Abstract
Virgin coconut oil (VCO) has been the subject of several studies which have aimed to alleviate Alzheimer’s disease (AD) pathology, focusing on in vitro antioxidant and acetylcholinesterase (AChE) inhibitory activities. Here, we studied an underutilized and lesser-valued part of the coconut tree, specifically the leaves, using in vitro and in vivo approaches. Coconut leaf extract (CLE) was screened for antioxidant and AChE inhibitory properties in vitro and therapeutic effects in two strains of transgenic Caenorhabditis elegans expressing amyloid-β1–42 (Aβ1-42) in muscle cells. CLE demonstrated free radical scavenging activity with an EC50 that is 79-fold less compared to ascorbic acid, and an AChE inhibitory activity that is 131-fold less compared to Rivastigmine. Surprisingly, in spite of its low antioxidant activity and AChE inhibition, CLE reduced Aβ deposits by 30.31% in CL2006 in a dose-independent manner, and reduced the percentage of paralyzed nematodes at the lowest concentration of CLE (159.38 μg/mL), compared to dH2O/vehicle (control). Phytochemical analysis detected glycosides, anthocyanins, and hydrolyzable tannins in CLE, some of which are known to be anti-amyloidogenic. Taken together, these findings suggest that CLE metabolites alternatively decrease AB1–42 aggregation and paralysis prevalence independently of free radical scavenging and AChE inhibition, and this warrants further investigation on the bioactive compounds of CLE.
1. Introduction
Alzheimer’s disease (AD) afflicts more than 26 million people globally, and is said to be the most common neurodegenerative disease worldwide. It is mainly idiopathic, with late-onset affecting more than 90% of cases [1]. It is classified, across its pathological hallmarks, as a tauopathy-characterized by hyperphosphorylated, filamentous tau aggregates prior to microtubule collapse—a major requisite for the formation of neurofibrillary tangles [2,3]. The presence of amyloid-β (Aβ) plaques are thought to constitute the main biomarkers for AD. The combined criteria from CERAD, Braak NFT, and Thal, for instance, maintain the definition of AD as a procession from a complex of clinically pathological diagnoses of Aβ aggregates, neurofibrillary tangles, and cognitive dysfunction; however, recent case series showing AD-diagnosed patients lacking Aβ deposits challenge the generalizability of the criteria, as well as the role of protein aggregates in the development and progression of AD [4]. Nonetheless, the combined presence of tangles and plaques, which are associated with progressive dementia and neurodegeneration, is the most widely accepted view [5].
Interestingly, the presence of Aβ and tau pathologies in AD are said to associate with inflammatory responses, the latter paving the way to the development of the disease in question. The soluble tau oligomers, which are hypothesized to bring about more drastic adverse events related to tangle formation, were shown to co-localize with inflammation-associated astrocytes, microglia, and related cytokines [3]. In addition, a recent study by Laurent et al. showed that T-cells from the hippocampus mediate the inflammation process and promote cognitive decline [6]. What is alarming is the fact that tau proteins propagate their pathological assemblies in a prion-like manner [7], which tends to aggravate the degree of inflammation and progression of AD.
Remarkably, the symptoms and general features of AD coincide with that of sporadic inclusion body myositis (sIBM)—a form of skeletal muscle disease. As with AD, sIBM is by consensus a combination of muscular degeneration and inflammation [8], often characterized by slow-onset atrophy, lethargy, and dysphagia, with hallmark biomarkers of filamentous inclusions and intracellular Aβ deposits in muscle cells [9]. As with AD, sIBM is late-onset, with the highest prevalence occurring in older age groups, especially beginning at the age of 50 [8,10]. Thus, it is seen that both degenerative diseases contribute to lessening the quality of life of the elderly, aggravated by the lack of a known cure.
There have been many researches on VCO and its potential to salvage neurons from amyloid-induced degeneration, reduce inflammation, and provide ketone bodies for therapeutic effects and increased cognitive function [11,12,13,14]. However, it is proposed that the anti-amyloidogenic and anti-aggregatory properties are to be found in the phenolic compounds of the plant [15,16], which motivated the present study. Here, we demonstrate the antioxidant and acetylcholinesterase (AChE) inhibitory properties of coconut leaf extract (CLE) using 2,2-diphenyl-1-picryl-hydrazyl (DPPH) scavenging and AChE inhibition assays. We then demonstrated that CLE reduced Aβ aggregation and paralysis in vivo. Phytochemical analysis then revealed that glycosides, anthocyanins, and hydrolyzable tannins were present in the extract, warranting further investigation on these bioactive compounds in CLE.
2. Materials and Methods
2.1. Leaf Harvesting and Crude Ethanolic Extraction of Cocos nucifera Leaves
Leaves were harvested from mature coconut (Cocos nucifera) trees in ecologically acceptable proportions at San Nicolas, Gapas City of the Nueva Ecija province, Philippines. For confirmation and the correct classification of plant identity, samples were sent to the botany division of The National Museum at Ermita, Manila, Philippines, and to the Industrial Technology Development Institute (ITDI) of the Department of Science and Technology (Taguig, Philippines) for standardized crude ethanolic extraction. For phytochemical analysis, the same outsource implemented the procedure, as requested.
2.2. Caenorhabditis elegans Strains
All strains were obtained from and provided by the Caenorhabditis Genetics Center (CGC) of the University of Minnesota, which is funded by the NIH Office of Research Infrastructure Programs (P40 OD010440). For the present study, the following strains were used:
- N2—wild type
- C. elegans var. Bristol
- CL4176—expresses Aβ1–42
- dvIs27 [myo-3p::A-β(1-42)::let-851 3′UTR]
- CL2006—expresses Aβ1–42; temperature-sensitive
- dvIs2 [pCL12(unc-54/human Aβ peptide 1-42 minigene) + pRF4]
CL2006 strains were maintained at 20 °C, while CL4176 strains were kept at 16 °C, and both were periodically transferred to OP50-incubated nematode growth media (NGM) plates. For phenotypic activation in CL4176, heat-sensitive nematodes were continually exposed at 25 °C post-treatment. All plates were prepared according to the protocol of Steirnagle [17].
2.3. Antioxidant and Acetylcholinesterase Inhibitory Activities of Crude CLE Extracts
Leaf extracts were tested for their antioxidant properties and capability of inhibiting AChE via the DPPH and AChE inhibition assays, respectively. For the DPPH scavenging assay, five treatments of CLE with progressing concentrations (in μg/mL) and one control were prepared, as follows. For the positive control, ascorbic acid at the same concentrations (in μg/mL) as the CLEs were tested. On the other hand, methanol was used as the negative control. Absorbance was then taken after 30 min at 517 nm and dose-response curves for both CLE and ascorbic acid were graphed, from which the EC50 value was directly obtained. The EC50 is the effective concentration where a half-maximal effect is observed. The AChE inhibition assay was similarly performed, consisting of 11 treatment groups. The positive control used in this assay was Rivastigmine, which is a known AChE inhibitory drug and is currently used as a treatment for patients with AD [18]. Meanwhile, the protocol for the AChE inhibitory assay was adopted from Ellman et al. [19]. Enzyme activity was obtained at 25-second intervals for 31 readings at 420 nm, using the Thermoscientific Multiskan. Both assays were done in triplicate.
