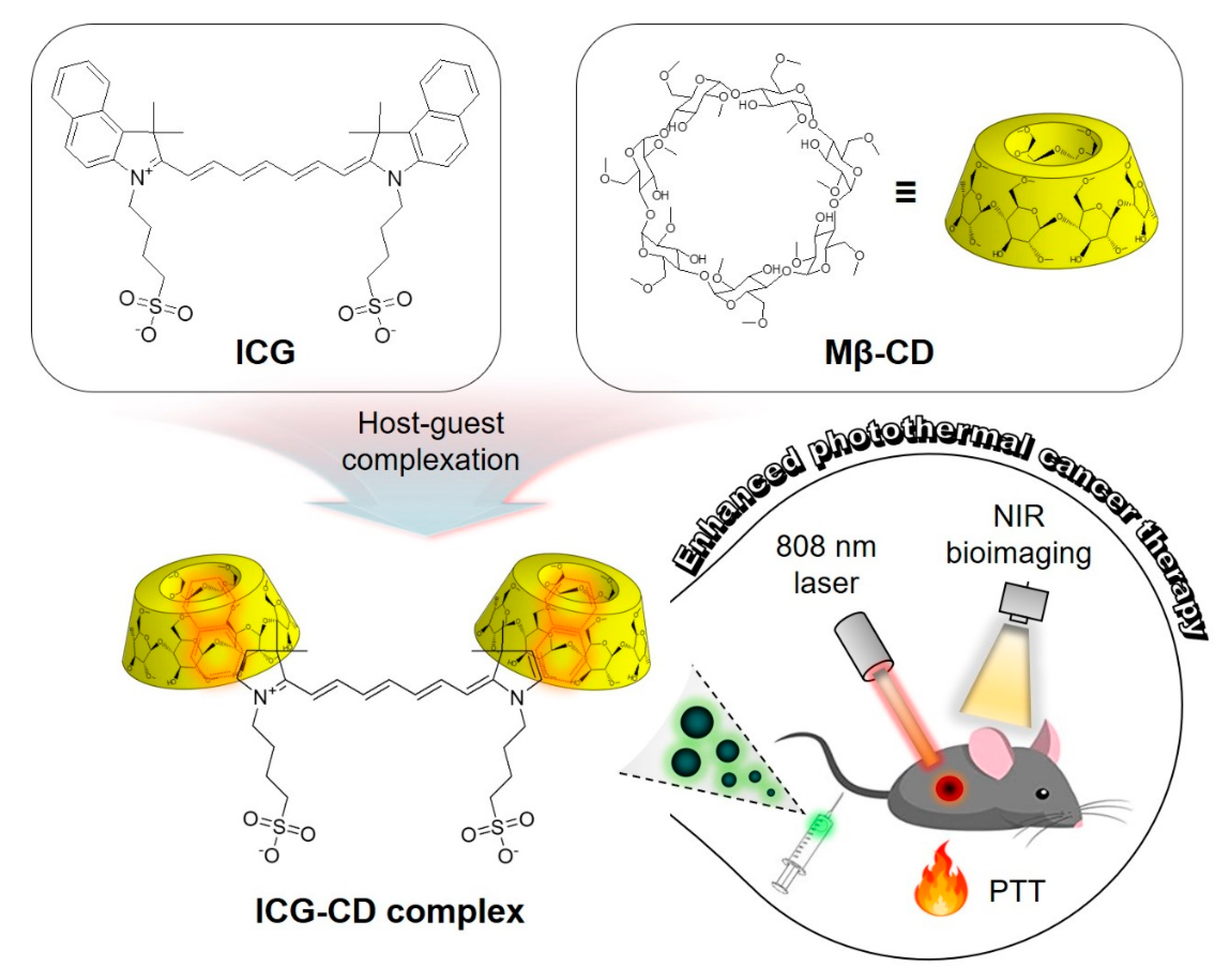
Indocyanine Green and Methyl-?-Cyclodextrin Complex for Enhanced Photothermal Cancer Therapy
Abstract
:1. Introduction
2. Experimental Section
2.1. Preparation of ICG-CD Complex
2.2. Analysis of Optical Properties
2.3. HT-29 Xenograft Mouse Model
2.4. In Vivo Tumor Imaging
2.5. Assessment of In Vivo Photothermal Effect
2.6. Statistical Analysis
2.7. Histological Analysis
3. Results and Discussion
3.1. Preparation and Characterization of the ICG-CD Complex
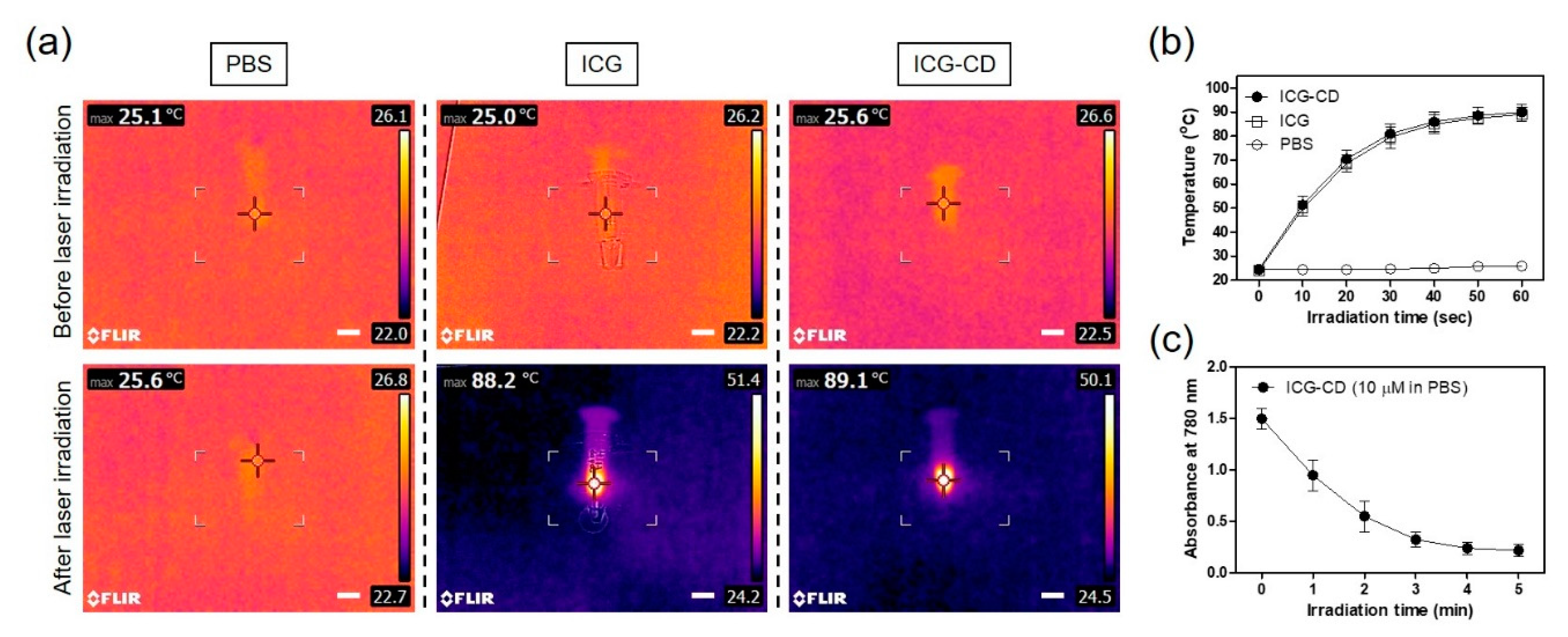
3.2. Assessment of In Vitro Photothermal Effect
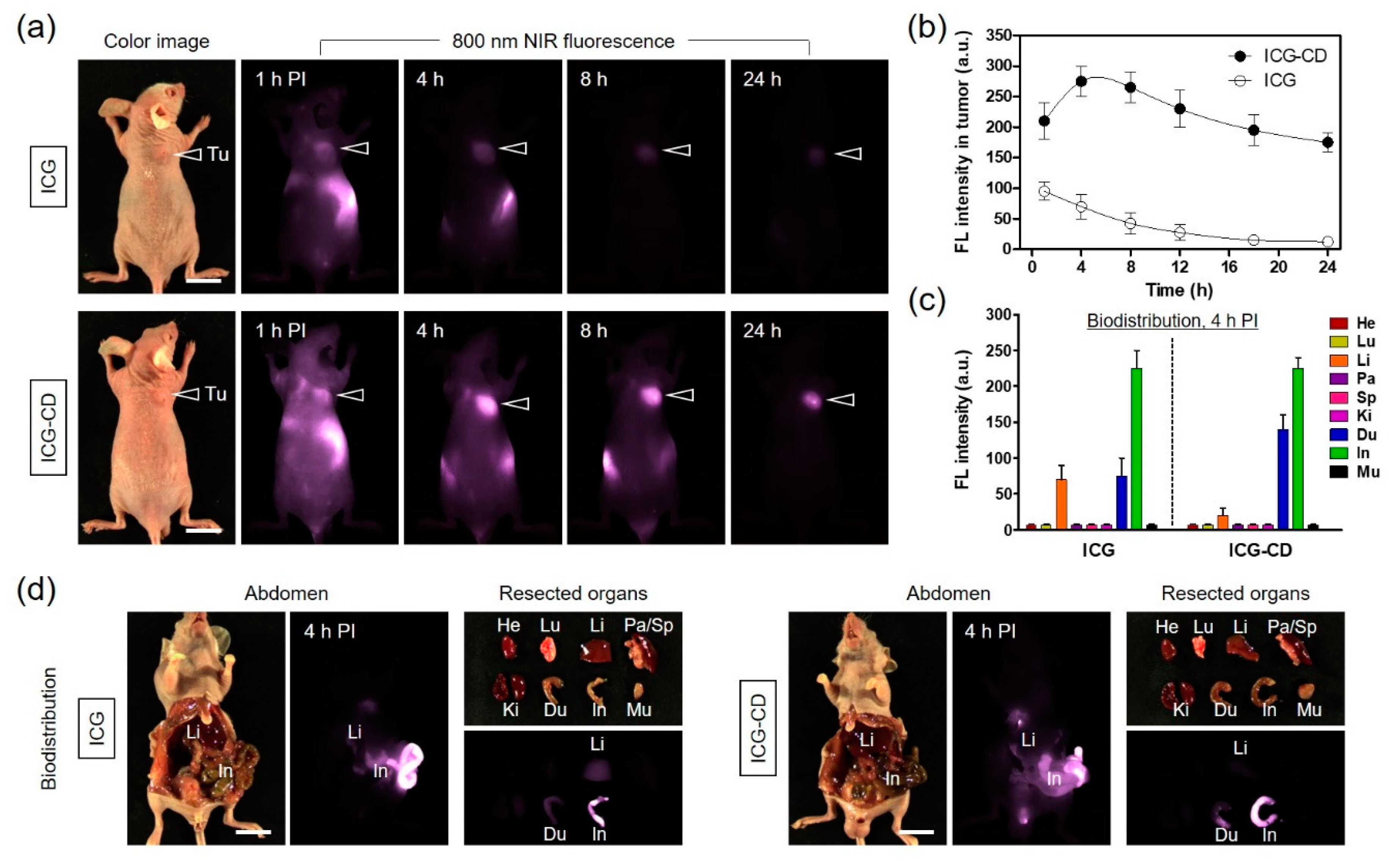
3.3. In Vivo NIR Fluorescence Imaging for Tumor Targetability
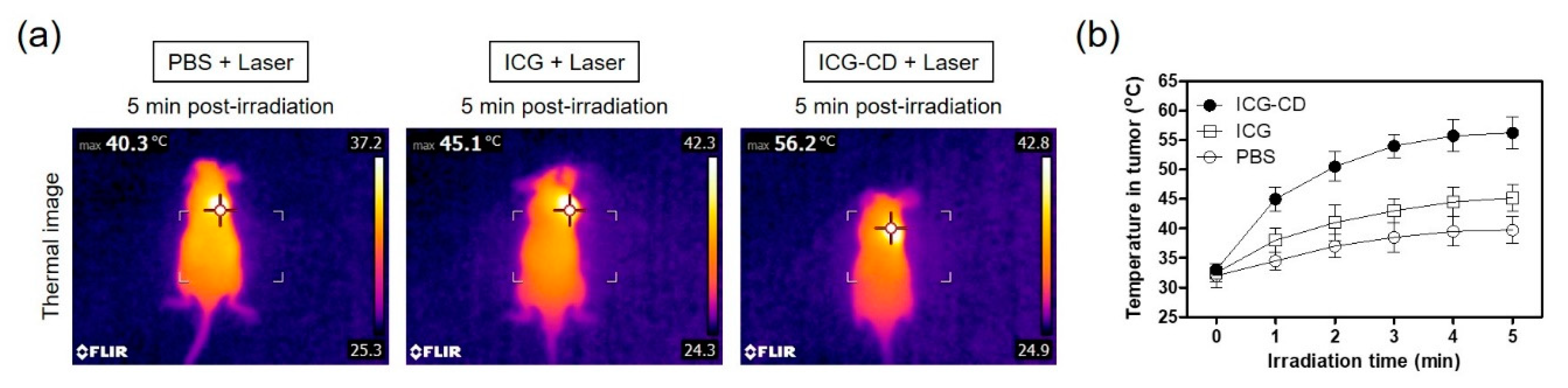
3.4. Assessment of In Vivo Photothermal Effect
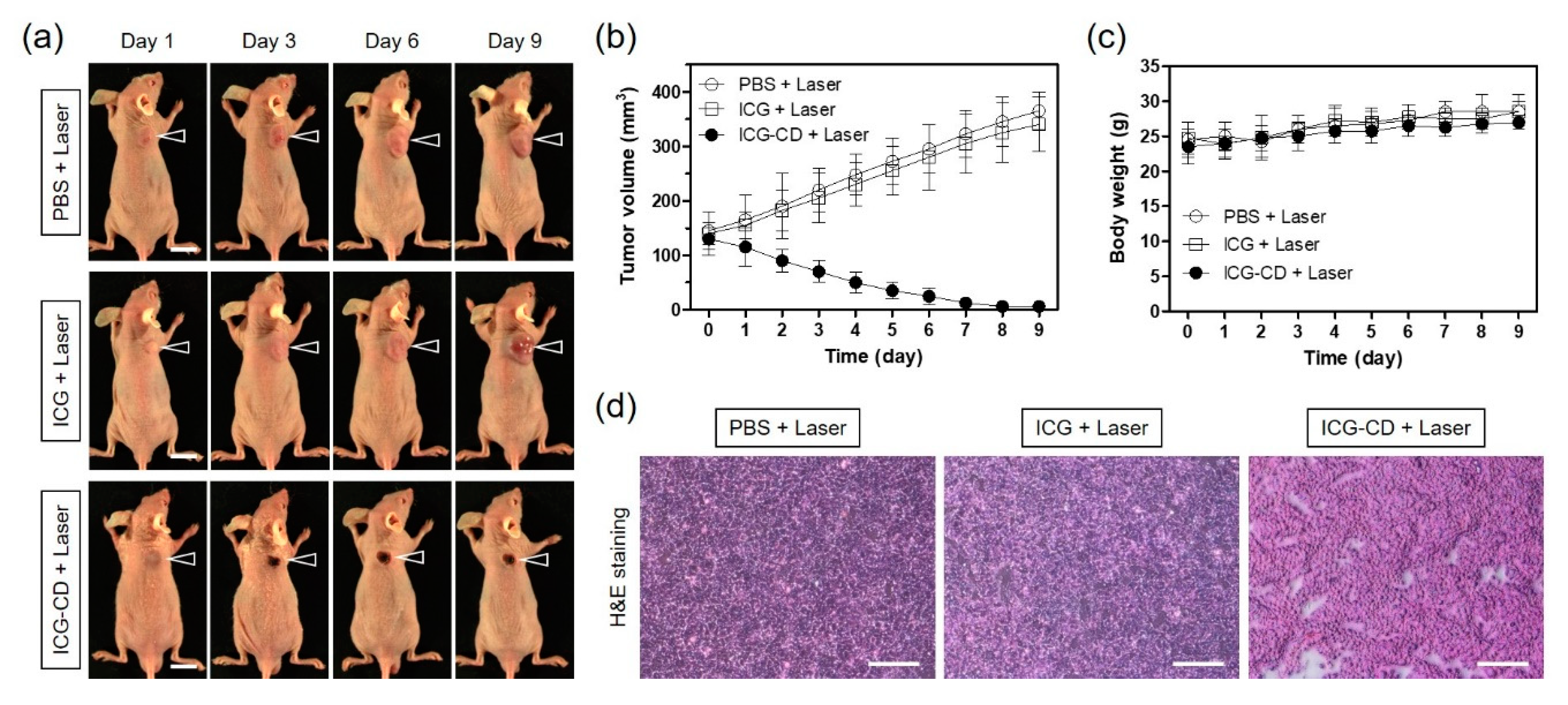
3.5. In Vivo PTT Efficacy
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhu, H.; Cheng, P.; Chen, P.; Pu, K. Recent progress in the development of near-infrared organic photothermal and photodynamic nanotherapeutics. Biomater. Sci. 2018, 6, 746–765. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Pu, K. Development of organic semiconducting materials for deep-tissue optical imaging, phototherapy and photoactivation. Chem. Soc. Rev. 2019, 48, 38–71. [Google Scholar] [CrossRef]
