SNX10 Is Involved in Ovarian Cancer Cell Metastasis by Repolarizing Tumor-Associated Macrophages Through mTOR1/Lysosomes Pathway
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture and Co-Culture of Tumor Cells and Macrophages
2.2. Lentivirus Transfection
2.3. Flow Cytometry Analysis
2.4. RNA Sequencing Analysis
2.5. Western Blotting Assay
2.6. Cell Viability Assay
2.7. Real-Time Reverse Transcription–PCR Assay
2.8. Transwell Assays
2.9. BODIPY Staining and Oil Red O Staining
2.10. Lysosome pH Detection
2.11. Database Analysis
2.12. Statistical Analysis
3. Results
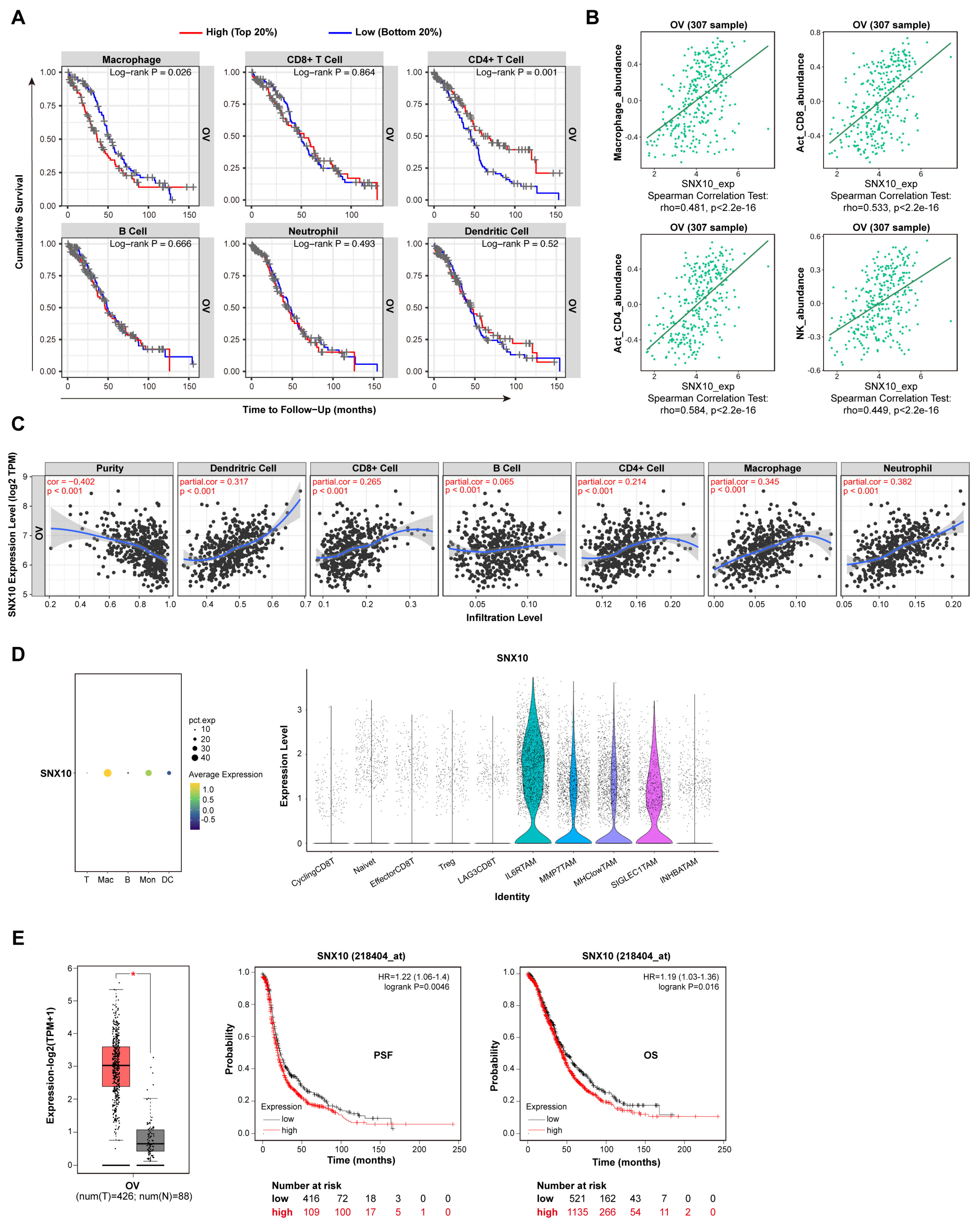
3.1. SNX10 Is Highly Expressed in Macrophages of Ovarian Cancer Tissue and Affects Prognosis
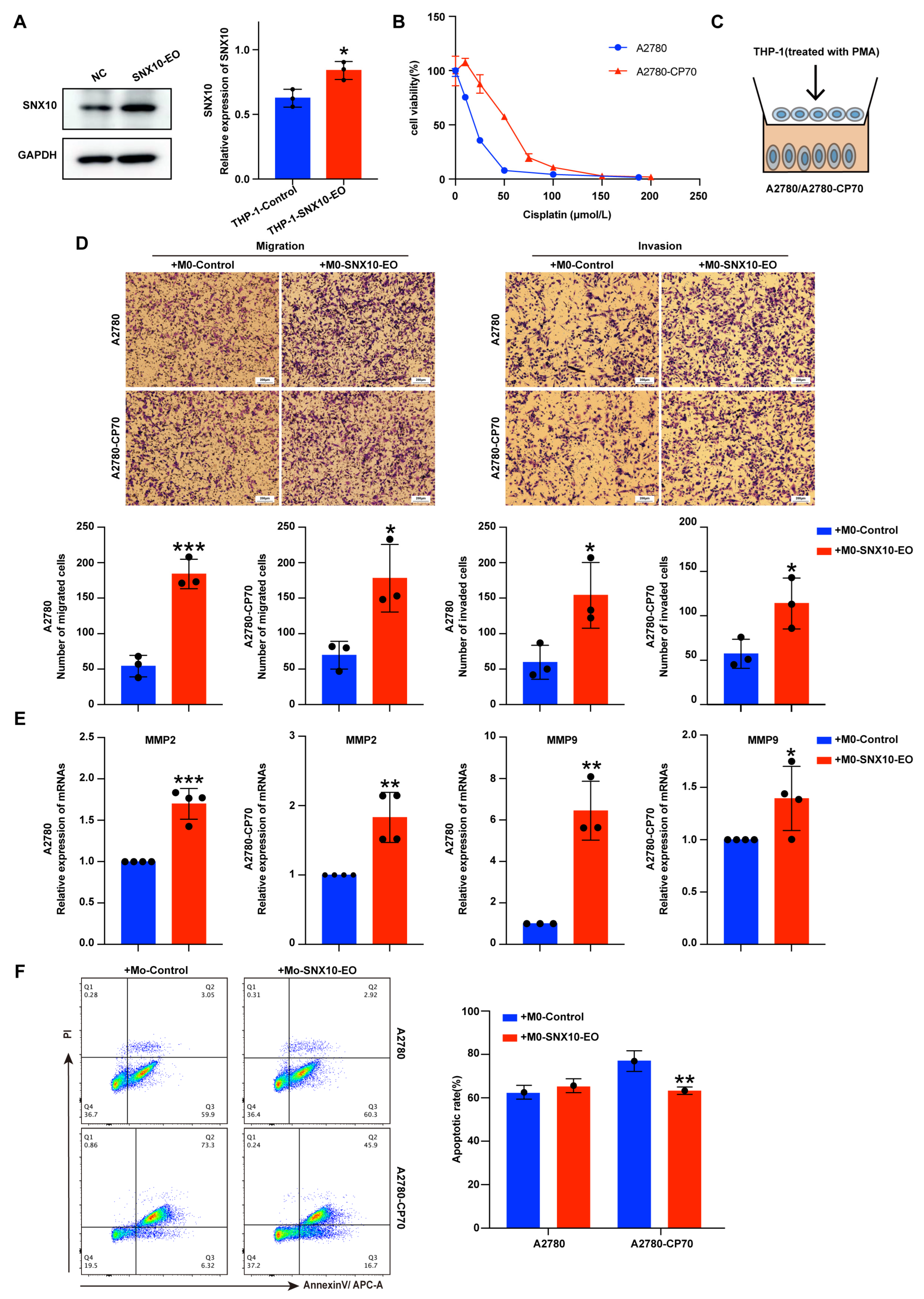
3.2. Overexpression of SNX10 in Macrophages Increases the Migration and Invasion Ability of Ovarian Cancer Cells
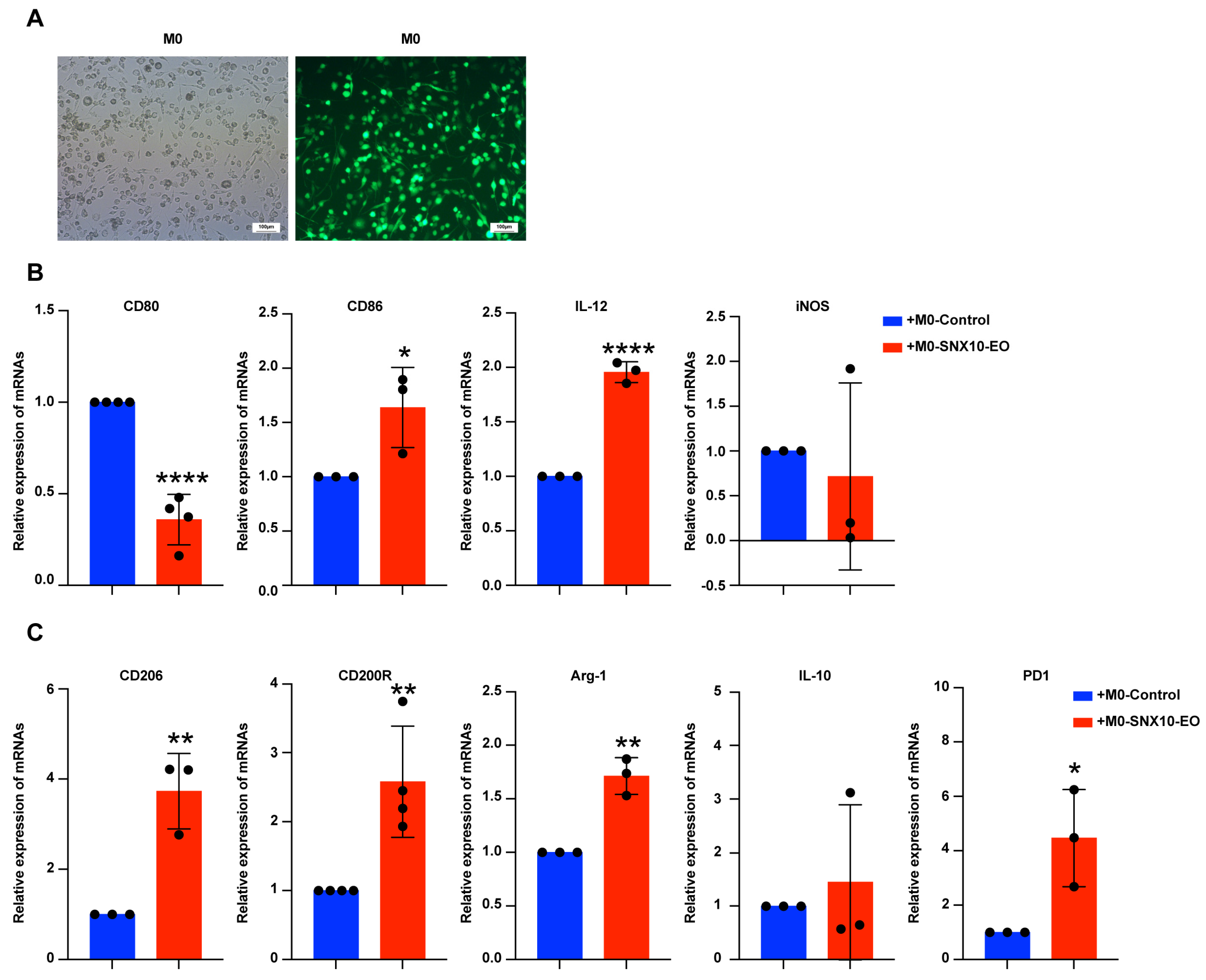
3.3. SNX10 Overexpression Promotes M2-like Macrophage Polarization
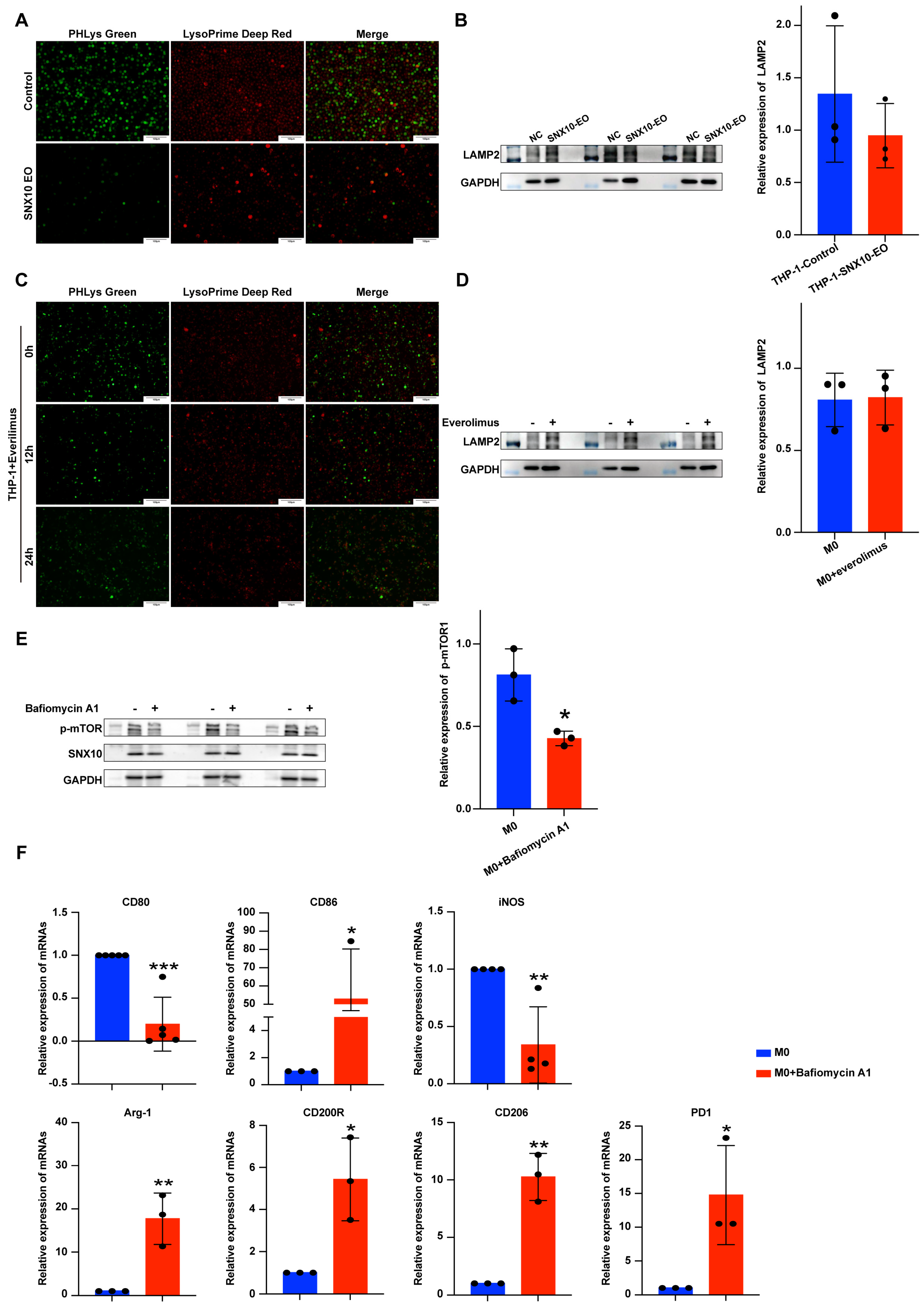
3.4. SNX10 Regulates Macrophage Polarization Through mTORC1-Mediated Lipid Metabolic Reprogramming
3.5. Overexpression of SNX10 Decreases the Number of Lysosomes and Induces Acidification Disorders to Promote M2 Macrophage Polarization
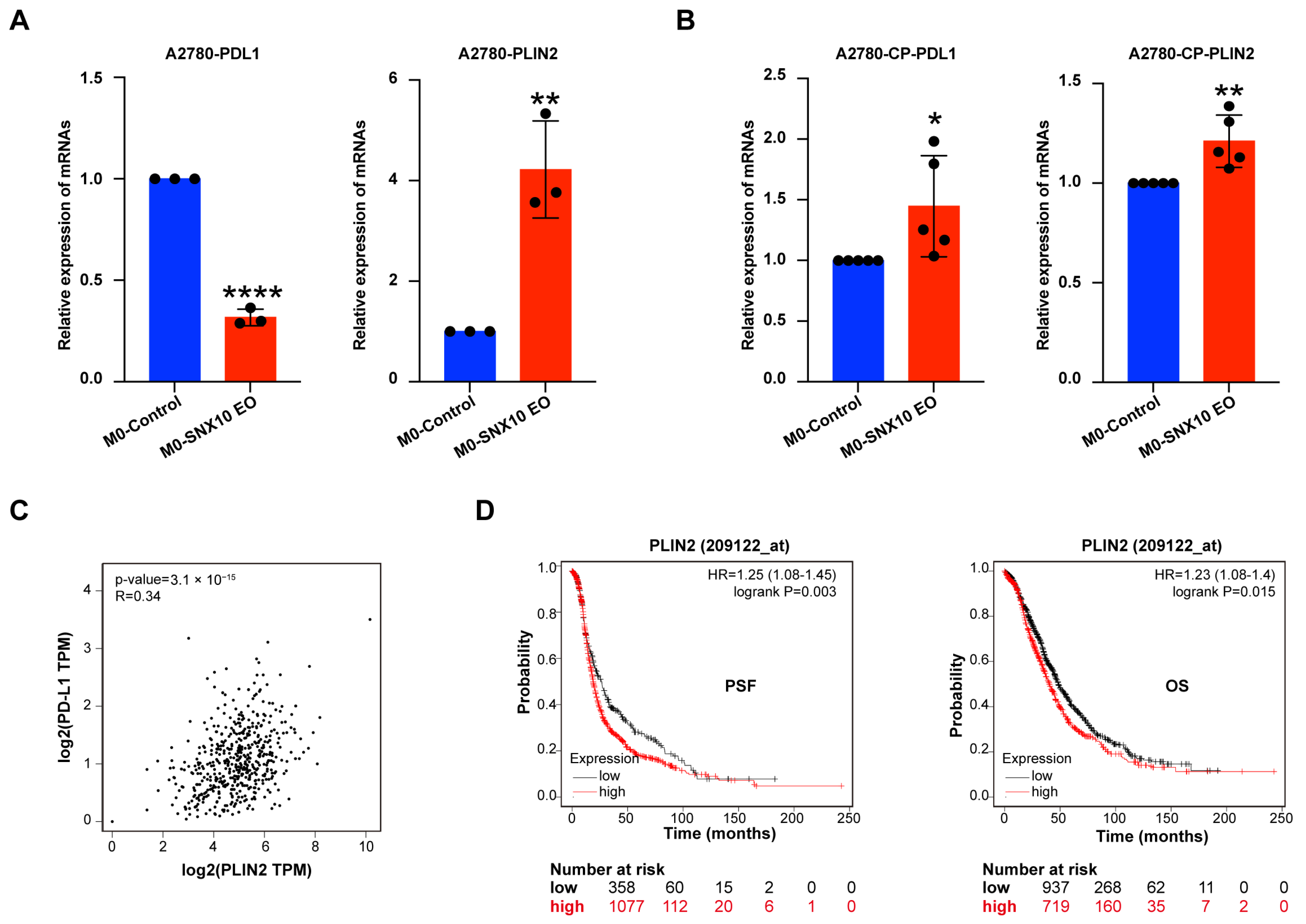
3.6. SNX10-Overexpressing Macrophages Modulate PD-L1 Expression and Lipid Metabolism in Ovarian Cancer Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TAMs | Tumor-associated macrophages |
| M0 macrophages | THP-1 cells after PMA stimulation |
| M0-control macrophages | M0 macrophages with SNX10 control expression |
| M0-SNX10-EO macrophages | M0 macrophages with SNX10 overexpressed |
References
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhao, J.; Zhang, L.; Wei, F.; Lian, Y.; Wu, Y.; Gong, Z.; Zhang, S.; Zhou, J.; Cao, K.; et al. Role of tumor microenvironment in tumorigenesis. J. Cancer 2017, 8, 761–773. [Google Scholar] [CrossRef]
- Xue, J.; Schmidt, S.V.; Sander, J.; Draffehn, A.; Krebs, W.; Quester, I.; De Nardo, D.; Gohel, T.D.; Emde, M.; Schmidleithner, L.; et al. Transcriptome-based network analysis reveals a spectrum model of human macrophage activation. Immunity 2014, 40, 274–288. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, T.; Natoli, G. Transcriptional regulation of macrophage polarization: Enabling diversity with identity. Nat. Rev. Immunol. 2011, 11, 750–761. [Google Scholar] [CrossRef]
