1. Introduction
The skin is the largest organ in the body and occupies about 16% of the total body weight of an adult and has a surface area of about 2 m
2 [
1]. It is a complex arrangement of structures and has a multifunctional role—provide a physical barrier to the environment by acting as a protective barrier against the ingress of foreign material, maintains homeostasis and thermoregulation by limiting the loss of water, electrolytes, and heat and prevents microbial colonization [
2,
3]. Although skin act as a shield against bacterial invasion, bacteria can still invade the epidermis and dermis to produce localized infections and cause a variety of pathologic changes in the skin (impetigo, furuncles, subcutaneous abscesses) [
4]. Microbial infections of the skin and underlying tissues are among the most frequent conditions found in ambulatory care patients [
5].
Staphylococcus aureus (
S. aureus) is one of the most important human and veterinary pathogens and is the causative agent for the majority of primary skin infections [
6]. It causes infections ranging from benign to life-threatening diseases [
7]. Skin and soft tissue infections (SSTIs) encompass a wide variety of clinical outcomes, ranging from mild cases of cellulitis, erysipelas, trauma, subcutaneous tissue infections, wound-related infections to complicated deep-seated infections with a systemic sign of sepsis [
8]. SSTIs may lead to severe complications and hospital admission when associated with co-morbidities and/or bacteremia. The most commonly reported cause of SSTIs is
S. aureus followed by β-hemolytic streptococci (BHS) [
9,
10]. The
S. aureus can internalize by a variety of nonphagocytic host cells and can contribute to the development of persistent or chronic infections and may lead to deeper tissue infections or dissemination [
11,
12,
13].
Wound management is a prevalent clinical problem as wound healing involves a series of complex processes including the inflammation phase, proliferative phase (formation of granulation tissue, re-epithelialization, and matrix formation), and remodeling phase [
14]. Each phase of wound healing is well defined, although they overlap with the next [
15]. The process of wound healing becomes delayed when wounds are colonized, and the colonizing agent is sustained [
16]. In the patient with a weak immune system, bacterial contamination can prolong wound healing [
17,
18] and colonization of bacteria in wounds is a serious threat. Open wounds are also at high risk of invasive wound infections, which can further lead to amputation and disability [
19]. SSTIs and wound healing all rely on efficient antibiotic therapies. Temporary eradication of
S. aureus with antibiotics often leads to clinical improvement. Antimicrobial resistance is one of the biggest challenges in the global health sector [
20]. The high incidence of methicillin resistance in hospitals complicates the prevention and treatment of serious infections due to staphylococci [
21]. As infections due to multi-resistant Gram-positive organisms are increasing day by day, their early recognition, treatment, and proper management are greatly required. Several antimicrobial agents in different dosage forms are available for the treatment of SSTIs. Topical application of antibiotic agents have several benefits over oral and systemic therapy [
22]—localized and targeted delivery can provide the required concentration for antibiotic activity more efficiently at the skin target site, can avoid unnecessary exposure of gut flora that may exert selection for resistance, can avoid side-effect and allergic reactions associated with systemic antibiotic treatment. Therefore, topical applications may highly influence the treatment efficiency and can increase patient compliance.
In such a scenario, the development of a new treatment strategy is crucial to deal with the emerging issues of skin infections. The topical delivery of antibacterial agents of medicinal plants can be considered as a source for new therapeutic agents aimed at the treatment and management of skin infections. Thymoquinone (TQ, 2-isopropyl-5-methyl-1,4-benzoquinone) is the main constituent of
Nigella sativa (Black cumin) seeds [
23]. TQ is a yellow crystalline molecule and has a basic quinone structure consisting of a para-substituted dione conjugated to a benzene ring to which a methyl and an isopropyl side chain groups are added in positions 2 and 5, respectively. TQ has many pharmacological properties such as anticancer, antimicrobial, anti-inflammatory, antioxidant, anti-asthmatic, and immunomodulatory effects [
24]. Thus far, several research groups have reported the compound’s anticancer and brain targeting properties. Odeh et al. loaded TQ in a liposome system and tested on breast cancer cell lines (MCF-7 and T47D) to evaluate TQ anticancer properties [
23], Ng et al. prepared TQ loaded nanostructured lipid carrier and showed its effectiveness towards breast cancer and cervical cancer cell lines [
25], and Ahmad et al. evaluated TQ-loaded mucoadhesive nano-emulsion for the treatment of cerebral ischemia [
26]. However, there have been no reports on TQ delivery from a topical delivery system and its effectiveness for the treatment of wound and
