Lysosomal Stress in Cardiovascular Diseases: Therapeutic Potential of Cardiovascular Drugs and Future Directions
Abstract
1. Introduction
2. Lysosomal Dysfunction, NLRP3 Inflammasome, and CVDs: Mechanisms and Therapeutic Insights

2.1. Cardiovascular Drugs Targeting Lysosomal Stress and NLRP3 Inflammasome Activation
2.1.1. Statins
2.1.2. SGLT2 Inhibitors
2.1.3. Vitamin E
2.2. Regulatory Complex and CVD
2.3. Uric Acid, Lysosomal Dysfunction, and NLRP3 Inflammasome Activation in Cardiovascular Disease
2.4. PCSK9 Inhibition and Its Potential Impact on Lysosomal Function
3. TFEB: A Key Player in Lysosomal Stress and Cardiovascular Therapy
3.1. Role of TFEB in Atherosclerosis and Endothelial Damage
3.2. TFEB in Vascular Smooth Muscle Cells and Plaque Stability
3.3. Cardiovascular Drugs Enhancing TFEB Activity
3.3.1. Statins
3.3.2. TRPML1 Agonists
3.3.3. Trehalose
3.3.4. Resveratrol (RSV)
3.3.5. Curcumin (Cur)
3.3.6. Traditional Chinese Medicine: Dehydroandrographolide (DA)
3.4. Eicosapentaenoic Acid (EPA) and Lysosomal Homeostasis
4. Ferroptosis in Cardiovascular Disease: From Plaque Destabilization to Myocardial Injury
4.1. Cardiovascular Drugs Targeting Ferroptosis and Lysosomal Stress
4.1.1. GLS1 Activators
4.1.2. Icariin
4.1.3. Cur
4.1.4. Other Therapeutic Approaches
5. Future Challenges in Bridging Experimental and Clinical Research on Lysosomal Dysfunction
5.1. Advancing Lysosome-Targeted Therapies
5.2. Overcoming Challenges in NLRP3 Inflammasome Inhibition
5.3. Addressing the Role of Ferroptosis in Cardiovascular Disease
5.4. Future Perspectives and Translational Research
- Clinical Trials and Drug Optimization: Expanding human trials for lysosome-modulating drugs, including TFEB activators, TRPML1 agonists, and ferroptosis inhibitors, is crucial for clinical translation.
- Personalized Medicine Approaches: Identifying patient subgroups with heightened lysosomal stress or inflammasome activation could allow for targeted interventions tailored to specific cardiovascular conditions.
- Biomarker Development: Establishing reliable biomarkers for lysosomal dysfunction, NLRP3 activation, and ferroptosis will aid in disease diagnosis, treatment monitoring, and therapy selection.
- NLRP3 Inflammasome Inhibition: Statins, SGLT2 inhibitors, and Vitamin E suppress NLRP3 activation, thereby reducing inflammation.
- TFEB Activation: Statins, TRPML1 agonists, trehalose, resveratrol, curcumin, and dehydroandrographolide (DA) enhance lysosomal biogenesis and autophagic clearance.
- Ferroptosis Modulation: GLS1 activators, icariin, and curcumin, regulate iron-dependent cell death, thereby protecting against cardiovascular damage.
- Lysosomal Repair Mechanisms: TRPML1 activation and lysophagy modulation stabilize lysosomal membranes and restore function.
6. Lysosomal Dysfunction and Cellular Senescence: Implications for Cardiovascular Health
6.1. Lysosomal Dysfunction in Cellular Senescence
6.2. Cardiovascular Drugs Targeting Senescence via Lysosomal Pathways
- Statins: These are used to lower lipid levels. Statins have demonstrated the ability to inhibit mTORC1 activation by reducing intracellular cholesterol levels. This indirect modulation of SASP secretion may help mitigate chronic atherosclerotic inflammation.
- SGLT2 Inhibitors: By activating autophagic flux, SGLT2 inhibitors restore lysosomal function, reduce oxidative stress, and enhance cellular resilience against inflammation, offering potential benefits to patients with CVDs and diabetes [112].
- mTOR Inhibitors: Drugs such as rapamycin directly inhibit mTORC1 activity, reduce SASP secretion, and promote autophagy and lysosomal biogenesis, making them significant candidates for CVD treatment.
6.3. Targeting GLS1 for Cardiovascular Benefits
7. Lysosomal Stress and the Role of V-ATPase in Lipid-Induced Cardiac Dysfunction
8. Apolipoprotein M (ApoM) and Lysosomal Function in Cardiovascular Disease
9. Conclusions
9.1. Pharmacological Approaches
9.2. Emerging Therapeutic Targets
10. Future Directions in Lysosome-Targeted Cardiovascular Therapies
10.1. Advancing Lysosomal-Targeted Pharmacological Therapies
10.2. Clinical Trials on Lysosome-Targeted Therapies in CVD
10.3. Exploring Novel Biomarkers and Diagnostic Tools for Lysosomal Dysfunction
10.4. Translating Lysosome-Targeted Strategies into Clinical Practice
10.5. Integrating Lysosomal Dysfunction into the Broader Landscape of CVD Pathophysiology
| Category | Key Focus Areas |
|---|---|
| Advancing Lysosomal-Targeted Pharmacological Therapies | Clinical validation of TFEB activators, TRPML1 agonists, and NLRP3 inhibitors. Development of combination therapies with existing cardiovascular drugs. Optimization of dosing strategies to prevent maladaptive autophagy. |
