Management of Acquired Hypothalamic Obesity After Childhood-Onset Craniopharyngioma—A Narrative Review
Abstract
1. Introduction
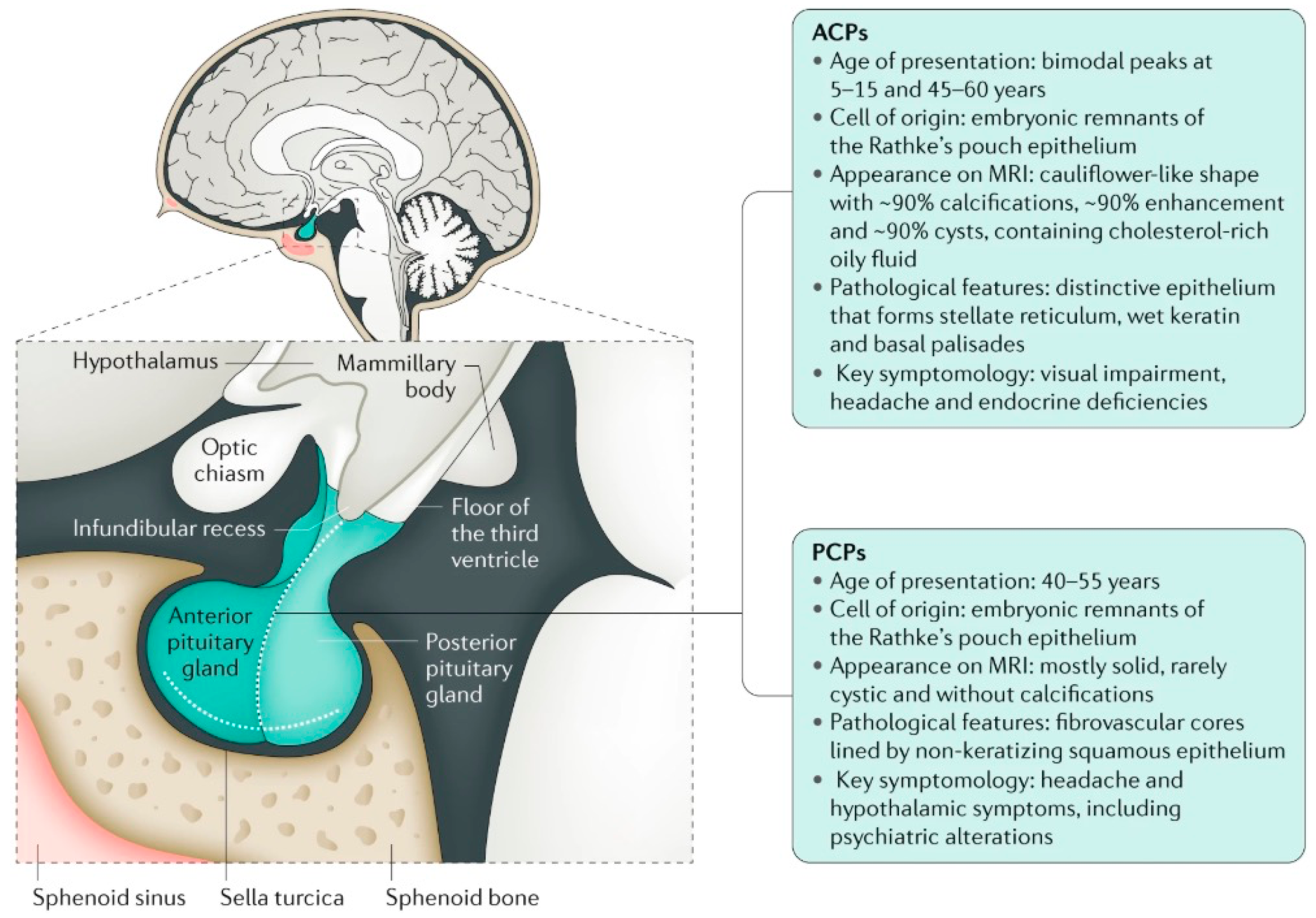
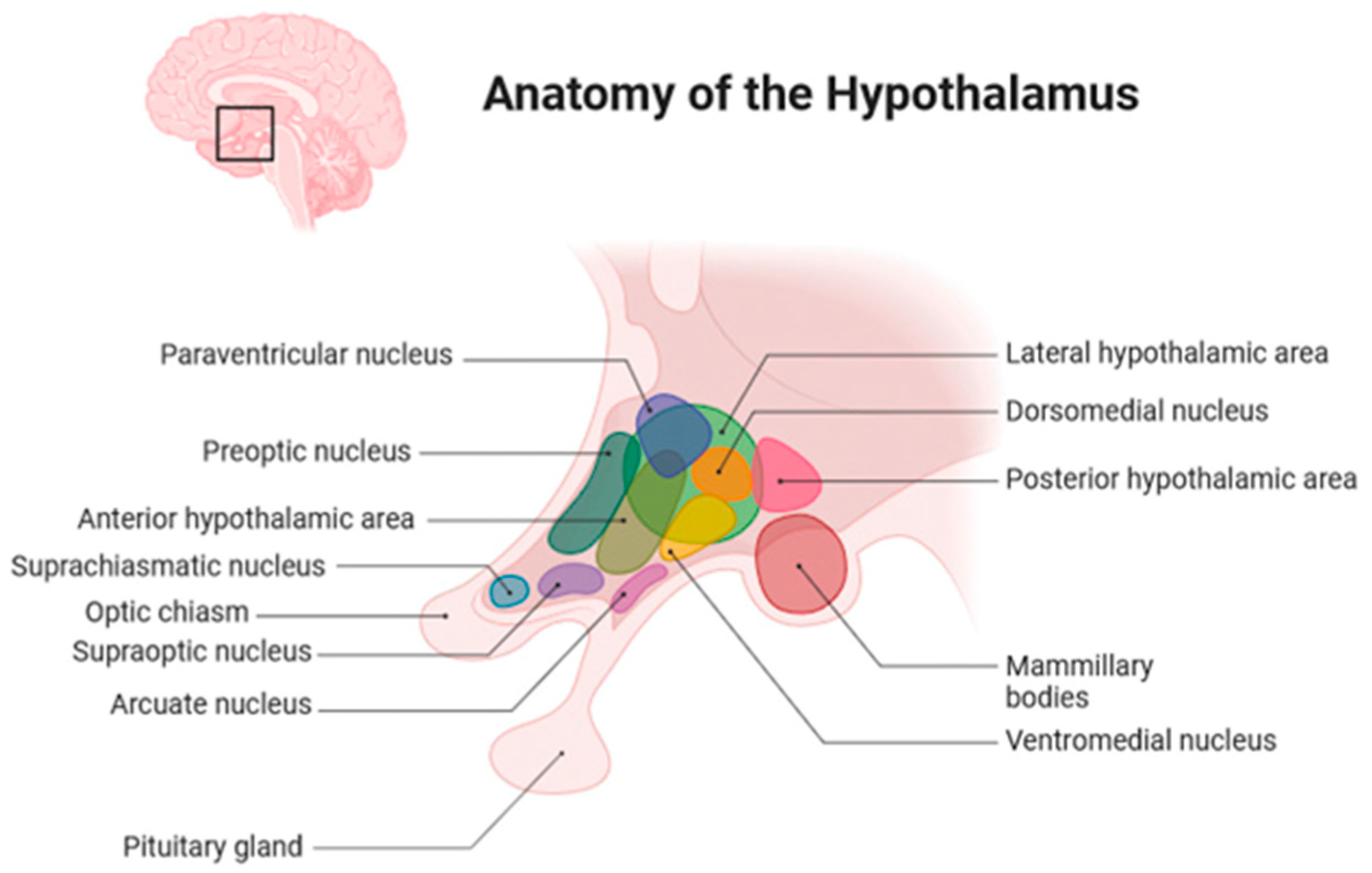
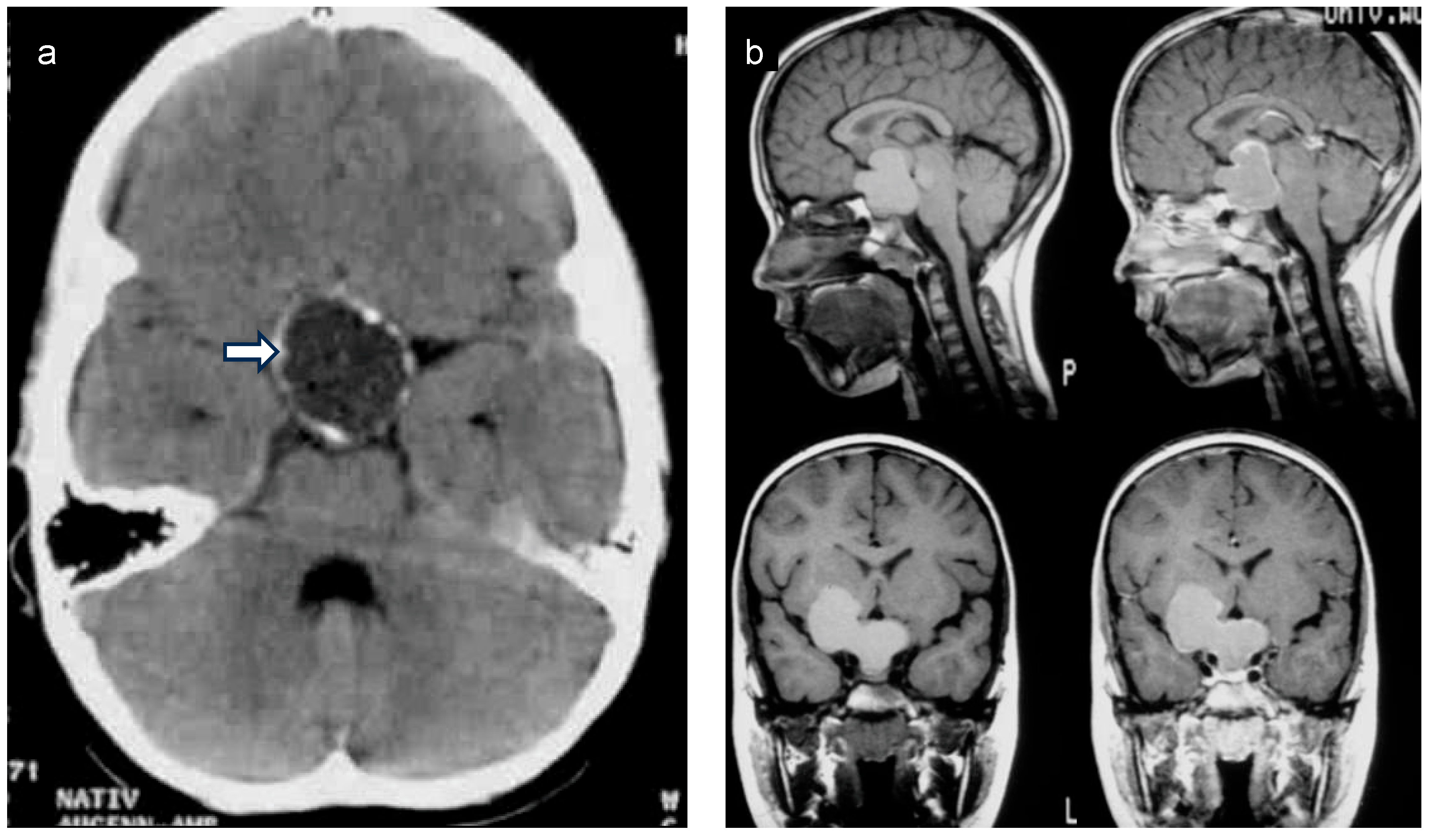
2. Anatomical Location of Childhood-Onset Craniopharyngioma Close to the Hypothalamus
3. Diagnosis, Treatment, and Follow-Up Care of Patients with Childhood-Onset Craniopharyngioma
4. Survival After Childhood-Onset Craniopharyngioma
5. Hypothalamic Syndrome
- Behavioral disorders include compulsive symptoms, obsessive behavior, rage, and hoarding [39].
- Dysregulation of circadian rhythms and sleep issues include sleep apnea, hypersomnia, increased daytime sleepiness, insomnia, and fatigue [37]. Fatigue is a multidimensional symptom present if exhaustion or tiredness cannot be related to a certain activity [40]. Patients with hypothalamic syndrome might report fatigue symptoms; however, fatigue is also a recognized late effect after other pediatric cancers and can be present in patients without sleep disorders (e.g., sleep apnea and narcolepsy) [41]. Accordingly, it can also be associated with CP treatment (e.g., irradiation).
- Disorders of pubertal development such as central precocious puberty or delayed pubertal development and deficiencies of growth hormone, TSH, ACTH, and LH/FSH are endocrine dysfunctions described for hypothalamic syndrome [37]. Arginine vasopressin deficiency (AVD, formerly central diabetes insipidus) with or without adequate thirst feeling can be included [37].
