Idebenone Protects against Spontaneous Chronic Murine Colitis by Alleviating Endoplasmic Reticulum Stress and Inflammatory Response
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Study Experimental Design and Drug Treatment
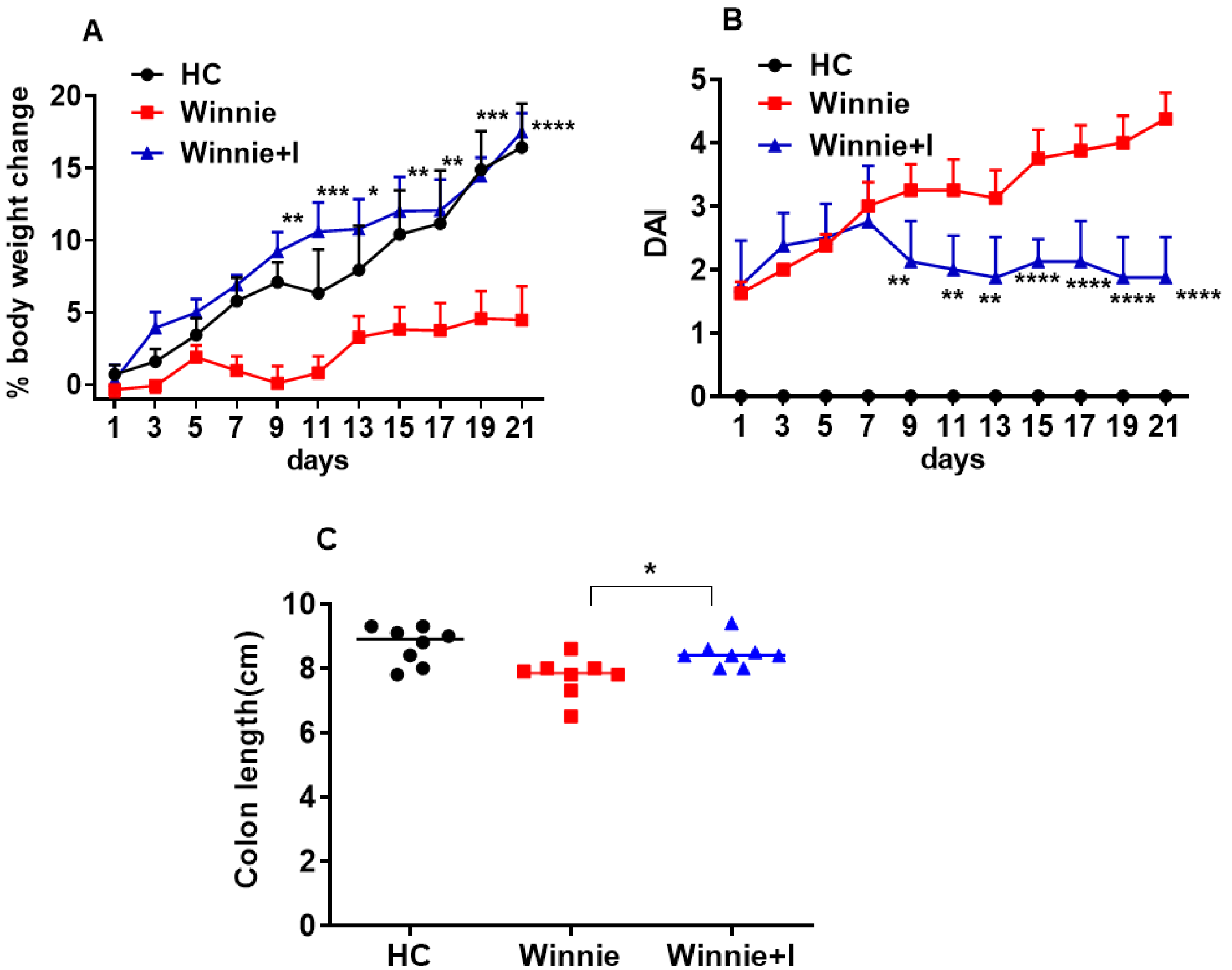
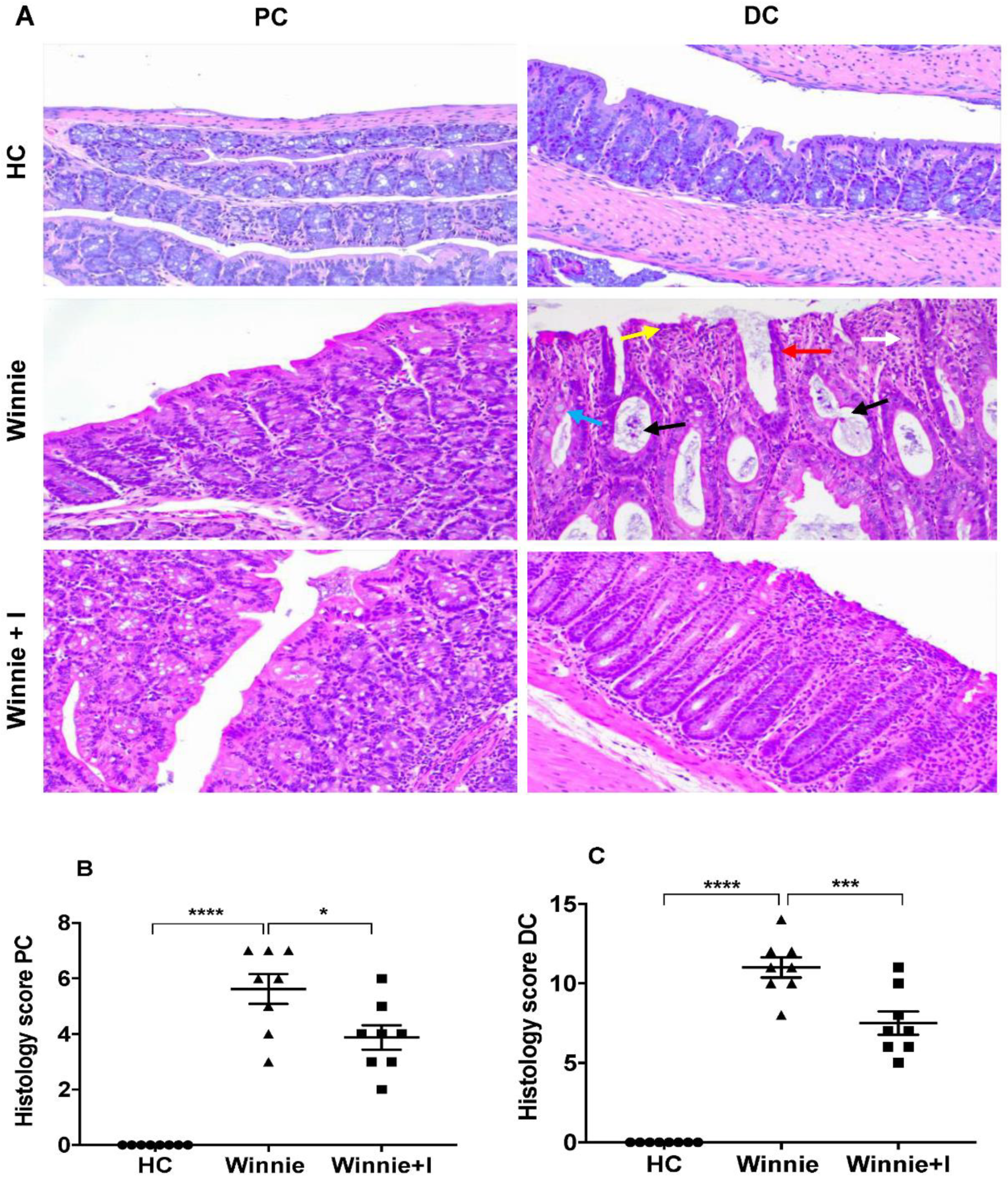
2.3. Histopathological Analysis and Clinical Scoring
2.4. Immunohistochemistry
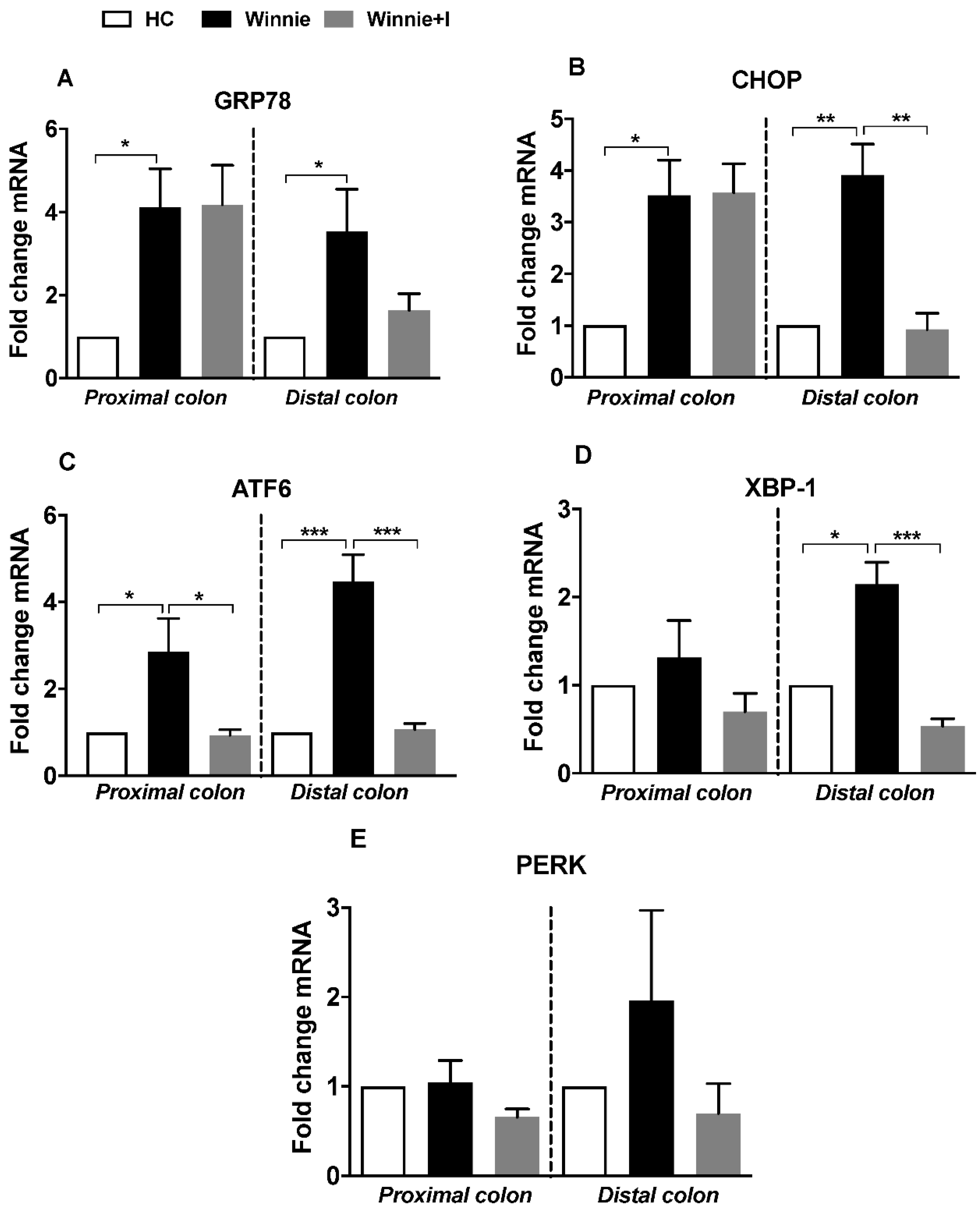
2.5. RNA Extraction and Gene Expression Analysis
2.6. Lipid Peroxidation
2.7. Western Blotting Analysis
2.8. Cytokine Measurement
2.9. Statistical Analysis
3. Results
3.1. Idebenone Improves Clinical Symptoms in the Spontaneous Winnie Model of Chronic Colitis
3.2. Idebenone Reduces Histopathology in Chronic Colitis
3.3. Idebenone Restores Mucin (MUC2) Expression in Goblet Cells
3.4. Idebenone Reduces Expression of ER Stress Markers
3.5. Idebenone Exerts Anti-ER Stress but Not Antioxidant Activity in the Winnie Model
3.6. Idebenone Reduces Pro-Inflammatory Cytokine Levels
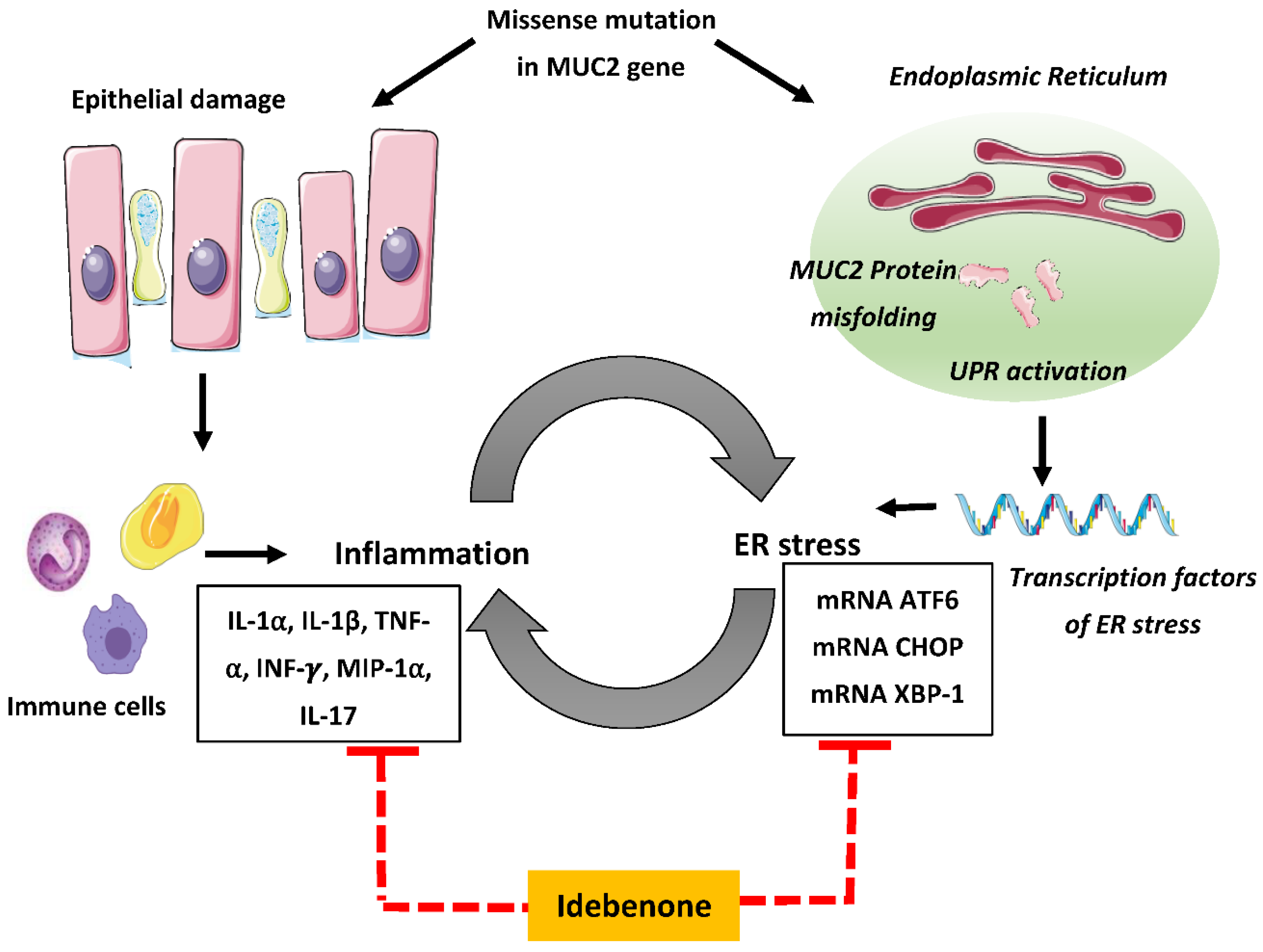
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hanauer, S.B. Inflammatory bowel disease: Epidemiology, pathogenesis, and therapeutic opportunities. Inflamm. Bowel Dis. 2006, 12, S3–S9. [Google Scholar] [CrossRef]
- Khor, B.; Gardet, A.; Xavier, R.J. Genetics and pathogenesis of inflammatory bowel disease. Nature 2011, 474, 307–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghosh, S.; Mitchell, R. Impact of inflammatory bowel disease on quality of life: Results of the European Federation of Crohn’s and Ulcerative Colitis Associations (EFCCA) patient survey. J. Crohns Colitis 2007, 1, 10–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernstein, C.N.; Blanchard, J.F.; Rawsthorne, P.; Yu, N. The prevalence of extraintestinal diseases in inflammatory bowel disease: A population-based study. Am. J. Gastroenterol. 2001, 96, 1116–1122. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, C.N.; Wajda, A.; Blanchard, J.F. The Clustering of Other Chronic Inflammatory Diseases in Inflammatory Bowel Disease: A Population-Based Study. Gastroenterology 2005, 129, 827–836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faubion, W.A., Jr.; Loftus, E.V., Jr.; Harmsen, W.S.; Zinsmeister, A.R.; Sandborn, W.J. The natural history of corticosteroid therapy for inflammatory bowel disease: A population-based study. Gastroenterology 2001, 121, 255–260. [Google Scholar] [CrossRef] [Green Version]
- Rutgeerts, P.; Sandborn, W.J.; Feagan, B.G.; Reinisch, W.; Olson, A.; Johanns, J.; Travers, S.; Rachmilewitz, D.; Hanauer, S.B.; Lichtenstein, G.R.; et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 2005, 353, 2462–2476. [Google Scholar] [CrossRef] [Green Version]
- Zenlea, T.; Peppercorn, M.A. Immunosuppressive therapies for inflammatory bowel disease. World J. Gastroenterol. 2014, 20, 3146–3152. [Google Scholar] [CrossRef]