2.4. Nematode Toxicity Assay
To eliminate the confounding factor of toxicity in the procedure, nematodes – wild-type (WT) and transgenic (TG) – were exposed to increasing concentrations of CLE (12.75, 127.5, 1275 μg/mL). Further, nematodes were exposed to sterile dH2O as the negative control. All treatment groups were exposed to intervention for 24 h, adopted from Qiao et al. [20], after which the percent mortality was obtained using a stereoscope from the Department of Biochemistry and Molecular Biology, College of Medicine, University of the Philippines Manila (Manila, Philippines). The toxicity assay aforementioned was done in triplicate.
2.5. Amyloid-β Aggregation and Paralysis Tests
To test the protective effect of CLE against AD and sIBM in vivo, two C. elegans strains (CL2006 and CL4176) were exposed to varying concentrations of CLE. In all procedures, sterile dH2O acted as the negative control. For CL2006, nematodes were divided into groups and exposed to treatment at 20 °C for five days. Nematodes were then sampled via worm-picking and placed on 2% agarose pads with two drops of ~80% glycerol, before being placed on a microscope slide. At this point, some of the worms may perish, so the glycerol may be lowered to ~50% or be viewed within five minutes or less to prevent confounding degradation. Aβ deposits were viewed under bright-field microscopy to improve protein deposit scoring. For CL4176, treatment was administered prior to heat activation. Nematodes were divided into groups and exposed at different concentrations of CLE at 16 °C for 36 h. The temperature was then raised to 25 °C for two hours, and the proportion of paralyzed nematodes was obtained every 12 for 36 h. Likewise, the aggregation assay was employed using similar conditions to the paralysis assay (Table 1). For both strains, the assays were done in triplicate.

Table 1.
Coconut leaf extract (CLE) treatment groups for Aβ1–42 aggregation and paralysis assays.
2.6. Statistical Analyses
The effects of intervention and control were compared via analysis of variance (ANOVA) and IC50 and EC50 were determined using GraphPad Prism 6.0 (GraphPad Software, Inc., La Jolla, CA, USA). For all of the statistical tests, a p-value of p < 0.05 was accepted as statistically significant. To further test the validity of the results, post-hoc two-tailed t-tests (in least significant difference) were computed where applicable.
3. Results
3.1. Coconut Leaf Extract (CLE) Neutralizes DPPH Radicals in a Dose-Dependent Manner
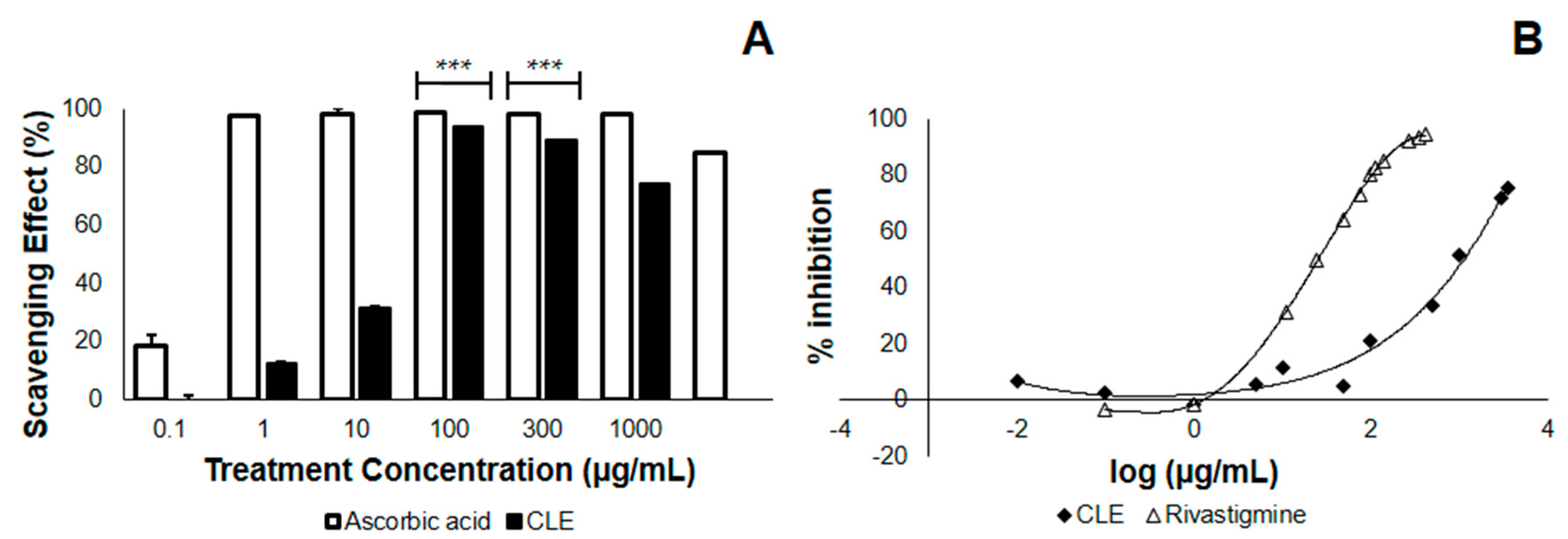
In the study conducted, leaves from Cocos nucifera were first tested in terms of their antioxidant property and AChE inhibitory activity, before being tested in vivo, for practical purposes. Here, we checked whether the CLE would neutralize the DPPH radical, thus lowering its absorbance at 517 nm. We found that at the λmax of DPPH, CLE neutralizes DPPH with an EC50 of 18.11 μg/mL (Table 2), less than one-fifth of the concentration of the 4th treatment group (Figure 1A). This implies that at a relatively low concentration, CLE elicits an antioxidant effect. Further, we found that the antioxidant property of CLE in vitro was dose-dependent, in that higher concentrations of CLE led to greater losses of DPPH absorbance at 517 nm (Figure 1A). Apparently, there was no difference in the antioxidant activity between 100–1000 μg/mL, as confirmed by one-way analysis of variance (ANOVA). However, the antioxidant activity at these concentrations might have already been saturated. In terms of its EC50, the antioxidant activity of CLE was about 79-fold less—implying a possible contribution from antioxidant activity against Aβ that is less manifold than if it were ascorbic acid.

Table 2.
Scavenged DPPH free radicals and inhibited AChE at various concentrations of CLE.
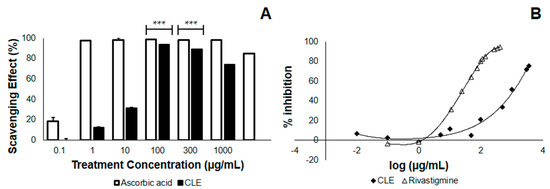
Figure 1.
2,2-diphenyl-1-picryl-hydrazyl (DPPH) scavenging and acetylcholinesterase (AChE) inhibition by ethanolic coconut (Cocos nucifera) leaf extract in vitro. The CLE was tested at varying concentrations in vitro for antioxidant activity and the ability to inhibit standard AChE. (A) DPPH scavenging effect of CLE compared with the control (ascorbic acid). At 100 μg/mL, CLE neutralized DPPH radicals by 93.85% (n = 3). *** p < 0.0001 when compared with the control at the same concentration using a two-tailed t-test. p < 0.05 was used to compare treatment groups 4 to 6 (100–1000 μg/mL) using one-way ANOVA; (B) Dose-response curves comparing the AChE inhibitory activity of CLE and positive control (Rivastigmine) in vitro.
