Herbal Tea for the Management of Pharyngitis: Inhibition of Streptococcus pyogenes Growth and Biofilm Formation by Herbal Infusions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Collection of Plant Materials
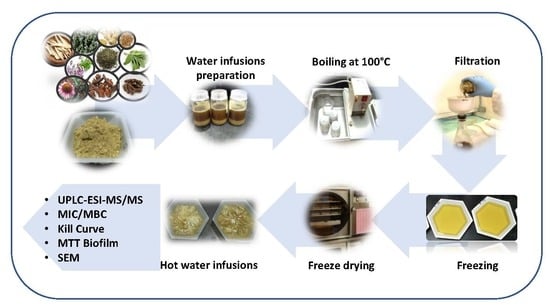
2.3. Preparation of Hot Water Infusions (HWIs)
2.4. Characterization of Phytochemicals in HWIs
2.4.1. Determination of Total Phenolic and Total Carotenoids Contents
2.4.2. Characterization of Phytochemical Profile
2.5. Bacteria and Growth Conditions
2.6. Anti-Bacterial Activity
2.6.1. Screening for Inhibitory Antibacterial Effects of HWIs
2.6.2. Determination of Minimum Inhibitory Concentrations (MICs)
2.6.3. Determination of Minimum Bactericidal Concentrations (MBCs)
2.6.4. Time-Kill Curves
2.7. Anti-Biofilm Formation Activity
2.7.1. Determination of Minimum Biofilm Inhibitory Concentrations (MBIC) and Biofilm Quantification by MTT Assay
2.7.2. SEM Visualization of Biofilms
2.8. Statistical Analysis
3. Results
3.1. Characterization of HWIs Using UPLC-ESI-MS/MS
3.2. HWIs Inhibits S.Pyogenes Planktonic Growth
3.3. Time to Kill Analysis of HWIs Against S. Pyogenes
3.4. HWIs Possesses Anti-Biofilm Formation Activity
3.5. HWIs Cause Morphological Changes of S. pyogenes Biofilms
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cirilli, A.R. Emergency evaluation and management of the sore throat. Emerg. Med. Clin. N. Am. 2013, 31, 501–515. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, N.; Leonard, E.; Martin, J.M. Prevalence of streptococcal pharyngitis and streptococcal carriage in children: A meta-analysis. Pediatrics 2010, 126, e557–e564. [Google Scholar] [CrossRef] [PubMed]
- Moreira, D.d.L.; Teixeira, S.S.; Monteiro, M.H.D.; De-Oliveira, A.C.A.X.; Paumgartten, F.J.R. Traditional use and safety of herbal medicines1. Rev. Bras. De Farmacogn. 2014, 24, 248–257. [Google Scholar] [CrossRef]
- Oyebode, O.; Kandala, N.-B.; Chilton, P.J.; Lilford, R.J. Use of traditional medicine in middle-income countries: A WHO-SAGE study. Health Policy Plan. 2016, 31, 984–991. [Google Scholar] [CrossRef] [PubMed]
- Rashrash, M.; Schommer, J.C.; Brown, L.M. Prevalence and predictors of herbal medicine use among adults in the United States. J. Patient Exp. 2017, 4, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Moerman, D.E. An analysis of the food plants and drug plants of native North America. J. Ethnopharmacol. 1996, 52, 1–22. [Google Scholar] [CrossRef]
- Nafiu, M.O.; Hamid, A.A.; Muritala, H.F.; Adeyemi, S.B. Chapter 7—Preparation, standardization, and quality control of medicinal plants in Africa. In Medicinal Spices and Vegetables from Africa; Kuete, V., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 171–204. [Google Scholar]
- Wijesundara, N.M.; Sekhon-Loodu, S.; Rupasinghe, H.P.V. Phytochemical-rich medicinal plant extracts suppress bacterial antigens-induced inflammation in human tonsil epithelial cells. PeerJ 2017, 5, e3469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wijesundara, N.M.; Rupasinghe, H.P.V. Bactericidal and anti-biofilm activity of ethanol extracts derived from selected medicinal plants against Streptococcus pyogenes. Molecules 2019, 24. [Google Scholar] [CrossRef] [PubMed]
- Wijesundara, N.M.; Rupasinghe, H.P.V. Essential oils from Origanum vulgare and Salvia officinalis exhibit antibacterial and anti-biofilm activities against Streptococcus pyogenes. Microb. Pathog. 2018, 117, 118–127. [Google Scholar] [CrossRef] [PubMed]
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically: Approved Standard. Available online: https://simpleshowoflove.weebly.com/uploads/1/4/0/7/14073276/agar_dilution_assay.pdf (accessed on 23 August 2019).
