Insulators to Improve the Safety of Retroviral Vectors for HIV Gene Therapy
Abstract
:1. Introduction

2. Insulators
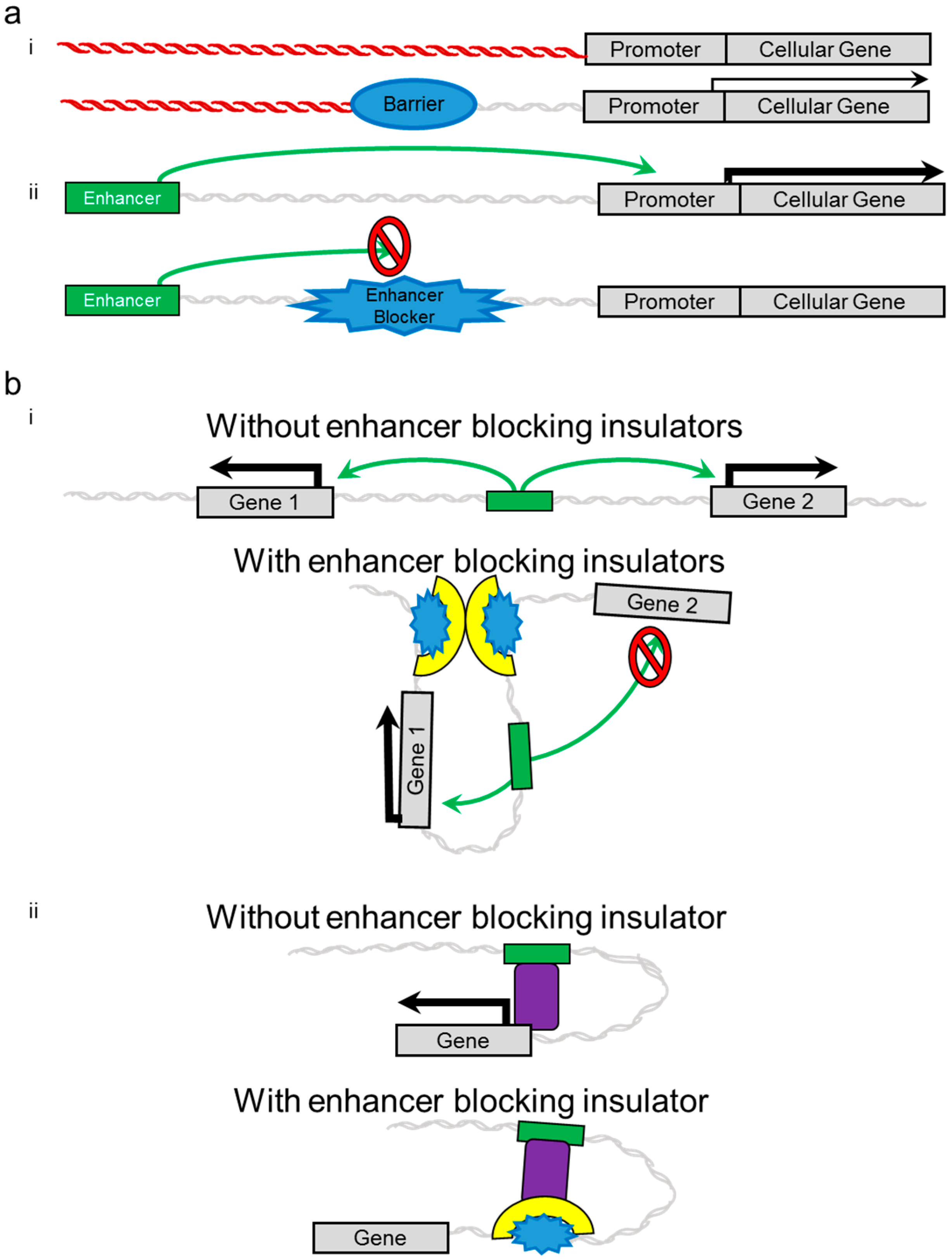
3. Evidence of Genotoxicity and Adverse Side Effects in Clinical Trials
| Clinical Trial | # Participants | # Adverse | Integration Associated with Mutagenesis | Reference | |||
|---|---|---|---|---|---|---|---|
| Oncogene | Position | kbp to TSS | +/− | ||||
| SCID-X1 | 20 | 5 | LMO2 | 1st intron | 2.0 | − | [12] |
| Upstream | 2.9 | + | [12] | ||||
| 2nd intron | 10.6 | − | [12] | ||||
| Upstream | 35.0 | − | [50] | ||||
| BML1 * | Upstream | 49.5 | + | [12] | |||
| CCDN2 | Upstream | 2.4 | − | [12] | |||
| WAS# | 10 | 7 | LMO2 ** | Upstream | 20.6 | − | [17] |
| Upstream | 32.3 | − | [17] | ||||
| Upstream | 33.0 | − | [17] | ||||
| Upstream | 1.5 | − | [17] | ||||
| 1st intron | 8.7 | − | [17] | ||||
| 1st intron *** | 24.7 | − | [17] | ||||
| MN1 | 2nd intron ** | 351.7 | − | [17] | |||
| MDS1 ** | 2nd intron | 299.5 | − | [17] | |||
| CGD | 17 | 3 | MDS1 | Downstream | NR | NR | [51,52] |
3.1. SCID-X1
3.2. WAS
3.3. CGD
3.4. β-Thalassemia
4. Development of Insulated Vectors

5. Insulated Retroviral Vectors
5.1. Gammaretroviral Vectors
5.2. Lentiviral Vectors
5.3. Foamy Viral Vectors
6. Considerations for the Development of Insulated Anti-HIV Retroviral Vectors
7. Future Perspectives and Unique Opportunities for Anti-HIV Gene Therapy
8. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Iwakuma, T.; Cui, Y.; Chang, L.J. Self-inactivating lentiviral vectors with U3 and U5 modifications. Virology 1999, 261, 120–132. [Google Scholar] [CrossRef] [PubMed]
- Kraunus, J.; Schaumann, D.H.; Meyer, J.; Modlich, U.; Fehse, B.; Brandenburg, G.; von Laer, D.; Klump, H.; Schambach, A.; Bohne, J.; et al. Self-inactivating retroviral vectors with improved RNA processing. Gene Ther. 2004, 11, 1568–1578. [Google Scholar] [CrossRef] [PubMed]
- Trobridge, G. Improved foamy virus vectors with minimal viral sequences. Mol. Ther. 2002, 6, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Kang, E.M.; de Witte, M.; Malech, H.; Morgan, R.A.; Carter, C.; Leitman, S.F.; Childs, R.; Barrett, A.J.; Little, R.; Tisdale, J.F. Gene therapy-based treatment for HIV-positive patients with malignancies. J. Hematother. Stem Cell Res. 2002, 11, 809–816. [Google Scholar] [CrossRef] [PubMed]
- Mitsuyasu, R.T.; Merigan, T.C.; Carr, A.; Zack, J.A.; Winters, M.A.; Workman, C.; Bloch, M.; Lalezari, J.; Becker, S.; Thornton, L.; et al. Phase 2 gene therapy trial of an anti-HIV ribozyme in autologous CD34+ cells. Nat. Med. 2009, 15, 285–292. [Google Scholar] [CrossRef] [PubMed]
- DiGiusto, D.L.; Krishnan, A.; Li, L.; Li, H.; Li, S.; Rao, A.; Mi, S.; Yam, P.; Stinson, S.; Kalos, M.; et al. RNA-based gene therapy for HIV with lentiviral vector-modified CD34+ cells in patients undergoing transplantation for aids-related lymphoma. Sci. Transl. Med. 2010, 2. [Google Scholar] [CrossRef] [PubMed]
