Silymarin Inhibits Glutamate Release and Prevents against Kainic Acid-Induced Excitotoxic Injury in Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Reagents
2.2. Animals
2.3. Synaptosomes
2.4. Glutamate Release
2.5. Intrasynaptosomal Ca2+ Concentration
2.6. Synaptosomal Plasma Membrane Potential
2.7. Animal Procedures and Histological Analyses
2.8. Determination of Glutamate by High-Performance Liquid Chromatography
2.9. Western Blot
2.10. Statistical Analyses
3. Results
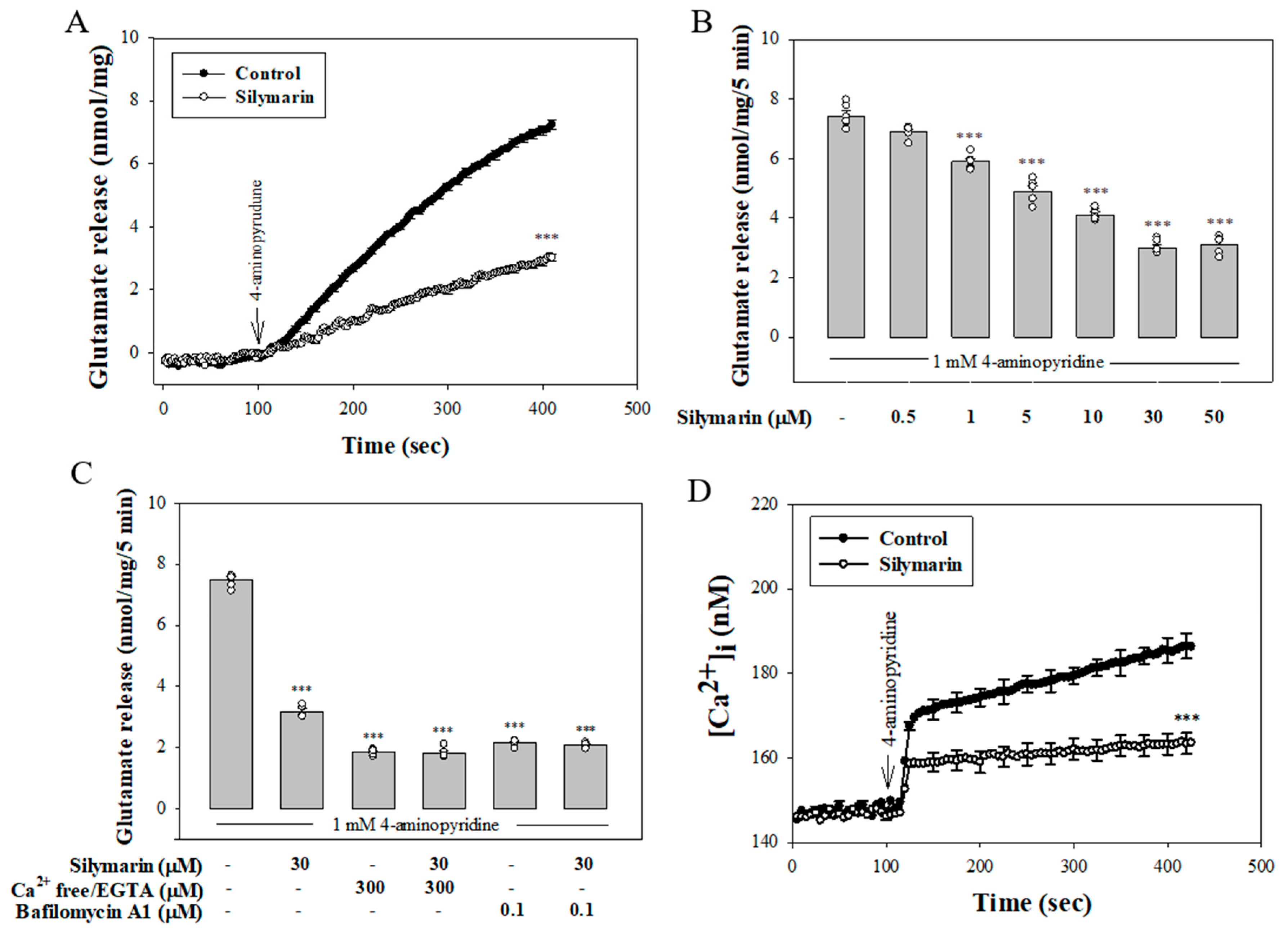
3.1. Silymarin Decreases 4-Aminopyridine-Evoked Glutamate Release from Rat Cerebrocortical Synaptosomes by Reducing Exocytotic Mechanism
3.2. Reduced Ca2+ Influx through N- and P/Q-Type Ca2+ Channels Is Involved in the Silymarin Effect
3.3. Silymarin Does Not Affect Synaptosomal Membrane Potential
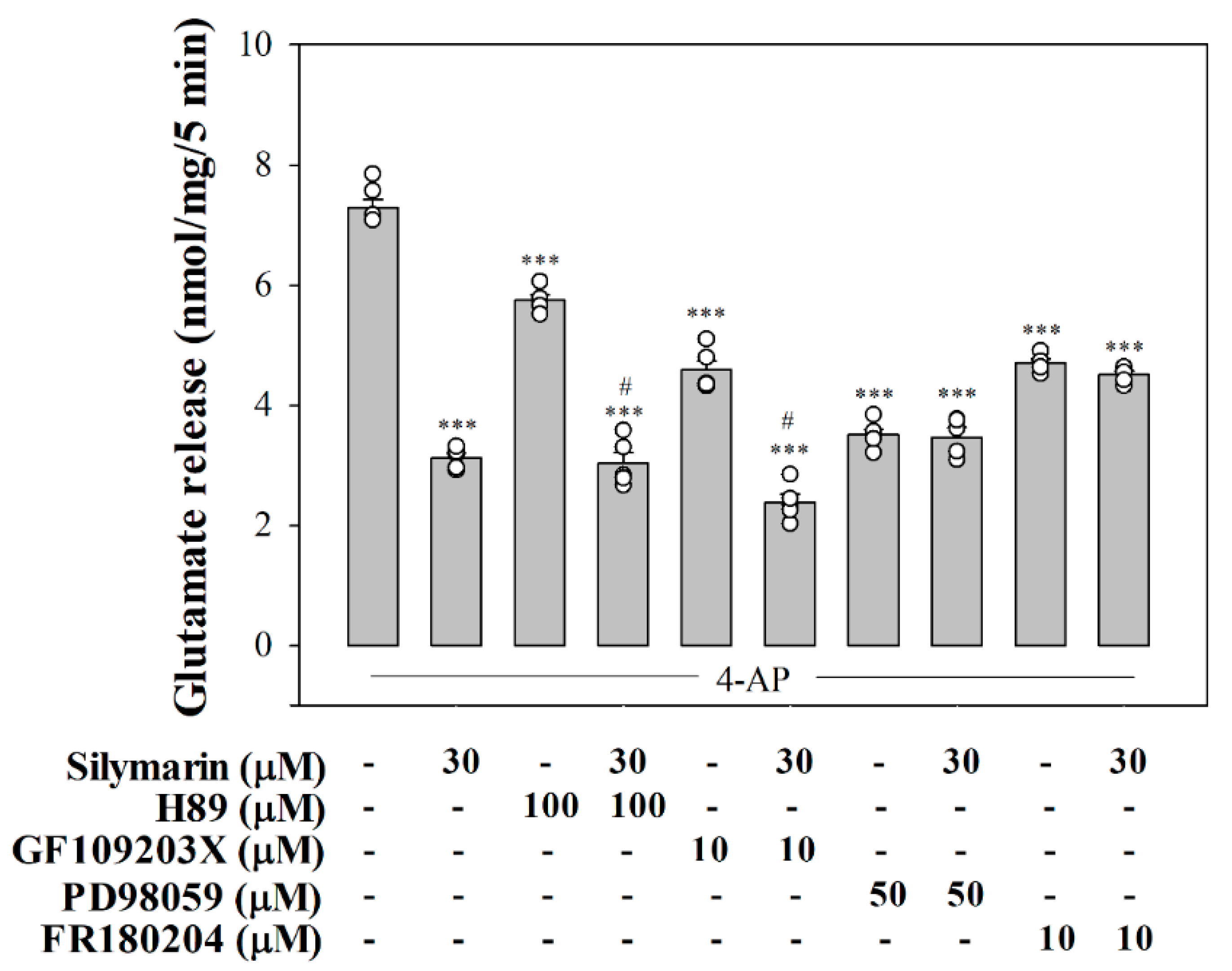
3.4. Suppressed MAPK Pathway Is Involved in Silymarin-Mediated Inhibition of Glutamate Release
3.5. Silymarin Attenuats Seizures and Glutamate Concentration Elevation in KA-Treated Rats
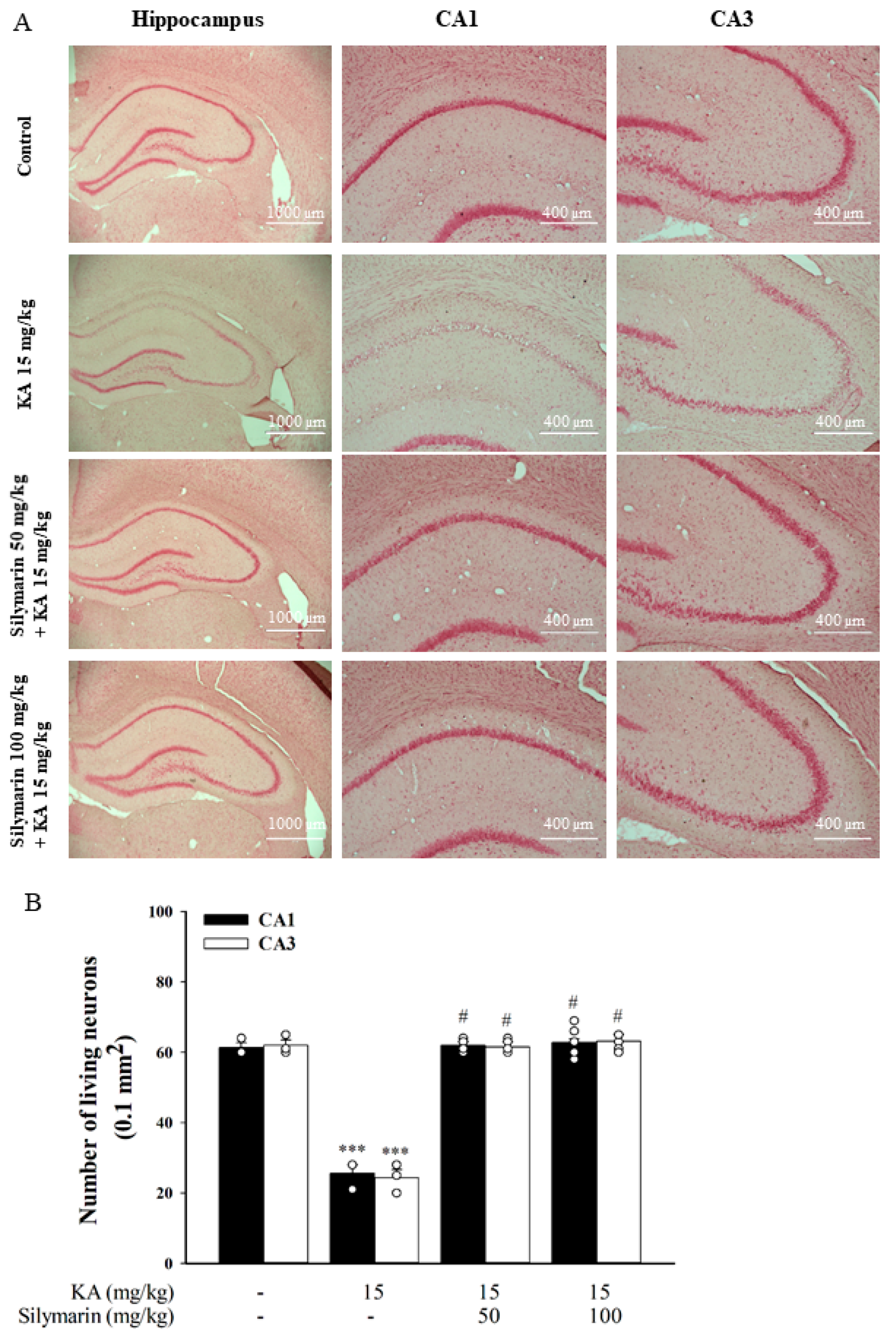
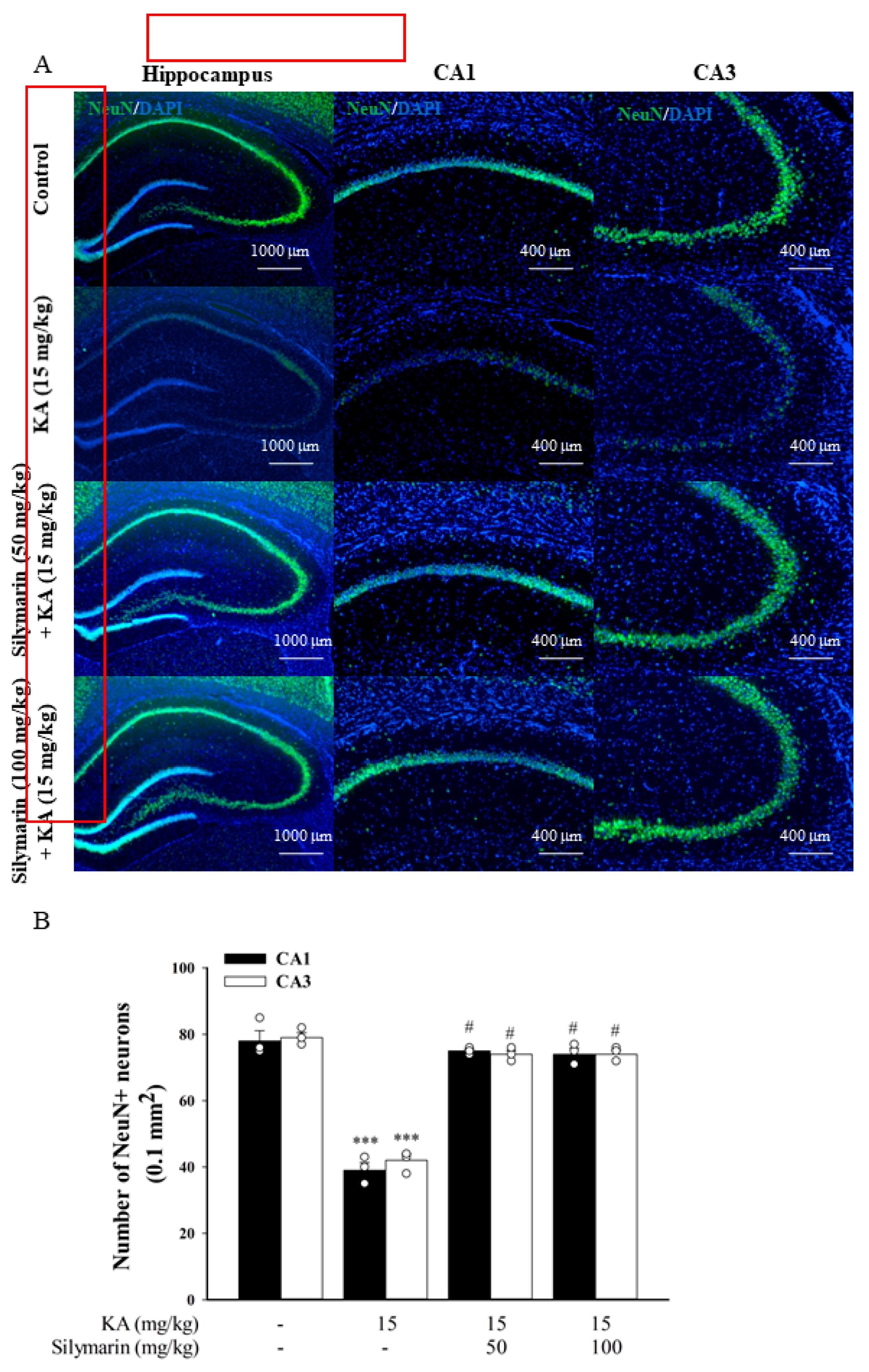
3.6. Silymarin Attenuats Hippocampal Neuronal Damage in KA-Treated Rats
3.7. Silymarin Attenuates Microglia and Astrocyte Activations in KA-Treated Hippocampus
3.8. Silymarin Inhibits HSP70 Expression and Increases Akt Phosphorylation in the Hippocampus of KA-Treated Rats