| Exploring Novel Biomarkers and Diagnostic Tools | Identification of circulating lysosomal biomarkers (cathepsins, LAMP2, TFEB activity). Development of advanced imaging techniques (PET tracers, MRI-based lysosomal assessment). |
| Translating Strategies into Clinical Practice | Investigation of lysosomal dysfunction in heart failure subtypes (HFpEF vs. HFrEF). Evaluation of lysosomal-targeted therapies in randomized clinical trials. Integration of lysosomal biomarkers into cardiovascular risk stratification. |
| Expanding the Role of Lysosomal Dysfunction in CVD Pathophysiology | Examination of the interplay between lysosomal stress, ferroptosis, and cellular senescence. Investigation of lysosomal impairment in age-related cardiovascular diseases (e.g., atherosclerosis, diabetic cardiomyopathy). |
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AMPK | Adenosine monophosphate-activated protein kinase |
| CHAOS | Cambridge Heart Antioxidant Study |
| CVD | Cardiovascular disease |
| ER | Endoplasmic reticulum |
| HF | Heart failure |
| ROS | Reactive oxygen species |
| SASP | Senescence-associated secretory phenotype |
| UA | Uric acid |
References
- Benjamin, E.J.; Muntner, P.; Alonso, A.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Das, S.R.; et al. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation 2019, 139, e56–e528. [Google Scholar] [CrossRef]
- Shirakawa, K.; Yan, X.; Shinmura, K.; Endo, J.; Kataoka, M.; Katsumata, Y.; Yamamoto, T.; Anzai, A.; Isobe, S.; Yoshida, N.; et al. Obesity accelerates T cell senescence in murine visceral adipose tissue. J. Clin. Investig. 2016, 126, 4626–4639. [Google Scholar] [CrossRef]
- Otoda, T.; Takamura, T.; Misu, H.; Ota, T.; Murata, S.; Hayashi, H.; Takayama, H.; Kikuchi, A.; Kanamori, T.; Shima, K.R.; et al. Proteasome dysfunction mediates obesity-induced endoplasmic reticulum stress and insulin resistance in the liver. Diabetes 2013, 62, 811–824. [Google Scholar] [CrossRef] [PubMed]
- Szendroedi, J.; Phielix, E.; Roden, M. The role of mitochondria in insulin resistance and type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2011, 8, 92–103. [Google Scholar] [CrossRef]
- Kim, J.; Kim, S.H.; Kang, H.; Lee, S.; Park, S.Y.; Cho, Y.; Lim, Y.M.; Ahn, J.W.; Kim, Y.H.; Chung, S.; et al. TFEB-GDF15 axis protects against obesity and insulin resistance as a lysosomal stress response. Nat. Metab. 2021, 3, 410–427. [Google Scholar] [CrossRef] [PubMed]
- Saftig, P.; Klumperman, J. Lysosome biogenesis and lysosomal membrane proteins: Trafficking meets function. Nat. Rev. Mol. Cell Biol. 2009, 10, 623–635. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. Inflammation in atherosclerosis. Nature 2002, 420, 868–874. [Google Scholar] [CrossRef]
- Mizushima, N.; Komatsu, M. Autophagy: Renovation of cells and tissues. Cell 2011, 147, 728–741. [Google Scholar] [CrossRef]
- Ballabio, A.; Bonifacino, J.S. Lysosomes as dynamic regulators of cell and organismal homeostasis. Nat. Rev. Mol. Cell Biol. 2020, 21, 101–118. [Google Scholar] [CrossRef]
- Inpanathan, S.; Botelho, R.J. The Lysosome Signaling Platform: Adapting With the Times. Front. Cell Dev. Biol. 2019, 7, 113. [Google Scholar] [CrossRef]
- Serrano-Puebla, A.; Boya, P. Lysosomal membrane permeabilization in cell death: New evidence and implications for health and disease. Ann. N. Y. Acad. Sci. 2016, 1371, 30–44. [Google Scholar] [CrossRef]
- Yang, H.; Tan, J.X. Lysosomal quality control: Molecular mechanisms and therapeutic implications. Trends Cell Biol. 2023, 33, 749–764. [Google Scholar] [CrossRef]
- Gupta, A.; Luthra, S.R.; Luthra, S. Fabry Disease in a Female: A Unique Case Highlighting the Variability in Clinical Presentation. Cureus 2024, 16, e70406. [Google Scholar] [CrossRef] [PubMed]
- Roh, K.; Noh, J.; Kim, Y.; Jang, Y.; Kim, J.; Choi, H.; Lee, Y.; Ji, M.; Kang, D.; Kim, M.S.; et al. Lysosomal control of senescence and inflammation through cholesterol partitioning. Nat. Metab. 2023, 5, 398–413. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, R.E.; Zoncu, R. The lysosome as a cellular centre for signalling, metabolism and quality control. Nat. Cell Biol. 2019, 21, 133–142. [Google Scholar] [CrossRef]
- Sciarretta, S.; Forte, M.; Frati, G.; Sadoshima, J. The complex network of mTOR signalling in the heart. Cardiovasc. Res. 2022, 118, 424–439. [Google Scholar] [CrossRef]
- Aits, S.; Jaattela, M. Lysosomal cell death at a glance. J. Cell Sci. 2013, 126, 1905–1912. [Google Scholar] [CrossRef] [PubMed]
- Duewell, P.; Kono, H.; Rayner, K.J.; Sirois, C.M.; Vladimer, G.; Bauernfeind, F.G.; Abela, G.S.; Franchi, L.; Nunez, G.; Schnurr, M.; et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 2010, 464, 1357–1361. [Google Scholar] [CrossRef] [PubMed]
- Karasawa, T.; Kawashima, A.; Usui-Kawanishi, F.; Watanabe, S.; Kimura, H.; Kamata, R.; Shirasuna, K.; Koyama, Y.; Sato-Tomita, A.; Matsuzaka, T.; et al. Saturated Fatty Acids Undergo Intracellular Crystallization and Activate the NLRP3 Inflammasome in Macrophages. Arter. Thromb. Vasc. Biol. 2018, 38, 744–756. [Google Scholar] [CrossRef]