- Savkovic, B.; Macpherson, J.L.; Zaunders, J.; Kelleher, A.D.; Knop, A.E.; Pond, S.; Evans, L.; Symonds, G.; Murray, J.M. T-lymphocyte perturbation following large-scale apheresis and hematopoietic stem cell transplantation in HIV-infected individuals. Clin. Immunol. 2012, 144, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Van Lunzen, J.; Glaunsinger, T.; Stahmer, I.; von Baehr, V.; Baum, C.; Schilz, A.; Kuehlcke, K.; Naundorf, S.; Martinius, H.; Hermann, F.; et al. Transfer of autologous gene-modified T cells in HIV-infected patients with advanced immunodeficiency and drug-resistant virus. Mol. Ther. 2007, 15, 1024–1033. [Google Scholar] [CrossRef] [PubMed]
- Tebas, P.; Stein, D.; Tang, W.W.; Frank, I.; Wang, S.Q.; Lee, G.; Spratt, S.K.; Surosky, R.T.; Giedlin, M.A.; Nichol, G.; et al. Gene editing of CCR5 in autologous CD4 T cells of persons infected with HIV. N. Engl. J. Med. 2014, 370, 901–910. [Google Scholar] [CrossRef] [PubMed]
- Savkovic, B.; Nichols, J.; Birkett, D.; Applegate, T.; Ledger, S.; Symonds, G.; Murray, J.M. A quantitative comparison of anti-HIV gene therapy delivered to hematopoietic stem cells versus CD4+ T cells. PLoS Comput. Biol. 2014, 10, e1003681. [Google Scholar] [CrossRef] [PubMed]
- Hoxie, J.A.; June, C.H. Novel cell and gene therapies for HIV. Cold Spring Harb. Perspect. Med. 2012, 2, a007179. [Google Scholar] [CrossRef] [PubMed]
- Hacein-Bey-Abina, S.; Garrigue, A.; Wang, G.P.; Soulier, J.; Lim, A.; Morillon, E.; Clappier, E.; Caccavelli, L.; Delabesse, E.; Beldjord, K.; et al. Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of scid-x1. J. Clin. Invest. 2008, 118, 3132–3142. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Dullmann, J.; Schiedlmeier, B.; Schmidt, M.; von Kalle, C.; Meyer, J.; Forster, M.; Stocking, C.; Wahlers, A.; Frank, O.; et al. Murine leukemia induced by retroviral gene marking. Science 2002, 296, 497. [Google Scholar] [CrossRef] [PubMed]
- Daniel, R.; Smith, J.A. Integration site selection by retroviral vectors: Molecular Mechanism and Clinical Consequences. Hum. Gene Ther. 2008, 19, 557–568. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.B.; Beard, B.C.; Trobridge, G.D.; Wood, B.L.; Sale, G.E.; Sud, R.; Humphries, R.K.; Kiem, H.P. High incidence of leukemia in large animals after stem cell gene therapy with a hoxb4-expressing retroviral vector. J. Clin. Invest. 2008, 118, 1502–1510. [Google Scholar] [CrossRef] [PubMed]
- Li, C.L.; Xiong, D.; Stamatoyannopoulos, G.; Emery, D.W. Genomic and functional assays demonstrate reduced gammaretroviral vector genotoxicity associated with use of the cHS4 chromatin insulator. Mol. Ther. 2009, 17, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Braun, C.J.; Boztug, K.; Paruzynski, A.; Witzel, M.; Schwarzer, A.; Rothe, M.; Modlich, U.; Beier, R.; Gohring, G.; Steinemann, D.; et al. Gene therapy for wiskott-aldrich syndrome—long-term efficacy and genotoxicity. Sci. Transl. Med. 