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Meldrum, B.S. Glutamate as a Neurotransmitter in the Brain: Review of Physiology and Pathology. J. Nutr. 2000, 130, 1007S–1015S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.; Danbolt, N.C. Glutamate as a neurotransmitter in the healthy brain. J. Neural Transm. (Vienna) 2014, 121, 799–817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, D.W. Glutamate neurotoxicity and diseases of the nervous system. Neuron 1988, 1, 623–634. [Google Scholar] [CrossRef]
- Lewerenz, J.; Maher, P. Chronic glutamate toxicity in neurodegenerative diseases-what is the evidence? Front. Neurosci. 2015, 9, 469. [Google Scholar] [CrossRef]
- Burd, I.; Welling, J.; Kannan, G.; Johnston, M.V. Excitotoxicity as a common mechanism for fetal neuronal injury with hypoxia and intrauterine inflammation. Adv. Pharmacol. 2016, 76, 85–101. [Google Scholar]
- Lazarevic, V.; Yang, Y.; Ivanova, D.; Fejtova, A.; Svenningsson, P. Riluzole attenuates the efficacy of glutamatergic transmission by interfering with the size of the readily releasable neurotransmitter pool. Neuropharmacology 2018, 143, 38–48. [Google Scholar] [CrossRef]
- Lazarevic, V.; Mantas, I.; Flais, I.; Svenningsson, P. Fluoxetine suppresses glutamate- and GABA-mediated neurotransmission by altering SNARE complex. Int. J. Mol. Sci. 2019, 20, 4247. [Google Scholar] [CrossRef] [Green Version]
- Chiu, K.M.; Lin, T.Y.; Lee, M.Y.; Lu, C.W.; Wang, M.J.; Wang, S.J. Dexmedetomidine protects neurons from kainic acid-induced excitotoxicity by activating BDNF signaling. Neurochem. Int. 2019, 129, 104493. [Google Scholar] [CrossRef]
- Rehman, M.U.; Wali, A.F.; Ahmad, A.; Shakeel, S.; Rasool, S.; Ali, R.; Rashid, S.M.; Madkhali, H.; Ganaie, M.A.; Khan, R. Neuroprotective strategies for neurological disorders by natural products: An update. Curr. Neuropharmacol. 2019, 17, 247–267. [Google Scholar] [CrossRef]