S. aureus associated bacterial skin infections.
The objective of this study was to synthesize and characterize a biocompatible novel topical polymeric film and hydrogel system that has the potential to deliver antibacterial TQ agent directly at the skin target site that may be useful for the treatment and management of S. aureus related bacterial skin infections and for the wound management. To achieve this objective, TQ loaded polyvinyl pyrrolidone (PVP) films were prepared using a solvent casting method and TQ wound hydrogels were prepared using different polymers. The prepared films and hydrogels were characterized by physical parameters, permeability, and stability studies. Its biocompatibility was assessed, and the antibacterial efficacy of films and hydrogels were evaluated in vitro and ex vivo on selected strains of S. aureus. Further, in vitro scratch assay models using human dermal fibroblast (HDF) and human keratinocyte cell line (HaCaT) were used to demonstrate its wound healing properties. To evaluate its preclinical and in vivo efficacy, a biopsy punch wound infection animal model was used. This work demonstrates that the PVP/TQ film is effective in the control of bacterial infection and facilitating wound healing.
2. Materials and Methods
2.1. Materials
Thymoquinone (TQ), polyvinylpyrrolidone (PVP), dibutyl phthalate (DBP), hydroxypropyl methylcellulose (HPMC), potassium chloride, benzoic acid, gentamicin solution, formalin solution (10%), triethanolamine, propylene glycol (PG), dipropylene glycol (DiPG), alamarBlue® (resazurin) assay kit, high-performance liquid chromatography (HPLC) grade water and acetonitrile were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). Laurocapram (Azone) was purchased from BOC Sciences (Shirley, NY, USA). Phosphate-buffered saline tablets (PBS, pH 7.4) was purchased from MP Biomedicals, LLC (Solon, OH, USA), ethanol (EtOH) was purchased from Decon Labs, Inc. (King of Prussia, PA, USA), xanthan gum (XG) was purchased from Spectrum Chemical (New Brunswick, NJ, USA), hydroxypropyl cellulose (HPC) was purchased from Ashland (Wilmington, DE, USA). Carbopol 980 and ultrez 10 were purchased from Lubrizol (Cleveland, OH, USA) and drierite was purchased from Acros organics (Morris Plains, NJ, USA). Bacto tryptic soy broth, bacto agar, Dulbecco’s Modified Eagle’s Medium (DMEM), and Dulbecco’s phosphate-buffered saline (DPBS) were purchased from Fisher Scientific (Hampton, NH, USA). Human HaCaT cell line, HDF, and Pen/strep were purchased from Life Technologies (Carlsbad, CA, USA). S. aureus (ATCC 49230) was purchased from ATCC (Manassas, VA, USA). Isoflurane was purchased from Henry Schein (Dublin, OH, USA). Fetal bovine serum (FBS) was purchased from Atlanta Biologicals (Minneapolis, MN, USA). CellTiter 96® was purchased from Promega (Madison, WI, USA). Gentamicin sulfate cream USP, 0.1% was purchased from Perrigo (Allegan, MI, USA). Dermatomed human cadaver skins from the posterior torso were obtained from New York Firefighter Skin Bank (New York, NY, USA). Adult male BALB/c mice (9 weeks old) were purchased from Charles River (Wilmington, MA, USA).
2.2. Fourier Transform Infrared (FTIR) Analysis
FTIR spectra of samples were taken on a model Nicolet iS10 instrument Thermo Scientific (Waltham, MA, USA) to investigate the possible interaction between the drug and polymer. FTIR spectra of pure drug, polymer, physical mixture of drug and polymer in a ratio of 1:1, films with TQ and films without TQ were scanned in the range between 400 and 4000 cm−1.