2014, 6, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Hendrie, P.C.; Huo, Y.; Stolitenko, R.B.; Russell, D.W. A rapid and quantitative assay for measuring neighboring gene activation by vector proviruses. Mol. Ther. 2008, 16, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Weber, E.L.; Cannon, P.M. Promoter choice for retroviral vectors: transcriptional strength versus trans-activation potential. Hum. Gene Ther. 2007, 18, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Modlich, U.; Bohne, J.; Schmidt, M.; von Kalle, C.; Knoss, S.; Schambach, A.; Baum, C. Cell-culture assays reveal the importance of retroviral vector design for insertional genotoxicity. Blood 2006, 108, 2545–2553. [Google Scholar] [CrossRef] [PubMed]
- Montini, E.; Cesana, D.; Schmidt, M.; Sanvito, F.; Bartholomae, C.C.; Ranzani, M.; Benedicenti, F.; Sergi, L.S.; Ambrosi, A.; Ponzoni, M.; et al. The genotoxic potential of retroviral vectors is strongly modulated by vector design and integration site selection in a mouse model of HSC gene therapy. J. Clin. Invest. 2009, 119, 964–975. [Google Scholar] [CrossRef] [PubMed]
- Rae, D.T.; Trobridge, G.D. Retroviral genotoxicity. In Gene Therapy—Tool and Potential Applications; Martin, D.F., Ed.; InTech: Haverhill, MA, USA, 2013. [Google Scholar]
- Emery, D.W. The use of chromatin insulators to improve the expression and safety of integrating gene transfer vectors. Hum. Gene Ther. 2011, 22, 761–774. [Google Scholar] [CrossRef] [PubMed]
- Felsenfeld, G. Chromatin structure and the expression of globin-encoding genes. Gene 1993, 135, 119–124. [Google Scholar] [CrossRef]
- Chung, J.H.; Whiteley, M.; Felsenfeld, G. A 5′ element of the chicken beta-globin domain serves as an insulator in human erythroid cells and protects against position effect in drosophila. Cell 1993, 74, 505–514. [Google Scholar] [CrossRef]
- Bell, A.C.; West, A.G.; Felsenfeld, G. Insulators and boundaries: Versatile regulatory elements in the eukaryotic genome. Science 2001, 291, 447–450. [Google Scholar] [CrossRef] [PubMed]
- West, A.G.; Gaszner, M.; Felsenfeld, G. Insulators: Many Functions, Many Mechanisms. Genes Dev. 2002, 16, 271–288. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.L.; Elgin, S.C. Putting boundaries on silence. Cell 1999, 99, 459–462. [Google Scholar] [CrossRef]
- Groth, A.C.; Liu, M.; Wang, H.; Lovelett, E.; Emery, D.W. Identification and characterization of enhancer-blocking insulators to reduce retroviral vector genotoxicity. PLoS ONE 2013, 8, e76528. [Google Scholar] [CrossRef] [PubMed]
- Browning, D.; Collins, C.; Leap, D.; Hocum, J.; Rae, D.; Trobridge, G. Insualted foamy viral vectors. Hum. Gene Ther. 2015. [Google Scholar] [CrossRef] [PubMed]
- Bell, A.C.; West, A.G.; Felsenfeld, G. The protein CTCF is required for the enhancer-blocking activity of vertebrate insulators. Cell 1999, 98, 387–396. [Google Scholar] [CrossRef]