- Deepak, P.; Prativa, B.; Suri, K.A. Isolation of berberine from Berberis vulgaris Linn. and standardization of aqueous extract by RP-HPLC. Int. J. Herb. Med. 2013, 1, 106–111. [Google Scholar]
- Yun, S.M.; Lee, M.H.; Lee, K.J.; Ku, H.O.; Son, S.W.; Joo, Y.S. Quantitative analysis of eugenol in clove extract by a validated HPLC method. J. AOAC Int. 2010, 93, 1806–1810. [Google Scholar] [PubMed]
- Brito, R.E.; González-Rodríguez, J.; Montoya, M.R.; Rodríguez Mellado, J.M. Comparison of the volatile antioxidant contents in the aqueous and methanolic extracts of a set of commercial spices and condiments. Eur. Food Res. Technol. 2017, 243, 1439–1445. [Google Scholar] [CrossRef]
- Sahin, S.; Eulenburg, V.; Heinlein, A.; Villmann, C.; Pischetsrieder, M. Identification of eugenol as the major determinant of GABAA-receptor activation by aqueous Syzygium aromaticum L. (clove buds) extract. J. Funct. Foods 2017, 37, 641–649. [Google Scholar] [CrossRef]
- Erenler, R.; Telci, I.; Ulutas, M.; Demirtas, I.; Gul, F.; Elmastas, M.; Kayir, O. Chemical constituents, quantitative analysis and antioxidant activities of Echinacea purpurea (L.) Moench and Echinacea pallida (Nutt.) Nutt. J. Food Biochem. 2015, 39, 622–630. [Google Scholar] [CrossRef]
- Brown, P.N.; Chan, M.; Paley, L.; Betz, J.M. Determination of major phenolic compounds in Echinacea spp. raw materials and finished products by high-performance liquid chromatography with ultraviolet detection: Single-laboratory validation matrix extension. J. AOAC Int. 2011, 94, 1400–1410. [Google Scholar] [CrossRef] [PubMed]
- Mølgaard, P.; Johnsen, S.; Christensen, P.; Cornett, C. HPLC method validated for the simultaneous analysis of cichoric acid and alkamides in Echinacea purpurea plants and products. J. Agric. Food Chem. 2003, 51, 6922–6933. [Google Scholar] [CrossRef] [PubMed]
- Senica, M.; Mlinsek, G.; Veberic, R.; Mikulic-Petkovsek, M. Which plant part of purple coneflower (Echinacea purpurea (L.) Moench) should be used for tea and which for tincture? J. Med. Food 2018, 22, 102–108. [Google Scholar] [CrossRef]
- Nile, S.H.; Park, S.W. Chromatographic analysis, antioxidant, anti-inflammatory, and xanthine oxidase inhibitory activities of ginger extracts and its reference compounds. Ind. Crop. Prod. 2015, 70, 238–244. [Google Scholar] [CrossRef]
- Krüger, S.; Bergin, A.; Morlock, G.E. Effect-directed analysis of ginger (Zingiber officinale) and its food products, and quantification of bioactive compounds via high-performance thin-layer chromatography and mass spectrometry. Food Chem. 2018, 243, 258–268. [Google Scholar] [CrossRef]
- Komes, D.; Belščak-Cvitanović, A.; Jurić, S.; Bušić, A.; Vojvodić, A.; Durgo, K. Consumer acceptability of liuorice root (Glycyrrhiza glabra L.) as an alternative sweetener and correlation with its bioactive content and biological activity. Int. J. Food Sci. Nutr. 2016, 67, 53–66. [Google Scholar] [CrossRef]