- Stallmach, A.; Hagel, S.; Bruns, T. Adverse effects of biologics used for treating IBD. Best Pract. Res. Clin. Gastroenterol. 2010, 24, 167–182. [Google Scholar] [CrossRef]
- Govani, S.M.; Higgins, P.D. Combination of thiopurines and allopurinol: Adverse events and clinical benefit in IBD. J. Crohns Colitis 2010, 4, 444–449. [Google Scholar] [CrossRef] [Green Version]
- Meyer, L.; Simian, D.; Lubascher, J.; Acuña, R.; Figueroa, C.; Silva, G.; Brahm, J.; Quera, R. Adverse events associated with the treatment of inflammatory bowel disease. Rev. Med. Chile 2015, 143, 7–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ott, S.J.; Musfeldt, M.; Wenderoth, D.F.; Hampe, J.; Brant, O.; Folsch, U.R.; Timmis, K.N.; Schreiber, S. Reduction in diversity of the colonic mucosa associated bacterial microflora in patients with active inflammatory bowel disease. Gut 2004, 53, 685–693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaser, A.; Martínez-Naves, E.; Blumberg, R.S. Endoplasmic reticulum stress: Implications for inflammatory bowel disease pathogenesis. Curr. Opin. Gastroenterol. 2010, 26, 318–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Targan, S.R.; Karp, L.C. Defects in mucosal immunity leading to ulcerative colitis. Immunol. Rev. 2005, 206, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Wang, Z.; Zhang, J. Pathomechanisms of Oxidative Stress in Inflammatory Bowel Disease and Potential Antioxidant Therapies. Oxid. Med. Cell. Longev. 2017, 2017, 4535194. [Google Scholar] [CrossRef] [PubMed]
- Antoni, L.; Nuding, S.; Wehkamp, J.; Stange, E.F. Intestinal barrier in inflammatory bowel disease. World J. Gastroenterol. 2014, 20, 1165–1179. [Google Scholar] [CrossRef]
- Bianco, A.M.; Girardelli, M.; Tommasini, A. Genetics of inflammatory bowel disease from multifactorial to monogenic forms. World J. Gastroenterol. 2015, 21, 12296–12310. [Google Scholar] [CrossRef]
- Heazlewood, C.K.; Cook, M.C.; Eri, R.; Price, G.R.; Tauro, S.B.; Taupin, D.; Thornton, D.J.; Png, C.W.; Crockford, T.L.; Cornall, R.J. Aberrant mucin assembly in mice causes endoplasmic reticulum stress and spontaneous inflammation resembling ulcerative colitis. PLoS Med. 2008, 5, e54. [Google Scholar] [CrossRef] [Green Version]
- Gersemann, M.; Becker, S.; Kübler, I.; Koslowski, M.; Wang, G.; Herrlinger, K.R.; Griger, J.; Fritz, P.; Fellermann, K.; Schwab, M.; et al. Differences in goblet cell differentiation between Crohn’s disease and ulcerative colitis. Differ. Res. Biol. Divers. 2009, 77, 84–94. [Google Scholar] [CrossRef]
- Ma, X.; Dai, Z.; Sun, K.; Zhang, Y.; Chen, J.; Yang, Y.; Tso, P.; Wu, G.; Wu, Z. Intestinal Epithelial Cell Endoplasmic Reticulum Stress and Inflammatory Bowel Disease Pathogenesis: An Update Review. Front. Immunol. 2017, 8, 1271. [Google Scholar] [CrossRef] [Green Version]