- Lobanenkov, V.V.; Nicolas, R.H.; Adler, V.V.; Paterson, H.; Klenova, E.M.; Polotskaja, A.V.; Goodwin, G.H. A novel sequence-specific DNA binding protein which interacts with three regularly spaced direct repeats of the CCCTC-motif in the 5′-flanking sequence of the chicken c-myc gene. Oncogene 1990, 5, 1743–1753. [Google Scholar] [PubMed]
- Vostrov, A.A.; Quitschke, W.W. The zinc finger protein CTCF binds to the apbbeta domain of the amyloid beta-protein precursor promoter. Evidence for a role in transcriptional activation. J. Biol. Chem. 1997, 272, 33353–33359. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Quitschke, W.W.; Vostrov, A.A.; Brewer, G.J. CTCF is essential for up-regulating expression from the amyloid precursor protein promoter during differentiation of primary hippocampal neurons. J. Neurochem. 1999, 73, 2286–2298. [Google Scholar] [CrossRef] [PubMed]
- Chao, W.; Huynh, K.D.; Spencer, R.J.; Davidow, L.S.; Lee, J.T. CTCF, a candidate trans-acting factor for X-inactivation choice. Science 2002, 295, 345–347. [Google Scholar] [CrossRef] [PubMed]
- Ohlsson, R.; Renkawitz, R.; Lobanenkov, V. CTCF is a uniquely versatile transcription regulator linked to epigenetics and disease. Trends Genet. 2001, 17, 520–527. [Google Scholar] [CrossRef]
- Yusufzai, T.M.; Felsenfeld, G. The 5′-HS4 chicken β-globin insulator is a CTCF-dependent nuclear matrix-associated element. Proc. Natl. Acad. Sci. USA 2004, 101, 8620–8624. [Google Scholar] [CrossRef] [PubMed]
- Wallace, J.A.; Felsenfeld, G. We gather together: Insulators and genome organization. Curr. Opin. Genet. Dev. 2007, 17, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Splinter, E.; Heath, H.; Kooren, J.; Palstra, R.J.; Klous, P.; Grosveld, F.; Galjart, N.; de Laat, W. CTCF mediates long-range chromatin looping and local histone modification in the beta-globin locus. Genes Dev. 2006, 20, 2349–2354. [Google Scholar] [CrossRef] [PubMed]
- Farrell, C.M.; West, A.G.; Felsenfeld, G. Conserved CTCF insulator elements flank the mouse and human beta-globin loci. Mol. Cell. Biol. 2002, 22, 3820–3831. [Google Scholar] [CrossRef] [PubMed]
- Holwerda, S.J.; de Laat, W. CTCF: The protein, the binding partners, the binding sites and their chromatin loops. Philos. Trans. R Soc. B Biol. Sci. 2013, 368. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, P.I.; Urbinati, F.; Velu, C.S.; Higashimoto, T.; Grimes, H.L.; Malik, P. The 3′ region of the chicken hypersensitive site-4 insulator has properties similar to its core and is required for full insulator activity. PLoS ONE 2009, 4, e6995. [Google Scholar] [CrossRef] [PubMed]