- Zhou, J.X.; Braun, M.S.; Wetterauer, P.; Wetterauer, B.; Wink, M. Antioxidant, cytotoxic, and antimicrobial activities of Glycyrrhiza glabra L., Paeonia lactiflora Pall., and Eriobotrya japonica (Thunb.) Lindl. extracts. Medicines 2019, 6, 43. [Google Scholar] [CrossRef] [PubMed]
- Méabed, E.M.H.; El- Sayed, N.M.; Abou-Sreea, A.I.B.; Roby, M.H.H. Chemical analysis of aqueous extracts of Origanum majorana and Foeniculum vulgare and their efficacy on Blastocystis spp. cysts. Phytomedicine 2018, 43, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Roby, M.H.H.; Sarhan, M.A.; Selim, K.A.-H.; Khalel, K.I. Evaluation of antioxidant activity, total phenols and phenolic compounds in thyme (Thymus vulgaris L.), sage (Salvia officinalis L.), and marjoram (Origanum majorana L.) extracts. Ind. Crop. Prod. 2013, 43, 827–831. [Google Scholar] [CrossRef]
- Savarese, M.; De Marco, E.; Sacchi, R. Characterization of phenolic extracts from olives (Olea europaea cv. Pisciottana) by electrospray ionization mass spectrometry. Food Chem. 2007, 105, 761–770. [Google Scholar] [CrossRef]
- Silva, S.; Gomes, L.; Leitão, F.; Coelho, A.; Boas, L. Phenolic compounds and antioxidant activity of Olea europaea L. fruits and leaves. Food Sci. Technol. Int. 2006, 12, 385–395. [Google Scholar] [CrossRef]
- Quirantes-Piné, R.; Lozano-Sánchez, J.; Herrero, M.; Ibáñez, E.; Segura-Carretero, A.; Fernández-Gutiérrez, A. HPLC–ESI–QTOF–MS as a powerful analytical tool for characterising phenolic compounds in olive-leaf extracts. Phytochem. Anal. 2013, 24, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Boukhris, M.; Simmonds, M.S.; Sayadi, S.; Bouaziz, M. Chemical composition and biological activities of polar extracts and essential oil of rose-scented geranium, Pelargonium graveolens. Phytother Res. 2013, 27, 1206–1213. [Google Scholar] [CrossRef]
- Martins, N.; Barros, L.; Santos-Buelga, C.; Henriques, M.; Silva, S.; Ferreira, I.C.F.R. Evaluation of bioactive properties and phenolic compounds in different extracts prepared from Salvia officinalis L. Food Chem. 2015, 170, 378–385. [Google Scholar] [CrossRef]
- Fecka, I.; Turek, S. Determination of water-soluble polyphenolic compounds in commercial herbal teas from lamiaceae: Peppermint, melissa, and sage. J. Agric. Food Chem. 2007, 55, 10908–10917. [Google Scholar] [CrossRef]
- Barsett, H.; Smestad Paulsen, B. Separation, isolation and characterization of acidic polysaccharides from the inner bark of Ulmus glabra Huds. Carbohydr. Polym. 1992, 17, 137–144. [Google Scholar] [CrossRef]
- Fecka, I.; Turek, S. Determination of polyphenolic compounds in commercial herbal drugs and spices from Lamiaceae: Thyme, wild thyme and sweet marjoram by chromatographic techniques. Food Chem. 2008, 108, 1039–1053. [Google Scholar] [CrossRef] [PubMed]
