Preliminary Study on ?3-Adrenoreceptor as Predictor Marker of Relapse in Ewing Sarcoma Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Human Blood Samples and Biopsies
2.2. Preparation of Blood Samples for Cytofluorimetric Analysis
2.3. Biopsies Samples
2.4. In Vitro Analysis of CD99 Expression after β3-ARs Blockade
2.5. Statistical Analysis
3. Results
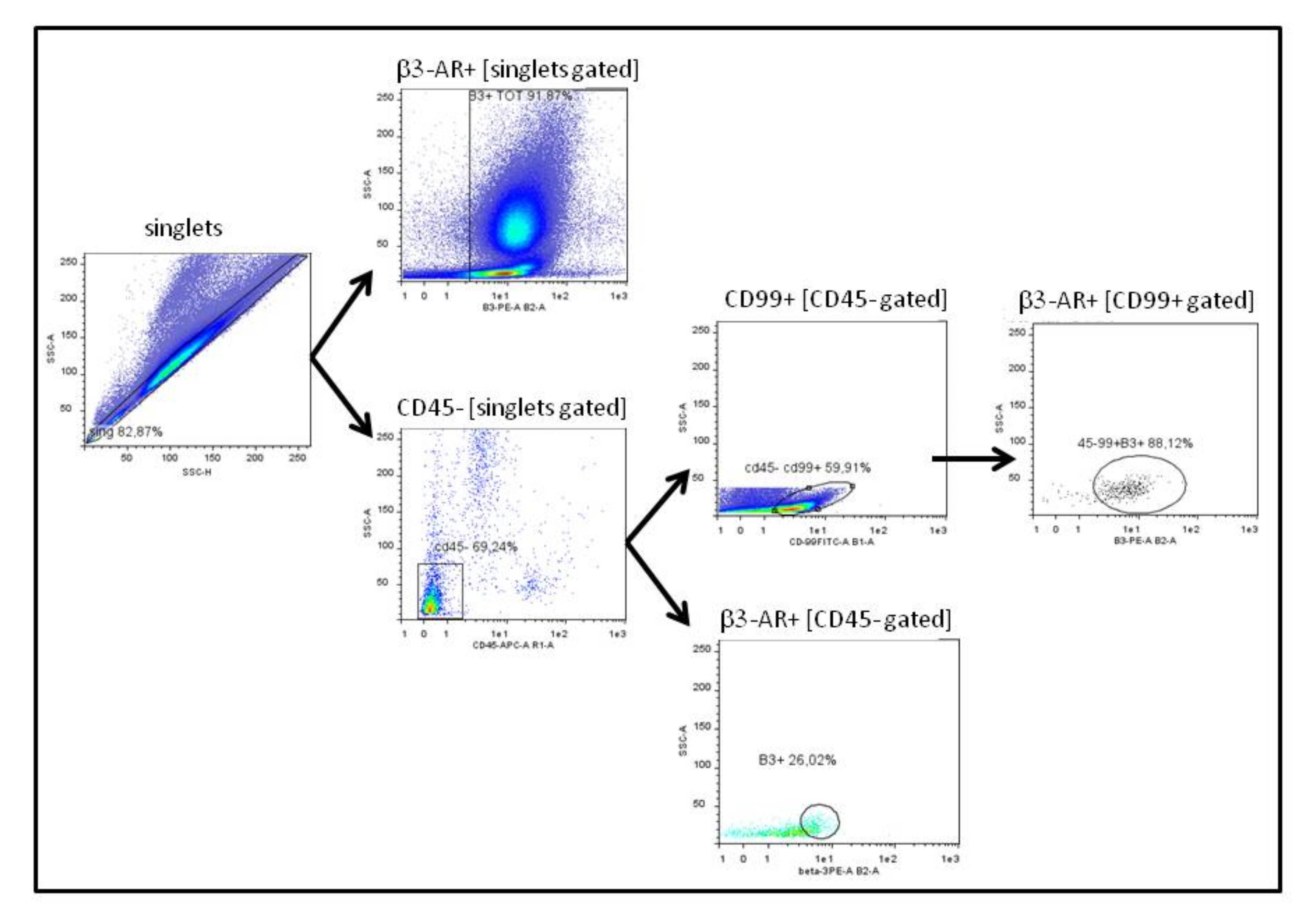
3.1. Analysis of Peripheral Blood Derived Cells
3.2. Analysis of Bone Marrow Derived Cells
3.3. Analysis of Biopsies Derived Cells
3.4. Analysis of CD99 Expression on Ewing Cell Lines after β3-ARs Blockade
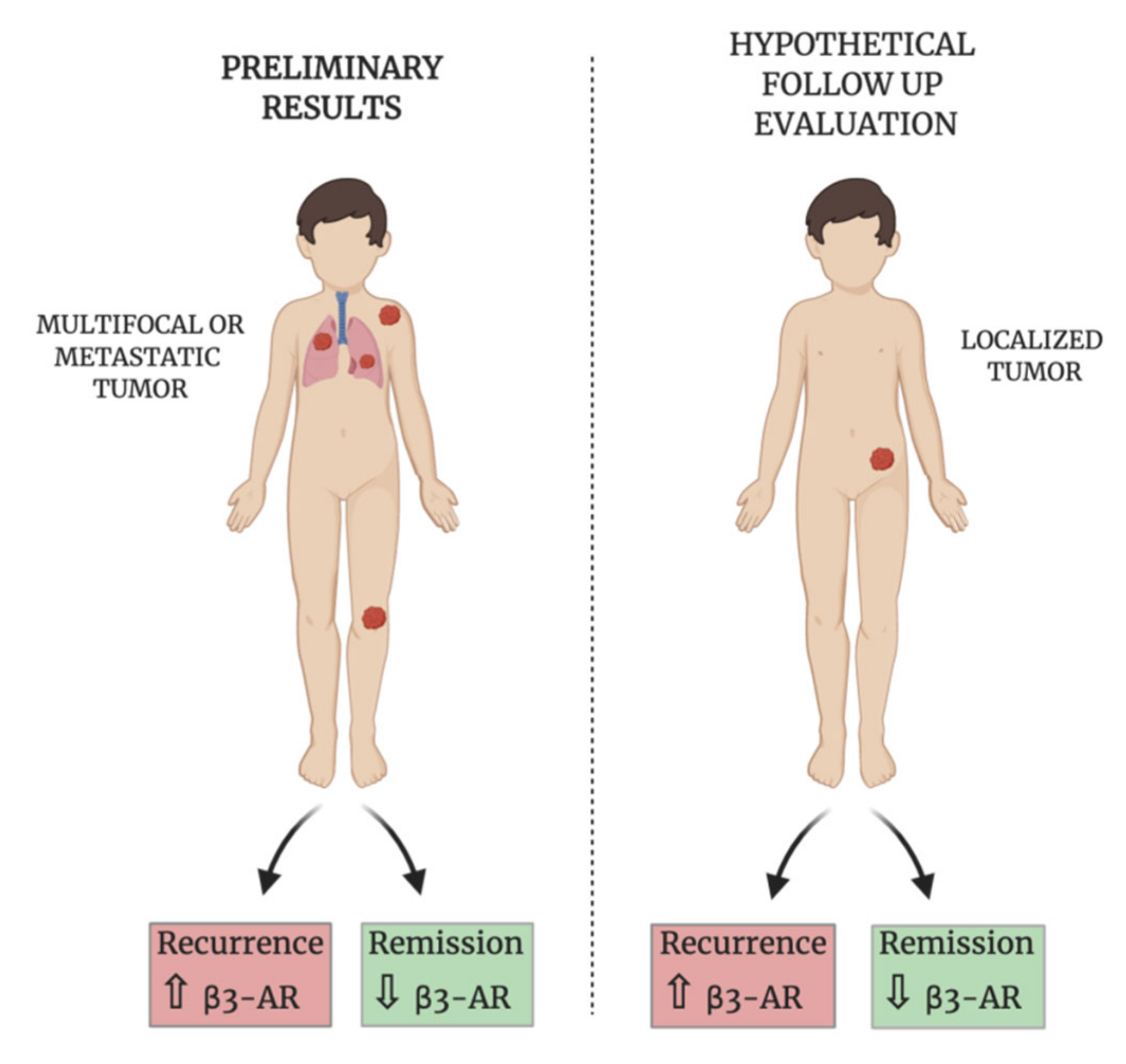
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dasgupta, A.; Trucco, M.; Rainusso, N.; Bernardi, R.J.; Shuck, R.; Kurenbekova, L.; Loeb, D.M.; Yustein, J.T. Metabolic modulation of Ewing sarcoma cells inhibits tumor growth and stem cell properties. Oncotarget 2017, 8, 77292–77308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoang, N.T.; Acevedo, L.A.; Mann, M.J.; Tolani, B. A review of soft-tissue sarcomas: Translation of biological advances into treatment measures. Cancer Manag. Res. 2018, 10, 1089–1114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaspar, N.; Hawkins, D.S.; Dirksen, U.; Lewis, I.J.; Ferrari, S.; Le Deley, M.-C.; Kovar, H.; Grimer, R.J.; Whelan, J.; Claude, L.; et al. Ewing Sarcoma: Current Management and Future Approaches Through Collaboration. J. Clin. Oncol. 2015, 33, 3036–3046. [Google Scholar] [CrossRef] [PubMed]
- Riggi, N.; Stamenkovic, I. The Biology of Ewing sarcoma. Cancer Lett. 2007, 254, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Karski, E.E.; Matthay, K.K.; Neuhaus, J.M.; Goldsby, R.E.; Dubois, S.G. Characteristics and outcomes of patients with Ewing sarcoma over 40 years of age at diagnosis. Cancer Epidemiol. 2013, 37, 29–33. [Google Scholar] [CrossRef] [Green Version]
- Fagioli, F.; Zecca, M.; Locatelli, F.; Lanino, E.; Uderzo, C.; Di Bartolomeo, P.; Berger, M.; Favre, C.; Rondelli, R.; Pession, A.; et al. Allogeneic Stem Cell Transplantation for Children with Acute Myeloid Leukemia in Second Complete Remission. J. Pediatr. Hematol. 2008, 30, 575–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scotlandi, K.; Perdichizzi, S.; Bernard, G.; Nicoletti, G.; Nanni, P.; Lollini, P.-L.; Curti, A.; Manara, M.C.; Benini, S.; Bernard, A.; et al. Targeting CD99 in association with doxorubicin: An effective combined treatment for Ewing’s sarcoma. Eur. J. Cancer 2006, 42, 91–96. [Google Scholar] [CrossRef]