- Soleimani, V.; Delghandi, P.S.; Moallem, S.A.; Karimi, G. Safety and toxicity of silymarin, the major constituent of milk thistle extract: An updated review. Phytother. Res. 2019, 33, 1627–1638. [Google Scholar] [CrossRef]
- Surai, P.F. Silymarin as a natural antioxidant: An overview of the current evidence and perspectives. Antioxidants 2015, 4, 204–247. [Google Scholar] [CrossRef] [Green Version]
- Vargas-Mendoza, N.; Morales-González, Á.; Morales-Martínez, M.; Soriano-Ursúa, M.A.; Delgado-Olivares, L.; Sandoval-Gallegos, E.M.; Madrigal-Bujaidar, E.; Álvarez-González, I.; Madrigal-Santillán, E.; Morales-Gonzalez, J.A. Flavolignans from Silymarin as Nrf2 bioactivators and their therapeutic applications. Biomedicines 2020, 8, 122. [Google Scholar] [CrossRef]
- Bakhshaee, M.; Jabbari, F.; Hoseini, S.; Farid, R.; Sadeghian, M.H.; Rajati, M.; Mohamadpoor, A.H.; Movahhed, R.; Zamani, M.A. Effect of silymarin in the treatment of allergic rhinitis. Otolaryngol. Head Neck Surg. 2011, 145, 904–909. [Google Scholar] [CrossRef]
- Forghani, P.; Khorramizadeh, M.R.; Waller, E.K. Silibinin inhibits accumulation of myeloid-derived suppressor cells and tumor growth of murine breast cancer. Cancer Med. 2014, 3, 215–224. [Google Scholar] [CrossRef]
- Lovelace, E.S.; Maurice, N.J.; Miller, H.W.; Slichter, C.K.; Harrington, R.; Magaret, A.; Prlic, M.; De Rosa, S.; Polyak, S.J. Silymarin suppresses basal and stimulus-induced activation, exhaustion, differentiation, and inflammatory markers in primary human immune cells. PLoS ONE 2017, 12, e0171139. [Google Scholar] [CrossRef]