- Adil, M.; Khan, R.; Rupasinghe, H.P.V. Application of medicinal plants as a source for therapeutic agents against Streptococcus pyogenes infections. Curr. Drug Metab. 2018, 19, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Mpala, L.; Chikowe, G.; Cock, I.E. Brachychiton rupestris (T.Mitch. ex Lindl.) K. Schum. Extracts Inhibit the Growth of Streptococcus pyogenes. Pharmacogn. Commun. 2019, 9, 27–33. [Google Scholar] [CrossRef] [Green Version]
- Wright, M.H.; Arnold, M.S.J.; Lee, C.J.; Courtney, R.; Greene, A.C.; Cock, I.E. Qualitative phytochemical analysis and antibacterial activity evaluation of indian Terminalia spp. against the pharyngitis causing pathogen Streptococcus pyogenes. Pharmacogn. Commun. 2016, 6, 85–92. [Google Scholar] [CrossRef]
- Wajima, T.; Kinugawa, R.; Yamada, T.; Ikoshi, H.; Noguchi, N. Panax Notoginseng extract possesses significant antibacterial activity against pathogenic Streptococci. Pharmacology 2019, 103, 221–227. [Google Scholar] [CrossRef]
- Mehreen, A.; Waheed, M.; Liaqat, I.; Arshad, N. Phytochemical, antimicrobial, and toxicological evaluation of traditional herbs used to treat sore throat. Biomed. Res. Int. 2016, 2016, 8503426. [Google Scholar] [CrossRef] [PubMed]
- Cowan, M.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef]
- Macé, S.; Truelstrup Hansen, L.; Rupasinghe, H.P.V. Anti-bacterial activity of phenolic compounds against Streptococcus pyogenes. Medicines 2017, 4, 25. [Google Scholar] [CrossRef]
- Lemos, A.S.O.; Campos, L.M.; Melo, L.; Guedes, M.C.M.R.; Oliveira, L.G.; Silva, T.P.; Melo, R.C.N.; Rocha, V.N.; Aguiar, J.A.K.; Apolônio, A.C.M.; et al. Antibacterial and antibiofilm activities of psychorubrin, a pyranonaphthoquinone isolated From Mitracarpus frigidus (Rubiaceae). Front. Microbiol. 2018, 9, 724. [Google Scholar] [CrossRef]
- Sara, G.Y.; Dauda, S.; Emmanuel, A.; Bhutto, Y.Y.; Joseph, I. Phytochemical screening and antimicrobial activity of leaf and stem-bark aqueous extracts of Diospyros mespiliformis. Int. J. Biochem. Res. Rev. 2018, 22, 1–8. [Google Scholar] [CrossRef]
- Sfeir, J.; Lefrancois, C.; Baudoux, D.; Derbre, S.; Licznar, P. In vitro antibacterial activity of essential oils against Streptococcus pyogenes. Evid.-Based Complement. Altern. Med. 2013, 2013, 9. [Google Scholar] [CrossRef] [PubMed]
- Fournomiti, M.; Kimbaris, A.; Mantzourani, I.; Plessas, S.; Theodoridou, I.; Papaemmanouil, V.; Kapsiotis, I.; Panopoulou, M.; Stavropoulou, E.; Bezirtzoglou, E.E.; et al. Antimicrobial activity of essential oils of cultivated oregano (Origanum vulgare), sage (Salvia officinalis), and thyme (Thymus vulgaris) against clinical isolates of Escherichia coli, Klebsiella oxytoca, and Klebsiella pneumoniae. Microb. Ecol. Health Dis. 2015, 26, 23289. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Chen, J.; Li, Y.J.; Zheng, Y.F.; Li, P. Antioxidant and anti-inflammatory activities of six flavonoids separated from licorice. Food Chem. 2013, 141, 1063–1071. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, T.; Terao, Y.; Okuni, H.; Ninomiya, K.; Sakata, H.; Ikebe, K.; Maeda, Y.; Kawabata, S. Biofilm formation or internalization into epithelial cells enable Streptococcus pyogenes to evade antibiotic eradication in patients with pharyngitis. Microb. Pathog. 2011, 51, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Post, J.C.; Stoodley, P.; Hall-Stoodley, L.; Ehrlich, G.D. The role of biofilms in otolaryngologic infections. Curr. Opin. Otolaryngol. Head Neck Surg. 2004, 12, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Darsini, D.T.P.; Srinivasan, P.; Guna, G.; Manimekalai, K.; Dineshbabu, J. In vitro anti-biofilm activity of Piper longum and Piper nigrum against clinical isolates of S. pyogenes isolated from pharyngitis patients. Int. J. Pharm. 2015, 6, 122–132. [Google Scholar] [CrossRef]
- Abachi, S.; Lee, S.; Rupasinghe, H.P.V. Molecular mechanisms of inhibition of Streptococcus species by phytochemicals. Molecules 2016, 21, 215. [Google Scholar] [CrossRef] [PubMed]
- Fiedler, T.; Riani, C.; Koczan, D.; Standar, K.; Kreikemeyer, B.; Podbielski, A. Protective mechanisms of respiratory tract Streptococci against Streptococcus pyogenes biofilm formation and epithelial cell infection. Appl. Environ. Microbiol. 2013, 79, 1265–1276. [Google Scholar] [CrossRef]
- North American Trends in the Consumer Health Product Industry. Available online: https://www.chpcanada.ca/wp-system/uploads/2017/12/CHPC-CHPA-RCC-Submission-2016-FINAL.pdf (accessed on 23 August 2019).
- Wang, L.; Yang, R.; Yuan, B.; Liu, Y.; Liu, C. The antiviral and antimicrobial activities of licorice, a widely-used Chinese herb. Acta Pharm. Sin. B 2015, 5, 310–315. [Google Scholar] [CrossRef] [Green Version]
- Ahn, S.-J.; Cho, E.-J.; Kim, H.-J.; Park, S.-N.; Lim, Y.-K.; Kook, J.-K. The antimicrobial effects of deglycyrrhizinated licorice root extract on Streptococcus mutans UA159 in both planktonic and biofilm cultures. Anaerobe 2012, 18, 590–596. [Google Scholar] [CrossRef]
- Nzeako, B.C.; Al-Kharousi, Z.S.; Al-Mahrooqui, Z. Antimicrobial activities of clove and thyme extracts. Sultan. Qaboos. Univ. Med. J. 2006, 6, 33–39. [Google Scholar] [PubMed]
- Kogiannou, D.A.A.; Kalogeropoulos, N.; Kefalas, P.; Polissiou, M.G.; Kaliora, A.C. Herbal infusions; their phenolic profile, antioxidant and anti-inflammatory effects in HT29 and PC3 cells. Food Chem. Toxicol. 2013, 61, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Sekita, Y.; Murakami, K.; Yumoto, H.; Hirao, K.; Amoh, T.; Fujiwara, N.; Hirota, K.; Fujii, H.; Matsuo, T.; Miyake, Y.; et al. Antibiofilm and anti-inflammatory activities of Houttuynia cordata decoction for oral care. Evid.-Based Complement. Altern. Med. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Weller, C.L. Recent advances in extraction of nutraceuticals from plants. Trends Food Sci. Technol. 2006, 17, 300–312. [Google Scholar] [CrossRef]