2.3. Fabrication of Films
The matrix-type polymeric films containing TQ were prepared by the solvent casting method. Accurately weighted TQ (1%, w/w) was dissolved in ethanol and was sonicated for 30 min to ensure solubilization. DBP was used as a plasticizer and Azone was used as a penetration enhancer. The weighted amount of PVP, DBP (4%, w/w), and Azone (5%, w/w) was added in the drug solution. The mixture was stirred at 200 rpm at 25 °C for 20 min. The solution was poured on a Teflon dish (15 cm2) and placed in an oven maintained at 60 ± 5 °C. To allow complete evaporation, the system was left undisturbed for 3-h and 20 min. The formed films were completely removed from the Teflon dish and punched out into 0.64 cm2 pieces. Control films were prepared without TQ but containing PVP, plasticizer, and penetration enhancer.
2.4. Preparation of TQ Hydrogel Formulations
Drug-loaded hydrogels were prepared using gelling agents, preservatives, penetration enhancers, and vehicles. Different concentrations of various polymers (gelling agents) with or without xanthan gum were dispersed slowly in an aqueous-based solution containing TQ (0.2%
w/
w), 1:1 concentration of propylene glycol, and dipropylene glycol (20%
w/
w, as a vehicle), benzoic acid (0.1%
w/
w, as a preservative), ethanol (5%
w/
w, as a penetration enhancer), using an overhead mechanical stirrer at a moderate speed. Triethanolamine was used to adjust the pH of Carbopol and Ultrez 10. The prepared hydrogels were packed in a wide-mouth jar covered with screw-capped plastic lid and kept in dark and at laboratory ambient temperature. The composition of different prepared TQ hydrogel formulations is given in
Table S1.
2.5. Field Emission Scanning Electron Microscopic (FESEM) Studies
The surface morphology of the film was recorded with a Zeiss field emission scanning electron microscopy (FESEM) (FSD PRE-AMP 4CH, Oberkochen, Germany). The film sample was mounted on an aluminium stub with the double-sided adhesive band then gold was sputtered on the specimen (20 nm) to ensure sufficient electrical conductivity. An accelerating voltage of 5 kV was applied, and the image was photographed by a secondary electron detector.
2.6. Physicochemical Characterization of Films
2.6.1. Film Thickness
Film thickness was measured using a digital caliper (Fisher Scientific, Portsmouth, NH, USA) at three different places, and the mean value was calculated.
2.6.2. Drug Content Uniformity
A prepared film was dissolved in 10 mL ethanol and stirred continuously for 24 h. The drug content was analyzed using the HPLC method described in
Section 2.10.
2.6.3. Weight Variation
Weight variation was studied by individually weighing five randomly selected films.
2.6.4. Flatness
Each film was cut into three sections (left, center, and right). The length of each section was measured to examine the variation in length that can arise from nonuniformity in flatness and was calculated as a percentage of constriction, with 0% constriction equivalent to 100% flatness.
where L1 = the initial length of each strip and L2 = the final length of each strip.
2.6.5. Folding Endurance
To determine the folding endurance the film was folded repeatedly at the same place until it broke. The folding endurance value represents the number of times the film could be folded at the same place without breaking.
2.6.6. Percentage of Moisture Content
The prepared films were marked, then weighed individually and kept in a desiccator containing drierite at room temperature for 24 h. The individual weights of the films were measured from time to time until a constant weight was observed. The difference between initial and final weight with respect to final weight was used to measure the percentage of moisture content.
2.6.7. Percentage of Moisture Uptake
The films were weighed and kept in a desiccator at room temperature for 24 h. Films were taken out and placed in a desiccator containing 100 mL of a saturated solution of potassium chloride to maintain 84% relative humidity until a constant weight for the films were obtained. The percentage of moisture uptake was calculated as the difference between final and initial weight with respect to the initial weight.
2.7. Physicochemical Characterization of the Prepared Hydrogels
2.7.1. Visual Inspection
TQ hydrogels were examined visually for their color and homogeneity (appearance and presence of any aggregates).
2.7.2. pH Determination
The pH of various TQ hydrogel formulations was determined using pH meter (Symphony B10P pH meter, VWR, Radnor, PA, USA). One g of TQ hydrogel was dissolved in 10 g of DI water. After 2 h pH was determined at room temperature.