- McGuckin, M.A.; Eri, R.; Simms, L.A.; Florin, T.H.; Radford-Smith, G. Intestinal barrier dysfunction in inflammatory bowel diseases. Inflamm. Bowel Dis. 2009, 15, 100–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asker, N.; Axelsson, M.A.; Olofsson, S.O.; Hansson, G.C. Dimerization of the human MUC2 mucin in the endoplasmic reticulum is followed by a N-glycosylation-dependent transfer of the mono- and dimers to the Golgi apparatus. J. Biol. Chem. 1998, 273, 18857–18863. [Google Scholar] [CrossRef] [Green Version]
- Hasnain, S.Z.; Tauro, S.; Das, I.; Tong, H.; Chen, A.C.; Jeffery, P.L.; McDonald, V.; Florin, T.H.; McGuckin, M.A. IL-10 promotes production of intestinal mucus by suppressing protein misfolding and endoplasmic reticulum stress in goblet cells. Gastroenterology 2013, 144, 357–368.e9. [Google Scholar] [CrossRef] [PubMed]
- Das, I.; Png, C.W.; Oancea, I.; Hasnain, S.Z.; Lourie, R.; Proctor, M.; Eri, R.D.; Sheng, Y.; Crane, D.I.; Florin, T.H. Glucocorticoids alleviate intestinal ER stress by enhancing protein folding and degradation of misfolded proteins. J. Exp. Med. 2013, 210, 1201–1216. [Google Scholar] [CrossRef] [Green Version]
- Kaser, A.; Lee, A.H.; Franke, A.; Glickman, J.N.; Zeissig, S.; Tilg, H.; Nieuwenhuis, E.E.; Higgins, D.E.; Schreiber, S.; Glimcher, L.H.; et al. XBP1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease. Cell 2008, 134, 743–756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, J.; Chen, X.; Hendershot, L.; Prywes, R. ER Stress Regulation of ATF6 Localization by Dissociation of BiP/GRP78 Binding and Unmasking of Golgi Localization Signals. Dev. Cell 2002, 3, 99–111. [Google Scholar] [CrossRef] [Green Version]
- Cox, J.S.; Shamu, C.E.; Walter, P. Transcriptional induction of genes encoding endoplasmic reticulum resident proteins requires a transmembrane protein kinase. Cell 1993, 73, 1197–1206. [Google Scholar] [CrossRef]
- Haze, K.; Yoshida, H.; Yanagi, H.; Yura, T.; Mori, K. Mammalian transcription factor ATF6 is synthesized as a transmembrane protein and activated by proteolysis in response to endoplasmic reticulum stress. Mol. Biol. Cell 1999, 10, 3787–3799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Korennykh, A.V.; Behrman, S.L.; Walter, P. Mammalian endoplasmic reticulum stress sensor IRE1 signals by dynamic clustering. Proc. Natl. Acad. Sci. USA 2010, 107, 16113–16118. [Google Scholar] [CrossRef] [Green Version]
- McGuckin, M.A.; Eri, R.D.; Das, I.; Lourie, R.; Florin, T.H. ER stress and the unfolded protein response in intestinal inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 298, G820–G832. [Google Scholar] [CrossRef] [Green Version]
- Hu, H.; Tian, M.; Ding, C.; Yu, S. The C/EBP Homologous Protein (CHOP) Transcription Factor Functions in Endoplasmic Reticulum Stress-Induced Apoptosis and Microbial Infection. Front. Immunol. 2019, 9, 3083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, S.S.; Kaufman, R.J. Unfolded protein response. Curr. Biol. 2012, 22, R622–R626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, H.; Matsui, T.; Yamamoto, A.; Okada, T.; Mori, K. XBP1 mRNA Is Induced by ATF6 and Spliced by IRE1 in Response to ER Stress to Produce a Highly Active Transcription Factor. Cell 2001, 107, 881–891. [Google Scholar] [CrossRef] [Green Version]