- Hornung, V.; Bauernfeind, F.; Halle, A.; Samstad, E.O.; Kono, H.; Rock, K.L.; Fitzgerald, K.A.; Latz, E. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat. Immunol. 2008, 9, 847–856. [Google Scholar] [CrossRef]
- Ono, H.; Ohta, R.; Kawasaki, Y.; Niwa, A.; Takada, H.; Nakahata, T.; Ohga, S.; Saito, M.K. Lysosomal membrane permeabilization causes secretion of IL-1beta in human vascular smooth muscle cells. Inflamm. Res. 2018, 67, 879–889. [Google Scholar] [CrossRef] [PubMed]
- Kurz, T.; Terman, A.; Gustafsson, B.; Brunk, U.T. Lysosomes in iron metabolism, ageing and apoptosis. Histochem. Cell Biol. 2008, 129, 389–406. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Choe, J.Y.; Kim, J.W.; Park, K.Y. HMG-CoA Reductase Inhibitors Suppress Monosodium Urate-Induced NLRP3 Inflammasome Activation through Peroxisome Proliferator-Activated Receptor-gamma Activation in THP-1 Cells. Pharmaceuticals 2023, 16, 522. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.T.; Koka, S.; Zhang, Y.; Hussain, T.; Li, X. Simvastatin improves lysosome function via enhancing lysosome biogenesis in endothelial cells. Front. Biosci. 2020, 25, 283–298. [Google Scholar] [CrossRef]
- Heerspink, H.J.L.; Stefansson, B.V.; Correa-Rotter, R.; Chertow, G.M.; Greene, T.; Hou, F.F.; Mann, J.F.E.; McMurray, J.J.V.; Lindberg, M.; Rossing, P.; et al. Dapagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2020, 383, 1436–1446. [Google Scholar] [CrossRef]
- Yurista, S.R.; Nguyen, C.T.; Rosenzweig, A.; de Boer, R.A.; Westenbrink, B.D. Ketone bodies for the failing heart: Fuels that can fix the engine? Trends Endocrinol. Metab. 2021, 32, 814–826. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.W.; Que, J.Q.; Liu, S.; Huang, K.Y.; Qian, L.; Weng, Y.B.; Rong, F.N.; Wang, L.; Zhou, Y.Y.; Xue, Y.J.; et al. Sodium-Glucose Co-transporter-2 Inhibitor of Dapagliflozin Attenuates Myocardial Ischemia/Reperfusion Injury by Limiting NLRP3 Inflammasome Activation and Modulating Autophagy. Front. Cardiovasc. Med. 2021, 8, 768214. [Google Scholar] [CrossRef]
- Traber, M.G.; Atkinson, J. Vitamin E, antioxidant and nothing more. Free Radic. Biol. Med. 2007, 43, 4–15. [Google Scholar] [CrossRef]
- Princen, H.M.; van Duyvenvoorde, W.; Buytenhek, R.; van der Laarse, A.; van Poppel, G.; Gevers Leuven, J.A.; van Hinsbergh, V.W. Supplementation with low doses of vitamin E protects LDL from lipid peroxidation in men and women. Arter. Thromb. Vasc. Biol. 1995, 15, 325–333. [Google Scholar] [CrossRef]
- Stephens, N.G.; Parsons, A.; Schofield, P.M.; Kelly, F.; Cheeseman, K.; Mitchinson, M.J. Randomised controlled trial of vitamin E in patients with coronary disease: Cambridge Heart Antioxidant Study (CHAOS). Lancet 1996, 347, 781–786. [Google Scholar] [CrossRef]
- Boaz, M.; Smetana, S.; Weinstein, T.; Matas, Z.; Gafter, U.; Iaina, A.; Knecht, A.; Weissgarten, Y.; Brunner, D.; Fainaru, M.; et al. Secondary prevention with antioxidants of cardiovascular disease in endstage renal disease (SPACE): Randomised placebo-controlled trial. Lancet 2000, 356, 1213–1218. [Google Scholar] [CrossRef] [PubMed]
- Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: Results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico. Lancet 1999, 354, 447–455. [Google Scholar]
- Lonn, E.; Yusuf, S.; Dzavik, V.; Doris, C.; Yi, Q.; Smith, S.; Moore-Cox, A.; Bosch, J.; Riley, W.; Teo, K.; et al. Effects of ramipril and vitamin E on atherosclerosis: The study to evaluate carotid ultrasound changes in patients treated with ramipril and vitamin E (SECURE). Circulation 2001, 103, 919–925. [Google Scholar] [CrossRef] [PubMed]
- Chiabrando, C.; Avanzini, F.; Rivalta, C.; Colombo, F.; Fanelli, R.; Palumbo, G.; Roncaglioni, M.C.; PPP Collaborative Group on the antioxidant effect of vitamin E. Long-term vitamin E supplementation fails to reduce lipid peroxidation in people at cardiovascular risk: Analysis of underlying factors. Curr. Control. Trials Cardiovasc. Med. 2002, 3, 5. [Google Scholar] [CrossRef]
- Levy, A.P.; Friedenberg, P.; Lotan, R.; Ouyang, P.; Tripputi, M.; Higginson, L.; Cobb, F.R.; Tardif, J.C.; Bittner, V.; Howard, B.V. The effect of vitamin therapy on the progression of coronary artery atherosclerosis varies by haptoglobin type in postmenopausal women. Diabetes Care 2004, 27, 925–930. [Google Scholar] [CrossRef][Green Version]
- Bar-Peled, L.; Sabatini, D.M. Regulation of mTORC1 by amino acids. Trends Cell Biol. 2014, 24, 400–406. [Google Scholar] [CrossRef]
- Yonehara, R.; Nada, S.; Nakai, T.; Nakai, M.; Kitamura, A.; Ogawa, A.; Nakatsumi, H.; Nakayama, K.I.; Li, S.; Standley, D.M.; et al. Structural basis for the assembly of the Ragulator-Rag GTPase complex. Nat. Commun. 2017, 8, 1625. [Google Scholar] [CrossRef]