6. Pharmacological Treatment of Patients with Acquired Hypothalamic Obesity
7. Bariatric Surgery
8. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACTH | Adrenocorticotropic hormone |
| MSH | -melanocyte-stimulating hormone |
| BMI | Body mass index |
| CP | Craniopharyngioma |
| CT | Computed tomography |
| DBS | Deep brain stimulation |
| FSH | Follicle-stimulating hormone |
| GLP-1 | Glucagon-like peptide |
| LAGB | Laparoscopic adjusted gastric banding |
| LEPR | Leptin receptor |
| LH | Luteinizing hormone |
| MC4R | Melanocortin-4 receptor |
| MRI | Magnetic resonance imaging |
| POMC | Pro-opiomelanocortin |
| QOL | Quality of life |
| RCT | Randomized controlled trial |
| RYGB | Roux-en-Y gastric bypass |
| SDS | Standard deviation score |
| SG | Sleeve gastrectomy |
| TBI | Traumatic brain injury |
| TSH | Thyroid-stimulating hormone |
| TWL | Total weight loss |
References
- Santagata S., K.-D.B.; Komori, T.; Müller, H.L.; Pietsch, T. Adamantinomatous craniopharyngioma. In WHO Classification of Tumours Editorial Board; Brat, D.J.G.A., Wesseling, P., Eds.; Tumours of the Sellar Region; Central Nervous System Tumours; WHO Classification of Tumours Series; International Agency for Research on Cancer: Lyon, France, 2021; Volume 6, pp. 393–396. [Google Scholar]
- Sterkenburg, A.S.; Hoffmann, A.; Gebhardt, U.; Warmuth-Metz, M.; Daubenbuchel, A.M.; Muller, H.L. Survival, Hypothalamic Obesity, and Neuropsychological/Psychosocial Status after Childhood-Onset Craniopharyngioma: Newly Reported Long-Term Outcomes. Neuro Oncol. 2015, 17, 1029–1038. [Google Scholar] [CrossRef] [PubMed]
- Muller, H.L.; Gebhardt, U.; Teske, C.; Faldum, A.; Zwiener, I.; Warmuth-Metz, M.; Pietsch, T.; Pohl, F.; Sorensen, N.; Calaminus, G. Post-Operative Hypothalamic Lesions and Obesity in Childhood Craniopharyngioma: Results of the Multinational Prospective Trial Kraniopharyngeom 2000 after 3-year Follow-Up. Eur. J. Endocrinol. 2011, 165, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Müller, H.L.; Tauber, M.; Lawson, E.A.; Özyurt, J.; Bison, B.; Martinez-Barbera, J.-P.; Puget, S.; Merchant, T.E.; van Santen, H.M. Hypothalamic Syndrome. Nat. Rev. Dis. Primers 2022, 8, 24. [Google Scholar] [CrossRef] [PubMed]
- Müller, H.L.; Merchant, T.E.; Warmuth-Metz, M.; Martinez-Barbera, J.-P.; Puget, S. Craniopharyngioma. Nat. Rev. Dis. Primers 2019, 5, 75. [Google Scholar] [CrossRef]
- Beckhaus, J. Diagnostic Determinants and Long-Term Outcomes of Childhood-Onset Craniopharyngioma; Carl von Ossietzky Universität Oldenburg: Oldenburg, Germany, 2024. [Google Scholar]
- Popp, M.-G.; Crivii, C.B.; Opincariu, I. Introduction to the Hypothalamus: Correlated from Animal Studies. In The Human Hypothalamus, 1st ed.; Uwaifo, G.I., Ed.; Humana Cham: Cham, Switzerland, 2020; pp. 3–6. [Google Scholar] [CrossRef]
- Brown, C.H.; Bains, J.S.; Ludwig, M.; Stern, J.E. Physiological Regulation of Magnocellular neurosecretory CELL Activity: Integration of Intrinsic, Local and Afferent Mechanisms. J. Neuroendocrinol. 2013, 25, 678–710. [Google Scholar] [CrossRef]
- Pang, J.C.; Chung, D.D.; Wang, J.; Abiri, A.; Lien, B.V.; Himstead, A.S.; Ovakimyan, A.; Kim, M.G.; Hsu, F.P.K.; Kuan, E.C. Characteristics and Outcomes in Pediatric Versus Adult Craniopharyngiomas: A Systematic Review and Meta-Analysis. Neurosurgery 2023, 92, 1112–1129. [Google Scholar] [CrossRef]
- Muller, H.L.; Emser, A.; Faldum, A.; Bruhnken, G.; Etavard-Gorris, N.; Gebhardt, U.; Oeverink, R.; Kolb, R.; Sorensen, N. Longitudinal Study on Growth and Body Mass Index before and after Diagnosis of Childhood Craniopharyngioma. J. Clin. Endocrinol. Metab. 2004, 89, 3298–3305. [Google Scholar] [CrossRef]
- Hoffman, L.M.; Jaimes, C.; Mankad, K.; Mirsky, D.M.; Tamrazi, B.; Tinkle, C.L.; Kline, C.; Ramasubramanian, A.; Malbari, F.; Mangum, R.; et al. Response Assessment in Pediatric Craniopharyngioma: Recommendations from the Response Assessment in Pediatric Neuro-Oncology (Rapno) Working Group. Neuro Oncol. 2023, 25, 224–233. [Google Scholar] [CrossRef]
- Warmuth-Metz, M.; Gnekow, A.K.; Muller, H.; Solymosi, L. Differential Diagnosis of Suprasellar Tumors in Children. Klin. Padiatr. 2004, 216, 323–330. [Google Scholar] [CrossRef]
- Ji, C.; Cheng, H.; Zhou, X.; Cao, X.; Qiao, N.; Shi, C.; Zhang, Y.; Ye, Z.; Zhao, Y. Ectopic Recurrence Craniopharyngioma: Series Report and Literature Review. Chin. Neurosurg. J. 2023, 9, 13. [Google Scholar] [CrossRef]
- Puget, S.; Garnett, M.; Wray, A.; Grill, J.; Habrand, J.L.; Bodaert, N.; Zerah, M.; Bezerra, M.; Renier, D.; Pierre-Kahn, A.; et al. Pediatric Craniopharyngiomas: Classification and Treatment According to the Degree of Hypothalamic Involvement. J. Neurosurg. 2007, 106, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Muller, H.L.; Gebhardt, U.; Faldum, A.; Warmuth-Metz, M.; Pietsch, T.; Pohl, F.; Calaminus, G.; Sorensen, N. Xanthogranuloma, Rathke’s Cyst, and Childhood Craniopharyngioma: Results of Prospective Multinational Studies of Children and Adolescents with Rare Sellar Malformations. J. Clin. Endocrinol. Metab. 2012, 97, 3935–3943. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Barbera, J.P.; Andoniadou, C.L. Biological Behaviour of Craniopharyngiomas. Neuroendocrinology 2020, 110, 797–804. [Google Scholar] [CrossRef]
- Whelan, R.; Prince, E.; Gilani, A.; Hankinson, T. The Inflammatory Milieu of Adamantinomatous Craniopharyngioma and Its Implications for Treatment. J. Clin. Med. 2020, 9, 519. [Google Scholar] [CrossRef]