3.2. CLE Inhibits AChE Less Effectively than Does Rivastigmine In Vitro
We then checked whether CLE inhibits AChE, which is postulated to confer protection from complications in AD and is therefore a desired effect. The results showed that when CLE is homogenized with AChE and incubated at 25 °C for 15 min, it inhibits the activity on acetylcholine metabolism with an IC50 of 3218.56 μg/mL. Using the standard of treatment (Rivastigmine), the IC50 value was found to be 24.52 μg/mL (Figure 1B). This implies the presence of an inhibitory activity only at manifold higher concentrations, as compared with vehicle (Table 2).
3.3. CLE is Non-Lethal to Wild-type and Transgenic C. elegans CL2006 and CL4176
To eliminate the possibility that CLE has intrinsically deleterious effects in vivo, we exposed strains N2, CL2006, and CL4176 at concentrations progressing to three orders of magnitude (12.75, 127.5, 1275 μg/mL). Observations post-treatment showed a 100% survival rate for all nematodes, indicating that at these concentrations, any lethal effects that are observed are solely due to Aβ1–42 expression.
3.4. CLE Significantly Reduces Aβ Aggregate Deposits in Transgenic C. elegans Strains CL2006 and CL4176
We next determined whether CLE would elicit protective effects in vivo. Varying concentrations of CLE were administered to groups of transgenic C. elegans after a maturation period of three days; then, measurements were successively taken post-treatment, according to established protocol. For testing the therapeutic effects of CLE, a C. elegans strain population continuously expressing Aβ aggregates in the muscle cell walls (CL2006) was used.
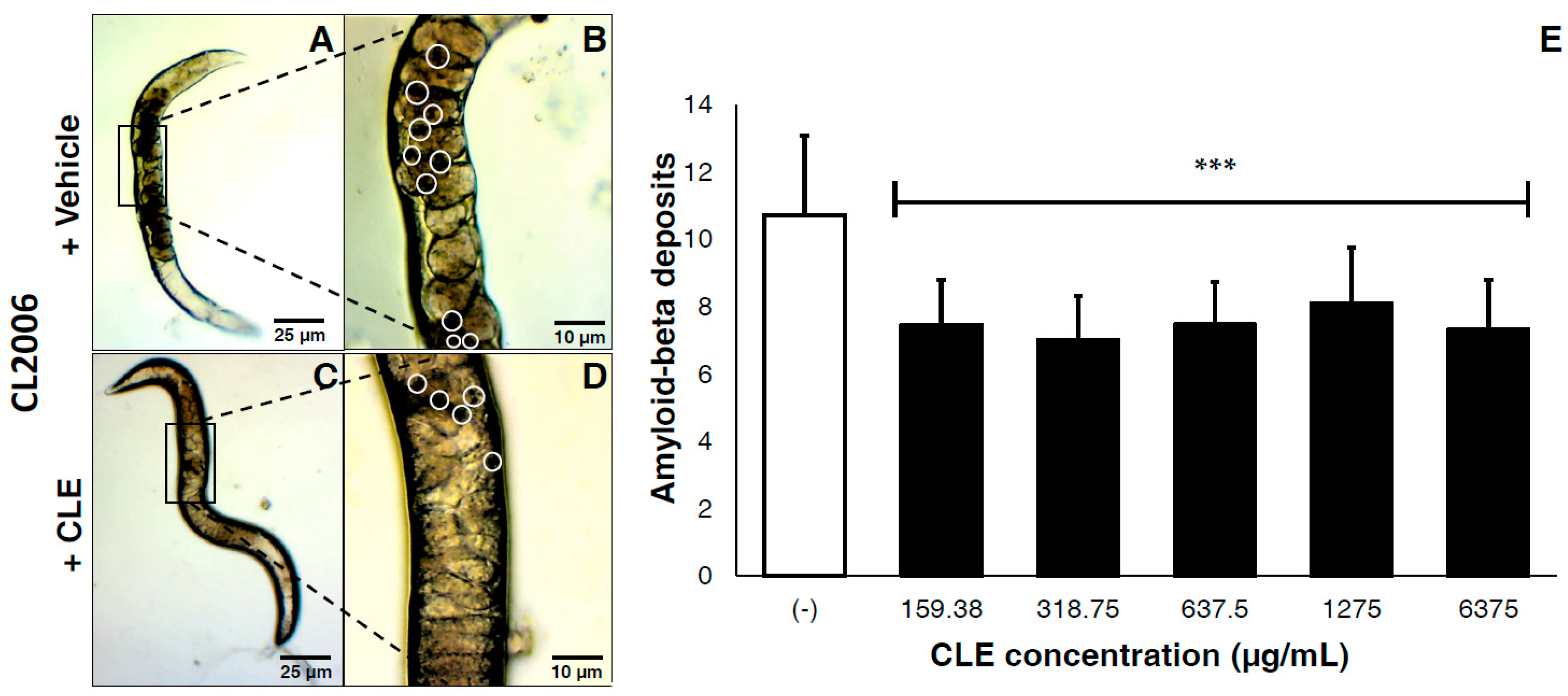
This tested the efficacy of CLEs against plaque deposition after the formation of Aβ proteins. In vivo, CLE protected against Aβ toxicity by reducing the deposits of visible aggregates in the cell walls of CL2006 (Figure 2A–D). Compared to vehicle, a mean reduction of 30.31% in Aβ deposits was observed. Two-tailed t-tests confirmed this observation as highly statistically significant at p < 0.0001 (Figure 2E). Further, there were no significant differences observed on the deposition of Aβ aggregates, regardless of the CLE concentration administered. This implies that the efficacy of CLE is somewhat dose-independent.
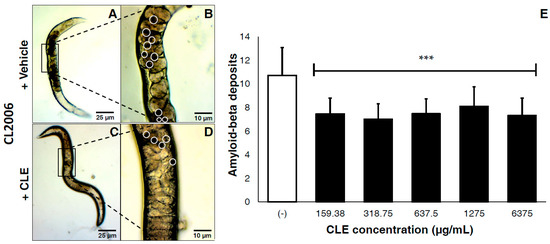
Figure 2.
CLE significantly reduces amyloid-β plaque deposits in transgenic C. elegans strains CL2006, independently of concentration. Nematodes were transferred to OP50-incubated NGM plates and allowed to mature for three days at 20 °C, after which they were fed with either vehicle or CLE at varying concentrations ad libitum. The amyloid-β deposits in CL2006 were then counted five days post-treatment, and were pooled in triplicate. Dark portions in the cell wall of C. elegans indicate the protein deposits. CL2006 strains were fed with either (A) vehicle or (C) CLE. Detailed images of the body walls of nematodes fed with (B) vehicle or (D) CLE are shown. White circles identify the aggregate deposits; (E) Aβ deposits were counted per treatment group at varying concentrations of CLE (in μg/mL). *** p < 0.0001 when compared with vehicle using two-tailed t-test. p < 0.05 was used to test for significance between treatment groups using one-way ANOVA.