- Reinartz, S.; Schumann, T.; Finkernagel, F.; Wortmann, A.; Jansen, J.M.; Meissner, W.; Krause, M.; Schwörer, A.M.; Wagner, U.; Müller-Brüsselbach, S.; et al. Mixed-polarization phenotype of ascites-associated macrophages in human ovarian carcinoma: Correlation of CD163 expression, cytokine levels and early relapse. Int. J. Cancer 2014, 134, 32–42. [Google Scholar] [CrossRef]
- Dietze, R.; Hammoud, M.K.; Gómez-Serrano, M.; Unger, A.; Bieringer, T.; Finkernagel, F.; Sokol, A.M.; Nist, A.; Stiewe, T.; Reinartz, S.; et al. Phosphoproteomics identify arachidonic-acid-regulated signal transduction pathways modulating macrophage functions with implications for ovarian cancer. Theranostics 2021, 11, 1377–1395. [Google Scholar] [CrossRef]
- Qian, B.Z.; Pollard, J.W. Macrophage diversity enhances tumor progression and metastasis. Cell 2010, 141, 39–51. [Google Scholar] [CrossRef]
- Wang, H.; Yung, M.M.H.; Ngan, H.Y.S.; Chan, K.K.L.; Chan, D.W. The Impact of the Tumor Microenvironment on Macrophage Polarization in Cancer Metastatic Progression. Int. J. Mol. Sci. 2021, 22, 6560. [Google Scholar] [CrossRef]
- Ran, X.M.; Yang, J.; Wang, Z.Y.; Xiao, L.Z.; Deng, Y.P.; Zhang, K.Q. M2 macrophage-derived exosomal circTMCO3 acts through miR-515-5p and ITGA8 to enhance malignancy in ovarian cancer. Commun. Biol. 2024, 7, 583. [Google Scholar] [CrossRef]
- Mantovani, A.; Savino, B.; Locati, M.; Zammataro, L.; Allavena, P.; Bonecchi, R. The chemokine system in cancer biology and therapy. Cytokine Growth Factor. Rev. 2010, 21, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.; Martinez, F.O. Alternative activation of macrophages: Mechanism and functions. Immunity 2010, 32, 593–604. [Google Scholar] [CrossRef] [PubMed]
- Cullen, P.J.; Korswagen, H.C. Sorting nexins provide diversity for retromer-dependent trafficking events. Nat. Cell Biol. 2011, 14, 29–37. [Google Scholar] [CrossRef]
- Teasdale, R.D.; Collins, B.M. Insights into the PX (phox-homology) domain and SNX (sorting nexin) protein families: Structures, functions and roles in disease. Biochem. J. 2012, 441, 39–59. [Google Scholar] [CrossRef]