- Gabay, S.; Merchant, T.E.; Boop, F.A.; Roth, J.; Constantini, S. Shifting Strategies in the Treatment of Pediatric Craniopharyngioma. Curr. Oncol. Rep. 2023, 25, 1497–1513. [Google Scholar] [CrossRef]
- Friedrich, C.; Boekhoff, S.; Bischoff, M.; Beckhaus, J.; Sowithayasakul, P.; Calaminus, G.; Eveslage, M.; Valentini, C.; Bison, B.; Harrabi, S.B.; et al. Outcome after Proton Beam Therapy Versus Photon-Based Radiation Therapy in Childhood-Onset Craniopharyngioma Patients-Results of Kraniopharyngeom 2007. Front. Oncol. 2023, 13, 1180993. [Google Scholar] [CrossRef]
- Merchant, T.E.; Hoehn, M.E.; Khan, R.B.; Sabin, N.D.; Klimo, P.; Boop, F.A.; Wu, S.; Li, Y.; Burghen, E.A.; Jurbergs, N.; et al. Proton Therapy and Limited Surgery for Paediatric and Adolescent Patients with Craniopharyngioma (RT2CR): A Single-Arm, Phase 2 Study. Lancet Oncol. 2023, 24, 523–534. [Google Scholar] [CrossRef]
- Harat, M.; Rudas, M.; Zielinski, P.; Birska, J.; Sokal, P. Nucleus Accumbens Stimulation in Pathological Obesity. Neurol. Neurochir. Pol. 2016, 50, 207–210. [Google Scholar] [CrossRef]
- Dassen, A.R.; van Schaik, J.; van den Munckhof, P.; Schuurman, P.R.; Hoving, E.W.; van Santen, H.M. Could Deep Brain Stimulation Be a Possible Solution for Acquired Hypothalamic Obesity? Heliyon 2023, 9, e14411. [Google Scholar] [CrossRef]
- Nuijts, M.A.; Veldhuis, N.; Stegeman, I.; van Santen, H.M.; Porro, G.L.; Imhof, S.M.; Schouten-van Meeteren, A.Y.N. Visual Functions in Children with Craniopharyngioma at Diagnosis: A Systematic Review. PLoS ONE 2020, 15, e0240016. [Google Scholar] [CrossRef]
- Caldarelli, M.; Massimi, L.; Tamburrini, G.; Cappa, M.; Di Rocco, C. Long-Term Results of the Surgical Treatment of Craniopharyngioma: The Experience at the Policlinico Gemelli, Catholic University, Rome. Childs Nerv. Syst. 2005, 21, 747–757. [Google Scholar] [CrossRef] [PubMed]
- Elliott, R.E.; Jane, J.A., Jr.; Wisoff, J.H. Surgical Management of Craniopharyngiomas in Children: Meta-Analysis and Comparison of Transcranial and Transsphenoidal Approaches. Neurosurgery 2011, 69, 630–643; discussion 643. [Google Scholar] [CrossRef] [PubMed]
- Prieto, R.; Pascual, J.M.; Barrios, L. Optic Chiasm Distortions Caused by Craniopharyngiomas: Clinical and Magnetic Resonance Imaging Correlation and Influence on Visual Outcome. World Neurosurg. 2015, 83, 500–529. [Google Scholar] [CrossRef] [PubMed]
- Heymsfield, S.B.; Clement, K.; Dubern, B.; Goldstone, A.P.; Haqq, A.M.; Kuhnen, P.; Richards, J.; Roth, C.L.; Akker, E.; Wabitsch, M.; et al. Defining Hyperphagia for Improved Diagnosis and Management of Mc4r Pathway-Associated Disease: A Roundtable Summary. Curr. Obes. Rep. 2025, 14, 13. [Google Scholar] [CrossRef]
- Muller, H.L.; Muller-Stover, S.; Gebhardt, U.; Kolb, R.; Sorensen, N.; Handwerker, G. Secondary Narcolepsy May Be a Causative Factor of Increased Daytime Sleepiness in Obese Childhood Craniopharyngioma Patients. J. Pediatr. Endocrinol. Metab. 2006, 19 (Suppl. 1), 423–429. [Google Scholar] [CrossRef]
- Muller, H.L.; Handwerker, G.; Wollny, B.; Faldum, A.; Sorensen, N. Melatonin Secretion and Increased Daytime Sleepiness in Childhood Craniopharyngioma Patients. J. Clin. Endocrinol. Metab. 2002, 87, 3993–3996. [Google Scholar] [CrossRef]
- Gan, H.W.; Morillon, P.; Albanese, A.; Aquilina, K.; Chandler, C.; Chang, Y.C.; Drimtzias, E.; Farndon, S.; Jacques, T.S.; Korbonits, M.; et al. National UK Guidelines for the Management of Paediatric Craniopharyngioma. Lancet Diabetes Endocrinol. 2023, 11, 694–706. [Google Scholar] [CrossRef]
- DeVile, C.J.; Grant, D.B.; Hayward, R.D.; Stanhope, R. Growth and Endocrine Sequelae of Craniopharyngioma. Arch. Dis. Child. 1996, 75, 108–114. [Google Scholar] [CrossRef]
- Van Roessel, I.; de Graaf, J.P.; Biermasz, N.R.; Charmandari, E.; van Santen, H.M. Acquired Hypothalamic Dysfunction in Childhood: ‘What Do Patients Need?’—An Endo-Ern Survey. Endocr. Connect. 2023, 12, e230147. [Google Scholar] [CrossRef]
- Lucas, J.T., Jr.; Faught, A.M.; Hsu, C.Y.; Wilson, L.J.; Guo, Y.; Li, Y.; Khan, R.; Becksfort, J.B.; LeVine, D.A.; Ismael, Y.; et al. Pre- and Posttherapy Risk Factors for Vasculopathy in Pediatric Patients with Craniopharyngioma Treated With Surgery and Proton Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2022, 113, 152–160. [Google Scholar] [CrossRef]
- Newhauser, W.D.; Durante, M. Assessing the Risk of Second Malignancies after Modern Radiotherapy. Nat. Rev. Cancer 2011, 11, 438–448. [Google Scholar] [CrossRef] [PubMed]
- Beckhaus, J.; Friedrich, C.; Muller, H.L. Vascular Morbidity and Mortality in Craniopharyngioma Patients-A Scoping Review. Cancers 2024, 16, 1099. [Google Scholar] [CrossRef] [PubMed]
- Van Schaik, J.; Hoving, E.W.; Muller, H.L.; van Santen, H.M. Hypothalamic-Pituitary Outcome after Treatment for Childhood Craniopharyngioma. Front. Horm. Res. 2021, 54, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Van Santen, H.M.; van Schaik, J.; van Roessel, I.M.; Beckhaus, J.; Boekhoff, S.; Müller, H.L. Diagnostic Criteria for the Hypothalamic Syndrome in Childhood. Eur. J. Endocrinol. 2023, 188, 214–225. [Google Scholar] [CrossRef]
- Van Iersel, L.; Brokke, K.E.; Adan, R.A.H.; Bulthuis, L.C.M.; van den Akker, E.L.T.; van Santen, H.M. Pathophysiology and Individualized Treatment of Hypothalamic Obesity Following Craniopharyngioma and Other Suprasellar Tumors: A Systematic Review. Endocr. Rev. 2019, 40, 193–235. [Google Scholar] [CrossRef]