2.7.3. Spreadability Test
A 10 mg sample was placed on top of a microscopic slide and covered with another slide, 50 gm of standardized weight was put on it and after 1 min the diameter of the sample was taken in mm.
2.7.4. Drug Content Uniformity
Three samples of a specific quantity (100 µL) of each prepared hydrogel were taken and dissolved in 10 mL of ethanol solvent. To ensure drug solubility the 20 mL glass vial containing the gel solution was put on a magnetic stirrer at 600 rpm at 25 °C overnight. The drug content was then determined using HPLC. The variability of TQ content in hydrogels was reported as % RSD:
2.8. Rheological Characterization of the Hydrogel Formulation
Rheological characterization was performed on a Malvern Kinexus Ultra + rheometer (Malvern, Worcestershire, UK) equipped with a 25 mm flat stainless-steel plate. All tests were done at 32 °C and a gap of 1 mm. The following tests were carried out:
2.8.1. Oscillation Stress Sweep
The samples were subjected to increasing stress (0.1–500 Pa) at a constant frequency of 1 Hz. This test allows determination of the linear viscoelastic region (LVR) of the sample, and therefore the consequent choice of the stress value to use in the subsequent oscillation test.
2.8.2. Frequency Sweep
All the prepared samples were subjected to an increasing frequency of 0.1–50 rad/s at constant stress (5 Pa) obtained from LVR. The effect of stress on elastic modulus (G′) and viscous modulus (G′′) was monitored.
2.9. In Vitro Skin Permeation Studies
Franz diffusion cell (FDC, Permegear Inc., Hellertown, PA, USA) and dermatomed human cadaver skin (~500 µm thickness) were used to perform in vitro skin permeation studies. Skin samples were prepared by slowly thawing at room temperature, cutting into appropriate pieces, and then soaking in filtered PBS (pH 7.4) for 15 min. After that, they were mounted on FDC (0.64 cm2 donor area and receptor volume of 5.0 mL) with the epidermal side in contact with the formulation or donor compartment. Filtered PBS (pH 7.4) was used as a receptor solution to fill the receptor compartment of each cell. The cell temperature was maintained at 37 °C under synchronous continuous stirring using a magnetic stirrer at 600 rpm and left for 15 min to equilibrate the diffusional membranes. At time zero, formulated films and 100 µL of hydrogel were placed over the skin in the donor compartment of each cell. At each time point, 300 µL of receptor samples was withdrawn from the sampling port and replaced with an equal amount of receptor solution. The receptor aliquots of 300 µL were analyzed at the end of the experiment using a valid HPLC method described below.
2.10. High-Performance Liquid Chromatography (HPLC)
For this study, an Agilent 1100 series instrument (Agilent Technologies, Santa Clara, CA, USA) coupled with UV detection (DAD) and HP Chemstation software V. 32 was used to validate the HPLC method. To analyze the concentration of TQ, a mobile phase of 80% acetonitrile and 20% water was pumped through an Agilent Eclipse XDB-C18 5 µm, 250 × 4.6 mm column with an injection volume of 20 µL and flow rate of 1.0 mL/min. The column temperature was set to 23 °C with UV detection of 250 nm used, with a retention time of 4.2 min. At a concentration of 0.39–100 µg/mL, the method was linear with an R2 value of 0.99. Intra- and inter-day precision and accuracy of the method showed a % CV of 0.01 and 0.1, respectively, which is lower than the requirements of 2%.
2.11. Skin Deposition Study
At the end of the permeation study, the skin was removed from the diffusion cell. The skin samples were then cut around the diffusional area, air-dried, accurately weighed, and placed into bead bug tubes. To extract the drug, the skin samples inside the tube were cut into very small pieces using a pair of scissors, and 1 mL ethanol was added to each tube. These were then homogenized for 9 min (3 min of 3 cycles) using a BeadBugTM Microtube homogenizer, D1030 (Benchmark Scientific, Sayreville, NJ, USA). All the skin samples were agitated at 37 °C for 24 h using a Julabo SW22 shaker (Julabo USA Inc., Allentown, PA, USA). After that, they were centrifuged at 1200 rpm for 5 min, and each of the skin samples was filtered through a 0.45 µm polypropylene filter medium with polypropylene housing. TQ concentrations were expressed as ng of TQ per skin weight in mg.