- Cho, M.H.; Li, Y.; Lo, P.; Lee, H.; Choi, Y. Fucoidan-based theranostic nanogel for enhancing imaging and photodynamic therapy of cancer. Nano-Micro Lett. 2020, 12, 47. [Google Scholar] [CrossRef] [Green Version]
- Yoo, Y.; Jo, G.; Jung, J.S.; Yang, D.H.; Hyun, H. Multivalent sorbitol probes for near-infrared photothermal cancer therapy. Part. Part. Syst. Charact. 2020, 37, 1900490. [Google Scholar] [CrossRef]
- Lim, W.; Jo, G.; Kim, E.J.; Cho, H.; Park, M.H.; Hyun, H. Zwitterionic near-infrared fluorophore for targeted photothermal cancer therapy. J. Mater. Chem. B 2020, 8, 2589–2597. [Google Scholar] [CrossRef] [PubMed]
- Park, M.H.; Jo, G.; Kim, E.J.; Jung, J.S.; Hyun, H. Tumor-targeted near-infrared fluorophore for fluorescence-guided phototherapy. Chem. Commun. 2020, 56, 4180–4183. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Jo, G.; Jung, J.S.; Yang, D.H.; Hyun, H. Near-infra-red fluorescent chitosan oligosaccharide lactate for targeted cancer imaging and photothermal therapy. Artif. Cells Nanomed. Biotechnol. 2020, 48, 1144–1152. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Jung, J.S.; Jo, G.; Yang, D.H.; Koh, Y.S.; Hyun, H. Near-infrared fluorescent sorbitol probe for targeted photothermal cancer therapy. Cancers 2019, 11, 1286. [Google Scholar] [CrossRef] [Green Version]