- Han, J.; Back, S.H.; Hur, J.; Lin, Y.H.; Gildersleeve, R.; Shan, J.; Yuan, C.L.; Krokowski, D.; Wang, S.; Hatzoglou, M.; et al. ER-stress-induced transcriptional regulation increases protein synthesis leading to cell death. Nat. Cell Biol. 2013, 15, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Hino, K.; Saito, A.; Asada, R.; Kanemoto, S.; Imaizumi, K. Increased susceptibility to dextran sulfate sodium-induced colitis in the endoplasmic reticulum stress transducer OASIS deficient mice. PLoS ONE 2014, 9, e88048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, F.; Edwards, R.; Dizon, D.; Afrasiabi, K.; Mastroianni, J.R.; Geyfman, M.; Ouellette, A.J.; Andersen, B.; Lipkin, S.M. Disruption of Paneth and goblet cell homeostasis and increased endoplasmic reticulum stress in Agr2-/- mice. Dev. Biol. 2010, 338, 270–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eri, R.; Adams, R.; Tran, T.; Tong, H.; Das, I.; Roche, D.; Oancea, I.; Png, C.W.; Jeffery, P.; Radford-Smith, G.L. An intestinal epithelial defect conferring ER stress results in inflammation involving both innate and adaptive immunity. Mucosal Immunol. 2011, 4, 354–364. [Google Scholar] [CrossRef]
- Robinson, A.M.; Rahman, A.A.; Carbone, S.E.; Randall-Demllo, S.; Filippone, R.; Bornstein, J.C.; Eri, R.; Nurgali, K. Alterations of colonic function in the Winnie mouse model of spontaneous chronic colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 312, G85–G102. [Google Scholar] [CrossRef] [Green Version]
- Rahman, A.A.; Robinson, A.M.; Jovanovska, V.; Eri, R.; Nurgali, K. Alterations in the distal colon innervation in Winnie mouse model of spontaneous chronic colitis. Cell Tissue Res. 2015, 362, 497–512. [Google Scholar] [CrossRef]
- Shinde, T.; Vemuri, R.; Shastri, S.; Perera, A.P.; Gondalia, S.V.; Beale, D.J.; Karpe, A.V.; Eri, R.; Stanley, R. Modulating the Microbiome and Immune Responses Using Whole Plant Fibre in Synbiotic Combination with Fibre-Digesting Probiotic Attenuates Chronic Colonic Inflammation in Spontaneous Colitic Mice Model of IBD. Nutrients 2020, 12, 2380. [Google Scholar] [CrossRef]
- Perera, A.P.; Fernando, R.; Shinde, T.; Gundamaraju, R.; Southam, B.; Sohal, S.S.; Robertson, A.A.B.; Schroder, K.; Kunde, D.; Eri, R. MCC950, a specific small molecule inhibitor of NLRP3 inflammasome attenuates colonic inflammation in spontaneous colitis mice. Sci. Rep. 2018, 8, 8618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Florin, T.; Movva, R.; Begun, J.; Duley, J.; Oancea, I.; Cuív, P.Ó. Colonic thioguanine pro-drug: Investigation of microbiome and novel host metabolism. Gut Microbes 2018, 9, 175–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brière, J.J.; Schlemmer, D.; Chretien, D.; Rustin, P. Quinone analogues regulate mitochondrial substrate competitive oxidation. Biochem. Biophys. Res. Commun. 2004, 316, 1138–1142. [Google Scholar] [CrossRef] [PubMed]