- Kimura, T.; Nada, S.; Takegahara, N.; Okuno, T.; Nojima, S.; Kang, S.; Ito, D.; Morimoto, K.; Hosokawa, T.; Hayama, Y.; et al. Polarization of M2 macrophages requires Lamtor1 that integrates cytokine and amino-acid signals. Nat. Commun. 2016, 7, 13130. [Google Scholar] [CrossRef]
- Hayama, Y.; Kimura, T.; Takeda, Y.; Nada, S.; Koyama, S.; Takamatsu, H.; Kang, S.; Ito, D.; Maeda, Y.; Nishide, M.; et al. Lysosomal Protein Lamtor1 Controls Innate Immune Responses via Nuclear Translocation of Transcription Factor EB. J. Immunol. 2018, 200, 3790–3800. [Google Scholar] [CrossRef]
- Tsujimoto, K.; Jo, T.; Nagira, D.; Konaka, H.; Park, J.H.; Yoshimura, S.I.; Ninomiya, A.; Sugihara, F.; Hirayama, T.; Itotagawa, E.; et al. The lysosomal Ragulator complex activates NLRP3 inflammasome in vivo via HDAC6. EMBO J. 2023, 42, e111389. [Google Scholar] [CrossRef]
- Martinon, F.; Petrilli, V.; Mayor, A.; Tardivel, A.; Tschopp, J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 2006, 440, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Braga, T.T.; Forni, M.F.; Correa-Costa, M.; Ramos, R.N.; Barbuto, J.A.; Branco, P.; Castoldi, A.; Hiyane, M.I.; Davanso, M.R.; Latz, E.; et al. Soluble Uric Acid Activates the NLRP3 Inflammasome. Sci. Rep. 2017, 7, 39884. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ma, Y.; Cao, R.; Wang, G.; Li, S.; Cao, Y.; Zhang, H.; Liu, M.; Liu, G.; Zhang, J.; et al. Soluble uric acid induces myocardial damage through activating the NLRP3 inflammasome. J. Cell. Mol. Med. 2020, 24, 8849–8861. [Google Scholar] [CrossRef] [PubMed]
- Neogi, T. Clinical practice. Gout. N. Engl. J. Med. 2011, 364, 443–452. [Google Scholar] [CrossRef]
- Lima, H., Jr.; Jacobson, L.S.; Goldberg, M.F.; Chandran, K.; Diaz-Griffero, F.; Lisanti, M.P.; Brojatsch, J. Role of lysosome rupture in controlling Nlrp3 signaling and necrotic cell death. Cell Cycle 2013, 12, 1868–1878. [Google Scholar] [CrossRef]
- Franklin, B.S.; Mangan, M.S.; Latz, E. Crystal Formation in Inflammation. Annu. Rev. Immunol. 2016, 34, 173–202. [Google Scholar] [CrossRef]
- Okada, M.; Matsuzawa, A.; Yoshimura, A.; Ichijo, H. The lysosome rupture-activated TAK1-JNK pathway regulates NLRP3 inflammasome activation. J. Biol. Chem. 2014, 289, 32926–32936. [Google Scholar] [CrossRef]
- Crisan, T.O.; Cleophas, M.C.; Oosting, M.; Lemmers, H.; Toenhake-Dijkstra, H.; Netea, M.G.; Jansen, T.L.; Joosten, L.A. Soluble uric acid primes TLR-induced proinflammatory cytokine production by human primary cells via inhibition of IL-1Ra. Ann. Rheum. Dis. 2016, 75, 755–762. [Google Scholar] [CrossRef]
- Zhong, Z.; Liang, S.; Sanchez-Lopez, E.; He, F.; Shalapour, S.; Lin, X.J.; Wong, J.; Ding, S.; Seki, E.; Schnabl, B.; et al. New mitochondrial DNA synthesis enables NLRP3 inflammasome activation. Nature 2018, 560, 198–203. [Google Scholar] [CrossRef]
- Johnson, R.J.; Lanaspa, M.A.; Gaucher, E.A. Uric acid: A danger signal from the RNA world that may have a role in the epidemic of obesity, metabolic syndrome, and cardiorenal disease: Evolutionary considerations. Semin. Nephrol. 2011, 31, 394–399. [Google Scholar] [CrossRef]
- O’Donoghue, M.L.; Fazio, S.; Giugliano, R.P.; Stroes, E.S.G.; Kanevsky, E.; Gouni-Berthold, I.; Im, K.; Lira Pineda, A.; Wasserman, S.M.; Ceska, R.; et al. Lipoprotein(a), PCSK9 Inhibition, and Cardiovascular Risk. Circulation 2019, 139, 1483–1492. [Google Scholar] [CrossRef] [PubMed]
- Mullard, A. Nine paths to PCSK9 inhibition. Nat. Rev. Drug Discov. 2017, 16, 299–301. [Google Scholar] [CrossRef] [PubMed]
- Oyama, K.; Furtado, R.H.M.; Fagundes, A., Jr.; Zelniker, T.A.; Tang, M.; Kuder, J.; Murphy, S.A.; Hamer, A.; Wang, H.; Keech, A.C.; et al. Effect of Evolocumab on Complex Coronary Disease Requiring Revascularization. J. Am. Coll. Cardiol. 2021, 77, 259–267. [Google Scholar] [CrossRef]
- Schwartz, G.G.; Steg, P.G.; Szarek, M.; Bittner, V.A.; Diaz, R.; Goodman, S.G.; Kim, Y.U.; Jukema, J.W.; Pordy, R.; Roe, M.T.; et al. Peripheral Artery Disease and Venous Thromboembolic Events After Acute Coronary Syndrome: Role of Lipoprotein(a) and Modification by Alirocumab: Prespecified Analysis of the Odyssey Outcomes Randomized Clinical Trial. Circulation 2020, 141, 1608–1617. [Google Scholar] [CrossRef]
- Ding, Z.; Wang, X.; Liu, S.; Zhou, S.; Kore, R.A.; Mu, S.; Deng, X.; Fan, Y.; Mehta, J.L. NLRP3 inflammasome via IL-1beta regulates PCSK9 secretion. Theranostics 2020, 10, 7100–7110. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, P.; Chen, Q.; Huang, Z.; Zou, D.; Zhang, J.; Gao, X.; Lin, Z. Mitochondrial ROS promote macrophage pyroptosis by inducing GSDMD oxidation. J. Mol. Cell Biol. 2019, 11, 1069–1082. [Google Scholar] [CrossRef]