- Qin, B.; He, M.; Chen, X.; Pei, D. Sorting nexin 10 induces giant vacuoles in mammalian cells. J. Biol. Chem. 2006, 281, 36891–36896. [Google Scholar] [CrossRef]
- Wang, X.; Ni, J.; You, Y.; Feng, G.; Zhang, S.; Bao, W.; Hou, H.; Li, H.; Liu, L.; Zheng, M.; et al. SNX10-mediated LPS sensing causes intestinal barrier dysfunction via a caspase-5-dependent signaling cascade. EMBO J. 2021, 40, e108080. [Google Scholar] [CrossRef]
- Bao, W.; Liu, X.; You, Y.; Hou, H.; Wang, X.; Zhang, S.; Li, H.; Feng, G.; Cao, X.; Jiang, H.; et al. Targeting sorting nexin 10 improves mouse colitis via inhibiting PIKfyve-mediated TBK1/c-Rel signaling activation. Pharmacol. Res. 2021, 169, 105679. [Google Scholar] [CrossRef]
- You, Y.; Zhou, C.; Li, D.; Cao, Z.L.; Shen, W.; Li, W.Z.; Zhang, S.; Hu, B.; Shen, X. Sorting nexin 10 acting as a novel regulator of macrophage polarization mediates inflammatory response in experimental mouse colitis. Sci. Rep. 2016, 6, 20630. [Google Scholar] [CrossRef]
- You, Y.; Bao, W.L.; Zhang, S.L.; Li, H.D.; Li, H.; Dang, W.Z.; Zou, S.L.; Cao, X.Y.; Wang, X.; Liu, L.X.; et al. Sorting Nexin 10 Mediates Metabolic Reprogramming of Macrophages in Atherosclerosis Through the Lyn-Dependent TFEB Signaling Pathway. Circ. Res. 2020, 127, 534–549. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.; Trapnell, C.; Donaghey, J.; Rinn, J.L.; Pachter, L. Improving RNA-Seq expression estimates by correcting for fragment bias. Genome Biol. 2011, 12, R22. [Google Scholar] [CrossRef] [PubMed]
- Anders, S.; Pyl, P.T.; Huber, W. HTSeq--a Python framework to work with high-throughput sequencing data. Bioinformatics 2015, 31, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- The Gene Ontology Consortium. The Gene Ontology Resource: 20 years and still GOing strong. Nucleic Acids Res. 2019, 47, D330–D338. [Google Scholar] [CrossRef]
- Kanehisa, M.; Araki, M.; Goto, S.; Hattori, M.; Hirakawa, M.; Itoh, M.; Katayama, T.; Kawashima, S.; Okuda, S.; Tokimatsu, T.; et al. KEGG for linking genomes to life and the environment. Nucleic Acids Res. 2008, 36, D480–D484. [Google Scholar] [CrossRef]
- Olalekan, S.; Xie, B.; Back, R.; Eckart, H.; Basu, A. Characterizing the tumor microenvironment of metastatic ovarian cancer by single-cell transcriptomics. Cell Rep. 2021, 35, 109165. [Google Scholar] [CrossRef]