- Suno, M.; Nagaoka, A. Inhibition of Lipid Peroxidation by a Novel Compound, Idebenone (CV-2619). Jpn. J. Pharmacol. 1984, 35, 196–198. [Google Scholar] [CrossRef] [Green Version]
- Becker, C.; Bray-French, K.; Drewe, J. Pharmacokinetic evaluation of idebenone. Expert Opin. Drug Metab. Toxicol. 2010, 6, 1437–1444. [Google Scholar] [CrossRef] [PubMed]
- Shastri, S.; Shinde, T.; Sohal, S.S.; Gueven, N.; Eri, R. Idebenone Protects against Acute Murine Colitis via Antioxidant and Anti-Inflammatory Mechanisms. Int. J. Mol. Sci. 2020, 21, 484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Rasheed, N.M.; Faddah, L.M.; Mohamed, A.M.; Abdel Baky, N.A.; Al-Rasheed, N.M.; Mohammad, R.A. Potential Impact of Quercetin and Idebenone against Immuno- inflammatory and Oxidative Renal Damage Induced in Rats by Titanium Dioxide Nanoparticles Toxicity. J. Oleo Sci. 2013, 62, 961–971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, A.; Liu, Z.; Song, L.; Wang, X.; Zhang, Y.; Wu, N.; Lin, J.; Liu, Y.; Liu, Z. Idebenone Alleviates Neuroinflammation and Modulates Microglial Polarization in LPS-Stimulated BV2 Cells and MPTP-Induced Parkinson’s Disease Mice. Front. Cell. Neurosci. 2018, 12, 529. [Google Scholar] [CrossRef]
- Gerhardt, E.; Gräber, S.; Szego, E.M.; Moisoi, N.; Martins, L.M.; Outeiro, T.F.; Kermer, P. Idebenone and resveratrol extend lifespan and improve motor function of HtrA2 knockout mice. PLoS ONE 2011, 6, e28855. [Google Scholar] [CrossRef] [Green Version]
- Shinde, T.; Perera, A.P.; Vemuri, R.; Gondalia, S.V.; Karpe, A.V.; Beale, D.J.; Shastri, S.; Southam, B.; Eri, R.; Stanley, R. Synbiotic Supplementation Containing Whole Plant Sugar Cane Fibre and Probiotic Spores Potentiates Protective Synergistic Effects in Mouse Model of IBD. Nutrients 2019, 11, 818. [Google Scholar] [CrossRef] [Green Version]
- Randall-Demllo, S.; Fernando, R.; Brain, T.; Sohal, S.S.; Cook, A.L.; Guven, N.; Kunde, D.; Spring, K.; Eri, R. Characterisation of colonic dysplasia-like epithelial atypia in murine colitis. World J. Gastroenterol. 2016, 22, 8334–8348. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Durrant, D.; Koka, S.; Salloum, F.N.; Xi, L.; Kukreja, R.C. Mammalian target of rapamycin (mTOR) inhibition with rapamycin improves cardiac function in type 2 diabetic mice: Potential role of attenuated oxidative stress and altered contractile protein expression. J. Biol. Chem. 2014, 289, 4145–4160. [Google Scholar] [CrossRef] [Green Version]
- Lean, Q.Y.; Eri, R.D.; Fitton, J.H.; Patel, R.P.; Gueven, N. Fucoidan extracts ameliorate acute colitis. PLoS ONE 2015, 10, e0128453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef] [Green Version]