- Cao, J.; Yu, H.; Wu, Y.; Wang, X. Occurrence and Biological Activities of Phenylpropionyl Iridoids. Mini Rev. Med. Chem. 2019, 19, 292–309. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.L.; Xiao, L.L.; Tang, Z.H.; Jiang, Z.S.; Liu, M.H. Role of PCSK9 in lipid metabolism and atherosclerosis. Biomed. Pharmacother. 2018, 104, 36–44. [Google Scholar] [CrossRef]
- Giunzioni, I.; Tavori, H.; Covarrubias, R.; Major, A.S.; Ding, L.; Zhang, Y.; DeVay, R.M.; Hong, L.; Fan, D.; Predazzi, I.M.; et al. Local effects of human PCSK9 on the atherosclerotic lesion. J. Pathol. 2016, 238, 52–62. [Google Scholar] [CrossRef]
- Ding, Z.; Liu, S.; Wang, X.; Deng, X.; Fan, Y.; Shahanawaz, J.; Shmookler Reis, R.J.; Varughese, K.I.; Sawamura, T.; Mehta, J.L. Cross-talk between LOX-1 and PCSK9 in vascular tissues. Cardiovasc. Res. 2015, 107, 556–567. [Google Scholar] [CrossRef]
- Kong, N.; Xu, Q.; Cui, W.; Feng, X.; Gao, H. PCSK9 inhibitor inclisiran for treating atherosclerosis via regulation of endothelial cell pyroptosis. Ann. Transl. Med. 2022, 10, 1205. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Tao, J.; Xi, L.; Wang, Z.; Liu, L. PCSK9 mediates the oxidative low--density lipoprotein--induced pyroptosis of vascular endothelial cells via the UQCRC1/ROS pathway. Int. J. Mol. Med. 2021, 47, 53. [Google Scholar] [CrossRef] [PubMed]
- Settembre, C.; Fraldi, A.; Medina, D.L.; Ballabio, A. Signals from the lysosome: A control centre for cellular clearance and energy metabolism. Nat. Rev. Mol. Cell Biol. 2013, 14, 283–296. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, G.; Ballabio, A. TFEB at a glance. J Cell Sci 2016, 129, 2475–2481. [Google Scholar] [CrossRef]
- Martini-Stoica, H.; Xu, Y.; Ballabio, A.; Zheng, H. The Autophagy-Lysosomal Pathway in Neurodegeneration: A TFEB Perspective. Trends Neurosci. 2016, 39, 221–234. [Google Scholar] [CrossRef]
- Nezich, C.L.; Wang, C.; Fogel, A.I.; Youle, R.J. MiT/TFE transcription factors are activated during mitophagy downstream of Parkin and Atg5. J. Cell Biol. 2015, 210, 435–450. [Google Scholar] [CrossRef]
- Sun, X.J.; Xiao, S.J.; Ma, W.Q.; Jin, H.; Ren, L.Q.; Yao, Y.Y.; Chen, Z.D.; Li, X.X.; Chen, T.; Liu, N.F. Activation of TFEB protects against diabetic vascular calcification by improving autophagic flux and activating Nrf2 antioxidant system. Am. J. Physiol. Endocrinol. Metab. 2025; in press. [Google Scholar] [CrossRef]
- Sun, J.; Lu, H.; Liang, W.; Zhao, G.; Ren, L.; Hu, D.; Chang, Z.; Liu, Y.; Garcia-Barrio, M.T.; Zhang, J.; et al. Endothelial TFEB (Transcription Factor EB) Improves Glucose Tolerance via Upregulation of IRS (Insulin Receptor Substrate) 1 and IRS2. Arter. Thromb. Vasc. Biol. 2021, 41, 783–795. [Google Scholar] [CrossRef]
- Liu, X.; Zheng, X.; Lu, Y.; Chen, Q.; Zheng, J.; Zhou, H. TFEB Dependent Autophagy-Lysosomal Pathway: An Emerging Pharmacological Target in Sepsis. Front. Pharmacol. 2021, 12, 794298. [Google Scholar] [CrossRef]
- Bhuiyan, M.S.; Pattison, J.S.; Osinska, H.; James, J.; Gulick, J.; McLendon, P.M.; Hill, J.A.; Sadoshima, J.; Robbins, J. Enhanced autophagy ameliorates cardiac proteinopathy. J. Clin. Investig. 2013, 123, 5284–5297. [Google Scholar] [CrossRef]
- Franco-Juarez, B.; Coronel-Cruz, C.; Hernandez-Ochoa, B.; Gomez-Manzo, S.; Cardenas-Rodriguez, N.; Arreguin-Espinosa, R.; Bandala, C.; Canseco-Avila, L.M.; Ortega-Cuellar, D. TFEB; Beyond Its Role as an Autophagy and Lysosomes Regulator. Cells 2022, 11, 3153. [Google Scholar] [CrossRef] [PubMed]
- Emanuel, R.; Sergin, I.; Bhattacharya, S.; Turner, J.; Epelman, S.; Settembre, C.; Diwan, A.; Ballabio, A.; Razani, B. Induction of lysosomal biogenesis in atherosclerotic macrophages can rescue lipid-induced lysosomal dysfunction and downstream sequelae. Arter. Thromb. Vasc. Biol. 2014, 34, 1942–1952. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Ouyang, C.; Zhang, H.; Gu, Y.; Deng, Y.; Du, C.; Cui, C.; Li, S.; Wang, W.; Kong, W.; et al. Vascular smooth muscle cell-derived hydrogen sulfide promotes atherosclerotic plaque stability via TFEB (transcription factor EB)-mediated autophagy. Autophagy 2022, 18, 2270–2287. [Google Scholar] [CrossRef]
- Bruiners, N.; Dutta, N.K.; Guerrini, V.; Salamon, H.; Yamaguchi, K.D.; Karakousis, P.C.; Gennaro, M.L. The anti-tubercular activity of simvastatin is mediated by cholesterol-driven autophagy via the AMPK-mTORC1-TFEB axis. J. Lipid Res. 2020, 61, 1617–1628. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Chou, W.C.; Tao, L.; Qiu, Z.; Gao, G. Atorvastatin Ameliorates Doxorubicin-Induced Cardiomyopathy by Regulating the Autophagy-Lysosome Pathway and Its Upstream Regulatory Factor Transcription Factor EB. J. Cardiovasc. Pharmacol. 2022, 80, 732–738. [Google Scholar] [CrossRef]
- Lee, C.; Lamech, L.; Johns, E.; Overholtzer, M. Selective Lysosome Membrane Turnover Is Induced by Nutrient Starvation. Dev. Cell 2020, 55, 289–297.e284. [Google Scholar] [CrossRef]