- Jiang, Y.; Ludwig, J.; Janku, F. Targeted therapies for advanced Ewing sarcoma family of tumors. Cancer Treat. Rev. 2015, 41, 391–400. [Google Scholar] [CrossRef] [Green Version]
- Messina, C.; Zecca, M.; Fagioli, F.; Rovelli, A.; Giardino, S.; Merli, P.; Porta, F.; Arico, M.; Sieni, E.; Basso, G.; et al. Outcomes of Children with Hemophagocytic Lymphohistiocytosis Given Allogeneic Hematopoietic Stem Cell Transplantation in Italy. Biol. Blood Marrow Transplant. 2018, 24, 1223–1231. [Google Scholar] [CrossRef] [Green Version]
- Ordóñez, J.L.; Osuna, D.; Herrero, D.; De Álava, E.; Madoz-Gúrpide, J. Advances in Ewing’s Sarcoma Research: Where Are We Now and What Lies Ahead? Cancer Res. 2009, 69, 7140–7150. [Google Scholar] [CrossRef] [Green Version]
- Herrero-Martin, D.; Fourtouna, A.; Niedan, S.; Riedmann, L.T.; Schwentner, R.; Aryee, D.N.T. Factors Affecting EWS-FLI1 Activity in Ewing’s Sarcoma. Sarcoma 2011, 2011, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, E.Y.; Park, W.S.; Jung, K.C.; Kim, S.H.; Kim, Y.Y.; Lee, W.J.; Park, S.H. Engagement of CD99 induces up-regulation of TCR and MHC class I and II molecules on the surface of human thymocytes. J. Immunol. 1998, 161, 749–754. [Google Scholar] [PubMed]
- Pasello, M.; Manara, M.C.; Scotlandi, K. CD99 at the crossroads of physiology and pathology. J. Cell Commun. Signal. 2018, 12, 55–68. [Google Scholar] [CrossRef] [Green Version]
- Benini, S.; Gamberi, G.; Cocchi, S.; Garbetta, J.; Alberti, L.; Righi, A.; Gambarotti, M.; Picci, P.; Ferrari, S. Detection of circulating tumor cells in liquid biopsy from Ewing sarcoma patients. Cancer Manag. Res. 2018, 10, 49–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engell, H.C. Cancer cells in the circulating blood; a clinical study on the occurrence of cancer cells in the peripheral blood and in venous blood draining the tumour area at operation. Acta Chir. Scand. Suppl. 1955, 201, 1–70. [Google Scholar]
