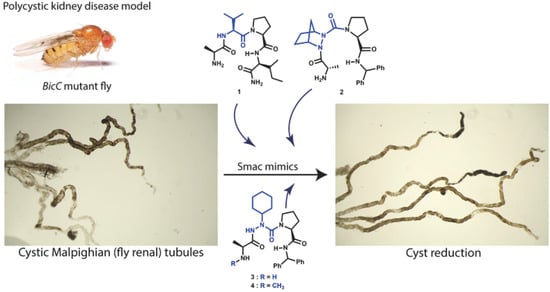
Cyst Reduction in a Polycystic Kidney Disease Drosophila Model Using Smac Mimics
Abstract
:1. Introduction
2. Experimental Section
2.1. Fly Lines and Genetics
2.2. In Vivo and Ex Vivo Assays
2.2.1. Cystic Index
2.2.2. Microscopy
2.3. General Synthetic Methods
3. Results
3.1. Chemistry
3.1.1. Effect of Smac Mimic Administration In Vivo
3.1.2. Smac Mimics Differentially Affect Distinct Regions of the MTs
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bergmann, C.; Guay-Woodford, L.M.; Harris, P.C.; Horie, S.; Peters, D.J.M.; Torres, V.E. Polycystic kidney disease. Nat. Rev. Dis. Primers. 2018, 80. [Google Scholar] [CrossRef] [PubMed]
- Happé, H.; Peters, J.D.M. Translational research in ADPKD: Lessons from animal models. Nat. Rev. Neph. 2014, 10, 587–601. [Google Scholar] [CrossRef] [PubMed]
- Gamberi, C.; Hipfner, D.R.; Trudel, M.; Lubell, W.D. Bicaudal C mutation causes myc and TOR pathway up-regulation and polycystic kidney disease-like phenotypes in Drosophila. PLoS Gen. 2017, 13, e1006694. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Kim, J.; Shrier, R.W.; Edelstein, C.L. Rapamycin markedly slows disease progression in a rat model of polycystic kidney disease. J. Am. Soc. Nephrol. 2005, 16, 46–51. [Google Scholar] [CrossRef]
- Shillingford, J.M.; Murcia, N.S.; Larson, C.H.; Low, S.H.; Hedgepeth, R.; Brown, N.; Flask, C.A.; Novick, A.C.; Goldfarb, D.A.; Kramer-Zucker, A.; et al. The mTOR pathway is regulated by polycystin-1, and its inhibition reverses renal cystogenesis in polycystic kidney disease. Proc. Natl. Acad. Sci. USA 2006, 103, 5466–5471. [Google Scholar] [CrossRef] [Green Version]
- Wahl, P.R.; Serra, A.L.; Le Hir, M.; Molle, K.D.; Hall, M.N.; Wuthrich, R.P. Inhibition of mTOR with sirolimus slows disease progression in Han:SPRD rats with autosomal dominant polycystic kidney disease (ADPKD). Nephrol. Dial. Transplant 2006, 21, 598–604. [Google Scholar] [CrossRef]
- Wu, M.; Wahl, P.R.; Le Hir, M.; Wackerie-Men, Y.; Wuthrich, R.P. Everolimus retards cyst growth and preserves kidney function in a rodent model for polycystic kidney disease. Kidney Blood Press. Res. 2007, 30, 253–259. [Google Scholar] [CrossRef]
- Shillingford, J.M.; Piontek, K.B.; Germino, G.G.; Weimbs, T. Rapamycin ameliorates PKD resulting from conditional inactivation of Pkd1. J. Am. Soc. Nephrol. 2010, 21, 489–497. [Google Scholar] [CrossRef]
- Millet-Boureima, C.; Marroquin, J.P.; Gamberi, C. Modeling renal disease “on the fly”. Biomed. Res. Int. 2018, 2018, 13. [Google Scholar] [CrossRef]
- Wang, J.; Kean, L.; Yang, J.; Allan, A.K.; Davies, S.A.; Herzyk, P.; Dow, J.A.T. Function-informed transcriptome analysis of Drosophila renal tubule. Genome Biology 2004, 5, R69. [Google Scholar] [CrossRef]
- Lalaoui, N.; Vaux, D.L. Recent advances in understanding inhibitor of apoptosis proteins. F1000Res. 2018, 7, 30631429. [Google Scholar] [CrossRef] [PubMed]
- Cong, H.; Xu, L.; Wu, Y.; Qu, Z.; Bian, T.; Zhang, W.; Xing, C.; Zhuang, C. Inhibitor of apoptosis protein (IAP) antagonists in anticancer agent discovery: Current status and perspectives. J. Med. Chem. 2019, 62, 5750–5772. [Google Scholar] [CrossRef] [PubMed]