- Weber, L.M.; Hippen, A.A.; Hickey, P.F.; Berrett, K.C.; Gertz, J.; Doherty, J.A.; Greene, C.S.; Hicks, S.C. Genetic demultiplexing of pooled single-cell RNA-sequencing samples in cancer facilitates effective experimental design. Gigascience 2021, 10, giab062. [Google Scholar] [CrossRef]
- You, Y.; Li, W.Z.; Zhang, S.; Hu, B.; Li, Y.X.; Li, H.D.; Tang, H.H.; Li, Q.W.; Guan, Y.Y.; Liu, L.X.; et al. SNX10 mediates alcohol-induced liver injury and steatosis by regulating the activation of chaperone-mediated autophagy. J. Hepatol. 2018, 69, 129–141. [Google Scholar] [CrossRef]
- Thelen, A.M.; Zoncu, R. Emerging Roles for the Lysosome in Lipid Metabolism. Trends Cell Biol. 2017, 27, 833–850. [Google Scholar] [CrossRef]
- Ebner, M.; Puchkov, D.; López-Ortega, O.; Muthukottiappan, P.; Su, Y.; Schmied, C.; Zillmann, S.; Nikonenko, I.; Koddebusch, J.; Dornan, G.L.; et al. Nutrient-regulated control of lysosome function by signaling lipid conversion. Cell 2023, 186, 5328–5346.e5326. [Google Scholar] [CrossRef] [PubMed]
- de Visser, K.E.; Joyce, J.A. The evolving tumor microenvironment: From cancer initiation to metastatic outgrowth. Cancer Cell 2023, 41, 374–403. [Google Scholar] [CrossRef] [PubMed]
- Cassetta, L.; Pollard, J.W. A timeline of tumour-associated macrophage biology. Nat. Rev. Cancer 2023, 23, 238–257. [Google Scholar] [CrossRef] [PubMed]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage plasticity, polarization, and function in health and disease. J. Cell Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef]
- Yan, C.; Li, K.; Meng, F.; Chen, L.; Zhao, J.; Zhang, Z.; Xu, D.; Sun, J.; Zhou, M. Integrated immunogenomic analysis of single-cell and bulk tissue transcriptome profiling unravels a macrophage activation paradigm associated with immunologically and clinically distinct behaviors in ovarian cancer. J. Adv. Res. 2023, 44, 149–160. [Google Scholar] [CrossRef]
- Xu, T.; Xu, J.; Ye, Y.; Wang, Q.; Shu, X.; Pei, D.; Liu, J. Structure of human SNX10 reveals insights into its role in human autosomal recessive osteopetrosis. Proteins 2014, 82, 3483–3489. [Google Scholar] [CrossRef]
- Zhang, M.; Cui, D.; Yang, H. The Distributional Characteristics of M2 Macrophages in the Placental Chorionic Villi are Altered Among the Term Pregnant Women With Uncontrolled Type 2 Diabetes Mellitus. Front. Immunol. 2022, 13, 837391. [Google Scholar] [CrossRef]