- Nakamura, S.; Shigeyama, S.; Minami, S.; Shima, T.; Akayama, S.; Matsuda, T.; Esposito, A.; Napolitano, G.; Kuma, A.; Namba-Hamano, T.; et al. LC3 lipidation is essential for TFEB activation during the lysosomal damage response to kidney injury. Nat. Cell Biol. 2020, 22, 1252–1263. [Google Scholar] [CrossRef]
- Sergin, I.; Evans, T.D.; Zhang, X.; Bhattacharya, S.; Stokes, C.J.; Song, E.; Ali, S.; Dehestani, B.; Holloway, K.B.; Micevych, P.S.; et al. Exploiting macrophage autophagy-lysosomal biogenesis as a therapy for atherosclerosis. Nat. Commun. 2017, 8, 15750. [Google Scholar] [CrossRef]
- Jeong, S.J.; Stitham, J.; Evans, T.D.; Zhang, X.; Rodriguez-Velez, A.; Yeh, Y.S.; Tao, J.; Takabatake, K.; Epelman, S.; Lodhi, I.J.; et al. Trehalose causes low-grade lysosomal stress to activate TFEB and the autophagy-lysosome biogenesis response. Autophagy 2021, 17, 3740–3752. [Google Scholar] [CrossRef]
- Sciarretta, S.; Yee, D.; Nagarajan, N.; Bianchi, F.; Saito, T.; Valenti, V.; Tong, M.; Del Re, D.P.; Vecchione, C.; Schirone, L.; et al. Trehalose-Induced Activation of Autophagy Improves Cardiac Remodeling After Myocardial Infarction. J. Am. Coll. Cardiol. 2018, 71, 1999–2010. [Google Scholar] [CrossRef]
- Fan, E.; Zhang, L.; Jiang, S.; Bai, Y. Beneficial effects of resveratrol on atherosclerosis. J. Med. Food 2008, 11, 610–614. [Google Scholar] [CrossRef] [PubMed]
- Shao, R.; Shi, J.; Du, K.; Wang, N.; Cai, W.; Liu, S.; Ding, Z.; Wang, Y.; Li, D. Resveratrol promotes lysosomal function via ER calcium-dependent TFEB activation to ameliorate lipid accumulation. Biochem. J. 2021, 478, 1159–1173. [Google Scholar] [CrossRef] [PubMed]
- Medina, D.L.; Di Paola, S.; Peluso, I.; Armani, A.; De Stefani, D.; Venditti, R.; Montefusco, S.; Scotto-Rosato, A.; Prezioso, C.; Forrester, A.; et al. Lysosomal calcium signalling regulates autophagy through calcineurin and TFEB. Nat. Cell Biol. 2015, 17, 288–299. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Yang, J.; Zhou, M.; Zhang, Y.; Liu, Y.; Hou, P.; Zeng, X.; Yi, L.; Mi, M. Resveratrol attenuates endothelial oxidative injury by inducing autophagy via the activation of transcription factor EB. Nutr. Metab. 2019, 16, 42. [Google Scholar] [CrossRef]
- Li, X.; Zhu, R.; Jiang, H.; Yin, Q.; Gu, J.; Chen, J.; Ji, X.; Wu, X.; Fu, H.; Wang, H.; et al. Autophagy enhanced by curcumin ameliorates inflammation in atherogenesis via the TFEB-P300-BRD4 axis. Acta Pharm. Sin. B 2022, 12, 2280–2299. [Google Scholar] [CrossRef]
- Duan, Y.; Huang, P.; Sun, L.; Wang, P.; Cai, Y.; Shi, T.; Li, Y.; Zhou, Y.; Yu, S. Dehydroandrographolide ameliorates doxorubicin-mediated cardiotoxicity by regulating autophagy through the mTOR-TFEB pathway. Chem. Biol. Interact. 2024, 399, 111132. [Google Scholar] [CrossRef]
- Bhatt, D.L.; Steg, P.G.; Miller, M.; Brinton, E.A.; Jacobson, T.A.; Ketchum, S.B.; Doyle, R.T., Jr.; Juliano, R.A.; Jiao, L.; Granowitz, C.; et al. Cardiovascular Risk Reduction with Icosapent Ethyl for Hypertriglyceridemia. N. Engl. J. Med. 2019, 380, 11–22. [Google Scholar] [CrossRef]
- Nicholls, S.J.; Lincoff, A.M.; Garcia, M.; Bash, D.; Ballantyne, C.M.; Barter, P.J.; Davidson, M.H.; Kastelein, J.J.P.; Koenig, W.; McGuire, D.K.; et al. Effect of High-Dose Omega-3 Fatty Acids vs Corn Oil on Major Adverse Cardiovascular Events in Patients at High Cardiovascular Risk: The STRENGTH Randomized Clinical Trial. JAMA 2020, 324, 2268–2280. [Google Scholar] [CrossRef]
- Doi, T.; Langsted, A.; Nordestgaard, B.G. A possible explanation for the contrasting results of REDUCE-IT vs. STRENGTH: Cohort study mimicking trial designs. Eur. Heart J. 2021, 42, 4807–4817. [Google Scholar] [CrossRef]
- Hsu, H.C.; Chen, C.Y.; Chiang, C.H.; Chen, M.F. Eicosapentaenoic acid attenuated oxidative stress-induced cardiomyoblast apoptosis by activating adaptive autophagy. Eur. J. Nutr. 2014, 53, 541–547. [Google Scholar] [CrossRef]
- Bao, X.; Luo, X.; Bai, X.; Lv, Y.; Weng, X.; Zhang, S.; Leng, Y.; Huang, J.; Dai, X.; Wang, Y.; et al. Cigarette tar mediates macrophage ferroptosis in atherosclerosis through the hepcidin/FPN/SLC7A11 signaling pathway. Free Radic. Biol. Med. 2023, 201, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhou, H.; Hua, L.; Hou, C.; Jia, Q.; Chen, J.; Zhang, S.; Wang, Y.; He, S.; Jia, E. Verification of ferroptosis and pyroptosis and identification of PTGS2 as the hub gene in human coronary artery atherosclerosis. Free Radic. Biol. Med. 2021, 171, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Ardehali, H.; Min, J.; Wang, F. The molecular and metabolic landscape of iron and ferroptosis in cardiovascular disease. Nat. Rev. Cardiol. 2023, 20, 7–23. [Google Scholar] [CrossRef]
- Bai, T.; Li, M.; Liu, Y.; Qiao, Z.; Wang, Z. Inhibition of ferroptosis alleviates atherosclerosis through attenuating lipid peroxidation and endothelial dysfunction in mouse aortic endothelial cell. Free Radic. Biol. Med. 2020, 160, 92–102. [Google Scholar] [CrossRef]