- Fagnou, C.; Michon, J.; Peter, M.; Bernoux, A.; Oberlin, O.; Zucker, J.M.; Magdelenat, H.; Delattre, O. Presence of tumor cells in bone marrow but not in blood is associated with adverse prognosis in patients with ewing’s tumor. Sociètè Francaise d’Oncologie Pèdiatrique. J. Clin. Oncol. 1998, 16, 1707–1711. [Google Scholar] [CrossRef]
- Pfleiderer, C.; Zoubek, A.; Gruber, B.; Kronberger, M.; Ambros, P.F.; Lion, T.; Fink, F.-M.; Gadner, H.; Kovar, H. Detection of tumour cells in peripheral blood and bone marrow from ewing tumour patients by rt-pcr. Int. J. Cancer 1995, 64, 135–139. [Google Scholar] [CrossRef]
- Schleiermacher, G.; Peter, M.; Oberlin, O.; Philip, T.; Rubie, H.; Mechinaud, F.; Sommelet-Olive, D.; Landman-Parker, J.; Bours, D.; Michon, J.; et al. Increased Risk of Systemic Relapses Associated with Bone Marrow Micrometastasis and Circulating Tumor Cells in Localized Ewing Tumor. J. Clin. Oncol. 2003, 21, 85–91. [Google Scholar] [CrossRef]
- Przybyl, J.; Kozak, K.; Kosela, H.; Falkowski, S.; Switaj, T.; Lugowska, I.; Szumera-Ciećkiewicz, A.; Ptaszynski, K.; Grygalewicz, B.; Chechlinska, M.; et al. Gene expression profiling of peripheral blood cells: New insights into Ewing sarcoma biology and clinical applications. Med. Oncol. 2014, 31, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Pasha, A.; Vignoli, M.; Subbiani, A.; Nocentini, A.; Selleri, S.; Gratteri, P.; Dabraio, A.; Casini, T.; Filippi, L.; Fotzi, I.; et al. β3-Adrenoreceptor Activity Limits Apigenin Efficacy in Ewing Sarcoma Cells: A Dual Approach to Prevent Cell Survival. Int. J. Mol. Sci. 2019, 20, 2149. [Google Scholar] [CrossRef] [Green Version]
- Bartness, T.J.; Vaughan, C.H.; Song, C.K. Sympathetic and sensory innervation of brown adipose tissue. Int. J. Obes. 2010, 34, S36–S42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calvani, M.; Cavallini, L.; Tondo, A.; Spinelli, V.; Ricci, L.; Pasha, A.; Bruno, G.; Buonvicino, D.; Bigagli, E.; Vignoli, M.; et al. β3-Adrenoreceptors Control Mitochondrial Dormancy in Melanoma and Embryonic Stem Cells. Oxidative Med. Cell. Longev. 2018, 2018, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monte, M.D.; Calvani, M.; Cammalleri, M.; Favre, C.; Filippi, L.; Bagnoli, P. β-Adrenoceptors as drug targets in melanoma: Novel preclinical evidence for a role of β 3 -adrenoceptors. Br. J. Pharmacol. 2018, 176, 2496–2508. [Google Scholar] [CrossRef] [PubMed]