| Plant Name | Family | Parts Used | Potential Major Phytochemicals ((M–H+)−, RT in min) | References | |
|---|---|---|---|---|---|
| Common | Botanical | ||||
| Barberry | Berberis vulgaris L. | Berberidaceae | Roots | Gingerol (293.38, 8.06), Caffeic acid (179.16, 3.22), Naringin (579.54, 5.99), Naringenin (271.26, 7.32), and Rosmerinic acid (359.32, 2.01). | [12] |
| Clove | Syzygium aromaticum L. | Myrtaceae | Flower buds | Eugenol (163.20, 4.40), Eugenyl acetate (205.24, 8.43), and β-Ocimene (135.25, 4.25). | [13,14,15] |
| Echinacea | Echinacea purpurea L. | Asteraceae | Flowers | Caftaric acid (311.23, 2.44), Chlorogenic acid (353.31, 2.78), Caffeic acid (179.16, 3.27), Cynarin (515.46, 6.02), Echinacoside (785.73, 6.56), Cichoric acid (473.37, 4.78), and β-Sitosterol (413.70, 4.61). | [16,17,18,19] |
| Stems | Quercetin (301.23, 6.12) and Eugenyl acetate (205.24, 8.43). | ||||
| Leaves | Caftaric acid (311.23, 2.44), Cichoric acid (473.37, 4.78), and Caffeic acid (179.16, 3.27). | ||||
| Ginger | Zingiber officinale L. | Zingiberaceae | Rhizomes | Gingerol (273.38, 7.18), α-Humulene (203. 24, 2.50), Gingerol (293.38, 7.18), α-Thujone/β-Thujone/camphor (151.23, 4.35), α or β-Caryophyllene (203.35, 2.52), Caffetic acid (311.23, 10.07), and Liquirtin (419.39, 6.21). | [20,21] |
| Licorice | Glycyrrhiza glabra L. | Papilionaceae | Roots | Glycyrrhizin (821.94, 6.86), Glabridin (323.97, 7.54), Thymohydroquinone (165.22, 2.81), Naringenin (271.26, 7.32), Asparegene (131.12, 1.63), Liquirtin (417.39, 6.34), 5 -Methoxyhydnocarpin (493.45, 6.28), Cynarin (515.46, 6.02), Quercetin (301.23, 6.12), p-Cyemene (133.21, 0.82), Generdiol (153.23, 3.47), α-Humulene (203.35, 2.53), and Kaempferol (285.23, 6.42). | [22,23] |
| Oregano | Origanum vulgare L. | Lamiaceae | Flowering shoots | Rosmerinic acid (359.32, 6.20), Oleanolic acid (455.71, 7.23), ρ-Cymene (133.21, 1.04), and 5-Methoxyhydnocarpin (493.45, 6.25). | [24,25] |
| Olive | Olea europeus L. | Oleaceae | Leaves | Hydroxytyrosol, Rutin, Luteolin-7-glucoside, Oleuropein glucoside, Luteolin-4′-glucoside, Oleuropein, and Oleuropein aglycon. | [26,27,28] |
| Rose geranium | Pelargonium graveolens L. | Geraniaceae | Leaves | Geraniol (151.24, 6.62) | [29] |
| Sage | Salvia officinalis L. | Lamiaceae | Leaves | 1,8-Cineole (153.24, 2.18 Borneol and/or Linalool and/or α-Terpineol and/or β-Pinene (153.24, 4.01), β-carotene (535.87, 3.46), γ-Terpinene and/or Mycrene and/or β-Pinene and/or α-Pinene (135.24, 4.24), Asparegene (131.12, 3.65), and α-Terpine (135.24, 4,42). | [25,30,31] |
| Slippery elm | Ulmus rubra Muhl. | Ulmaceae | Inner barks | Ursolic acid /Betulinic acid (455.71, 9.5) and β-carotene (535.87, 5.29) | [32] |
| Thyme | Thymus vulgaris L. | Lamiaceae | Flowering shoots | Thymol and Carvacrol (149.21, 6.65), Thymohydroquinone (165.22, 7.17), γ-Terpinene, Myrcene, and α-Pinene (135.24, 4.24), Gingerol (293.38, 7.68), and Kaempferol (285.23, 6.42) | [25,33] |