- Nakahashi, H.; Kwon, K.R.; Resch, W.; Vian, L.; Dose, M.; Stavreva, D.; Hakim, O.; Pruett, N.; Nelson, S.; Yamane, A.; et al. A genome-wide map of CTCF multivalency redefines the CTCF code. Cell. Rep. 2013, 3, 1678–1689. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Maurano, M.T.; Wang, H.; Qi, H.; Song, C.Z.; Navas, P.A.; Emery, D.W.; Stamatoyannopoulos, J.A.; Stamatoyannopoulos, G. Genomic discovery of potent chromatin insulators for human gene therapy. Nat. Biotechnol. 2015, 33, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Weth, O.; Renkawitz, R. CTCF function is modulated by neighboring DNA binding factors. Biochem. Cell. Biol. 2011, 89, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Thrasher, A.J. Gene therapy for PIDs: Progress, pitfalls and prospects. Gene 2013, 525, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Petz, L.D.; Burnett, J.C.; Li, H.; Li, S.; Tonai, R.; Bakalinskaya, M.; Shpall, E.J.; Armitage, S.; Kurtzberg, J.; Regan, D.M.; et al. Progress toward curing HIV infection with hematopoietic cell transplantation. Stem Cells Cloning 2015, 8, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Ott, M.G.; Schmidt, M.; Schwarzwaelder, K.; Stein, S.; Siler, U.; Koehl, U.; Glimm, H.; Kuhlcke, K.; Schilz, A.; Kunkel, H.; et al. Correction of X-linked chronic granulomatous disease by gene therapy, augmented by insertional activation of MDS1-EVI1, PRDM16 or SETBP1. Nat. Med. 2006, 12, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Cavazzana-Calvo, M.; Payen, E.; Negre, O.; Wang, G.; Hehir, K.; Fusil, F.; Down, J.; Denaro, M.; Brady, T.; Westerman, K.; et al. Transfusion independence and HMGA2 activation after gene therapy of human β-thalassaemia. Nature 2010, 467, 318–322. [Google Scholar] [CrossRef] [PubMed]
- Howe, S.J.; Mansour, M.R.; Schwarzwaelder, K.; Bartholomae, C.; Hubank, M.; Kempski, H.; Brugman, M.H.; Pike-Overzet, K.; Chatters, S.J.; de Ridder, D.; et al. Insertional mutagenesis combined with acquired somatic mutations causes leukemogenesis following gene therapy of SCID-X1 patients. J. Clin. Invest. 2008, 118, 3143–3150. [Google Scholar] [CrossRef] [PubMed]
- Siler, U.; Paruzynski, A.; Holtgreve-Grez, H.; Kuzmenko, E.; Koehl, U.; Renner, E.D.; Alhan, C.; de Loosdrecht, A.A.; Schwable, J.; Pfluger, T.; et al. Successful combination of sequential gene therapy and rescue Allo-HSCT in two children with X-CGD—importance of timing. Curr. Gene Ther. 2015, 15, 416–427. [Google Scholar] [CrossRef] [PubMed]
- Stein, S.; Ott, M.G.; Schultze-Strasser, S.; Jauch, A.; Burwinkel, B.; Kinner, A.; Schmidt, M.; Kramer, A.; Schwable, J.; Glimm, H.; et al. Genomic instability and myelodysplasia with monosomy 7 consequent to evi1 activation after gene therapy for chronic granulomatous disease. Nat. Med. 2010, 16, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Thrasher, A.J.; Hacein-Bey-Abina, S.; Gaspar, H.B.; Blanche, S.; Davies, E.G.; Parsley, K.; Gilmour, K.; King, D.; Howe, S.; Sinclair, J.; et al. Failure of SCID-X1 gene therapy in older patients. Blood 2005, 105, 4255–4257. [Google Scholar] [CrossRef] [PubMed]