- Li, L.; Ma, S.R.; Yu, Z.L. Targeting the lipid metabolic reprogramming of tumor-associated macrophages: A novel insight into cancer immunotherapy. Cell Oncol. 2024, 47, 415–428. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, D.; Yang, L.; Zhang, Y. Metabolic reprogramming in the immunosuppression of tumor-associated macrophages. Chin. Med. J. 2022, 135, 2405–2416. [Google Scholar] [CrossRef]
- Qiao, X.; Hu, Z.; Xiong, F.; Yang, Y.; Peng, C.; Wang, D.; Li, X. Lipid metabolism reprogramming in tumor-associated macrophages and implications for therapy. Lipids Health Dis. 2023, 22, 45. [Google Scholar] [CrossRef]
- Wang, R.; Hu, Q.; Wu, Y.; Guan, N.; Han, X.; Guan, X. Intratumoral lipid metabolic reprogramming as a pro-tumoral regulator in the tumor milieu. Biochim. Biophys. Acta Rev. Cancer 2023, 1878, 188962. [Google Scholar] [CrossRef] [PubMed]
- Martin-Perez, M.; Urdiroz-Urricelqui, U.; Bigas, C.; Benitah, S.A. The role of lipids in cancer progression and metastasis. Cell Metab. 2022, 34, 1675–1699. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, H.; Zhao, Z.B.; Zhu, W.; Feng, P.P.; Zhu, X.W.; Gong, J.P. SIRT4 silencing in tumor-associated macrophages promotes HCC development via PPARδ signalling-mediated alternative activation of macrophages. J. Exp. Clin. Cancer Res. 2019, 38, 469. [Google Scholar] [CrossRef] [PubMed]
- Masetti, M.; Carriero, R.; Portale, F.; Marelli, G.; Morina, N.; Pandini, M.; Iovino, M.; Partini, B.; Erreni, M.; Ponzetta, A.; et al. Lipid-loaded tumor-associated macrophages sustain tumor growth and invasiveness in prostate cancer. J. Exp. Med. 2022, 219, e20210564. [Google Scholar] [CrossRef]
- Xiao, J.; Wang, S.; Chen, L.; Ding, X.; Dang, Y.; Han, M.; Zheng, Y.; Shen, H.; Wu, S.; Wang, M.; et al. 25-Hydroxycholesterol regulates lysosome AMP kinase activation and metabolic reprogramming to educate immunosuppressive macrophages. Immunity 2024, 57, 1087–1104.e1087. [Google Scholar] [CrossRef]
- Sag, D.; Cekic, C.; Wu, R.; Linden, J.; Hedrick, C.C. The cholesterol transporter ABCG1 links cholesterol homeostasis and tumour immunity. Nat. Commun. 2015, 6, 6354. [Google Scholar] [CrossRef]
- Xu, H.; Zhou, S.; Tang, Q.; Xia, H.; Bi, F. Cholesterol metabolism: New functions and therapeutic approaches in cancer. Biochim. Biophys. Acta Rev. Cancer 2020, 1874, 188394. [Google Scholar] [CrossRef]