3.5. Paralysis of CL4176 C. elegans Strains Was Partially Relieved by Exposure to CLE Prior to Heat Activation of Aβ Expression
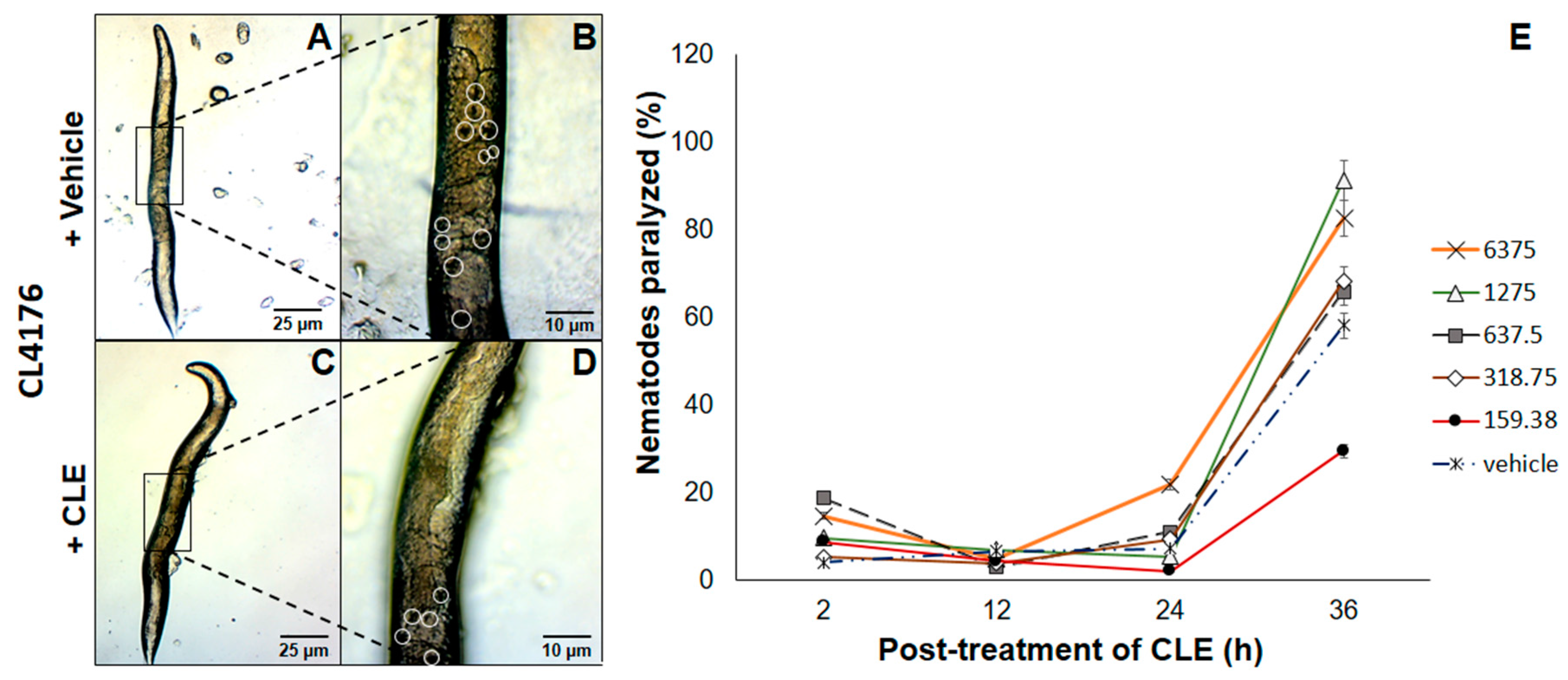
The next question to ask was whether CLEs elicited beneficial effects when administered at an earlier time point. Therefore, a strain only expressing Aβ proteins upon exposure to a temperature of 25 °C and above proved the utility. To this end, CL4176 C. elegans strains were exposed to CLE for 36 h, before allowing Aβ production. This is in stark contrast with CL2006, primarily because the prophylactic effect of CLE was now being tested. In all of triplicates examined, nematodes assigned to treatment groups with CLE exposure had a lower prevalence of paralysis at the 12th hour post-induction, which then led to paralysis no better than or worse than the control, except at the concentration of 159.38 μg/mL (Figure 3E).
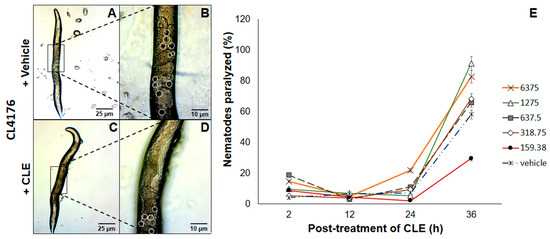
Figure 3.
CLE reduces paralysis prevalence in C. elegans strain CL4176. Nematodes were exposed to (A) vehicle or (C) CLE in concentrations similar to the Aβ aggregation assay in vivo. Aβ expression was induced at 25 °C after 36 h post-treatment of CLE or vehicle at 16 °C for 36 h. Detailed images of the body walls of nematodes fed with (B) vehicle or (D) CLE are shown. White circles identify the aggregate deposits; (E) The proportion of paralyzed nematodes were gathered at 2, 12, 24, and 36 h post-induction. To determine the link of aggregation in paralysis, the aggregation assay was done in CL4176 using the paralysis assay conditions.
To assess the link between aggregation and paralysis, the CL4176 strain was used for the aggregation assay at the concentration with the lowest apparent effect in CL4176 (1275 μg/mL) at the 36th hour, with conditions similar to the paralysis assay. As the results showed, aggregates were lessened by up to 54.55% compared to vehicle at 12 h post-induction. To this end, it is likely that paralysis reduction was drastic at this time point due to a high reduction in Aβ deposits—however, the lack of a trend between paralysis and Aβ aggregation at the 24th and 36th hours suggests that CLE can only delay paralysis, and that Aβ aggregation is not the only determinant of paralysis in CL4176, since the concentration that worked was the lowest (159.38 μg/mL), which should also have the lowest antioxidant and AChE inhibitory activities of all the CLE treatment groups. The observed proportion of nematodes relieved of motor deficits was statistically confirmed with a value of p < 0.05.
There was a certain difficulty in distinguishing the proportion of relieved nematodes using the CL2006 strains, so the paralysis assay was only done on CL4176. This difficulty is in fact warranted, due to the inherent resistance of CL2006 to paralysis—indeed, the variability of paralysis between nematodes was found to be unpredictable, with some nematodes never becoming paralyzed [21]. Hence, the need to use CL4176 for paralysis assays related to Aβ expression maintains its essentiality.
3.6. Phytochemicals Present in CLE Possibly Interact with Aβ1–42 Peptide
In the phytochemical analysis, screening of the compounds resulted in the presence of glycoside compounds, flavonoids (particularly anthocyanins), and hydrolyzable tannins (Table 3). Due to the presence of hydrolyzable tannins in the CLE, it was postulated that the metabolites of such tannins could also contribute to the efficacy of the leaf extract. Urolithin A, a derivative of ellagitannin that is known to induce mitophagy and increase the lifespan of C. elegans [22,23,24,25]; gallic acid, a part of hydrolyzable tannins with known antimicrobial, antioxidant, and neuroprotective effects [26,27,28]; and pyrogallol, a derivative of gallic acid whose moiety is associated with β-secretase (BACE1) inhibition [29] and together with gallic acid is known to be anti-amyloidogenic [30], could be possible contributors to the protective effect of CLE in vivo. It was noted that cardiac glycosides may accumulate in the central nervous system [31], warranting investigation on its alternative yet promising effect on Aβ1–42 aggregation.

Table 3.
Phytochemical analysis of crude ethanolic CLE.