- Feng, B.; Niu, Z.; Hou, B.; Zhou, L.; Li, Y.; Yu, H. Enhancing triple negative breast cancer immunotherapy by ICG-templated self-assembly of paclitaxel nanoparticles. Adv. Funct. Mater. 2020, 30, 1906605. [Google Scholar] [CrossRef]
- Quan, Y.H.; Oh, C.H.; Jung, D.; Lim, J.Y.; Choi, B.H.; Rho, J.; Choi, Y.; Han, K.N.; Kim, B.M.; Kim, C.; et al. Evaluation of intraoperative near-infrared fluorescence visualization of the lung tumor margin with indocyanine green inhalation. JAMA Surg. 2020, 155, 732–740. [Google Scholar] [CrossRef]
- Yu, S.; Cheng, B.B.; Yao, T.F.; Xu, C.C.; Nguyen, K.T.; Hong, Y.; Yuan, B.H. New generation ICG-based contrast agents for ultrasound-switchable fluorescence imaging. Sci. Rep. 2016, 6, 35942. [Google Scholar] [CrossRef] [PubMed]
- Shan, W.J.; Chen, R.H.; Zhang, Q.; Zhao, J.; Chen, B.B.; Zhou, X.; Ye, S.F.; Bi, S.L.; Nie, L.M.; Ren, L. Improved stable indocyanine green (ICG)-mediated cancer optotheranostics with naturalized hepatitis B core particles. Adv. Mater. 2018, 30, 1707567. [Google Scholar] [CrossRef] [PubMed]