- Damgaard, R.B.; Nachbur, U.; Yabal, M.; Wong, W.W.; Fiil, B.K.; Kastirr, M.; Rieser, E.; Rickard, J.A.; Bankovacki, A.; Peschel, C.; et al. The ubiquitin ligase XIAP recruits LUBAC for NOD2 signaling in inflammation and innate immunity. Mol. Cell 2012, 46, 746–758. [Google Scholar] [CrossRef] [PubMed]
- Damgaard, R.B.; Fiil, B.K.; Speckmann, C.; Yabal, M.; zur Stadt, U.; Bekker-Jensen, S.; Jost, P.J.; Ehl, S.; Mailand, N.; Gyrd-Hansen, M. Disease-causing mutations in the XIAPBIR2 domain impair NOD2-dependent immune signalling. EMBO Mol. Med. 2013, 5, 1278–1295. [Google Scholar] [CrossRef]
- Fulda, S.; Vucic, D. Targeting IAP proteins for therapeutic intervention in cancer. Nat. Rev. Drg. Disc. 2012, 11, 109–124. [Google Scholar] [CrossRef]
- Duckett, C.S.; Nava, V.E.; Gedrich, R.W.; Clem, R.J.; Van Dongen, J.L.; Gilfillan, M.C.; Shiels, H.; Hardwick, J.; Thompson, C.B. A conserved family of cellular genes related to the baculovirus iap gene and encoding apoptosis inhibitors. EMBO J. 1996, 15, 2685–2694. [Google Scholar] [CrossRef]
- Hay, B.A.; Wassarman, D.A.; Rubin, G.M. Drosophila homologs of baculovirus inhibitor of apoptosis proteins function to block cell death. Cell 1995, 83, 1253–1262. [Google Scholar] [CrossRef]
- Jones, G.; Jones, D.; Zhou, L.; Steller, H.; Chu, Y. Deterin, a new inhibitor of apoptosis from Drosophila melanogaster. J. Biol. Chem. 2000, 275, 22157–22165. [Google Scholar] [CrossRef]
- Vernooy, S.Y.; Chow, V.; Su, J.; Verbrugghe, K.; Yang, J.; Cole, S.; Olson, M.R.; Hay, B.A. Drosophila Bruce can potently suppress Rpr- and Grim-dependent but not Hid-dependent cell death. Curr. Biol. 2002, 12, 1164–1168. [Google Scholar] [CrossRef]
- White, K.; Grether, M.E.; Abrams, J.M.; Young, L.; Farrell, K.; Steller, H. Genetic control of programmed cell death in Drosophila. Science 1994, 264, 677–683. [Google Scholar] [CrossRef]
- Grether, M.E.; Abrams, J.M.; Agapite, J.; White, K.; Steller, H. The head involution defective gene of Drosophila melanogaster functions in programmed cell death. Genes Dev. 1995, 9, 1694–1708. [Google Scholar]
- Chen, P.; Nordstrom, W.; Gish, B.; Abrams, J.M. grim, a novel cell death gene in Drosophila. Genes Dev. 1996, 10, 1773–1782. [Google Scholar]
- Verhagen, A.M.; Vaux, D.L. Cell death regulation by the mammalian IAP antagonist Diablo/Smac. Apoptosis 2002, 7, 163–166. [Google Scholar] [CrossRef] [PubMed]
- Tenev, T.; Zachariou, A.; Wilson, R.; Paul, A.; Meier, P. Jafrac2 is an IAP antagonist that promotes cell death by liberating Dronc from DIAP1. EMBO J. 2002, 21, 5118–5129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christich, A.; Kauppila, S.; Chen, P.; Sogame, N.; Ho, S.; Abrams, J.M. The damage-responsive Drosophila gene sickle encodes a novel IAP binding protein similar to but distinct from reaper, grim, and hid. Curr. Biol. 2002, 12, 137–140. [Google Scholar] [CrossRef]
- Srinivasula, S.M.; Datta, P.; Kobayashi, M.; Wu, J.; Fujioka, M.; Hegde, R.; Zhang, Z.; Mukattash, R.; Fernandes-Alnemri, T.; Shi, Y.; et al. sickle, a novel Drosophila death gene in the reaper/hid/grim region, encodes an IAP-inhibitory protein. Curr. Biol. 2002, 12, 125–130. [Google Scholar] [CrossRef]