4. Discussion
4.1. C. elegans as a Model for Alzheimer’s Disease (AD) and Sporadic Inclusion Body Myositis (sIBM)
In the study conducted, Aβ aggregation and its toxicity in the form of paralysis were treated in vivo with ethanolic extracts from coconut (Cocos nucifera) leaves. The transgenic Caenorhabditis elegans strains used served as biological models of AD and sIBM—the former in terms of Aβ overexpression and aggregation, and the latter in terms of expression in the muscle cell walls. Further, the two strains modelled the effects of administration at two different time points-before the induction of Aβ expression and after it was expressed. While higher forms of animal models would generate greater external validity, the use of transgenic nematodes for this study holds several advantages. For one, human orthologs of disease genes such as tau, relevant neuronal cells, ion channels, and transporters are conserved in C. elegans, which are important in neurodegenerative disease studies.
To this end, nematodes have been utilized in pathway analysis and drug screening for diseases such as AD, Parkinson’s disease (PD), and Huntington’s (HD) [32,33,34]. Further, the conservation of 12 over 17 signalling cascades, a short generation time, and a life cycle of two to three weeks, as well as the ease of observation provided by its transparent body lining, provide efficient tools for scoring protein aggregation [35]. While it is arguable that aggregation in muscle walls does not entirely represent the multiple factors associated with AD, expression in muscle cells grants a larger picture of protein formation, and is useful for studies specifically targeting insoluble Aβ deposits, since it is easier to visualize than if it were in neuronal cells and is actually the tissue of choice for studies involving such proteins [36,37].
4.2. Reduction of Aβ1–42 Aggregation by CLE Is Independent of Free Radical Scavenging and AChE Inhibition
In the DPPH assay, the EC50 value for radical scavenging was found to be 18.11 μg/mL, and as the concentration increased further, the antioxidant activity remained the same, as confirmed by ANOVA. However, this antioxidant activity may or may not play a part in the amelioration of insoluble Aβ deposits, due to the fact that CLE has a ~79-fold greater EC50 as compared to ascorbic acid (Table 2).
In the AChE inhibition assay, CLE showed even less effective results. In preventing the activity of standard AChE, the IC50 of CLE was found to be more manifold than that of Rivastigmine (3218.56 μg/mL versus 24.52 μg/mL), implying a dose-dependent activity whose efficacy is observed at much higher concentrations. The critical results were manifested in the Aβ aggregation assay in CL2006. In all treatment groups (159.38.5 to 6375 μg/mL), a steady decrease in protein deposition of 30.31% was observed. Strikingly, this opposed the dose-dependent activity of CLE against AChE, since at every concentration from 160 μg/mL, the efficacy of the extract was consistently maintained. Were AChE inhibition to contribute significantly, the beneficial effect should be observed between treatments 4 and 5 (1275 and 6375 μg/mL)—however, this was not the case. Hence, CLE acted against Aβ deposits through a pathway that involved more than just AChE inhibition.
Further, the results opposed, in some way, the antioxidant activity of CLE against DPPH. CL2006 displayed a 30.31% Aβ reduction from 187.5 μg/mL of CLE, which was also dose-independent and was statistically the same throughout all the concentrations. While this concentration is about nine times that of the EC50 value in vitro, we argue that: (1) the composition of CLE as it acts in vivo would likely change due to metabolism by the C. elegans machinery; and (2) it is expected that at high concentrations, vitamin C, as well as other antioxidants, should become pro-oxidant in vitro and in vivo [22,23,24]—therefore, at least at treatments 3 to 5 (with concentrations 35 to 350 times higher than EC50 in vitro), Aβ was expected to increase. It is worth noting that previous in vivo studies demonstrated the therapeutic effects of vitamin C at moderate doses [38,39], which at larger doses of about 600 mg/kg, resulted in increased neurodegeneration and neuroinflammation in AD rat models [40]. These findings reflect the duality of vitamin C in terms of its oxidizing ability [41,42], which may promote Aβ deposition by reacting with metal ions similar to how copper (Cu2+) induces Aβ-mediated H2O2 generation upon binding to His residues and undergoing redox reactions [43]. Since CLE is non-inferior to ascorbic acid in terms of its antioxidant activity at high concentrations, a similar trend to previous studies should be reflected in the treatment groups, which were 9–350 times higher than the DPPH EC50 of CLE. Strikingly, this was not the case, and Aβ deposition neither increased nor decreased significantly at higher CLE concentrations in vivo. To this end, we hypothesize that CLE is also acting in a way that opposes the free radical scavenging duality at high concentrations.
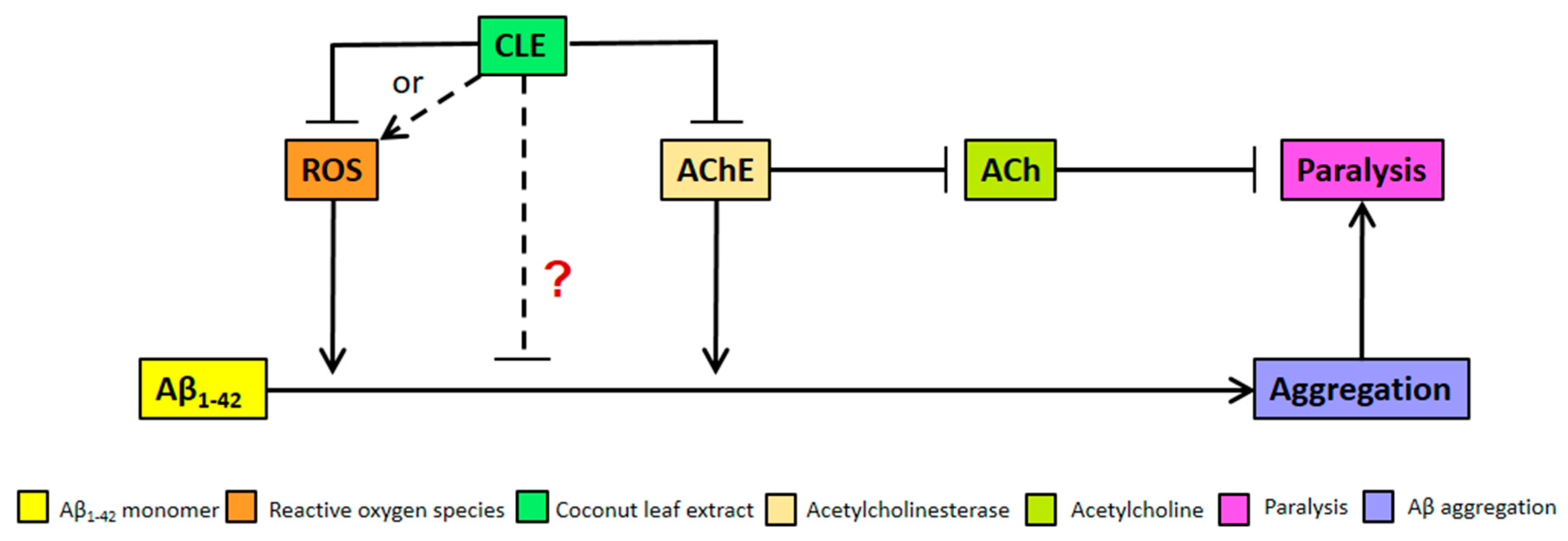
We clarify that the alternative anti-aggregatory effect of CLE might be dose-dependent, but is being masked by the increasing pro-oxidant activity of antioxidants at higher concentrations of CLE (Figure 4). This hypothesis is not unlikely, since recent studies have also shown neuroprotective effects that are either independent of antioxidant activity or address a more complex interplay of factors that constitute an antioxidant, such as lipophilicity and molecular weight [44,45]. These remarkable results point out the possible mode of action of CLE metabolites apart from conventional criteria, such as interactions with Aβ-related proteins like the peroxisome proliferator-activated receptor gamma (PPARγ) and pro-apoptotic proteins—respectively known to be upregulated and downregulated by natural products such as epigallocatechin gallate (EGCG) in green tea [29]. Further, CLE may instead be interacting with known proteins affecting Aβ deposition in C. elegans, such as DAF-2 and FOXO [46,47], all of which warrant further investigation on the possible mechanisms on CLE metabolites.