- Di Prospero, N.A.; Sumner, C.J.; Penzak, S.R.; Ravina, B.; Fischbeck, K.H.; Taylor, J.P. Safety, tolerability, and pharmacokinetics of high-dose idebenone in patients with Friedreich ataxia. Arch. Neurol. 2007, 64, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Hanski, C.; Born, M.; Foss, H.D.; Marowski, B.; Mansmann, U.; Arastéh, K.; Bachler, B.; Papenfuss, M.; Niedobitek, F. Defective post-transcriptional processing of MUC2 mucin in ulcerative colitis and in Crohn’s disease increases detectability of the MUC2 protein core. J. Pathol. 1999, 188, 304–311. [Google Scholar] [CrossRef]
- Bertolotti, A.; Wang, X.; Novoa, I.; Jungreis, R.; Schlessinger, K.; Cho, J.H.; West, A.B.; Ron, D. Increased sensitivity to dextran sodium sulfate colitis in IRE1beta-deficient mice. J. Clin. Investig. 2001, 107, 585–593. [Google Scholar] [CrossRef] [Green Version]
- Bogaert, S.; De Vos, M.; Olievier, K.; Peeters, H.; Elewaut, D.; Lambrecht, B.; Pouliot, P.; Laukens, D. Involvement of endoplasmic reticulum stress in inflammatory bowel disease: A different implication for colonic and ileal disease? PLoS ONE 2011, 6, e25589. [Google Scholar] [CrossRef] [Green Version]
- Ye, J.; Rawson, R.B.; Komuro, R.; Chen, X.; Davé, U.P.; Prywes, R.; Brown, M.S.; Goldstein, J.L. ER Stress Induces Cleavage of Membrane-Bound ATF6 by the Same Proteases that Process SREBPs. Mol. Cell 2000, 6, 1355–1364. [Google Scholar] [CrossRef]
- Senkal, C.E.; Ponnusamy, S.; Bielawski, J.; Hannun, Y.A.; Ogretmen, B. Antiapoptotic roles of ceramide-synthase-6-generated C16-ceramide via selective regulation of the ATF6/ CHOP arm of ER-stress-response pathways. FASEB J. 2010, 24, 296–308. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, H.; Okada, T.; Haze, K.; Yanagi, H.; Yura, T.; Negishi, M.; Mori, K. ATF6 Activated by Proteolysis Binds in the Presence of NF-Y (CBF) Directly to the Cis-Acting Element Responsible for the Mammalian Unfolded Protein Response. Mol. Cell. Biol. 2000, 20, 6755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamoto, K.; Sato, T.; Matsui, T.; Sato, M.; Okada, T.; Yoshida, H.; Harada, A.; Mori, K. Transcriptional Induction of Mammalian ER Quality Control Proteins Is Mediated by Single or Combined Action of ATF6α and XBP1. Dev. Cell 2007, 13, 365–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, H.; Niemeijer, M.; van de Water, B.; Beltman, J.B. ATF6 Is a Critical Determinant of CHOP Dynamics during the Unfolded Protein Response. iScience 2020, 23, 100860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Shen, J.; Prywes, R. The luminal domain of ATF6 senses endoplasmic reticulum (ER) stress and causes translocation of ATF6 from the ER to the Golgi. J. Biol. Chem. 2002, 277, 13045–13052. [Google Scholar] [CrossRef] [Green Version]