- Wing, J.P.; Karres, J.S.; Ogdahl, J.L.; Zhou, L.; Schwartz, L.M.; Nambu, J.R. Drosophila sickle is a novel grim-reaper cell death activator. Curr. Biol. 2002, 12, 131–135. [Google Scholar] [CrossRef]
- Challa, M.; Malladi, S.; Pellock, B.J.; Dresnek, D.; Varadarajan, S.; Yin, Y.W.; White, K.; Bratton, S.B. Drosophila Omi, a mitonchondrial-localized IAP antagonist and proapoptotic serine protease. EMBO J. 2007, 26, 3144–3156. [Google Scholar] [CrossRef]
- Igaki, T.; Suzuki, Y.; Tokushige, N.; Aonuma, H.; Takahashi, R.; Miura, M. Evolution of mitochondrial cell death pathway: Proapoptotic role of HtrA2/Omi in Drosophila. Biochem. Biophys. Res. Commun. 2007, 356, 993–997. [Google Scholar] [CrossRef]
- Khan, F.S.; Fujioka, M.; Datta, P.; Fernandes-Alnemri, T.; Jaynes, J.B.; Alnemri, E.S. The interaction of DIAP1 with dOmi/HtrA2 regulates cell death in Drosophila. Cell Death Diff. 2008, 15, 1073–1083. [Google Scholar] [CrossRef]
- Du, C.; Fang, M.; Li, Y.; Li, L.; Wang, X. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell 2000, 102, 33–42. [Google Scholar] [CrossRef]
- Verhagen, A.M.; Ekert, P.G.; Pakusch, M.; Silke, J.; Connolly, L.M.; Reid, G.E.; Moritz, R.L.; Simpson, R.J.; Vaux, D.L. Identification of DIABLO, a mammalian protein that promotes apoptosis by binding to and antagonizing IAP proteins. Cell 2000, 102, 43–53. [Google Scholar] [CrossRef]
- van Loo, G.; van Gurp, M.; Depuydt, B.; Srinivasula, S.M.; Rodriguez, I.; Alnemri, E.S.; Gevaert, K.; Vandekerckhove, J.; Declercq, W.; Vandenabeele, P. The serine protease Omi/HtrA2 is released from mitochondria during apoptosis. Omi interacts with caspase-inhibitor XIAP and induces enhanced caspase activity. Cell Death Diff. 2002, 9, 20–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gottfried, Y.; Rotem, A.; Lotan, R.; Steller, H.; Larisch, S. The mitochondrial ARTS protein promotes apoptosis through targeting XIAP. EMBO J. 2004, 23, 1627–1635. [Google Scholar] [CrossRef] [PubMed]
- Liston, P.; Fong, W.G.; Kelly, N.L.; Toji, S.; Miyazaki, T.; Conte, D.; Tamai, K.; Craig, C.G.; McBurney, M.W.; Korneluk, R.G. Identification of XAF1 as an antagonist of XIAP anti-caspase activity. Nat. Cell. Biol. 2001, 3, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Wing, J.P.; Schwartz, L.M.; Nambu, J.R. The RHG motifs of Drosophila Reaper and Grim are important for their distinct cell death-inducing abilities. Mechan. Dev. 2001, 102, 193–203. [Google Scholar] [CrossRef]
- Saita, S.; Nolte, H.; Fiedler, K.U.; Kashkar, H.; Venne, A.S.; Zahedi, R.P.; Kruger, M.; Langer, T. PARL mediates Smac proteolytic maturation in mitochondria to promote apoptosis. Nat. Cell Biol. 2017, 19, 318–328. [Google Scholar] [CrossRef]
- Xu, D.; Woodfield, S.E.; Lee, T.V.; Fan, Y.; Antonio, C.; Bergmann, A. Genetic control of programmed cell death (apoptosis) in Drosophila. Fly 2009, 3, 78–90. [Google Scholar] [CrossRef]
- Brenner, D.; Blaser, H.; Mak, T.W. Regulation of tumour necrosis factor signaling: Live or let die. Nat. Rev. Immunol. 2015, 15, 362–374. [Google Scholar] [CrossRef]