- Pradel, L.C.; Mitchell, A.J.; Zarubica, A.; Dufort, L.; Chasson, L.; Naquet, P.; Broccardo, C.; Chimini, G. ATP-binding cassette transporter hallmarks tissue macrophages and modulates cytokine-triggered polarization programs. Eur. J. Immunol. 2009, 39, 2270–2280. [Google Scholar] [CrossRef]
- Barnea-Zohar, M.; Winograd-Katz, S.E.; Shalev, M.; Arman, E.; Reuven, N.; Roth, L.; Golani, O.; Stein, M.; Thalji, F.; Kanaan, M.; et al. An SNX10-dependent mechanism downregulates fusion between mature osteoclasts. J. Cell Sci. 2021, 134, jcs254979. [Google Scholar] [CrossRef]
- Deng, R.R.; Yuan, Y.P. Ropivacaine inhibits the proliferation and metastasis of gastric cancer cells via the SNX10/SRC/STAT3 pathway. Chem. Biol. Drug Des. 2024, 103, e14405. [Google Scholar] [CrossRef]
- Settembre, C.; Perera, R.M. Lysosomes as coordinators of cellular catabolism, metabolic signalling and organ physiology. Nat. Rev. Mol. Cell Biol. 2024, 25, 223–245. [Google Scholar] [CrossRef] [PubMed]
- Bonam, S.R.; Wang, F.; Muller, S. Lysosomes as a therapeutic target. Nat. Rev. Drug Discov. 2019, 18, 923–948. [Google Scholar] [CrossRef] [PubMed]
- Bourdenx, M.; Daniel, J.; Genin, E.; Soria, F.N.; Blanchard-Desce, M.; Bezard, E.; Dehay, B. Nanoparticles restore lysosomal acidification defects: Implications for Parkinson and other lysosomal-related diseases. Autophagy 2016, 12, 472–483. [Google Scholar] [CrossRef] [PubMed]
- Nicoli, E.R.; Weston, M.R.; Hackbarth, M.; Becerril, A.; Larson, A.; Zein, W.M.; Baker, P.R., 2nd; Burke, J.D.; Dorward, H.; Davids, M.; et al. Lysosomal Storage and Albinism Due to Effects of a De Novo CLCN7 Variant on Lysosomal Acidification. Am. J. Hum. Genet. 2019, 104, 1127–1138. [Google Scholar] [CrossRef]
- Hosios, A.M.; Wilkinson, M.E.; McNamara, M.C.; Kalafut, K.C.; Torrence, M.E.; Asara, J.M.; Manning, B.D. mTORC1 regulates a lysosome-dependent adaptive shift in intracellular lipid species. Nat. Metab. 2022, 4, 1792–1811. [Google Scholar] [CrossRef]
- Liu, G.Y.; Sabatini, D.M. mTOR at the nexus of nutrition, growth, ageing and disease. Nat. Rev. Mol. Cell Biol. 2020, 21, 183–203. [Google Scholar] [CrossRef]
- Laplante, M.; Sabatini, D.M. mTOR signaling in growth control and disease. Cell 2012, 149, 274–293. [Google Scholar] [CrossRef]