- Stolf, A.M.; Cardoso, C.C.; Acco, A. Effects of silymarin on diabetes mellitus complications: A review. Phytother. Res. 2017, 31, 366–374. [Google Scholar] [CrossRef]
- Nencini, C.; Giorgi, G.; Micheli, L. Protective effect of silymarin on oxidative stress in rat brain. Phytomedicine 2007, 14, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Raza, S.S.; Khan, M.M.; Ashafaq, M.; Ahmad, A.; Khuwaja, G.; Khan, A.; Siddiqui, M.S.; Safhi, M.M.; Islam, F. Silymarin protects neurons from oxidative stress associated damages in focal cerebral ischemia: A behavioral, biochemical and immunohistological study in Wistar rats. J. Neurol. Sci. 2011, 309, 45–54. [Google Scholar] [CrossRef]
- Pérez, H.J.; Carrillo, S.C.; García, E.; Ruiz-Mar, G.; Pérez-Tamayo, R.; Chavarría, A. Neuroprotective effect of silymarin in a MPTP mouse model of Parkinson’s disease. Toxicology 2014, 319, 38–43. [Google Scholar] [CrossRef]
- Hirayama, K.; Oshima, H.; Yamashita, A.; Sakatani, K.; Yoshino, A.; Katayama, Y. Neuroprotective effects of silymarin on ischemia-induced delayed neuronal cell death in rat hippocampus. Brain Res. 2016, 1646, 297–303. [Google Scholar] [CrossRef]
- Shen, L.; Liu, L.; Li, X.Y.; Ji, H.F. Regulation of gut microbiota in Alzheimer’s disease mice by silibinin and silymarin and their pharmacological implications. Appl. Microbiol. Biotechnol. 2019, 103, 7141–7149. [Google Scholar] [CrossRef]
- Wang, Q.; Yu, S.; Simonyi, A.; Sun, G.Y.; Sun, A.Y. Kainic acid-mediated excitotoxicity as a model for neurodegeneration. Mol. Neurobiol. 2005, 31, 3–16. [Google Scholar] [CrossRef]