- Calvani, M.; Pelon, F.; Comito, G.; Taddei, M.L.; Moretti, S.; Innocenti, S.; Nassini, R.; Gerlini, G.; Borgognoni, L.; Bambi, F.; et al. Norepinephrine promotes tumor microenvironment reactivity through β3-adrenoreceptors during melanoma progression. Oncotarget 2014, 6, 4615–4632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monte, M.D.; Casini, G.; Filippi, L.; Nicchia, G.P.; Svelto, M.; Bagnoli, P. Functional involvement of β3-adrenergic receptors in melanoma growth and vascularization. J. Mol. Med. 2013, 91, 1407–1419. [Google Scholar] [CrossRef]
- Montoya, A.; Amaya, C.N.; Belmont, A.; Diab, N.; Trevino, R.; Villanueva, G.; Rains, S.; Sanchez, L.A.; Badri, N.; Otoukesh, S.; et al. Use of non-selective β-blockers is associated with decreased tumor proliferative indices in early stage breast cancer. Oncotarget 2016, 8, 6446–6460. [Google Scholar] [CrossRef] [Green Version]
- Watkins, J.L.; Thaker, P.H.; Nick, A.M.; Ramondetta, L.M.; Kumar, S.; Ms, D.L.U.; Matsuo, K.; Squires, K.C.; Coleman, R.L.; Lutgendorf, S.K.; et al. Clinical impact of selective and nonselective beta-blockers on survival in patients with ovarian cancer. Cancer 2015, 121, 3444–3451. [Google Scholar] [CrossRef]
- Hwa, Y.L.; Shi, Q.; Kumar, S.K.; Lacy, M.Q.; Gertz, M.A.; Kapoor, P.; Buadi, F.K.; Leung, N.; Dingli, D.; Go, R.; et al. Beta-blockers improve survival outcomes in patients with multiple myeloma: A retrospective evaluation. Am. J. Hematol. 2016, 92, 50–55. [Google Scholar] [CrossRef]
- Calvani, M.; Bruno, G.; Monte, M.D.; Nassini, R.; Fontani, F.; Casini, A.; Cavallini, L.; Becatti, M.; Bianchini, F.; De Logu, F.; et al. β 3 -Adrenoceptor as a potential immuno-suppressor agent in melanoma. Br. J. Pharmacol. 2019, 176, 2509–2524. [Google Scholar] [CrossRef]
- Bruno, G.; Cencetti, F.; Pini, A.; Tondo, A.; Cuzzubbo, D.; Fontani, F.; Strinna, V.; Buccoliero, A.M.; Casazza, G.; Donati, C.; et al. β3-adrenorecepto blockade reduces tumor growth and increases neuronal differentiation in neuroblastoma via SK2/S1P2 modulation. Oncogene 2019, 39, 368–384. [Google Scholar] [CrossRef] [Green Version]