- Jin, H.; Zhang, C.; Nagenborg, J.; Juhasz, P.; Ruder, A.V.; Sikkink, C.; Mees, B.M.E.; Waring, O.; Sluimer, J.C.; Neumann, D.; et al. Genome-scale metabolic network of human carotid plaque reveals the pivotal role of glutamine/glutamate metabolism in macrophage modulating plaque inflammation and vulnerability. Cardiovasc. Diabetol. 2024, 23, 240. [Google Scholar] [CrossRef]
- Chen, Y.; Cui, Y.; Li, M.; Xia, M.; Xiang, Q.; Mao, Y.; Li, H.; Chen, J.; Zeng, W.; Zheng, X.; et al. A novel mechanism of ferroptosis inhibition-enhanced atherosclerotic plaque stability: YAP1 suppresses vascular smooth muscle cell ferroptosis through GLS1. FASEB J. 2024, 38, e23850. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, M.; Mao, C.; Zhang, C.; Ma, W.; Tang, J.; Xiang, D.; Qi, X. Icariin alleviates ferroptosis-related atherosclerosis by promoting autophagy in xo-LDL-induced vascular endothelial cell injury and atherosclerotic mice. Phytother. Res. 2023, 37, 3951–3963. [Google Scholar] [CrossRef]
- Zhao, S.T.; Qiu, Z.C.; Xu, Z.Q.; Tao, E.D.; Qiu, R.B.; Peng, H.Z.; Zhou, L.F.; Zeng, R.Y.; Lai, S.Q.; Wan, L. Curcumin attenuates myocardial ischemia--reperfusion--induced autophagy--dependent ferroptosis via Sirt1/AKT/FoxO3a signaling. Int. J. Mol. Med. 2025, 55, 51. [Google Scholar] [CrossRef]
- Fang, X.; Wang, H.; Han, D.; Xie, E.; Yang, X.; Wei, J.; Gu, S.; Gao, F.; Zhu, N.; Yin, X.; et al. Ferroptosis as a target for protection against cardiomyopathy. Proc. Natl. Acad. Sci. USA 2019, 116, 2672–2680. [Google Scholar] [CrossRef]
- Gao, M.; Monian, P.; Quadri, N.; Ramasamy, R.; Jiang, X. Glutaminolysis and Transferrin Regulate Ferroptosis. Mol. Cell 2015, 59, 298–308. [Google Scholar] [CrossRef]
- Li, N.; Wang, W.; Zhou, H.; Wu, Q.; Duan, M.; Liu, C.; Wu, H.; Deng, W.; Shen, D.; Tang, Q. Ferritinophagy-mediated ferroptosis is involved in sepsis-induced cardiac injury. Free Radic. Biol. Med. 2020, 160, 303–318. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zeng, X.; Li, X.; Mehta, J.L.; Wang, X. Role of NLRP3 inflammasome in the pathogenesis of cardiovascular diseases. Basic Res. Cardiol. 2018, 113, 5. [Google Scholar] [CrossRef]
- Qiu, C.; Xia, F.; Zhang, J.; Shi, Q.; Meng, Y.; Wang, C.; Pang, H.; Gu, L.; Xu, C.; Guo, Q.; et al. Advanced Strategies for Overcoming Endosomal/Lysosomal Barrier in Nanodrug Delivery. Research 2023, 6, 0148. [Google Scholar] [CrossRef]
- Sun, Y.; Sha, Y.; Cui, G.; Meng, F.; Zhong, Z. Lysosomal-mediated drug release and activation for cancer therapy and immunotherapy. Adv. Drug Deliv. Rev. 2023, 192, 114624. [Google Scholar] [CrossRef] [PubMed]
- Serrano, M.; Blasco, M.A. Putting the stress on senescence. Curr. Opin. Cell Biol. 2001, 13, 748–753. [Google Scholar] [CrossRef]
- Englund, D.A.; Jolliffe, A.M.; Hanson, G.J.; Aversa, Z.; Zhang, X.; Jiang, X.; White, T.A.; Zhang, L.; Monroe, D.G.; Robbins, P.D.; et al. Senotherapeutic drug treatment ameliorates chemotherapy-induced cachexia. JCI Insight 2024, 9, e169512. [Google Scholar] [CrossRef] [PubMed]
- Sapieha, P.; Mallette, F.A. Cellular Senescence in Postmitotic Cells: Beyond Growth Arrest. Trends Cell Biol. 2018, 28, 595–607. [Google Scholar] [CrossRef]
- Yoshimoto, S.; Loo, T.M.; Atarashi, K.; Kanda, H.; Sato, S.; Oyadomari, S.; Iwakura, Y.; Oshima, K.; Morita, H.; Hattori, M.; et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature 2013, 499, 97–101. [Google Scholar] [CrossRef]
- Baker, D.J.; Childs, B.G.; Durik, M.; Wijers, M.E.; Sieben, C.J.; Zhong, J.; Saltness, R.A.; Jeganathan, K.B.; Verzosa, G.C.; Pezeshki, A.; et al. Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature 2016, 530, 184–189. [Google Scholar] [CrossRef]
- Lagoumtzi, S.M.; Chondrogianni, N. Senolytics and senomorphics: Natural and synthetic therapeutics in the treatment of aging and chronic diseases. Free Radic. Biol. Med. 2021, 171, 169–190. [Google Scholar] [CrossRef]
- Otoda, T.; Sekine, A.; Uemoto, R.; Tsuji, S.; Hara, T.; Tamaki, M.; Yuasa, T.; Tamaki, T.; Matsuhisa, M.; Aihara, K.I. Albuminuria and Serum Tumor Necrosis Factor Receptor Levels in Patients with Type 2 Diabetes on SGLT2 Inhibitors: A Prospective Study. Diabetes Ther. 2024, 15, 127–143. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.J.; Wang, T.J.; Chen, S.D.; Lin, K.L.; Liou, C.W.; Lan, M.Y.; Chuang, Y.C.; Chuang, J.H.; Wang, P.W.; Lee, J.J.; et al. Two Birds One Stone: The Neuroprotective Effect of Antidiabetic Agents on Parkinson Disease-Focus on Sodium-Glucose Cotransporter 2 (SGLT2) Inhibitors. Antioxidants 2021, 10, 1935. [Google Scholar] [CrossRef] [PubMed]
- Johmura, Y.; Yamanaka, T.; Omori, S.; Wang, T.W.; Sugiura, Y.; Matsumoto, M.; Suzuki, N.; Kumamoto, S.; Yamaguchi, K.; Hatakeyama, S.; et al. Senolysis by glutaminolysis inhibition ameliorates various age-associated disorders. Science 2021, 371, 265–270. [Google Scholar] [CrossRef]