- Harris, P.C.; Watson, M.L. Autosomal dominant polycystic kidney disease: Neoplasia in disguise? Nephrol. Dial. Transplant 1997, 12, 1089–1090. [Google Scholar] [CrossRef]
- Grantham, J.J. Polycystic kidney disease: Neoplasia in disguise. Am. J. Kidney Dis. 1990, 15, 110–116. [Google Scholar] [CrossRef]
- Fan, L.X.; Zhou, X.; Sweeney, W.E.; Wallace, D.P.; Avner, E.D.; Grantham, J.J.; Li, X. Smac-mimetic-induced epithelial cell death reduces the growth of renal cysts. J. Am. Soc. Nephrol. 2013, 24, 2010–2022. [Google Scholar] [CrossRef] [PubMed]
- Igaki, T.; Kanda, H.; Yamamoto-Goto, Y.; Kanuka, H.; Kuranaga, E.; Aigaki, T.; Miura, M. Eiger, a TNF superfamily ligand that triggers the Drosophila JNK pathway. EMBO J. 2002, 21, 3009–3018. [Google Scholar] [CrossRef] [PubMed]
- Kanda, H.; Igaki, T.; Kanuka, H.; Yagi, T.; Miura, M. Wengen, a member of the Drosophila Tumor Necrosis Factor receptor superfamily, is required for Eiger signaling. J. Biol. Chem. 2002, 277, 28372–28375. [Google Scholar] [CrossRef]
- Moreno, E.; Yan, M.; Basler, K. Evolution of TNF signaling mechanisms: JNK-dependent apoptosis triggered by Eiger, the Drosophila homolog of the TNF superfamily. Curr. Biol. 2002, 12, 1263–1268. [Google Scholar] [CrossRef]
- Kauppila, S.; Maaty, W.S.A.; Chen, P.; Tomar, R.S.; Eby, M.T.; Chapo, J.; Chew, S.; Rathore, N.; Zachariah, S.; Sinha, S.K.; et al. Eiger and its receptor, Wengen, comprise a TNF-like system in Drosophila. Oncogene 2003, 22, 4860–4867. [Google Scholar] [CrossRef]
- Igaki, T.; Miura, M. The Drosophila TNF ortholog Eiger: Emerging physiological roles and evolution of the TNF system. Semin. Immunol. 2014, 26, 267–274. [Google Scholar] [CrossRef]
- Andersen, D.S.; Colombani, J.; Palmerini, V.; Chakrabandhu, K.; Boone, E.; Rothlisberger, M.; Toggweiler, J.; Basler, K.; Mapelli, M.; Hueber, A.; et al. The Drosophila TNF receptor Grindelwald couples loss of cell polarity and neoplastic growth. Nature 2015, 522, 482–486. [Google Scholar] [CrossRef]
- Chingle, R.; Ratni, S.; Claing, A.; Lubell, W.D. Application of constrained aza-valine analogs for Smac mimicry. J. Pept. Sci. 2016, 106, 235–244. [Google Scholar] [CrossRef]
- Wu, G.; Chai, J.; Suber, T.L.; Wu, J.; Du, C.; Wang, X.; Shi, Y. Structural basis of IAP recognition by Smac/DIABLO. Nature 2000, 408, 1008–1012. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, C.; Olejniczak, E.T.; Meadows, R.P.; Betz, S.F.; Oost, T.; Herrmann, J.; Wu, J.C.; Fesik, S.W. Structural basis for binding of Smac/DIABLO to the XIAP BIR3 domain. Nature 2000, 408, 1004–1008. [Google Scholar] [CrossRef] [PubMed]
- Fadeel, B.; Orrenius, S. Apoptosis: A basic biological phenomenon with wide-ranging implications in human disease. J. Int. Med. 2005, 258, 479–517. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Nikolovska-Coleska, Z.; Yang, C.-Y.; Qian, D.; Lu, J.; Qiu, S.; Bai, L.; Peng, Y.; Cai, Q.; Wang, S. Design of small-molecule peptidic and nonpeptidic Smac mimetics. Acc. Chem. Res. 2008, 41, 1264–1277. [Google Scholar] [CrossRef] [PubMed]