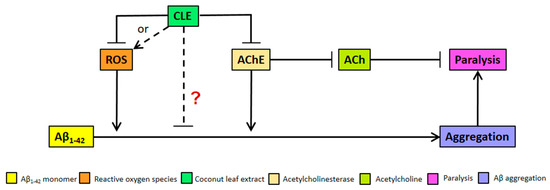
Figure 4.
Model for Aβ aggregation and paralysis reduction by CLE in transgenic C. elegans. CLE acts by inhibiting reactive oxygen species (ROS) and AChE activities in C. elegans, albeit at high concentrations—both of which would otherwise worsen Aβ-induced pathology. However, at high concentrations, a pro-oxidant effect is expected due to antioxidant excess, resulting to CLE-induced ROS production (broken arrow). At concentrations 9 to 350 times higher than the EC50 of CLE, the effect on aggregation was dose-independent, as shown in Figure 2. Therefore, it is possible that CLE is acting directly through compounds that are anti-aggregatory, which masks the pro-oxidant activity of antioxidants in CLE at higher extract concentrations (red “?”).
We hypothesize, therefore, that bioactive compounds in CLE directly act to inhibit Aβ1–42 aggregation (Figure 4), and if these anti-aggregatory compounds in associated CLE are isolated and separated from those that confer protection from oxidative stress, a much greater effect might be observed in vivo.
4.3. Reduction of Aβ-Induced Paralysis by CLE Is Independent of Free Radical Scavenging and AChE Inhibition
In the paralysis assay, CL4176 nematodes displayed a higher incidence of paralysis 2 h post-induction in treatment groups with CLE. The hypothesis that CLE is deleterious in terms of paralysis was tested by comparing the proportion of nematodes paralyzed 12 h post-induction. The results showed that this was not the case. In the assays performed, CL4176 nematodes were relieved of paralysis compared to the control at the lowest concentration of CLE (159.38 μg/mL). Since this concentration also exhibits the lowest antioxidant and AChE inhibitory activities, the effect observed against paralysis implies an action independent of antioxidant activity and AChE inhibition. From here, it may be inferred that the factors determining the incidence of paralysis are different from those that aggravate its progression. For instance, it is known that the lifespan of C. elegans is short (two to three weeks), more so with transgenic strains, and that as the nematodes age, the capacity to maintain proteostasis decreases, aggravating the accumulation of insoluble proteins that exist even in physiologic conditions [48,49]. Therefore, it is rational to hypothesize that the extracts might also be increasing the lifespan of the paralyzed nematodes. Should this be the case, then the succeeding cross-sectional observations would tend to obtain a higher proportion of paralyzed nematodes in the CLE treatment groups, because more nematodes survive long enough to be counted and these nematodes, due to an older age, develop a more profound form of paralysis. These results may have implications in the age and concentration at which CLE administration would elicit its maximum protective effect against AD and sIBM. Further, it is also possible that CLE has bioactive compounds which are cytotoxic but non-lethal to the nematodes at very high concentrations, but was not readily evident in the aggregation and nematode toxicity assays because of the suspected anti-aggregatory bioactive compound.
We further compared the effects of CLE exposure in CL2006 and CL4176, and found that the concentration of CLE does not matter in CL2006; rather, at any concentration, the nematodes benefited in terms of the reduction in Aβ deposits. With CL4176, this was not the case. This is implicative of two possibilities: (1) CLE contains a bioactive compound that is exclusively effective against Aβ1–42 aggregation; and (2) more factors other than Aβ aggregation, ROS production, and AChE action on ACh may exist in the progression of paralysis in C. elegans. We recommend using a greater sample size than n = 30 to assess whether the paralysis trend improves, as well as using higher animal models, such as mice.
4.4. Anthocyanins, Tannins and Glycosides Are Candidate Compounds Against Aβ1–42 Induced Pathology
Phytochemical screening of CLE showed the presence of anthocyanins, hydrolyzable tannins, and glycosides. It is important to note that the metabolism of CLE by C. elegans occurred throughout the study; hence, the isolation of a single compound might be more complex. For instance, secondary metabolites such as urolithin A, which has been shown to extend the lifespan of C. elegans and improve muscle strength [22,23,24,25], or gallic acid and its derivative pyrogallol that are known as anti-amyloidogenic compounds [30], are all known secondary metabolites of the phytochemicals detected and may in part explain the observed differences in the assays performed. Indeed, the metabolism of phytochemicals in vivo may play a role in the differential effects of CLE in vitro and in vivo.
5. Conclusions
AD and sIBM are neurodegenerative and inflammatory muscle diseases, respectively, but are related to each other due to known risk factors related to proteinopathies. One such factor is the aggregation of Aβ, which allows a “two birds with one stone” approach to drug or extract screening for cell-distinct diseases. The results of our study show that CLE has an antioxidant activity that is inferior by 79-fold to ascorbic acid, and an AChE inhibitory activity 131-fold less than Rivastigmine, a known AChE inhibitory drug being prescribed to AD patients. However, stark contrasts between the antioxidant and AChE inhibitory activities in vitro, and the protective effect of CLE against Aβ aggregation and paralysis in vivo, suggest that CLE may act against AD and sIBM in a way that is independent of free radical scavenging and acetylcholinesterase inhibition. However, the protective effects of CLE only delay the progression of paralysis and cannot fully salvage the nematodes from deleterious motor deficits. This warrants further investigations on the time-dependence of CLE administration and biofunctional activities apart from AChE inhibition and free radical scavenging. Lastly, the presence of anthocyanins, hydrolyzable tannins, and glycosides direct future researchers to a guided screening of compounds related to these in an effort to treat Aβ1–42-induced pathology.
Acknowledgments
All strains were obtained from and provided by the Caenorhabditis Genetics Center (CGC) of the University of Minnesota, which is funded by the NIH Office of Research Infrastructure Programs (P40 OD010440). We wish to thank the Department of Education of the Republic of the Philippines for the funding to do this research. Mary Lorraine Lorido is acknowledged for her valuable contribution in assisting Aza Lea and Maries Ann. Further, their colleagues at Juan R. Liwag Memorial High School are also acknowledged. No funds for covering the cost of open access publishing have been given.
Author Contributions
Paul Mark Medina conceived and designed the experiments, discussed the results, and designed the figures with Rafael Vincent Manalo, and was the overall supervisor of the experiments; Rafael Vincent Manalo wrote the paper, managed the C. elegans strains, did additional experiments with Paul Mark Medina for manuscript revisions, and contributed ideas in discussing the results; Maries Ann Silvestre and Aza Lea Anne Barbosa performed the experiments; In addition, all authors contributed to the analysis of the paper.