- Li, T.Y.; Gao, A.W.; Li, X.; Li, H.; Liu, Y.J.; Lalou, A.; Neelagandan, N.; Naef, F.; Schoonjans, K.; Auwerx, J. V-ATPase/TORC1-mediated ATFS-1 translation directs mitochondrial UPR activation in C. elegans. J. Cell Biol. 2023, 222, e202205045. [Google Scholar] [CrossRef]
- Lim, C.Y.; Davis, O.B.; Shin, H.R.; Zhang, J.; Berdan, C.A.; Jiang, X.; Counihan, J.L.; Ory, D.S.; Nomura, D.K.; Zoncu, R. ER-lysosome contacts enable cholesterol sensing by mTORC1 and drive aberrant growth signalling in Niemann-Pick type C. Nat. Cell Biol. 2019, 21, 1206–1218. [Google Scholar] [CrossRef]
- Zhang, J.; Zeng, W.; Han, Y.; Lee, W.R.; Liou, J.; Jiang, Y. Lysosomal LAMP proteins regulate lysosomal pH by direct inhibition of the TMEM175 channel. Mol. Cell 2023, 83, 2524–2539.e2527. [Google Scholar] [CrossRef]
- Handy, J.; Macintosh, G.C.; Jenny, A. Ups and downs of lysosomal pH: Conflicting roles of LAMP proteins? Autophagy 2024, 20, 437–440. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Chen, J.; Liu, S.; Xu, H. The Acid Gate in the Lysosome. Autophagy 2023, 19, 1368–1370. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xia, L.; Wu, Y.; Zhou, H.; Chen, X.; Li, H.; Xu, M.; Qi, Z.; Wang, Z.; Sun, H.; et al. Programmed death ligand-1 regulates angiogenesis and metastasis by participating in the c-JUN/VEGFR2 signaling axis in ovarian cancer. Cancer Commun. 2021, 41, 511–527. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Pan, B.; Jia, H.; Zhang, Y.; Wang, S.; Wang, Y.; Zhang, S.; Li, M.; Wang, A.; Wang, X.; et al. PD-L1 expression in ovarian clear cell carcinoma using the 22C3 pharmDx assay. Diagn. Pathol. 2024, 19, 82. [Google Scholar] [CrossRef]
- Kloosterman, D.J.; Erbani, J.; Boon, M.; Farber, M.; Handgraaf, S.M.; Ando-Kuri, M.; Sánchez-López, E.; Fontein, B.; Mertz, M.; Nieuwland, M.; et al. Macrophage-mediated myelin recycling fuels brain cancer malignancy. Cell 2024, 187, 5336–5356.e5330. [Google Scholar] [CrossRef]
- Wang, J.; Yang, Y.; Shao, F.; Meng, Y.; Guo, D.; He, J.; Lu, Z. Acetate reprogrammes tumour metabolism and promotes PD-L1 expression and immune evasion by upregulating c-Myc. Nat. Metab. 2024, 6, 914–932. [Google Scholar] [CrossRef]
