13040990
APA StyleSabit, H., Abouelnour, S., Hassen, B. M., Magdy, S., Yasser, A., Wadan, A.-H. S., Abdel-Ghany, S., Radwan, F., Alqosaibi, A. I., Hafiz, H., Awlya, O. F. A., & Arneth, B. (2025). Anticancer Potential of Prebiotics: Targeting Estrogen Receptors and PI3K/AKT/mTOR in Breast Cancer. Biomedicines, 13(4), 990. https://doi.org/10.3390/biomedicines13040990