- Ozyurt, J.; Thiel, C.M.; Lorenzen, A.; Gebhardt, U.; Calaminus, G.; Warmuth-Metz, M.; Muller, H.L. Neuropsychological Outcome in Patients with Childhood Craniopharyngioma and Hypothalamic Involvement. J. Pediatr. 2014, 164, 876–881.e4. [Google Scholar] [CrossRef]
- Van Deuren, S.; Boonstra, A.; van Dulmen-den Broeder, E.; Blijlevens, N.; Knoop, H.; Loonen, J. Severe Fatigue after Treatment for Childhood Cancer. Cochrane Database Syst. Rev. 2020, 3, Cd012681. [Google Scholar] [CrossRef]
- Helligsoe, A.S.L.; Weile, K.S.; Kenborg, L.; Henriksen, L.T.; Lassen-Ramshad, Y.; Amidi, A.; Wu, L.M.; Winther, J.F.; Pickering, L.; Mathiasen, R. Systematic Review: Sleep Disorders Based on Objective Data in Children and Adolescents Treated for a Brain Tumor. Front. Neurosci. 2022, 16, 808398. [Google Scholar] [CrossRef]
- Tran, L.T.; Park, S.; Kim, S.K.; Lee, J.S.; Kim, K.W.; Kwon, O. Hypothalamic Control of Energy Expenditure and Thermogenesis. Exp. Mol. Med. 2022, 54, 358–369. [Google Scholar] [CrossRef]
- Aldave, G.; Okcu, M.F.; Chintagumpala, M.; Ruggieri, L.; Minard, C.G.; Malbari, F.; Mash, L.E.; Paulino, A.C.; McGovern, S.; Ramaswamy, U.; et al. Comparison of Neurocognitive and Quality-of-Life Outcomes in Pediatric Craniopharyngioma Patients Treated with Partial Resection and Radiotherapy Versus Gross-Total Resection Only. J. Neurosurg. Pediatr. 2023, 31, 453–462. [Google Scholar] [CrossRef]
- Baqai, M.W.S.; Shah, Z.; Malik, M.J.A.; Zia, N.; Shafqat, S.; Zahid, N.; Shamim, M.S. Quality of Life of Pediatric Patients with Craniopharyngioma: A Retrospective Series from a Low-Middle-Income Country with More Than 4 Years Follow-Up. Surg. Neurol. Int. 2024, 15, 199. [Google Scholar] [CrossRef] [PubMed]
- Bogusz, A.; Boekhoff, S.; Warmuth-Metz, M.; Calaminus, G.; Eveslage, M.; Muller, H.L. Posterior Hypothalamus-Sparing Surgery Improves Outcome after Childhood Craniopharyngioma. Endocr. Connect. 2019, 8, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Kayadjanian, N.; Hsu, E.A.; Wood, A.M.; Carson, D.S. Caregiver Burden and Its Relationship to Health-Related Quality of Life in Craniopharyngioma Survivors. J. Clin. Endocrinol. Metab. 2023, 109, e76–e87. [Google Scholar] [CrossRef] [PubMed]
- Mann-Markutzyk, L.V.; Beckhaus, J.; Ozyurt, J.; Mehren, A.; Friedrich, C.; Muller, H.L. Daytime Sleepiness and Health-Related Quality of Life in Patients with Childhood-Onset Craniopharyngioma. Sci. Rep. 2025, 15, 9407. [Google Scholar] [CrossRef]
- Eveslage, M.; Calaminus, G.; Warmuth-Metz, M.; Kortmann, R.D.; Pohl, F.; Timmermann, B.; Schuhmann, M.U.; Flitsch, J.; Faldum, A.; Muller, H.L. The Postopera tive Quality of Life in Children and Adolescents with Craniopharyngioma. Dtsch. Arztebl. Int. 2019, 116, 321–328. [Google Scholar] [CrossRef]
- Craven, M.; Crowley, J.H.; Chiang, L.; Kline, C.; Malbari, F.; Hocking, M.C.; McCormack, S.E. A Survey of Patient-Relevant Outcomes in Pediatric Craniopharyngioma: Focus on Hypothalamic Obesity. Front. Endocrinol. 2022, 13, 876770. [Google Scholar] [CrossRef]
- Mandrell, B.N.; Guo, Y.; Li, Y.; Hancock, D.; Caples, M.; Ashford, J.M.; Merchant, T.E.; Conklin, H.M.; Crabtree, V.M. Internalizing Symptoms and Their Impact on Patient-Reported Health-Related Quality of Life and Fatigue among Patients with Craniopharyngioma during Proton Radiation Therapy. Children 2024, 11, 1159. [Google Scholar] [CrossRef]
- Niel, K.A.; Klages, K.L.; Merchant, T.E.; Wise, M.S.; Hancock, D.; Caples, M.; Mandrell, B.N.; Conklin, H.M.; Crabtree, V.M. Impact of Sleep, Neuroendocrine, and Executive Function on Health-Related Quality of Life in Young People with Craniopharyngioma. Dev. Med. Child. Neurol. 2021, 63, 984–990. [Google Scholar] [CrossRef]
- Sowithayasakul, P.; Beckhaus, J.; Boekhoff, S.; Friedrich, C.; Calaminus, G.; Muller, H.L. Vision-Related Quality of Life in Patients with Childhood-Onset Craniopharyngioma. Sci. Rep. 2023, 13, 19599. [Google Scholar] [CrossRef]
- Van Schaik, J.; Schouten-van Meeteren, A.Y.N.; Vos-Kerkhof, E.; Janssens, G.O.; Porro, G.L.; Fiocco, M.; Bakker, B.; Tissing, W.J.E.; Hoving, E.W.; van Santen, H.M. Treatment and outcome of the Dutch Childhood Craniopharyngioma Cohort Study: First Results after Centralization of Care. Neuro Oncol. 2023, 25, 2250–2261. [Google Scholar] [CrossRef]
- Gan, H.W.; Cerbone, M.; Dattani, M.T. Appetite- and Weight-Regulating Neuroendocrine Circuitry in Hypothalamic Obesity. Endocr. Rev. 2023, 45, 309–342. [Google Scholar] [CrossRef] [PubMed]
- Roth, C.L.; McCormack, S.E. Acquired Hypothalamic Obesity: A Clinical Overview and Update. Diabetes Obes. Metab. 2024, 26 (Suppl. 2), 34–45. [Google Scholar] [CrossRef] [PubMed]
- Lustig, R.H. Hypothalamic Obesity: Causes, Consequences, Treatment. Pediatr. Endocrinol. Rev. 2008, 6, 220–227. [Google Scholar]
- Dimitri, P. Treatment of Acquired Hypothalamic Obesity: Now and the Future. Front. Endocrinol. 2022, 13, 846880. [Google Scholar] [CrossRef]
- Rakhshani, N.; Jeffery, A.S.; Schulte, F.; Barrera, M.; Atenafu, E.G.; Hamilton, J.K. Evaluation of a Comprehensive Care Clinic Model for Children with Brain Tumor and Risk for Hypothalamic Obesity. Obesity 2010, 18, 1768–1774. [Google Scholar] [CrossRef]