- Boeglin, D.; Hamdan, F.F.; Melendez, R.E.; Cluzeau, J.; Laperriere, A.; Heroux, M.; Bouvier, M.; Lubell, W.D. Calcitonin gene-related peptide analogues with aza and indolizidinone amino acid residues reveal conformational requirements for antagonist activity at the human calcitonin gene-related peptide 1 receptor. J. Med. Chem. 2007, 50, 1401–1408. [Google Scholar] [CrossRef]
- Bourguet, C.B.; Goupil, E.; Tassy, D.; Hou, X.; Thouin, E.; Polyak, F.; Hebert, T.E.; Claing, A.; Laporte, S.A.; Chemtob, S.; et al. Targeting the prostaglandin F2α receptor for preventing preterm labor with azapeptide tocolytics. J. Med. Chem. 2011, 54, 6085–6097. [Google Scholar] [CrossRef]
- Mir, F.M.; Atmuri, N.D.P.; Bourguet, C.B.; Fores, J.R.; Hou, X.; Chemtob, S.; Lubell, W.D. Paired utility of aza-amino acyl proline and indolizidinone amino acid residues for peptide mimicry: Conception of prostaglandin F2a receptor allosteric modulators that delay preterm birth. J. Med. Chem. 2019, 62, 4500–4525. [Google Scholar] [CrossRef]
- Bourguet, C.B.; Boulay, P.; Claing, A.; Lubell, W.D. Design and synthesis of novel azapeptide activators of apoptosis mediated by caspase-9 in cancer cells. Bioorg. Med. Chem. Lett. 2014, 24, 3361–3365. [Google Scholar] [CrossRef]
- Chingle, R.; Mulumba, M.; Chung, N.N.; Nguyen, T.M.-D.; Ong, H.; Ballet, S.; Schiller, P.W.; Lubell, W.D. Solid-phase azopeptide Diels-Alder chemistry for aza-pipecolyl residue synthesis to study peptide conformation. J. Ord. Chem. 2019, 84, 6006–6016. [Google Scholar] [CrossRef]
- Chingle, R.; Lubell, W.D. Azopeptides: Synthesis and pericyclic chemistry. Org. Lett. 2015, 17, 5400–5403. [Google Scholar] [CrossRef]
- Oost, T.; Sun, C.; Armstrong, R.C.; Al-Assaad, A.-S.; Betz, S.F.; Deckwerth, T.L.; Ding, H.; Elmore, S.W.; Meadows, R.P.; Olejniczak, E.T.; et al. Discovery of potent antagonists of the antiapoptotic protein XIAP for the treatment of cancer. J. Med. Chem. 2004, 47, 4417–4426. [Google Scholar] [CrossRef]
- Chatterjee, J.; Gilon, C.; Hoffman, A.; Kessler, H. N-Methylation of peptides: A new perspective in medicinal chemistry. Acc. Chem. Res. 2008, 41, 1331–1342. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, J.; Rechenmacher, F.; Kessler, H. N-methylation of peptides and proteins: An important element for modulating biological functions. Angewandte Chemie. 2013, 52, 254–269. [Google Scholar] [CrossRef] [PubMed]
- Merlino, F.; Billard, E.; Yousif, A.M.; Di Maro, S.; Brancaccio, D.; Abate, L.; Carotenuto, A.; Bellavita, R.; d’Emmanuele di Villa Bianca, R.; Santicioli, P.; et al. Functional selectivity revealed by N-methylation scanning of human urotensin II and related peptides. J. Med. Chem. 2019, 62, 1455–1467. [Google Scholar] [CrossRef] [PubMed]
- Sozen, M.A.; Armstrong, J.D.; Yang, M.; Kaiser, K.; Dow, J.A.T. Functional domains are specified to single-cell resolution in a Drosophila epithelium. Proc. Natl. Acad. Sci. USA 1997, 94, 5207–5212. [Google Scholar] [CrossRef] [PubMed]
- Goilav, B. Apoptosis in polycystic kidney disease. BBA-Mol. Basis Dis. 2011, 1812, 1272–1280. [Google Scholar] [CrossRef] [Green Version]