Conflicts of Interest
The authors declare no conflict of interest. The funding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
References
- El Gaamouch, F.; Jing, P.; Xia, J.; Cai, D. Alzheimer’s Disease Risk Genes and Lipid Regulators. J. Alzheimer’s Dis. 2016, 53, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.L.; Narasimhan, S.; Changolkar, L.; He, Z.; Stieber, A.; Zhang, B.; Gathagan, R.J.; Iba, M.; McBride, J.D.; Trojanowski, J.Q.; et al. Unique pathological tau conformers from Alzheimer’s brains transmit tau pathology in nontransgenic mice. J. Exp. Med. 2016, 213, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Nilson, A.N.; English, K.C.; Gerson, J.E.; Whittle, T.B.; Crain, C.N.; Xue, J.; Sengupta, U.; Castillo-Carranza, D.L.; Zhang, W.; Gupta, P.; et al. Tau Oligomers Associate with Inflammation in the Brain and Retina of Tauopathy Mice and in Neurodegenerative Diseases. J. Alzheimer’s Dis. 2016, 55, 1083–1099. [Google Scholar] [CrossRef] [PubMed]
- Crary, J. Primary age-related tauopathy and the amyloid cascade hypothesis: The exception that proves the rule. J. Neurol. Neuromed. 2016, 1, 53–57. [Google Scholar]
- Hardy, J.; Selkoe, D.J. The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science 2002, 297, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Laurent, C.; Dorotheé, G.; Hunot, S.; Martin, E.; Monnet, Y.; Duchamp, M.; Dong, Y.; Légeron, F.P.; Leboucher, A.; Burnouf, S.; et al. Hippocampal T cell infiltration promotes neuroinflammation and cognitive decline in a mouse model of tauopathy. Brain 2016, 140, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Tardivel, M.; Bégard, S.; Bousset, L.; Dujardin, S.; Coens, A.; Melki, R.; Buée, L.; Colin, M. Tunneling nanotube (TNT)-mediated neuron-to neuron transfer of pathological Tau protein assemblies. Acta Neuropathol. Commun. 2016, 4, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Karpati, G.; O’Ferrall, E. Sporadic inclusion body myositis: Pathogenic considerations. Ann. Neurol. 2009, 65, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Dalakas, M.C. Sporadic inclusion body myositis—Diagnosis, pathogenesis and therapeutic strategies. Nat. Clin. Pract. Neurol. 2006, 2, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Oh, T.H.; Brumfield, K.A.; Kasperbauer, J.L.; Basford, J.R. Dysphagia in inclusion body myositis: Clinical features, management, and clinical outcome. Am. J. Phys. Med. Rehabil. 2008, 87, 883–889. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; de la Rubia Orti, J.E.; Selvi, S.; Sancho Castillo, S.; Rochina, M.J.; Manresa Ramon, R.; Montoya-Castilla, I. Coconut Oil: Non-Alternative Drug Treatment Against Alzheimer’s Disease. Nutr. Hosp. 2015, 32, 2822–2827. [Google Scholar]
- Nafar, F.; Mearow, K.M. Coconut oil attenuates the effects of amyloid-β on cortical neurons in vitro. J. Alzheimer’s Dis. 2014, 39, 233–237. [Google Scholar]
- Nonaka, Y.; Takagi, T.; Inai, M.; Nishimura, S.; Urashima, S.; Honda, K.; Aoyama, T.; Terada, S. Lauric Acid Stimulates Ketone Body Production in the KT-5 Astrocyte Cell Line. J. Oleo Sci. 2016, 65, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Dedea, L. Can coconut oil replace caprylidene for Alzheimer disease? J. Am. Acad. Phys. Assist. 2012, 25, 19. [Google Scholar] [CrossRef]
- Fernando, W.M.; Martins, I.J.; Goozee, K.G.; Brennan, C.S.; Jayasena, V.; Martins, R.N. The role of dietary coconut for the prevention and treatment of Alzheimer’s disease: Potential mechanisms of action. Br. J. Nutr. 2015, 114, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.W.C.; Lim, Y.Y.; Omar, M. Antioxidant and antibacterial activity of leaves of Etlingera species (Zingiberaceae) in Peninsular Malaysia. Food Chem. 2007, 104, 1586–1593. [Google Scholar] [CrossRef]
- Stiernagle, T. Maintenance of C. elegans. Available online: http://www.wormbook.org/chapters/www_strainmaintain/strainmaintain.html (accessed on 20 December 2016).
- Lefèvre, G.; Callegari, F.; Gstegier, S.; Xiong, Y. Effects of Renal Impairment on Steady-State Plasma Concentrations of Rivastigmine: A Population Pharmacokinetic Analysis of Capsule and Patch Formulations in Patients with Alzheimer’s Disease. Drugs Aging 2016, 33, 725–736. [Google Scholar] [CrossRef] [PubMed]
- George, L.; Ellman, K.; Courtney, D.; Andres, V., Jr.; Featherstone, R. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 90–95. [Google Scholar]
- Qiao, Y.; Zhao, Y.; Wu, Q.; Sun, L.; Ruan, Q.; Chen, Y.; Wang, M.; Duan, J.; Wang, D. Full toxicity assessment of Genkwa Flos and the underlying mechanism in nematode Caenorhabditis elegans. PLoS ONE 2014, 9, e91825. [Google Scholar] [CrossRef] [PubMed]
- Lublin, A.L.; Link, C.D. Alzheimer’s Disease Drug Discovery: In vivo screening using C. elegans as a model for β-amyloid peptide-induced toxicity. Drug Discov. Today Technol. 2013, 10, e115–e119. [Google Scholar] [CrossRef] [PubMed]
- Ryu, D.; Mouchiroud, L.; Andreux, P.A.; Katsyuba, E.; Moullan, N.; Nicolet-Dit-Felix, A.A.; Williams, E.G.; Jha, P.; Lo Sasso, G.; Huzard, D.; et al. Urolithin A induces mitophagy and prolongs lifespan in C. elegans and increases muscle function in rodents. Nat. Med. 2016, 22, 879–888. [Google Scholar] [PubMed]
- Valero, T. Mitochondrial biogenesis: Pharmacological approaches. Curr. Pharm. Des. 2014, 20, 5507–5509. [Google Scholar] [CrossRef] [PubMed]
- Tomas-Barberan, F.A.; Gonzales-Sarrias, A.; Garcia-Villalba, R.; Nunez-Sanchez, M.A.; Selma, M.V.; Garcia-Conesa, M.T.; Espin, J.C. Urolithins, the rescue of ‘old’ metabolites to understand a ‘new’ concept: Metabotypes as a nexus between phenolic metabolism, microbiota dysbiosis and host health status. Mol. Nutr. Food Res. 2016, 61, 1500901. [Google Scholar] [CrossRef] [PubMed]
- Seeram, N.P.; Aronson, W.J.; Zhang, Y.; Henning, S.M.; Moro, A.; Lee, R.P.; Sartippour, M.; Harris, D.M.; Rettig, M.; Suchard, M.A.; et al. Pomegrenate ellagitannin-derived metabolites inhibit prostate cancer growth and localize to the mouse prostate gland. J. Agric. Food Chem. 2007, 55, 7732–7737. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, M.T.; Farbood, Y.; Sameri, M.J.; Sarkaki, A.; Naghizadeh, B.; Rafeirad, M. Neuroprotective effects of oral gallic acid against oxidative stress induced by 6-hydroxydopamine in rats. Food Chem. 2013, 138, 1028–1033. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.X.; Li, Y.B.; Zhao, R.P. Epigallocatechin Gallate Attenuates β-Amyloid Generation and Oxidative Stress Involvement of PPARγ in N2a/APP695 Cells. Neurochem. Res. 2017, 42, 468–480. [Google Scholar] [CrossRef] [PubMed]