- Suresh, R.; Ali, S.; Ahmad, A.; Philip, P.A.; Sarkar, F.H. The Role of Cancer Stem Cells in Recurrent and Drug-Resistant Lung Cancer. Adv. Exper. Med. Biol. 2015, 890, 57–74. [Google Scholar] [CrossRef]
- Doherty, M.R.; Smigiel, J.M.; Junk, D.J.; Jackson, M.W. Cancer Stem Cell Plasticity Drives Therapeutic Resistance. Cancers 2016, 8, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbott, A. Cancer: The root of the problem. Nature 2006, 442, 742–743. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, C.; Leitz, M.R.; Oberdorf-Maass, S.; Lohse, M.J.; Klotz, K.N. Comparative pharmacology of human beta-adrenergic receptor subtypes—Characterization of stably transfected receptors in cho cells. Naunyn Schmiedebergs Arch. Pharmacol. 2004, 369, 151–159. [Google Scholar] [CrossRef]
- Baker, J.G. The selectivity of _-adrenoceptor agonists at human 1-, 2- and 3-adrenoceptors. Br. J. Pharmacol. 2010, 160, 1048–1061. [Google Scholar] [CrossRef] [Green Version]
- Sato, M.; Horinouchi, T.; Hutchinson, D.S.; Evans, B.A.; Summers, R.J. Ligand-Directed Signaling at the β3-Adrenoceptor Produced by 3-(2-Ethylphenoxy)-1-[(1,S)-1,2,3,4-tetrahydronapth-1-ylamino]-2S-2-propanol oxalate (SR59230A) Relative to Receptor Agonists. Mol. Pharmacol. 2007, 72, 1359–1368. [Google Scholar] [CrossRef]
- Gold, B.; Cankovic, M.; Furtado, L.V.; Meier, F.; Gocke, C.D. Do circulating tumor cells, exosomes, and circulating tumor nucleic acids have clinical utility? A report of the association for molecular pathology. J. Mol. Diagn. 2015, 17, 209–224. [Google Scholar] [CrossRef] [Green Version]
- Allard, W.J. Tumor Cells Circulate in the Peripheral Blood of All Major Carcinomas but not in Healthy Subjects or Patients With Nonmalignant Diseases. Clin. Cancer Res. 2004, 10, 6897–6904. [Google Scholar] [CrossRef] [Green Version]
- Bailly, R.A.; Bosselut, R.; Zucman-Rossi, J.; Cormier, F.; Delattre, O.; Roussel, M.F.; Thomas, G.; Ghysdael, J. DNA-binding and transcriptional activation properties of the EWS-FLI-1 fusion protein resulting from the t (11;22) translocation in Ewing sarcoma. Mol. Cell. Biol. 1994, 14, 3230–3241. [Google Scholar] [CrossRef] [Green Version]