- Kirchherr, A.K.; Briel, A.; Mäder, K. Stabilization of indocyanine green by encapsulation within micellar systems. Mol. Pharm. 2009, 6, 480–491. [Google Scholar] [CrossRef]
- Liu, R.; Yao, T.; Liu, Y.; Yu, S.; Ren, L.; Hong, Y.; Nguyen, K.T.; Yuan, B. Temperature-sensitive polymeric nanogels encapsulating with β-cyclodextrin and ICG complex for high-resolution deep-tissue ultrasound-switchable fluorescence imaging. Nano Res. 2020, 13, 1100–1110. [Google Scholar] [CrossRef]
- An, F.; Yang, Z.; Zheng, M.; Mei, T.; Deng, G.; Guo, P.; Li, Y.; Sheng, R. Rationally assembled albumin/indocyanine green nanocomplex for enhanced tumor imaging to guide photothermal therapy. J. Nanobiotechnol. 2020, 18, 49. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Li, S.; Wang, J.; Chu, C.; Zhang, Y.; Pang, X.; Lv, P.; Wang, X.; Zhao, Q.; Chen, J.; et al. A single-step multi-level supramolecular system for cancer sonotheranostics. Nanoscale Horiz. 2019, 4, 190–195. [Google Scholar] [CrossRef]
- Guerrini, L.; Hartsuiker, L.; Manohar, S.; Otto, C. Monomer adsorption of indocyanine green to gold nanoparticles. Nanoscale 2011, 3, 4247–4253. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Meng, L.; Sun, C.; Ye, W.; Chen, C.; Du, B. A “protective umbrella” nanoplatform for loading ICG and multi-modal imaging-guided phototherapy. Nanomedicine 2018, 14, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Kurahashi, T.; Iwatsuki, K.; Onishi, T.; Arai, T.; Teranishi, K.; Hirata, H. Near-infrared indocyanine dye permits real-time characterization of both venous and lymphatic circulation. J. Biomed. Opt. 2016, 21, 086009. [Google Scholar] [CrossRef]
- Szente, L.; Szejtli, J. Highly soluble cyclodextrin derivatives: Chemistry, properties, and trends in development. Adv. Drug Deliv. Rev. 1999, 36, 17–28. [Google Scholar] [CrossRef]
- Barros, T.C.; Toma, S.H.; Toma, H.E.; Bastos, E.L.; Baptista, M.S. Polymethine cyanine dyes in β-cyclodextrin solution: Multiple equilibria and chemical oxidation. J. Phys. Org. Chem. 2010, 23, 893–903. [Google Scholar] [CrossRef]
- Onodera, R.; Motoyama, K.; Okamatsu, A.; Higashi, T.; Arima, H. Potential use of folate-appended methyl-β-cyclodextrin as an anticancer agent. Sci. Rep. 2013, 3, 1104. [Google Scholar] [CrossRef] [Green Version]
- Loftsson, T.; Brewster, M.E. Pharmaceutical applications of cyclodextrins: Basic science and product development. J. Pharm. Pharmacol. 2010, 62, 1607–1621. [Google Scholar] [CrossRef] [PubMed]