| Plant-Source, Plant Part | ATCC 19615 | ATCC 49399 | Clinical Isolate | ||||||
|---|---|---|---|---|---|---|---|---|---|
| MIC (mg/mL) | MBC (mg/mL) | MBC/MIC | MIC (mg/mL) | MBC (mg/mL) | MBC/MIC | MIC (mg/mL) | MBC (mg/mL) | MBC/MIC | |
| Clove FB | 12.50 | 25.00 | 2 | 12.50 | 25.00 | 2 | 12.50 | 25.0 | 2 |
| Sage L | 12.50 | 25.00 | 12.50 | 25.00 | 12.50 | 25.0 | |||
| Ginger-Canada Rh | 50.00 | >50.00 | - | 50.00 | >50.00 | - | NA | NA | - |
| Ginger-Chinese Rh | 50.00 | >50.00 | - | 50.00 | >50.00 | - | NA | NA | - |
| Oregano FB | 3.13 | 6.25 | 2 | 3.13 | 6.25 | 2 | 3.13 | 6.25 | 2 |
| Thyme FB | 3.13 | 6.25 | 2 | 3.13 | 6.25 | 2 | 3.13 | 6.25 | 2 |
| Licorice R | 1.56 | 3.13 | 2 | 1.56 | 3.13 | 4 | 3.13 | 6.25 | 2 |
| Barberry R | 3.13 | 6.25 | 2 | 3.13 | 6.25 | 2 | 3.13 | 6.25 | 2 |
| Echinacea L | 50.00 | >50.00 | - | 50.00 | >50.00 | - | NA | NA | - |
| Echinacea S | 6.25 | 12.50 | 2 | 6.25 | 12.50 | 2 | 6.25 | 12.50 | 2 |
| Echinacea F | 50.00 | >50.00 | - | 50.00 | >50.00 | - | 50.00 | >50.00 | - |
| Geranium L | 25.00 | 50.00 | 2 | 25.00 | 50.00 | 2 | NA | NA | - |
| Slippery elm IB | >50.00 | >50.00 | - | >50.00 | >50.00 | - | NA | NA | - |
| Olive L | >50.00 | >50.00 | - | >50.00 | >50.00 | - | NA | NA | - |
| Penicillin G | 0.0078 | 0.0156 | 2 | 0.0078 | 0.0156 | 2 | 0.0078 | 0.0156 | 2 |
| Hot Water Infusions | MBIC (mg/mL) | ||
|---|---|---|---|
| ATCC 19615 | ATCC 49399 | Clinical | |
| Licorice Roots | 1.56 (1 × MIC) | 6.25 (4 × MIC) | 3.13 (2 × MIC) |
| Barberry Root | 6.25 (2 × MIC) | 6.25 (2 × MIC) | 6.25 (2 × MIC) |
| Oregano Flowering shoots | 6.25 (2 × MIC) | 6.25 (2 × MIC) | 6.25 (2 × MIC) |
| Thyme Flowering shoots | 6.25 (2 × MIC) | 6.25 (2 × MIC) | 6.25 (2 ×MIC) |
| Penicillin G | 0.0156 (2 × MIC) | 0.0625 (8 × MIC) | 0.0625 (8 × MIC) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wijesundara, N.M.; Rupasinghe, H.P.V. Herbal Tea for the Management of Pharyngitis: Inhibition of Streptococcus pyogenes Growth and Biofilm Formation by Herbal Infusions. Biomedicines 2019, 7, 63. https://doi.org/10.3390/biomedicines7030063
Wijesundara NM, Rupasinghe HPV. Herbal Tea for the Management of Pharyngitis: Inhibition of Streptococcus pyogenes Growth and Biofilm Formation by Herbal Infusions. Biomedicines. 2019; 7(3):63. https://doi.org/10.3390/biomedicines7030063
Chicago/Turabian StyleWijesundara, Niluni M., and H. P. Vasantha Rupasinghe. 2019. "Herbal Tea for the Management of Pharyngitis: Inhibition of Streptococcus pyogenes Growth and Biofilm Formation by Herbal Infusions" Biomedicines 7, no. 3: 63. https://doi.org/10.3390/biomedicines7030063
APA StyleWijesundara, N. M., & Rupasinghe, H. P. V. (2019). Herbal Tea for the Management of Pharyngitis: Inhibition of Streptococcus pyogenes Growth and Biofilm Formation by Herbal Infusions. Biomedicines, 7(3), 63. https://doi.org/10.3390/biomedicines7030063