- Huynh, K.; Klose, M.; Krogsgaard, K.; Drejer, J.; Byberg, S.; Madsbad, S.; Magkos, F.; Aharaz, A.; Edsberg, B.; Tfelt-Hansen, J.; et al. Randomized Controlled Trial of Tesomet for Weight Loss in Hypothalamic Obesity. Eur. J. Endocrinol. 2022, 186, 687–700. [Google Scholar] [CrossRef]
- Denzer, C.; Denzer, F.; Lennerz, B.S.; Vollbach, H.; Lustig, R.H.; Wabitsch, M. Treatment of Hypothalamic Obesity with Dextroamphetamine: A Case Series. Obes. Facts 2019, 12, 91–102. [Google Scholar] [CrossRef]
- Masarwa, R.; Brunetti, V.C.; Aloe, S.; Henderson, M.; Platt, R.W.; Filion, K.B. Efficacy and Safety of Metformin for Obesity: A Systematic Review. Pediatrics 2021, 147, e20201610. [Google Scholar] [CrossRef]
- Ng, V.W.W.; Gerard, G.; Koh, J.J.K.; Loke, K.Y.; Lee, Y.S.; Ng, N.B.H. The Role of Glucagon-Like Peptide 1 Receptor Agonists for Weight Control in Individuals with Acquired Hypothalamic Obesity-A Systematic Review. Clin. Obes. 2024, 14, e12642. [Google Scholar] [CrossRef]
- Shoemaker, A.H.; Silver, H.J.; Buchowski, M.; Slaughter, J.C.; Yanovski, J.A.; Elfers, C.; Roth, C.L.; Abuzzahab, M.J. Energy Balance in Hypothalamic Obesity in Response to Treatment with a Once-Weekly Glp-1 Receptor Agonist. Int. J. Obes. 2022, 46, 623–629. [Google Scholar] [CrossRef]
- Roth, C.L.; Perez, F.A.; Whitlock, K.B.; Elfers, C.; Yanovski, J.A.; Shoemaker, A.H.; Abuzzahab, M.J. A Phase 3 Randomized Clinical Trial Using a Once-Weekly Glucagon-Like Peptide-1 Receptor Agonist in Adolescents and Young Adults with Hypothalamic Obesity. Diabetes Obes. Metab. 2021, 23, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Gjersdal, E.; Larsen, L.B.; Ettrup, K.S.; Vestergaard, P.; Nielsen, E.H.; Karmisholt, J.S.; Muller, H.L.; Dal, J. Semaglutide as a Promising Treatment for Hypothalamic Obesity: A Six-Month Case Series on Four Females with Craniopharyngioma. Pituitary 2024, 27, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Dimitri, P.; Roth, C.L. Treatment of Hypothalamic Obesity with Glp-1 Analogs. J. Endocr. Soc. 2024, 9, bvae200. [Google Scholar] [CrossRef]
- Parks, M.; Rosebraugh, C. Weighing Risks and Benefits of Liraglutide—The Fda’s Review of a New Antidiabetic Therapy. N. Engl. J. Med. 2010, 362, 774–777. [Google Scholar] [CrossRef]
- Pasternak, B.; Wintzell, V.; Hviid, A.; Eliasson, B.; Gudbjornsdottir, S.; Jonasson, C.; Hveem, K.; Svanstrom, H.; Melbye, M.; Ueda, P. Glucagon-like Peptide 1 Receptor agonist Use and Risk of thyroid Cancer: Scandinavian Cohort Study. BMJ 2024, 385, e078225. [Google Scholar] [CrossRef]
- Hsu, E.A.; Miller, J.L.; Perez, F.A.; Roth, C.L. Oxytocin and Naltrexone Successfully Treat Hypothalamic Obesity in a Boy Post-Craniopharyngioma Resection. J. Clin. Endocrinol. Metab. 2018, 103, 370–375. [Google Scholar] [CrossRef]
- Hoffmann, A.; Ozyurt, J.; Lohle, K.; Reichel, J.; Thiel, C.M.; Muller, H.L. First Experiences with Neuropsychological Effects of Oxytocin Administration in Childhood-Onset Craniopharyngioma. Endocrine 2017, 56, 175–185. [Google Scholar] [CrossRef]
- Wabitsch, M.; Farooqi, S.; Fluck, C.E.; Bratina, N.; Mallya, U.G.; Stewart, M.; Garrison, J.; van den Akker, E.; Kuhnen, P. Natural History of Obesity Due to Pomc, Pcsk1, and Lepr Deficiency and the Impact of Setmelanotide. J. Endocr. Soc. 2022, 6, bvac057. [Google Scholar] [CrossRef]
- Roth, C.L.; Scimia, C.; Shoemaker, A.H.; Gottschalk, M.; Miller, J.; Yuan, G.; Malhotra, S.; Abuzzahab, M.J. Setmelanotide for the Treatment of Acquired Hypothalamic Obesity: A Phase 2, Open-Label, Multicentre Trial. Lancet Diabetes Endocrinol. 2024, 12, 380–389. [Google Scholar] [CrossRef]
- Van Santen, H.M.; Denzer, C.; Müller, H.L. Could Setmelanotide Be the Game-Changer for Acquired Hypothalamic Obesity? Front. Endocrinol. 2024, 14, 1307889. [Google Scholar] [CrossRef]
- Van Schaik, J.; Welling, M.S.; de Groot, C.J.; van Eck, J.P.; Juriaans, A.; Burghard, M.; Oude Ophuis, S.B.J.; Bakker, B.; Tissing, W.J.E.; Schouten-van Meeteren, A.Y.N.; et al. Dextroamphetamine Treatment in Children with Hypothalamic Obesity. Front. Endocrinol. 2022, 13, 845937. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, J.K.; Conwell, L.S.; Syme, C.; Ahmet, A.; Jeffery, A.; Daneman, D. Hypothalamic Obesity following Craniopharyngioma Surgery: Results of a Pilot Trial of Combined Diazoxide and Metformin Therapy. Int. J. Pediatr. Endocrinol. 2011, 2011, 417949. [Google Scholar] [CrossRef]
- Lustig, R.H.; Hinds, P.S.; Ringwald-Smith, K.; Christensen, R.K.; Kaste, S.C.; Schreiber, R.E.; Rai, S.N.; Lensing, S.Y.; Wu, S.; Xiong, X. Octreotide Therapy of Pediatric Hypothalamic Obesity: A Double-Blind, Placebo-Controlled Trial. J. Clin. Endocrinol. Metab. 2003, 88, 2586–2592. [Google Scholar] [CrossRef]
- Svendstrup, M.; Rasmussen, A.K.; Kistorp, C.; Klose, M.; Andreassen, M. Semaglutide Treatment of Hypothalamic Obesity—A Real-Life Data Study. Pituitary 2024, 27, 685–692. [Google Scholar] [CrossRef]
- Zoicas, F.; Droste, M.; Mayr, B.; Buchfelder, M.; Schofl, C. GLP-1 Analogues as a New Treatment Option for Hypothalamic Obesity in Adults: Report of Nine Cases. Eur. J. Endocrinol. 2013, 168, 699–706. [Google Scholar] [CrossRef]
- Lomenick, J.P.; Buchowski, M.S.; Shoemaker, A.H. A 52-Week Pilot Study of the Effects of Exenatide on Body Weight in Patients with Hypothalamic Obesity. Obesity 2016, 24, 1222–1225. [Google Scholar] [CrossRef]
- Wijnen, M.; Olsson, D.S.; van den Heuvel-Eibrink, M.M.; Wallenius, V.; Janssen, J.A.; Delhanty, P.J.; van der Lely, A.J.; Johannsson, G.; Neggers, S.J. Efficacy and Safety of Bariatric Surgery for Craniopharyngioma-Related Hypothalamic Obesity: A Matched Case-Control Study with 2 Years of Follow-Up. Int. J. Obes. 2017, 41, 210–216. [Google Scholar] [CrossRef]