- Raiteri, L.; Giovedì, S.; Benfenati, F.; Raiteri, M.; Bonanno, G. Cellular mechanisms of the acute increase of glutamate release induced by nerve growth factor in rat cerebral cortex. Neuropharmacology 2003, 44, 390–402. [Google Scholar] [CrossRef]
- Lu, C.W.; Lin, T.Y.; Huang, S.K.; Wang, S.J. Echinacoside inhibits glutamate release by suppressing voltage-dependent Ca2+ entry and protein kinase C in rat cerebrocortical nerve terminals. Int. J. Mol. Sci. 2016, 17, 1006. [Google Scholar] [CrossRef] [Green Version]
- Zappettini, S.; Grilli, M.; Salamone, A.; Fedele, E.; Marchi, M. Pre-synaptic nicotinic receptors evoke endogenous glutamate and aspartate release from hippocampal synaptosomes by way of distinct coupling mechanisms. Br. J. Pharmacol. 2010, 161, 1161–1171. [Google Scholar] [CrossRef] [Green Version]
- Nicholls, D.G.; Sihra, T.S. Synaptosomes possess an exocytotic pool of glutamate. Nature 1986, 321, 772–773. [Google Scholar] [CrossRef]
- Lin, T.Y.; Lu, C.W.; Wu, C.C.; Huang, S.K.; Wang, S.J. Palmitoylethanolamide inhibits glutamate release in rat cerebrocortical nerve terminals. Int. J. Mol. Sci. 2015, 16, 5555–5571. [Google Scholar] [CrossRef] [Green Version]
- Grynkiewicz, G.; Poenie, M.; Tsien, R.Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 1985, 260, 3440–3450. [Google Scholar]
- Lin, K.C.; Wang, C.C.; Wang, S.J. Bupropion attenuates kainic acid-induced seizures and neuronal cell death in rat hippocampus. Prog. Neuropsychopharmacol. Biol. Psychiatry 2013, 45, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Ho, Y.H.; Hung, C.F.; Kuo, J.R.; Wang, S.J. Xanthohumol, an active constituent from hope, affords protection against kainic acid-induced excitotoxicity in rats. Neurochem. Int. 2020, 133, 104629. [Google Scholar] [CrossRef]