- Tabas, I.; Ron, D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat. Cell Biol. 2011, 13, 184–190. [Google Scholar] [CrossRef]
- Guan, Y.; Li, J.; Zhan, T.; Wang, J.-W.; Yu, J.-B.; Yang, L. Idebenone Maintains Survival of Mutant Myocilin Cells by Inhibiting Apoptosis. Chin. Med. J. 2016, 129, 2001–2004. [Google Scholar] [CrossRef]
- McCord, J.M. The evolution of free radicals and oxidative stress. Am. J. Med. 2000, 108, 652–659. [Google Scholar] [CrossRef]
- Tu, W.; Wang, H.; Li, S.; Liu, Q.; Sha, H. The Anti-Inflammatory and Anti-Oxidant Mechanisms of the Keap1/Nrf2/ARE Signaling Pathway in Chronic Diseases. Aging Dis. 2019, 10, 637–651. [Google Scholar] [CrossRef] [Green Version]
- Strober, W.; Fuss, I.J. Proinflammatory cytokines in the pathogenesis of inflammatory bowel diseases. Gastroenterology 2011, 140, 1756–1767. [Google Scholar] [CrossRef] [Green Version]
- Kmieć, Z.; Cyman, M.; Ślebioda, T.J. Cells of the innate and adaptive immunity and their interactions in inflammatory bowel disease. Adv. Med. Sci. 2017, 62, 1–16. [Google Scholar] [CrossRef]
- Ligumsky, M.; Simon, P.L.; Karmeli, F.; Rachmilewitz, D. Role of interleukin 1 in inflammatory bowel disease--enhanced production during active disease. Gut 1990, 31, 686–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keshav, S.; Lawson, L.; Chung, L.P.; Stein, M.; Perry, V.H.; Gordon, S. Tumor necrosis factor mRNA localized to Paneth cells of normal murine intestinal epithelium by in situ hybridization. J. Exp. Med. 1990, 171, 327–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breese, E.J.; Michie, C.A.; Nicholls, S.W.; Murch, S.H.; Williams, C.B.; Domizio, P.; Walker-Smith, J.A.; MacDonald, T.T. Tumor necrosis factor alpha-producing cells in the intestinal mucosa of children with inflammatory bowel disease. Gastroenterology 1994, 106, 1455–1466. [Google Scholar] [CrossRef]
- Bisping, G.; Lügering, N.; Lütke-Brintrup, S.; Pauels, H.G.; Schürmann, G.; Domschke, W.; Kucharzik, T. Patients with inflammatory bowel disease (IBD) reveal increased induction capacity of intracellular interferon-gamma (IFN-gamma) in peripheral CD8+ lymphocytes co-cultured with intestinal epithelial cells. Clin. Exp. Immunol. 2001, 123, 15–22. [Google Scholar] [CrossRef]
- Banks, C.; Bateman, A.; Payne, R.; Johnson, P.; Sheron, N. Chemokine expression in IBD. Mucosal chemokine expression is unselectively increased in both ulcerative colitis and Crohn’s disease. J. Pathol. 2003, 199, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Rovedatti, L.; Kudo, T.; Biancheri, P.; Sarra, M.; Knowles, C.H.; Rampton, D.S.; Corazza, G.R.; Monteleone, G.; Di Sabatino, A.; MacDonald, T.T. Differential regulation of interleukin 17 and interferon γ production in inflammatory bowel disease. Gut 2009, 58, 1629. [Google Scholar] [CrossRef] [PubMed]