- Chinen, J.; Davis, J.; de Ravin, S.S.; Hay, B.N.; Hsu, A.P.; Linton, G.F.; Naumann, N.; Nomicos, E.Y.; Silvin, C.; Ulrick, J.; et al. Gene therapy improves immune function in preadolescents with X-linked severe combined immunodeficiency. Blood 2007, 110, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Boztug, K.; Schmidt, M.; Schwarzer, A.; Banerjee, P.P.; Diez, I.A.; Dewey, R.A.; Bohm, M.; Nowrouzi, A.; Ball, C.R.; Glimm, H.; et al. Stem-cell gene therapy for the wiskott-aldrich syndrome. N. Engl. J. Med. 2010, 363, 1918–1927. [Google Scholar] [CrossRef] [PubMed]
- Chandrakasan, S.; Malik, P. Gene therapy for hemoglobinopathies: The State of the Field and the Future. Hematol. Oncol. Clin. North. Am. 2014, 28, 199–216. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, P.I.; Higashimoto, T.; Urbinati, F.; Modlich, U.; Nestheide, S.; Xia, P.; Fox, C.; Corsinotti, A.; Baum, C.; Malik, P. Genotoxic potential of lineage-specific lentivirus vectors carrying the beta-globin locus control region. Mol. Ther. 2009, 17, 1929–1937. [Google Scholar] [CrossRef] [PubMed]
- An, W.; Telesnitsky, A. Frequency of direct repeat deletion in a human immunodeficiency virus type 1 vector during reverse transcription in human cells. Virology 2001, 286, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Urbinati, F.; Arumugam, P.; Higashimoto, T.; Perumbeti, A.; Mitts, K.; Xia, P.; Malik, P. Mechanism of reduction in titers from lentivirus vectors carrying large inserts in the 3′LTR. Mol. Ther. 2009, 17, 1527–1536. [Google Scholar] [CrossRef] [PubMed]
- Gaussin, A.; Modlich, U.; Bauche, C.; Niederlander, N.J.; Schambach, A.; Duros, C.; Artus, A.; Baum, C.; Cohen-Haguenauer, O.; Mermod, N. CTF/NF1 transcription factors act as potent genetic insulators for integrating gene transfer vectors. Gene Ther. 2012, 19, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Hanawa, H.; Yamamoto, M.; Zhao, H.; Shimada, T.; Persons, D.A. Optimized lentiviral vector design improves titer and transgene expression of vectors containing the chicken beta-globin locus hs4 insulator element. Mol. Ther. 2009, 17, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Suttiprapa, S.; Rinaldi, G.; Brindley, P.J. Prototypic chromatin insulator cHS4 protects retroviral transgene from silencing in schistosoma mansoni. Transgenic Res. 2012, 21, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, T.T.; Jakobsson, J.; Rosenqvist, N.; Lundberg, C. Incorporating double copies of a chromatin insulator into lentiviral vectors results in less viral integrants. BMC Biotechnol. 2009, 9, 13. [Google Scholar] [CrossRef] [PubMed]
- Emery, D.W.; Yannaki, E.; Tubb, J.; Stamatoyannopoulos, G. A chromatin insulator protects retrovirus vectors from chromosomal position effects. Proc. Natl. Acad. Sci. USA 2000, 97, 9150–9155. [Google Scholar] [CrossRef] [PubMed]
- Cesana, D.; Ranzani, M.; Volpin, M.; Bartholomae, C.; Duros, C.; Artus, A.; Merella, S.; Benedicenti, F.; Sergi Sergi, L.; Sanvito, F.; et al. Uncovering and dissecting the genotoxicity of self-inactivating lentiviral vectors in vivo. Mol. Ther. 2014, 22, 774–785. [Google Scholar] [CrossRef] [PubMed]