- Fan, Y.; Dan, W.; Wang, Y.; Ma, Z.; Jian, Y.; Liu, T.; Li, M.; Wang, Z.; Wei, Y.; Liu, B.; et al. Itaconate transporter SLC13A3 confers immunotherapy resistance via alkylation-mediated stabilization of PD-L1. Cell Metab. 2025, 37, 514–526.e515. [Google Scholar] [CrossRef]

| Gene Name | Primer Sequences (5′ to 3′) |
|---|---|
| PD-L1 | Forward: GCATTTGCTGAACGCATTTACT Reverse: ATTTCCCAGGGAGAGCTGGT |
| PD1 | Forward: CCAAGGCGCAGATCAAAGAG Reverse: TGGCTCCTATTGTCCCTCGTG |
| CD80 | Forward: GGGGAAATGCGCCTCTCTG Reverse: GTGGATTTAGTTTCACAGCTTGC |
| iNOS | Forward: TCCCGAGTCAGAGTCACCAT Reverse: CATGCAGACAACCTTGGGGT |
| CD206 | Forward: GTGATGGGACCCCTGTAACG Reverse: CTGCCCAGTACCCATCCTTG |
| IL-12A | Forward: AAAGATAAAACCAGCACAGTGGAG Reverse: CCAGGCAACTCCCATTAGTTAT |
| IL-10 | Forward: AGGGCACCCAGTCTGAGAAC Reverse: GGCAACCCAGGTAACCCTTAAAGT |
| VEGF | Forward: TAAGTCCTGGAGCGTTCCCT Reverse: ACGCGAGTCTGTGTTTTTGC |
| PLIN2 | Forward: ACAACCGAGTGTGGTGACTC Reverse: TCTTCACACCGTTCTCTGCC |
| MMP2 | Forward: AAGTATGGGAACGCCGATGG Reverse: GCCGTACTTGCCATCCTTCT |
| MMP9 | Forward: GGACAAGCTCTTCGGCTTCT Reverse: TCGCTGGTACAGGTCGAGTA |
| GAPDH | Forward: TGATGACATCAAGAAGGTGGTGAAG Reverse: TCCTTGGAGGCCATGTGGGCCAT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chai, R.; Zheng, K.; Xu, T.; Wang, H.; Cheng, X.; Lu, C.; Kang, Y. SNX10 Is Involved in Ovarian Cancer Cell Metastasis by Repolarizing Tumor-Associated Macrophages Through mTOR1/Lysosomes Pathway. Biomedicines 2025, 13, 1021. https://doi.org/10.3390/biomedicines13051021
Chai R, Zheng K, Xu T, Wang H, Cheng X, Lu C, Kang Y. SNX10 Is Involved in Ovarian Cancer Cell Metastasis by Repolarizing Tumor-Associated Macrophages Through mTOR1/Lysosomes Pathway. Biomedicines. 2025; 13(5):1021. https://doi.org/10.3390/biomedicines13051021
Chicago/Turabian StyleChai, Ranran, Kewei Zheng, Ting Xu, Hui Wang, Xiaobo Cheng, Chong Lu, and Yu Kang. 2025. "SNX10 Is Involved in Ovarian Cancer Cell Metastasis by Repolarizing Tumor-Associated Macrophages Through mTOR1/Lysosomes Pathway" Biomedicines 13, no. 5: 1021. https://doi.org/10.3390/biomedicines13051021
APA StyleChai, R., Zheng, K., Xu, T., Wang, H., Cheng, X., Lu, C., & Kang, Y. (2025). SNX10 Is Involved in Ovarian Cancer Cell Metastasis by Repolarizing Tumor-Associated Macrophages Through mTOR1/Lysosomes Pathway. Biomedicines, 13(5), 1021. https://doi.org/10.3390/biomedicines13051021