- Zenewicz, L.A.; Antov, A.; Flavell, R.A. CD4 T-cell differentiation and inflammatory bowel disease. Trends Mol. Med. 2009, 15, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Bettigole, S.E.; Glimcher, L.H. Endoplasmic Reticulum Stress in Immunity. Annu. Rev. Immunol. 2015, 33, 107–138. [Google Scholar] [CrossRef]
- Cao, S.S.; Luo, K.L.; Shi, L. Endoplasmic Reticulum Stress Interacts With Inflammation in Human Diseases. J. Cell. Physiol. 2016, 231, 288–294. [Google Scholar] [CrossRef]
- Hao, X.; Yao, A.; Gong, J.; Zhu, W.; Li, N.; Li, J. Berberine Ameliorates Pro-inflammatory Cytokine-Induced Endoplasmic Reticulum Stress in Human Intestinal Epithelial Cells In Vitro. Inflammation 2012, 35, 841–849. [Google Scholar] [CrossRef]
- Kim, S.R.; Kim, H.J.; Kim, D.I.; Lee, K.B.; Park, H.J.; Jeong, J.S.; Cho, S.H.; Lee, Y.C. Blockade of Interplay between IL-17A and Endoplasmic Reticulum Stress Attenuates LPS-Induced Lung Injury. Theranostics 2015, 5, 1343–1362. [Google Scholar] [CrossRef] [Green Version]
- Pahl, H.L.; Baeuerle, P.A. Activation of NF-kappa B by ER stress requires both Ca2+ and reactive oxygen intermediates as messengers. FEBS Lett. 1996, 392, 129–136. [Google Scholar] [CrossRef] [Green Version]
- Deng, J.; Lu, P.D.; Zhang, Y.; Scheuner, D.; Kaufman, R.J.; Sonenberg, N.; Harding, H.P.; Ron, D. Translational Repression Mediates Activation of Nuclear Factor Kappa B by Phosphorylated Translation Initiation Factor 2. Mol. Cell. Biol. 2004, 24, 10161. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Kaufman, R.J. From endoplasmic-reticulum stress to the inflammatory response. Nature 2008, 454, 455–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cláudio, N.; Dalet, A.; Gatti, E.; Pierre, P. Mapping the crossroads of immune activation and cellular stress response pathways. EMBO J. 2013, 32, 1214–1224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmitz, M.L.; Shaban, M.S.; Albert, B.V.; Gökçen, A.; Kracht, M. The Crosstalk of Endoplasmic Reticulum (ER) Stress Pathways with NF-κB: Complex Mechanisms Relevant for Cancer, Inflammation and Infection. Biomedicines 2018, 6, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shastri, S.; Shinde, T.; Perera, A.P.; Gueven, N.; Eri, R. Idebenone Protects against Spontaneous Chronic Murine Colitis by Alleviating Endoplasmic Reticulum Stress and Inflammatory Response. Biomedicines 2020, 8, 384. https://doi.org/10.3390/biomedicines8100384
Shastri S, Shinde T, Perera AP, Gueven N, Eri R. Idebenone Protects against Spontaneous Chronic Murine Colitis by Alleviating Endoplasmic Reticulum Stress and Inflammatory Response. Biomedicines. 2020; 8(10):384. https://doi.org/10.3390/biomedicines8100384
Chicago/Turabian StyleShastri, Sonia, Tanvi Shinde, Agampodi Promoda Perera, Nuri Gueven, and Rajaraman Eri. 2020. "Idebenone Protects against Spontaneous Chronic Murine Colitis by Alleviating Endoplasmic Reticulum Stress and Inflammatory Response" Biomedicines 8, no. 10: 384. https://doi.org/10.3390/biomedicines8100384