2.12. Stability Study
The films were put in a Petri dish and the dish was wrapped by aluminum foil and stored at 20 °C for 60 days. The samples were analyzed for physical changes such as color, texture, and other physical parameters. The FTIR spectra of stored films were compared with freshly prepared films. The films were also analyzed for drug content. On the other hand, all the hydrogel formulations were filled in glassware and covered with aluminum foil and were kept at laboratory ambient temperature for 8 months. The physical stability of the formulation was examined visually for appearance, color, and odor in every two weeks. After 8 months an antibacterial efficacy study was performed to confirm the formulation stability of hydrogel formulation.
2.13. In Vitro Antibacterial Activity of TQ Films and Hydrogels
The prepared control films and TQ loaded films were tested for their antibacterial activity against S. aureus (ATCC 49230) using the disc diffusion method. Briefly, Muller Hinton agar (MHA) plates were used for screening, prepared by pouring 15 mL of molten media into sterile Petri dishes. Then 150 µL of overnight cultured bacteria adjusted to OD concentration of 0.602 (OD 1 = 1 × 109/mL of bacteria) in sterile TSB (Tryptic soy broth) was spread on the surface of MHA agar plates with the help of a sterile spreader. The disc-shaped polymer film of 0.64 cm2 and 100 µL of TQ gel were then placed on the surface of the medium and incubated at 37 °C for 24 h. Gentamicin 500 µg/mL and 50 µg/mL was used as a positive control, UV irradiated filter paper was used as a negative control, and control film without TQ was used as a control. At the end of incubation, the inhibition zones were examined around the polymer disc films. The study was performed in triplicate.
2.14. Ex Vivo Antibacterial Activity of TQ Films and Hydrogels Using Human Cadaver Skin Explants
Human cadaver skin was thoroughly washed three times using sterile PBS. Using surgical gloves and sterile scissors they were cut into pieces (2 cm × 2 cm) and two skin samples were placed on each agar plate. 5 µL of 1 × 10⁶ CFU/mL was put onto each skin piece followed by the application of treatment. After overnight incubation at 37 °C, bacteria were extracted for counting using sterile PBS and 10 s of vortex. Serial dilutions of bacteria were prepared and were plated on TSB agar plates. Bacteria were counted after overnight incubation at 37 °C.
2.15. Cytocompatibility Study
AlamarBlue
® (resazurin) assay was used to evaluate the cytocompatibility of the TQ film using two cell lines, HaCaT (passage 8) and HDF (passage 5). The cells were counted and were seeded into the 6 well plates at a density of 200,000 cells/cm². After reaching confluency, the cells were treated with the samples for 24 h. Cells treated with media containing 1% Triton served as positive control and cells in media without any treatment acted as a negative control. After 24 h the cells were treated with alamarBlue
® and incubated for 4 h. The optical density was measured at an excitation-emission wavelength of 560–590 nm using Spark 10 M multimode microplate reader (Tecan, Männedorf, Switzerland). The percentage of cell viability was calculated using the formula given below:
2.16. Scratch Assay for Wound Closure Activity
The HDF (passages 2–4) cells were counted and were seeded into the 24-well plates at a density of 50,000 cells/well. After reaching confluency, the culture media was replaced with sterile base media (DMEM with 1%
P/
S), and scratch wounds were created in the cell monolayer using a 200 µL sterile pipette tip [
27]. TQ in DMEM without serum at different concentrations (1 ng/mL and 100 ng/mL) was put into the culture media and the experiment was continued for 24 h. Base media was used as a control. Images were taken at 0, 4, 8, 12, and 24 h. The HaCaT (passage 37–39) cells were counted and were seeded into the 24 well plates at a density of 250K cells/well. After reaching confluency, the culture media was replaced with sterile base media (DMEM with 1% FBS + 1%
P/
S), and scratch wounds were created in the cell monolayer using a sterile pipette tip. TQ in DMEM at different concentrations (1 ng/mL and 100 ng/mL) was put into the culture media and the experiment was continued for six days. Base media was used as a control. Images were taken at 0, 24, 48, 72, and 144 h.