- Berridge, M.J. Neuronal calcium signaling. Neuron 1998, 21, 13–26. [Google Scholar] [CrossRef] [Green Version]
- Millán, C.; Sánchez-Prieto, J. Differential coupling of N- and P/Q-type calcium channels to glutamate exocytosis in the rat cerebral cortex. Neurosci. Lett. 2002, 330, 29–32. [Google Scholar] [CrossRef]
- Nicholls, D.G. Presynaptic modulation of glutamate release. Prog. Brain Res. 1998, 116, 15–22. [Google Scholar]
- Jeon, B.T.; Lee, D.H.; Kim, K.H.; Kim, H.J.; Kang, S.S.; Cho, G.J.; Choi, W.S.; Roh, G.S. Ketogenic diet attenuates kainic acid-induced hippocampal cell death by decreasing AMPK/ACC pathway activity and HSP70. Neurosci. Lett. 2009, 453, 49–53. [Google Scholar] [CrossRef]
- Miltiadous, P.; Stamatakis, A.; Stylianopoulou, F. Neuroprotective effects of IGF-I following kainic acid-induced hippocampal degeneration in the rat. Cell. Mol. Neurobiol. 2010, 30, 347–360. [Google Scholar] [CrossRef]
- Takeda, A.; Itoh, H.; Tamano, H.; Yuzurihara, M.; Oku, N. Suppressive effect of Yokukansan on excessive release of glutamate and aspartate in the hippocampus of zinc-deficient rats. Nutr. Neurosci. 2008, 11, 41–46. [Google Scholar] [CrossRef]
- Duan, Y.; Wang, Z.; Zhang, H.; He, Y.; Fan, R.; Cheng, Y.; Sun, G.; Sun, X. Extremely low frequency electromagnetic field exposure causes cognitive impairment associated with alteration of the glutamate level, MAPK pathway activation and decreased CREB phosphorylation in mice hippocampus: Reversal by procyanidins extracted from the lotus seedpod. Food Funct. 2014, 5, 2289–2297. [Google Scholar]