- Gul, F.; Khan, K.M.; Adhikari, A.; Zafar, S.; Akram, M.; Khan, H.; Saeed, M. Antimicrobial and antioxidant activities of a new metabolite from Quercus incana. Nat. Prod. Res. 2016, 21, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.Y.; Bae, K.; Seong, Y.H.; Song, K.S. Green tea catechins as a BACE1 (β-secretase) inhibitor. Bioorg. Med. Chem. Lett. 2003, 13, 3905–3908. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.; Kantham, S.; Rao, V.M.; Palanivelu, M.K.; Pham, H.L.; Shaw, P.N.; McGeary, R.P.; Ross, B.P. Metal chelation radical scavenging and inhibition of Aβ42 fibrillation by food constituents in relation to Alzheimer’s disease. Food Chem. 2016, 199, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Smith, T. Pharmakokinetics, bioavailability and serum levels of cardiac glycosides. J. Am. Coll. Cardiol. 1985, 5, 43A–50A. [Google Scholar] [CrossRef]
- Alexander, A.; Marfil, V.; Li, C. Use of Caenorhabditis elegans as a model to study Alzheimer’s disease and other neurodegenerative diseases. Front. Genet. 2014, 5, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Diomede, L.; Rigacci, S.; Romeo, M.; Stefani, M.; Salmona, M. Oleuropein Aglycone Protects Transgenic C. elegans Strains Expressing Aβ42 by Reducing Plaque Load and Motor Deficit. PLoS ONE 2013, 8, e58893. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Barclay, J.; Burgoyne, R.; Morgan, A. Using C. elegans to discover therapeutic compounds for ageing-associated neurodegenerative diseases. Chem. Cent. J. 2015, 9, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Markaki, M.; Tavernarakis, N. Modeling human diseases in Caenorhabditis elegans. Biotechnol. J. 2010, 5, 1261–1276. [Google Scholar] [CrossRef] [PubMed]
- Link, C.D. Expression of human β-amyloid peptide in transgenic Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 1995, 92, 9368–9372. [Google Scholar] [CrossRef] [PubMed]
- David, D.; Ollikainen, N.; Trinidad, J.; Cary, M.; Burlingame, A.; Kenyon, C. Widespread Protein Aggregation as an Inherent Part of Aging in C. elegans. PLoS Biol. 2010, 8, e1000450. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.H.; Hyon-Lee; Lee, K.M. The Possible Role of Antioxidant Vitamin C in Alzheimer’s Disease Treatment and Prevention. Am. J. Alzheimer’s Dis. 2013, 28, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Mukherjee, A.; Swarnakar, S.; Das, N. Nanocapsulated Ascorbic Acid Combating Cerebral Ischemia Reperfusion-Induced Oxidative Injury in Rat Brain. Curr. Alzheimer Res. 2016, 13, 1363–1373. [Google Scholar] [CrossRef] [PubMed]
- Sil, S.; Ghosh, T.; Gupta, P.; Ghosh, R.; Kabir, S.N.; Roy, A. Dual Role of Vitamin C on the Neuroinflammation Mediated Neurodegeneration and Memory Impairments in Colchicine Induced Rat Model of Alzheimer’s Disease. J. Mol. Neurosci. 2016, 60, 421–435. [Google Scholar] [CrossRef] [PubMed]
- Rietjens, I.; Boersma, M.; de Haan, L.; Spenkelink, B.; Awad, H.; Cnubben, N.; van Zanden, J.; van der Woude, H.; Alink, G.; Koeman, J. The pro-oxidant chemistry of the natural antioxidants vitamin C, vitamin E, carotenoids and flavonoids. Environ. Toxicol. Pharmacol. 2002, 11, 321–333. [Google Scholar] [CrossRef]
- Putchala, M.C.; Ramani, P.; Sherlin, H.J.; Premkumar, P.; Natesan, A. Ascorbic acid and its pro-oxidant activity as a therapy for tumours of oral cavity—A systematic review. Arch. Oral. Biol. 2013, 58, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Ginotra, Y.P.; Ramteke, S.N.; Walke, G.R.; Rapole, S.; Kulkarni, P.P. Histidine availability is decisive in ROS-mediated cytotoxicity of copper complexes of Aβ1-16 peptide. Free Radic. Res. 2016, 50, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, K.; Wong, J.M.; Vann, P.H.; Sumien, N. Exercise, but not antioxidants, reversed the ApoE4-associated motor impairments in adult GFAP-ApoE mice. Behav. Brain Res. 2016, 305, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Ohlow, M.J.; Sohre, S.; Granold, M.; Schreckenberger, M.; Moosmann, B. Why Have Clinical Trials of Antioxidants to Prevent Neurodegeneration Failed?—A Cellular Investigation of Novel Phenothiazine-Type Antioxidants Reveals Competing Objectives for Pharmaceutical Neuroprotection. Pharm. Res. 2016. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-K.; Kim, T.H.; Lee, S.-J. Mechanisms of aging-related proteinopathies in Caenorhabditis elegans. Exp. Mol. Med. 2016, 48, e263. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, W.; Ebert, P.R. Metformin Attenuates Aβ Pathology Mediated Through Levamisole Sensitive Nicotinic Acetylcholine Receptors in a C. elegans Model of Alzheimer’s Disease. Mol. Neurobiol. 2016, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kikis, E. The struggle by Caenorhabditis elegans to maintain proteostasis during aging and disease. Biol. Direct 2016, 11, 58. [Google Scholar] [CrossRef] [PubMed]
- Cuanalo-Contreras, K.; Park, W.; Murkherjee, A.; Millán-Pérez, P.; Soto, C. Delaying aging in Caenorhabditis elegans with protein aggregation inhibitors. Biochem. Biophys. Res. Commun. 2017, 482, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Liu, X.; Zhang, X.; Jin, Q.; Li, J. Phenolic Profiles, Antioxidant Activities, and Neuroprotective Properties of Mulberry (Morus atropurpurea Roxb.) Fruit Extracts from Different Ripening Stages. J. Food Sci. 2016, 81, C2439–C2446. [Google Scholar] [CrossRef] [PubMed]
- Di Giovanni, S.; Eleuteri, S.; Paleologou, K.E.; Yin, G.; Zweckstetter, M.; Carrupt, P.A.; Lashuel, H.A. Entacapone and tolcapone, two catechol O-methyltransferase inhibitors, block fibril formation of α-synuclein and β-amyloid and protect against amyloid-induced toxicity. J. Biol. Chem. 2010, 285, 14941–14954. [Google Scholar] [CrossRef] [PubMed]
- Du, X.T.; Wang, L.; Wang, Y.J.; Andreasen, M.; Zhan, D.W.; Feng, Y.; Li, M.; Zhao, M.; Otzen, D.; Xue, D.; et al. Aβ1-16 can aggregate and induce the production of reactive oxygen species, nitric oxide, and inflammatory cytokines. J. Alzheimer’s Dis. 2011, 27, 401–413. [Google Scholar]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).