2.17. In Vivo Bacterial Skin Infection Study
Animal infection experiments were performed at the Nelson Biological Laboratories, Rutgers University (Piscataway, NJ, USA) following a protocol approved by the Rutgers University Institutional Animal Care and Use Committee (IACUC ID: PROTO201702583, approved on 24 September 2019).
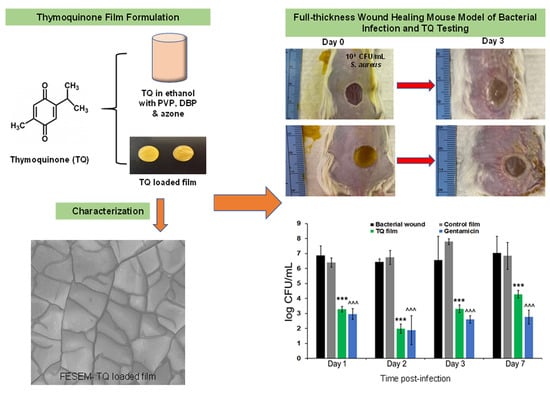
Figure 1 shows the in-vivo experimental design. Briefly, mice were housed under standard conditions of light and temperature and were fed a standard diet and water ad libitum. Adult male mice (BALB/c, 10 weeks) were used for all experiments after habituating for a week at laboratory conditions [
28,
29]. Before the experiment day, mice were anesthetized using 5% isoflurane and then maintained at 2–3% during the procedure. The hair over the dorsum (head to tail) was shaved with an electric clipper first and then the remaining hair was removed with a depilatory cream (Nair
TM hair remover lotion, Church & Dwight, Ewing Township, NJ, USA). Finally, the shaved area was washed with wet scrub, and the animals were returned to their cages. On the next day, a 10 mm biopsy punch was used to create the full-thickness wound on the dorsum of the mice. Immediately afterward bacterial infection at the wound site was initiated by placing a 10 µL droplet containing 10
8 CFU/mL of
S. aureus from an overnight bacterial culture in the stationary phase. Mice were divided into the following groups namely Control wound (10 mm biopsy skin wound), Bacterial wound (skin wound infected with bacteria), Control film (wound infected with bacteria and then treated with control film without TQ), TQ film (wound infected with bacteria and then treated with TQ loaded film), and Gentamicin (wound infected with bacteria and then treated with gentamicin marketed cream formulation). Films with or without TQ were applied at the wound infection site on Day 0, 1, 2, and 3 (only TQ film,
n = 2). Gentamicin was applied similarly to the TQ film. Wounds were covered using Tegaderm film (3M, Saint Paul, MN, USA). At each time point (Day 1, 2, 3, and 7) bacterial samples were collected by taking out the Tegaderm film from the wound site and kept in 2 mL microtube containing PBS. The tubes were then vortexed for 10 s to extract the bacteria. Different bacterial dilutions were made by adding 10 µL of the bacterial solution to the 990 µL of TSB solution and were plated on TSB agar plates. After overnight incubation at 37 °C bacteria were counted. The experiments were continued for 21 days. Wounds were visually monitored for local inflammatory reactions and photographed at Day 0, 3, 7, 10, 14, and 21 days. All the animals were euthanized at the end of day 21. The mice were observed at least once each day for signs of fatigue, stress, and aggressiveness. The mice were weighed at each time point.
2.18. Statistical Analysis
The cumulative amounts of TQ permeated per unit area were plotted against time. The flux was calculated by determination of the slope of the linear portion of the permeation profile. The % wound closure for each time interval was determined by the following formula and were calculated using NIH ImageJ software (NIH, Bethesda, MD, USA):
Results are reported as mean ± SD. The statistical analysis of the data was performed by using one-way analysis of variance (ANOVA) followed by post hoc Tukey HSD test and Student’s t-test. A p-value < 0.05 was considered statistically significant.