- Emery, D.W.; Yannaki, E.; Tubb, J.; Nishino, T.; Li, Q.; Stamatoyannopoulos, G. Development of virus vectors for gene therapy of beta chain hemoglobinopathies: Flanking with a chromatin insulator reduces γ-globin gene silencing in vivo. Blood 2002, 100, 2012–2019. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Osborne, C.S.; Bharadwaj, R.R.; Pasceri, P.; Sukonnik, T.; Pannell, D.; Recillas-Targa, F.; West, A.G.; Ellis, J. Retrovirus silencer blocking by the chs4 insulator is ctcf independent. Nucleic Acids Res. 2003, 31, 5317–5323. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.J.; Liu, X.; He, J. Lentiviral sirnas targeting multiple highly conserved rna sequences of human immunodeficiency virus type 1. Gene Ther. 2005, 12, 1133–1144. [Google Scholar] [CrossRef] [PubMed]
- Trobridge, G.D.; Miller, D.G.; Jacobs, M.A.; Allen, J.M.; Kiem, H.P.; Kaul, R.; Russell, D.W. Foamy virus vector integration sites in normal human cells. Proc. Natl. Acad. Sci. USA 2006, 103, 1498–1503. [Google Scholar] [CrossRef] [PubMed]
- Olszko, M.E.; Trobridge, G.D. Foamy virus vectors for HIV gene therapy. Viruses 2013, 5, 2585–2600. [Google Scholar] [CrossRef] [PubMed]
- Li, M.J.; Kim, J.; Li, S.; Zaia, J.; Yee, J.K.; Anderson, J.; Akkina, R.; Rossi, J.J. Long-term inhibition of HIV-1 infection in primary hematopoietic cells by lentiviral vector delivery of a triple combination of anti-HIV shRNA, anti-CCR5 ribozyme, and a nucleolar-localizing TAR decoy. Mol. Ther. 2005, 12, 900–909. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.P.; Vink, M.A.; Westerink, J.T.; Ramirez de Arellano, E.; Konstantinova, P.; Ter Brake, O.; Berkhout, B. Titers of lentiviral vectors encoding shrnas and mirnas are reduced by different mechanisms that require distinct repair strategies. RNA 2010, 16, 1328–1339. [Google Scholar] [CrossRef] [PubMed]
- Bahner, I.; Sumiyoshi, T.; Kagoda, M.; Swartout, R.; Peterson, D.; Pepper, K.; Dorey, F.; Reiser, J.; Kohn, D.B. Lentiviral vector transduction of a dominant-negative Rev gene into human CD34+ hematopoietic progenitor cells potently inhibits human immunodeficiency virus-1 replication. Mol. Ther. 2007, 15, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Mautino, M.R.; Morgan, R.A. Potent inhibition of human immunodeficiency virus type 1 replication by conditionally replicating human immunodeficiency virus-based lentiviral vectors expressing envelope antisense mrna. Hum. Gene Ther. 2000, 11, 2025–2037. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.; Vojtech, L.; Bahner, I.; Kohn, D.; von Laer, D.; Russell, D.W.; Richard, R. Foamy virus vectors expressing anti-HIV transgenes efficiently block HIV-1 replication. Mol. Ther. 2007, 16, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Kiem, H.P.; Wu, R.A.; Sun, G.; von Laer, D.; Rossi, J.J.; Trobridge, G.D. Foamy combinatorial anti-HIV vectors with MGMTP140K potently inhibit HIV-1 and SHIV replication and mediate selection in