- Van Santen, S.S.; Wolf, P.; Kremenevski, N.; Boguszewski, C.L.; Beiglbock, H.; Fiocco, M.; Wijnen, M.; Wallenius, V.R.; van den Heuvel-Eibrink, M.M.; van der Lely, A.J.; et al. Bariatric Surgery for Hypothalamic Obesity in Craniopharyngioma Patients: A Retrospective, Matched Case-Control Study. J. Clin. Endocrinol. Metab. 2021, 106, e4734–e4745. [Google Scholar] [CrossRef]
- Faucher, P.; Carette, C.; Jannot, A.S.; Gatta-Cherifi, B.; Van Straaten, A.; Piquet, M.A.; Raverot, G.; Alligier, M.; Batisse, T.; Ziegler, O.; et al. Five-Year Changes in Weight and Diabetes Status After Bariatric Surgery for Craniopharyngioma-Related Hypothalamic Obesity: A Case-Control Study. Obes. Surg. 2022, 32, 2321–2331. [Google Scholar] [CrossRef]
- Garrez, I.; Lapauw, B.; Van Nieuwenhove, Y. Bariatric Surgery for Treatment of Hypothalamic Obesity after Craniopharyngioma Therapy: A Matched Case-Control Study. Obes. Surg. 2020, 30, 2439–2444. [Google Scholar] [CrossRef]
- Bretault, M.; Boillot, A.; Muzard, L.; Poitou, C.; Oppert, J.M.; Barsamian, C.; Gatta, B.; Muller, H.; Weismann, D.; Rottembourg, D.; et al. Bariatric Surgery Following Treatment for Craniopharyngioma: A Systematic Review and Individual-Level Data Meta-analysis. J. Clin. Endocrinol. Metab. 2013, 98, 2239–2246. [Google Scholar] [CrossRef] [PubMed]
- Muller, H.L.; Gebhardt, U.; Maroske, J.; Hanisch, E. Long-Term Follow-Up of Morbidly Obese Patients with Childhood Craniopharyngioma after Laparoscopic Adjustable Gastric Banding (Lagb). Klin. Padiatr. 2011, 223, 372–373. [Google Scholar] [CrossRef] [PubMed]
- Weismann, D.; Pelka, T.; Bender, G.; Jurowich, C.; Fassnacht, M.; Thalheimer, A.; Allolio, B. Bariatric Surgery for Morbid Obesity in Craniopharyngioma. Clin. Endocrinol. 2013, 78, 385–390. [Google Scholar] [CrossRef]
- Trotta, M.; Da Broi, J.; Salerno, A.; Testa, R.M.; Marinari, G.M. Sleeve Gastrectomy Leads to Easy Management of Hormone Replacement Therapy and Good Weight Loss in Patients Treated for Craniopharyngioma. Updates Surg. 2017, 69, 95–99. [Google Scholar] [CrossRef]
- Gatta, B.; Nunes, M.L.; Bailacq-Auder, C.; Etchechoury, L.; Collet, D.; Tabarin, A. Is Bariatric Surgery Really Inefficient in Hypothalamic Obesity? Clin. Endocrinol. 2013, 78, 636–638. [Google Scholar] [CrossRef]
- Smith, D.K.; Sarfeh, J.; Howard, L. Truncal Vagotomy in Hypothalamic Obesity. Lancet 1983, 1, 1330–1331. [Google Scholar] [CrossRef]
- Muller, H.L. Bariatric Interventions in Craniopharyngioma Patients-Best Choice or Last Option for Treatment of Hypothalamic Obesity? J. Clin. Endocrinol. Metab. 2022, 107, e426–e428. [Google Scholar] [CrossRef]
- Styne, D.M.; Arslanian, S.A.; Connor, E.L.; Farooqi, I.S.; Murad, M.H.; Silverstein, J.H.; Yanovski, J.A. Pediatric Obesity-Assessment, Treatment, and Prevention: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2017, 102, 709–757. [Google Scholar] [CrossRef]
| Diagnosis | Pat. No. | Age at Craniopharyngioma Diagnosis (Years) | Follow-Up Interval (Years) | Treatment | Quality of Life/Outcome | Authors/Year of Publication |
|---|---|---|---|---|---|---|
| CO CP | 48 | GTR: Median 6.4 (range: 2.2–16.8) PR + RT: Median 8.5 (range: 3.8–16.4) | 10 years | 21 GTR, 22 PR + RT | No differences in the trajectory of intellectual functioning or QoL scale scores between the two groups (GTR vs. PR + RT). | Aldave et al., 2023 [43] |
| CO CP from a lower-middle-income country | 29 | Mean age 13.5 ± 4.2 SD | Mean FUP: 4.4 ± 2.2 SD | 15 GTR 11 Debulking 3 Reservoir and biopsy | PedsQL: GTR 56.6 ± 7.12 Debulking: 93.8 ± 3.37 Biopsy: 83.3 ± 5.69 | Baqai et al., 2024 [44] |
| CO CP with presurgical grade 2 HI [3] | 109 | Median 9.5 (range: 1.3–17.9) | Mean 6.1 (range: 3.0–10.2) | Surgery leading to 23 grade 0 HL 29 grade 1 HL 57 grade 2 HL | Worse PEDQOL for grade 3 patients in terms of physical, social, and emotional functionality when compared with HL grade 0 and 1. | Bogusz et al., 2019 [45] |
| Caregivers of CO CP patients | 82 | Mean age 9.3 ± 4.5 SD | 52.4% GTR | Survivor poly-symptomatology predicted caregiver burden. The study separated hyperphagia and obesity and identified hyperphagia and other hypothalamic dysfunction symptoms as understudied issues. | Kayadjanian et al., 2023 [46] | |
| Hypothalamic dysfunction + CO CP | 290 | n.a. | n. a. | n. a. | Worldwide online survey: Obesity (51%) and fatigue (48%). Needs for improvement in the domains of obesity, fatigue, and lifestyle. | Van Roessel et al., 2020 [32] |
| CO CP | 119 | Median 12 (range: 2–17) | Mean 10 (range: 1–39) | CR in 34 (29%) 6 HL Garde 0 23 HL grade 1 55 HL grade 2 | QoL (EORTC QLQ-C30) was negatively correlated with daytime sleepiness (ESS), the highest ESS in patients with HL grade 2. | Mann-Markutzyk et al., 2025 [47] |
| CO CP | 131 | Median 9.7 (range: 1.3–17.6) | 3 years | Complete Res.: 21 (18%) Incomplete Res.: 94 (82%) | Grade 2 HI, grade 2 HL, and complete surgical resection were associated with low QoL. | Eveslage et al., 2019 [48] |
| Caregivers of CO CP patients | 106 | <18 years | n. a. | 48 RT 134 surgical interventions | Online survey: reduced social functioning. | Craven et al., 2022 [49] |