- Nicholls, D.G.; Sihra, T.S.; Sanchez-Prieto, J. Calcium-dependent and -independent release of glutamate from synaptosomes monitored by continuous fluorometry. J. Neurochem. 1987, 49, 50–57. [Google Scholar] [CrossRef]
- McMahon, H.T.; Nicholls, D.G. Transmitter glutamate release from isolated nerve terminals: Evidence for biphasic release and triggering by localized Ca2+. J. Neurochem. 1991, 56, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, E.; Sánchez-Prieto, J. Presynaptic modulation of glutamate release targets different calcium channels in rat cerebrocortical nerve terminals. Eur. J. Neurosci. 1997, 9, 2009–2018. [Google Scholar] [CrossRef]
- Barrie, A.P.; Nicholls, D.G.; Sanchez-Prieto, J.; Sihra, T.S. An ion channel locus for the protein kinase C potentiation of transmitter glutamate release from guinea pig cerebrocortical synaptosomes. J. Neurochem. 1991, 57, 1398–1404. [Google Scholar] [CrossRef]
- Dickinson, J.A.; Kew, J.N.; Wonnacott, S. Presynaptic alpha 7- and beta 2-containing nicotinic acetylcholine receptors modulate excitatory amino acid release from rat prefrontal cortex nerve terminals via distinct cellular mechanisms. Mol. Pharmacol. 2008, 74, 348–359. [Google Scholar] [CrossRef] [Green Version]
- Lin, T.Y.; Lu, C.W.; Huang, S.K.; Wang, S.J. Curcumin inhibits glutamate release from rat prefrontal nerve endings by affecting vesicle mobilization. Int. J. Mol. Sci. 2012, 13, 9097–9109. [Google Scholar] [CrossRef] [Green Version]
- Jovanovic, J.N.; Benfenati, F.; Siow, Y.L.; Sihra, T.S.; Sanghera, J.S.; Pelech, S.L.; Greengard, P.; Czernik, A.J. Neurotrophins stimulate phosphorylation of synapsin I by MAP kinase and regulate synapsin I-actin interactions. Proc. Natl. Acad. Sci. USA 1996, 93, 3679–3683. [Google Scholar] [CrossRef] [Green Version]