- Liu, Y.; Steinbusch, L.K.M.; Nabben, M.; Kapsokalyvas, D.; van Zandvoort, M.; Schonleitner, P.; Antoons, G.; Simons, P.J.; Coumans, W.A.; Geomini, A.; et al. Palmitate-Induced Vacuolar-Type H(+)-ATPase Inhibition Feeds Forward Into Insulin Resistance and Contractile Dysfunction. Diabetes 2017, 66, 1521–1534. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Schianchi, F.; Neumann, D.; Wong, L.Y.; Sun, A.; van Nieuwenhoven, F.A.; Zeegers, M.P.; Strzelecka, A.; Col, U.; Glatz, J.F.C.; et al. Specific amino acid supplementation rescues the heart from lipid overload-induced insulin resistance and contractile dysfunction by targeting the endosomal mTOR-v-ATPase axis. Mol. Metab. 2021, 53, 101293. [Google Scholar] [CrossRef] [PubMed]
- Chirinos, J.A.; Zhao, L.; Jia, Y.; Frej, C.; Adamo, L.; Mann, D.; Shewale, S.V.; Millar, J.S.; Rader, D.J.; French, B.; et al. Reduced Apolipoprotein M and Adverse Outcomes Across the Spectrum of Human Heart Failure. Circulation 2020, 141, 1463–1476. [Google Scholar] [CrossRef]
- Hanff, T.C.; Cohen, J.B.; Zhao, L.; Javaheri, A.; Zamani, P.; Prenner, S.B.; Rietzschel, E.; Jia, Y.; Walsh, A.; Maranville, J.; et al. Quantitative Proteomic Analysis of Diabetes Mellitus in Heart Failure With Preserved Ejection Fraction. JACC Basic Transl. Sci. 2021, 6, 89–99. [Google Scholar] [CrossRef]
- Guo, Z.; Valenzuela Ripoll, C.; Picataggi, A.; Rawnsley, D.R.; Ozcan, M.; Chirinos, J.A.; Chendamarai, E.; Girardi, A.; Riehl, T.; Evie, H.; et al. Apolipoprotein M Attenuates Anthracycline Cardiotoxicity and Lysosomal Injury. JACC Basic Transl. Sci. 2023, 8, 340–355. [Google Scholar] [CrossRef]
- Zhao, D.; Xu, R.; Zhou, Y.; Wu, J.; Zhang, X.; Lin, H.; Wang, J.; Ding, Z.; Zou, Y. ORP5 promotes cardiac hypertrophy by regulating the activation of mTORC1 on lysosome. J. Adv. Res. 2024; in press. [Google Scholar] [CrossRef]
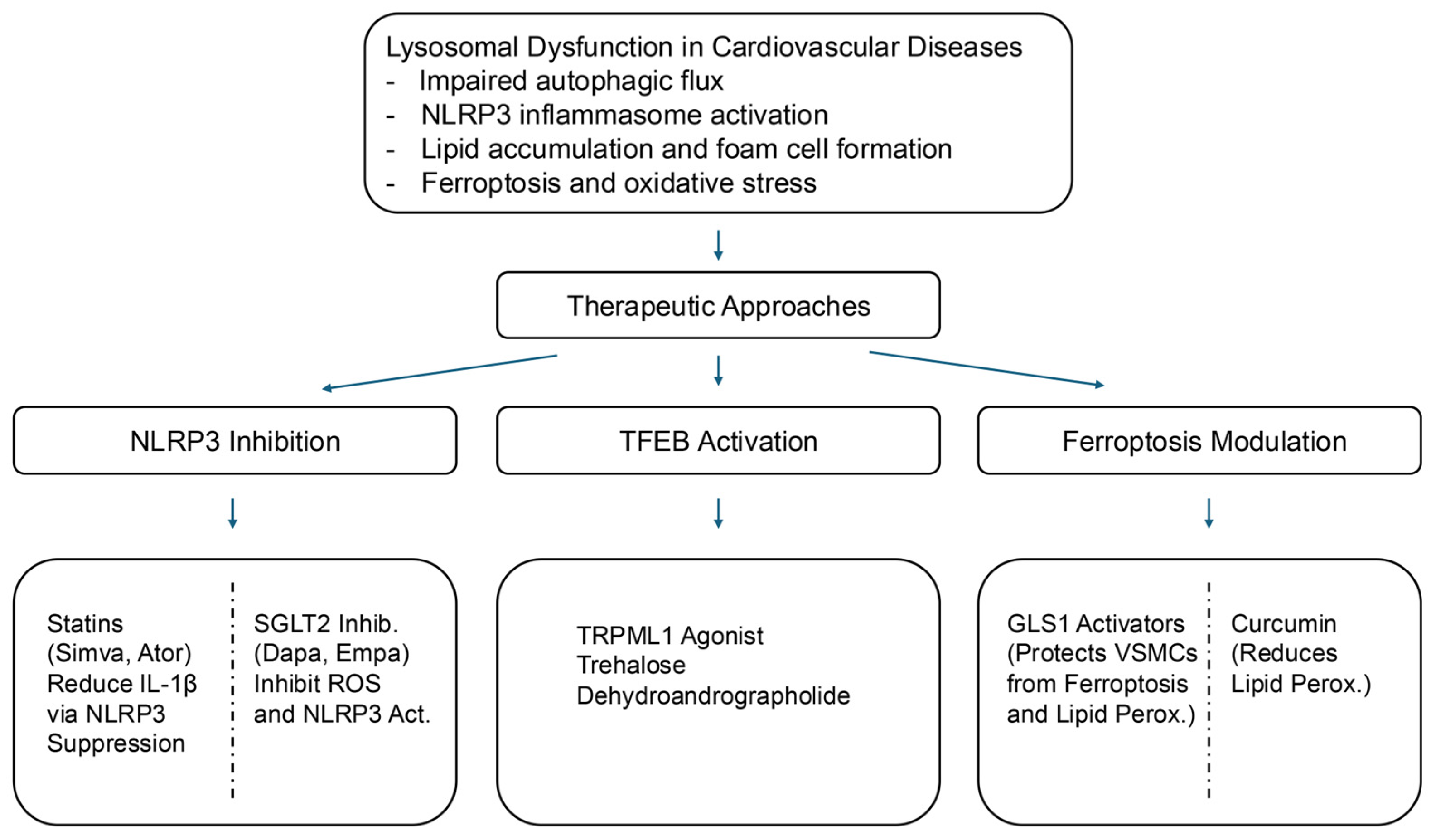

| Drug Class | Mechanism of Action | Clinical Status | Key Clinical Findings |
|---|---|---|---|
| NLRP3 Inflammasome Inhibitors | |||
| Statins | Inhibits NLRP3 inflammasome via AMPK activation, reducing inflammation | Clinical use | Lowers inflammatory burden in CVD |
| SGLT2 Inhibitors | Suppresses NLRP3 inflammasome activation enhances autophagy | Clinical use | Reduces myocardial infarction size, cardiac damage markers |
| Vitamin E | Inhibits Lamtor1-HDAC6 interaction, reducing NLRP3 activation | Observational and preclinical studies | Associated with reduced CVD risk |
| Cardiovascular Drugs Enhancing TFEB Activity | |||
| Statins | Enhances TFEB activity via mTORC1 inhibition, AMPK activation | Widely used in clinical practice | Improves lysosomal function, reduces lipid accumulation |
| TRPML1 Agonists | Activates TFEB via lysosomal calcium signaling | Preclinical | Protects against oxidative stress and autophagic defects |
| Trehalose | Promotes TFEB activation, enhances lysosomal biogenesis | Preclinical, under investigation | Reduces plaque burden, enhances autophagy |
| Resveratrol (RSV) | Stimulates ER-Ca2⁺ signaling and activates TFEB | Preclinical evidence | Improves lipid metabolism and autophagy regulation |
| Curcumin (Cur) | Promotes TFEB activation, enhances lipid catabolism | Preclinical studies | Reduces foam cell formation and inflammation |
| Dehydroandrographolide (DA) | Activates TFEB enhances the lysosomal function | Experimental models | Improves autophagic flux, reduces CVD progression |
| Ferroptosis Modulators | |||
| GLS1 Activators | Enhances glutaminolysis, reducing ferroptosis | Preclinical studies | Prevents oxidative stress-induced VSMC death |
| Icariin | Reduces ROS, promotes TFEB nuclear translocation, prevents ferroptosis | Preclinical models | Reduces atherosclerotic lesions |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Otoda, T.; Aihara, K.-i.; Takayama, T. Lysosomal Stress in Cardiovascular Diseases: Therapeutic Potential of Cardiovascular Drugs and Future Directions. Biomedicines 2025, 13, 1053. https://doi.org/10.3390/biomedicines13051053
Otoda T, Aihara K-i, Takayama T. Lysosomal Stress in Cardiovascular Diseases: Therapeutic Potential of Cardiovascular Drugs and Future Directions. Biomedicines. 2025; 13(5):1053. https://doi.org/10.3390/biomedicines13051053
Chicago/Turabian StyleOtoda, Toshiki, Ken-ichi Aihara, and Tadateru Takayama. 2025. "Lysosomal Stress in Cardiovascular Diseases: Therapeutic Potential of Cardiovascular Drugs and Future Directions" Biomedicines 13, no. 5: 1053. https://doi.org/10.3390/biomedicines13051053
APA StyleOtoda, T., Aihara, K.-i., & Takayama, T. (2025). Lysosomal Stress in Cardiovascular Diseases: Therapeutic Potential of Cardiovascular Drugs and Future Directions. Biomedicines, 13(5), 1053. https://doi.org/10.3390/biomedicines13051053