| CO CP | 92 | Mean age 10.5 ± 4.0 SD | n. a. | Proton beam therapy after surgical intervention | Fatigue, QoL, and brain tumor symptoms improved over time during proton beam therapy. | Mandrell et al., 2024 [50] |
| CO CP | 78 | Mean age 10.8 ± 3.11 SD | n. a. | 56 surgical resections 16 catheter implantation | Poorer parent-reported QoL; AVD directly predicted greater global executive functioning impairment. | Niel et al., 2021 [51] |
| CO CP and parents/caregivers | 120 | Median 10.0 (range: 1.3 –16.8) | 3 years | 25 complete resection 95 incomplete resection 61 RT | Reduced autonomy was found three years after diagnosis in self-assessments and parental assessments of QoL (PedQol). | Sowithayasakul et al., 2023 [52] |
| Irradiated CO CP | 99 | Median 9.5 (range: 1.6–17.9) | Median 6.4 (range: 0.9–14.7) | 64 proton beam therapy 35 photon-based RT | No significant difference between PBT and RT in terms of QoL (PedQol), functional capacity (FMH), and body mass index. | Friedrich et al., 2023 [19] |
| CO CP | 87 | Mean 7.39 ± 3.67 SD | Median 6.54 (IQR: 3.11–10.69) | 25% complete resection 44% incomplete resection 30% cyst drainage 46% RT | BMI at dgn and grade of HL were associated with hypothalamic obesity. | Van Schaik et al., 2023 [53] |
| Intervention | # | Age (Years) | BMI/Weight at Intervention | BMI/Weight Change During/After Intervention | Authors | |
|---|---|---|---|---|---|---|
| Pharmacological agent | Dextroamphetamine | 19 | 12.3 ± 4.0 | BMI 3.58 ± 0.85 SD | ΔBMI SDS −0.14 | van Schaik et al. [74] |
| Dextroamphetamine | 7 | 0.5, 11.1, 11.8, 12.5, 14.7, 14.8, 21.0 | BMI +3.17 ± 0.9 SD Range: +1.9 to +4.4 SD | Mean BMI SDS decelerated to −0.18 ± 0.12/year during the 1st year of treatment and stabilized at +0.05 ± 0.32/year during the 2nd year of treatment. | Denzer et al. [60] | |
| Diazoxide/metformin | 9 | 15.4 ± 2.9 | BMI +1.8–+2.96 SD | ΔBMI −0.3 ± 2.3 kg/m2 | Hamilton et al. [75] | |
| Octreotide (RCT) | 10 | 13.8 ± 1.2 | BMI 37.1 ± 1.3 kg/m2 | ΔBMI −0.2 ± 0.2 kg/m2 (vs. placebo +2.2 ± 0.5 kg/m2) | Lustig et al. [76] | |
| Semaglutide | 26 | 52 (18–65) | BMI 38 (28–58) kg/m2. | Mean TWL 13.4 kg (95% CI 10.3–16.5 kg) | Svendstrup et al. [77] | |
| Semaglutide | 4 | 22, 44, 57, 69 | BMI 48.0 (35.0–55.5) kg/m2 | ΔBMI 7.9 BMI (6.7–10.1); weight loss of 17.0% (11.3–22.4%) | Gjersdal et al. [65] | |
| Exenatide/liraglutide | 9 | 46 (22–49) | BMI 37.6 ± 7.2 kg/m2 | Exenatide: ΔBMI −6.1 to −2.8 kg/m2; liraglutide: Δweight −22 to −9 kg | Zoicas et al. [78] | |
| Exenatide | 8 | 27.5 ± 7.8 | BMI 47.5 ± 10.8 kg/m2 | Mean Δweight −1.4 kg | Lomenick et al. [79] | |
| Tesomet (tesofensine and metoprolol) | 18 | 45.4 ± 13.3 | BMI 37.3 ± 5.6 kg/m2 | Δweight: −6.3% (tesomet −6.6% vs. placebo −0.3%) | Huynh et al. [59] | |
| Setmelanotide | 18 | 15.0 ± 5.3 | BMI 38·0 ± 6·5 kg/m2 | ΔBMI: −15% (SDS 10%) after 4 mo; extension 12 mo (12 patients): −26% (12 SDS) | Roth et al. [72] | |
| Lifestyle modification | Regular visits to a comprehensive care clinic | 39 | 13.4 (4.3–18.2) | BMI 1.93 (0–3.2) SD | Median ΔBMI rate +4.5 kg/m2/y (−17.8 to +8.4); median ΔBMI SDS rate 0.0/y (−5.2 to +0.5) | Rakhshani et al. [58] |
| Bariatric surgery | SG (n = 3); RYGB (n = 5) | 8 | 33.4 ± 13.6 | BMI 43.3 ± 4.1 kg/m2 | SG (n = 3): mean Δweight −10%; RYGB (n = 5): mean Δweight −25% | Wijnen et al. [80] |
| RYGB (n = 12), SG (n = 4) | 16 | 26 ± 12 | BMI 46 ± 8 kg/m2 | Mean Δweight: −22% after 5 years | van Santen et al. [81] | |
| SG (39%), RYGB (61%) | 23 | 35 (25–43) | BMI 44.2 (40.7–51.0) kg/m2 | ΔTWL (%) − 39.0% (14.0; 53.3) | Faucher et al. [82] | |
| SG (n = 2), RYGB (n = 3) | 5 | 38 (27–47) | BMI 41.3 (37.9–46.3) kg/m2 | ΔTWL (%) −14.7% (23.7; 5.8) | Garrez et al. [83] | |
| LAGB (n = 6); SG (n = 8); RYGB (n = 6); BPD (n = 1) | 21 | 24 (12–54) | BMI 49.6 kg/m2 | TWL (%) LAGB: 10.5%; SG: 20.7%; RYGB: 20.2%; BPD: 24.8% | Bretault et al. [84] | |
| LAGB (n = 4) | 4 | 13, 17, 21, 23 | BMI +7.3 – +12.3 SD | ΔBMI +1.7 to +8.7 kg/m2 | Müller et al. [85] | |
| LAGB (n = 6); SG (n = 4); RYGB (n = 2) | 9 | 17 (12–30) | BMI 44.7 (40.2–61.6) kg/m2 | LAGB: no change; SG: no change; RYGB: mean Δweight −30% | Weismann et al. [86] | |
| SG | 3 | 21, 22, 24 | BMI 49.2 (41.6–58.1) kg/m2 | Mean ΔBMI −13.9 kg/m2; Δweight −17.6%, −25.0%, −41.1% | Trotta et al. [87] | |
| SG (n = 2); RYGB (n = 2) | 4 | 24, 30, 43, 51 | BMI 37.6, 37.7, 43.7, 51.0 kg/m2 | ΔBMI: SG −10, −3.6; RYGB: −6.2, +11.3 kg/m2 | Gatta et al. [88] | |
| Vagotomy | Truncal vagotomy | 1 | 19 | BMI 43.0 kg/m2 | Δweight −7.0 kg | Smit et al. [89] |
| DBS | Nucleus accumbens DBS | 1 | 19 | BMI 52.9 kg/m2 | ΔBMI −5.2 kg/m2 | Harat et al. [21] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Müller, H.L. Management of Acquired Hypothalamic Obesity After Childhood-Onset Craniopharyngioma—A Narrative Review. Biomedicines 2025, 13, 1016. https://doi.org/10.3390/biomedicines13051016
Müller HL. Management of Acquired Hypothalamic Obesity After Childhood-Onset Craniopharyngioma—A Narrative Review. Biomedicines. 2025; 13(5):1016. https://doi.org/10.3390/biomedicines13051016
Chicago/Turabian StyleMüller, Hermann L. 2025. "Management of Acquired Hypothalamic Obesity After Childhood-Onset Craniopharyngioma—A Narrative Review" Biomedicines 13, no. 5: 1016. https://doi.org/10.3390/biomedicines13051016
APA StyleMüller, H. L. (2025). Management of Acquired Hypothalamic Obesity After Childhood-Onset Craniopharyngioma—A Narrative Review. Biomedicines, 13(5), 1016. https://doi.org/10.3390/biomedicines13051016