- Kovar, H.; Aryee, D.; Zoubek, A. The Ewing family of tumors and the search for the Achilles’ heel. Curr. Opin. Oncol. 1999, 11, 275–284. [Google Scholar] [CrossRef]
- Hahm, K.-B.; Cho, K.; Lee, C.; Im, Y.-H.; Chang, J.; Choi, S.-G.; Sorensen, P.H.; Thiele, C.J.; Kim, S.-J. Repression of the gene encoding the TGF-β type II receptor is a major target of the EWS-FLI1 oncoprotein. Nat. Genet. 1999, 23, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, G.; Jain, S. Chemotherapy in Ewing’s sarcoma. Indian J. Orthop. 2010, 44, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Lowell, B.B.; Flier, J.S. Brown Adipose Tissue, β3-Adrenergic Receptors, and Obesity. Annu. Rev. Med. 1997, 48, 307–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calvani, M.; Vignoli, M.; Beltrami, G.; Pasha, A.; Scalini, P.; Mannurita, S.C.; Cardellicchio, S.; Coccoli, L.; Cecchi, C.; De Marco, E.; et al. Preliminary Study on ?3-Adrenoreceptor as Predictor Marker of Relapse in Ewing Sarcoma Patients. Biomedicines 2020, 8, 413. https://doi.org/10.3390/biomedicines8100413
Calvani M, Vignoli M, Beltrami G, Pasha A, Scalini P, Mannurita SC, Cardellicchio S, Coccoli L, Cecchi C, De Marco E, et al. Preliminary Study on ?3-Adrenoreceptor as Predictor Marker of Relapse in Ewing Sarcoma Patients. Biomedicines. 2020; 8(10):413. https://doi.org/10.3390/biomedicines8100413
Chicago/Turabian StyleCalvani, Maura, Marina Vignoli, Giovanni Beltrami, Amada Pasha, Perla Scalini, Sara Ciullini Mannurita, Stefania Cardellicchio, Luca Coccoli, Cecilia Cecchi, Emanuela De Marco, and et al. 2020. "Preliminary Study on ?3-Adrenoreceptor as Predictor Marker of Relapse in Ewing Sarcoma Patients" Biomedicines 8, no. 10: 413. https://doi.org/10.3390/biomedicines8100413
APA StyleCalvani, M., Vignoli, M., Beltrami, G., Pasha, A., Scalini, P., Mannurita, S. C., Cardellicchio, S., Coccoli, L., Cecchi, C., De Marco, E., Luti, L., Bernasconi, S., Filippi, L., Casazza, G., Tamburini, A., & Favre, C. (2020). Preliminary Study on ?3-Adrenoreceptor as Predictor Marker of Relapse in Ewing Sarcoma Patients. Biomedicines, 8(10), 413. https://doi.org/10.3390/biomedicines8100413