vivo. Gene Ther. 2010, 17, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Gijsbers, R.; Ronen, K.; Vets, S.; Malani, N.; de Rijck, J.; McNeely, M.; Bushman, F.D.; Debyser, Z. LEDGF hybrids efficiently retarget lentiviral integration into heterochromatin. Mol. Ther. 2010, 18, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Silvers, R.; Simth, J.; Schowalter, M.; Litwin, S.; Liang, Z.; Geary, K.; Daniel, R. Modification of integration site preferences of an HIV-1-based vector by expression of a novel synthetic protein. Hum. Gene Ther. 2010, 21, 337–349. [Google Scholar] [CrossRef] [PubMed]
- El Ashkar, S.; de Rijck, J.; Demeulemeester, J.; Vets, S.; Madlala, P.; Cermakova, K.; Debyser, Z.; Gijsbers, R. BET-independent MLV-based vectors target away from promoters and regulatory elements. Mol. Ther. Nucleic Acids 2014, 3. [Google Scholar] [CrossRef] [PubMed]
- Moolten, F.L. Tumor chemosensitivity conferred by inserted herpes thymidine kinase genes: Paradigm for a prospective cancer control strategy. Cancer Res. 1986, 46, 5276–5281. [Google Scholar] [PubMed]
- Mullen, C.A.; Kilstrup, M.; Blaese, R.M. Transfer of the bacterial gene for cytosine deaminase to mammalian cells confers lethal sensitivity to 5-fluorocytosine: A negative selection system. Proc. Natl. Acad. Sci. USA 1992, 89, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Ciceri, F.; Bonini, C.; Gallo-Stampino, C.; Bordignon, C. Modulation of gvhd by suicide-gene transduced donor t lymphocytes: Clinical applications in mismatched transplantation. Cytotherapy 2005, 7, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Niculescu-Duvaz, I.; Springer, C.J. Introduction to the background, principles, and state of the art in suicide gene therapy. Mol. Biotechnol. 2005, 30, 71–88. [Google Scholar] [CrossRef]
- Traversari, C.; Marktel, S.; Magnani, Z.; Mangia, P.; Russo, V.; Ciceri, F.; Bonini, C.; Bordignon, C. The potential immunogenicity of the TK suicide gene does not prevent full clinical benefit associated with the use of TK-transduced donor lymphocytes in HSCT for hematologic malignancies. Blood 2007, 109, 4708–4715. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Browning, D.L.; Trobridge, G.D. Insulators to Improve the Safety of Retroviral Vectors for HIV Gene Therapy. Biomedicines 2016, 4, 4. https://doi.org/10.3390/biomedicines4010004
Browning DL, Trobridge GD. Insulators to Improve the Safety of Retroviral Vectors for HIV Gene Therapy. Biomedicines. 2016; 4(1):4. https://doi.org/10.3390/biomedicines4010004
Chicago/Turabian StyleBrowning, Diana L., and Grant D. Trobridge. 2016. "Insulators to Improve the Safety of Retroviral Vectors for HIV Gene Therapy" Biomedicines 4, no. 1: 4. https://doi.org/10.3390/biomedicines4010004
APA StyleBrowning, D. L., & Trobridge, G. D. (2016). Insulators to Improve the Safety of Retroviral Vectors for HIV Gene Therapy. Biomedicines, 4(1), 4. https://doi.org/10.3390/biomedicines4010004