- Grosse, P.Y.; Bressolle, F.; Pinguet, F. Antiproliferative effect of methyl-b-cyclodextrin in vitro and in human tumour xenografted athymic nude mice. Br. J. Cancer 1998, 78, 1165–1169. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jo, G.; Lee, B.Y.; Kim, E.J.; Park, M.H.; Hyun, H. Indocyanine Green and Methyl-?-Cyclodextrin Complex for Enhanced Photothermal Cancer Therapy. Biomedicines 2020, 8, 476. https://doi.org/10.3390/biomedicines8110476
Jo G, Lee BY, Kim EJ, Park MH, Hyun H. Indocyanine Green and Methyl-?-Cyclodextrin Complex for Enhanced Photothermal Cancer Therapy. Biomedicines. 2020; 8(11):476. https://doi.org/10.3390/biomedicines8110476
Chicago/Turabian StyleJo, Gayoung, Bo Young Lee, Eun Jeong Kim, Min Ho Park, and Hoon Hyun. 2020. "Indocyanine Green and Methyl-?-Cyclodextrin Complex for Enhanced Photothermal Cancer Therapy" Biomedicines 8, no. 11: 476. https://doi.org/10.3390/biomedicines8110476
APA StyleJo, G., Lee, B. Y., Kim, E. J., Park, M. H., & Hyun, H. (2020). Indocyanine Green and Methyl-?-Cyclodextrin Complex for Enhanced Photothermal Cancer Therapy. Biomedicines, 8(11), 476. https://doi.org/10.3390/biomedicines8110476