- Ziehm, M.; Kaur, S.; Ivanov, D.K.; Ballester, P.J.; Marcus, D.; Partridge, L.; Thornton, J.M. Drug repurposing for aging research using model organisms. Aging Cell 2017, 16, 1006–1015. [Google Scholar] [CrossRef]







| Anterior Tubule | Posterior Tubule | |||||||
|---|---|---|---|---|---|---|---|---|
| Mimic | Cyst # Vehicle | Cyst # Treated | % Reduction | p Value | Cyst # Vehicle | Cyst # Treated | % Reduction | p Value |
| 1 | 228 | 127 | 44% | 0.0005 | 276 | 220 | 20% | 0.0457 |
| 2 | 216 | 165 | 24% | 0.0324 | 244 | 162 | 34% | 0.0017 |
| 3 | 195 | 153 | 21% | 0.1028 | 238 | 156 | 34% | 0.0014 |
| 4 | 134 | 93 | 31% | 0.0553 | 152 | 124 | 18% | 0.1877 |
| Anterior Tubule | Posterior Tubule | |||||||
|---|---|---|---|---|---|---|---|---|
| Mimic | Cyst # Vehicle | Cyst # Treated | % Reduction | p Value | Cyst # Vehicle | Cyst # Treated | % Reduction | p Value |
| 1 | 357 | 199 | 44% | <0.0001 | 433 | 272 | 37% | 0.0002 |
| 2 | 233 | 142 | 39% | 0.0011 | 302 | 202 | 33% | 0.0020 |
| 3 | 114 | 102 | 11% | 0.6317 | 155 | 122 | 21% | 0.2285 |
| 4 | 199 | 119 | 40% | 0.0005 | 243 | 151 | 38% | 0.0002 |
| Smac-Mimic | Anterior Tubule | Posterior Tubule | ||||
|---|---|---|---|---|---|---|
| Prox. | Int. | Term. | Prox. | Int. | Term. | |
| 1 (n = 50) | n/s | 34% p = 0.0381 | 53% p < 0.0001 | 5% p = 0.7531 | 26% p = 0.0449 | 22% p = 0.1405 |
| 2 (n = 50) | n/s | 5% p = 0.7599 | 41% p = 0.0006 | 47% p = 0.0057 | 29% p = 0.0389 | 32% p = 0.0125 |
| 3 (n = 50) | n/a | 2% p = 0.9072 | 40% p = 0.0041 | 21% p = 0.2431 | 30% p = 0.0465 | 45% p = 0.0007 |
| 4 (n = 50) | n/a | 36% p = 0.0461 | 20% p = 0.3377 | n/s p = 0.4769 | 33% p = 0.0130 | n/s p = 0.9245 |
| Smac-Mimic | Anterior Tubule | Posterior Tubule | ||||
|---|---|---|---|---|---|---|
| Prox. | Int. | Term. | Prox. | Int. | Term. | |
| 1 (n = 50) | n/s | 48% p < 0.0001 | 40% p = 0.0038 | 39% p = 0.0150 | 41% p = 0.0008 | 32% p = 0.0022 |
| 2 (n = 50) | n/a | 41% p = 0.0045 | 37% p = 0.0161 | 43% p = 0.0080 | 27% p = 0.0703 | 32% p = 0.0086 |
| 3 (n = 25) | n/a | 9% p = 0.7140 | 11% p = 0.6746 | 40% p = 0.1325 | 10% p = 0.7038 | 21% p = 0.2429 |
| 4 (n = 50) | n/s | 42% p = 0.0014 | 38% p = 0.0195 | 35% p = 0.1069 | 22% p = 0.1231 | 51% p < 0.0001 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Millet-Boureima, C.; Chingle, R.; Lubell, W.D.; Gamberi, C. Cyst Reduction in a Polycystic Kidney Disease Drosophila Model Using Smac Mimics. Biomedicines 2019, 7, 82. https://doi.org/10.3390/biomedicines7040082
Millet-Boureima C, Chingle R, Lubell WD, Gamberi C. Cyst Reduction in a Polycystic Kidney Disease Drosophila Model Using Smac Mimics. Biomedicines. 2019; 7(4):82. https://doi.org/10.3390/biomedicines7040082
Chicago/Turabian StyleMillet-Boureima, Cassandra, Ramesh Chingle, William D. Lubell, and Chiara Gamberi. 2019. "Cyst Reduction in a Polycystic Kidney Disease Drosophila Model Using Smac Mimics" Biomedicines 7, no. 4: 82. https://doi.org/10.3390/biomedicines7040082
APA StyleMillet-Boureima, C., Chingle, R., Lubell, W. D., & Gamberi, C. (2019). Cyst Reduction in a Polycystic Kidney Disease Drosophila Model Using Smac Mimics. Biomedicines, 7(4), 82. https://doi.org/10.3390/biomedicines7040082