- Fdez, E.; Hilfiker, S. Vesicle pools and synapsins: New insights into old enigmas. Brain Cell Biol. 2006, 35, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Chi, P.; Greengard, P.; Ryan, T.A. Synaptic vesicle mobilization is regulated by distinct synapsin I phosphorylation pathways at different frequencies. Neuron 2003, 38, 69–78. [Google Scholar] [CrossRef] [Green Version]
- Devi, K.P.; Malar, D.S.; Braidy, N.; Nabavi, S.M.; Nabavi, S.F. A Mini Review on the Chemistry and Neuroprotective Effects of Silymarin. Curr. Drug Targets 2017, 18, 1529–1536. [Google Scholar] [CrossRef]
- Śmiałowska, M.; Gołembiowska, K.; Kajta, M.; Zięba, B.; Dziubina, A.; Domin, H. Selective mGluR1 antagonist EMQMCM inhibits the kainate-induced excitotoxicity in primary neuronal cultures and in the rat hippocampus. Neurotox. Res. 2012, 21, 379–392. [Google Scholar] [CrossRef] [Green Version]
- Ben-Ari, Y. Limbic seizure and brain damage produced by kainic acid: Mechanisms and relevance to human temporal lobe epilepsy. Neuroscience 1985, 14, 375–403. [Google Scholar] [CrossRef]
- Sperk, G. Kainic acid seizures in the rat. Prog. Neurobiol. 1994, 42, 1–32. [Google Scholar] [CrossRef]
- Domin, H.; Zięba, B.; Gołembiowska, K.; Kowalska, M.; Dziubina, A.; Śmiałowska, M. Neuroprotective potential of mGluR5 antagonist MTEP: Effects on kainate-induced excitotoxicity in the rat hippocampus. Pharmacol. Rep. 2010, 62, 1051–1061. [Google Scholar] [CrossRef]
- Kim, D.H.; Yoon, B.H.; Jung, W.Y.; Kim, J.M.; Park, S.J.; Park, D.H.; Huh, Y.; Park, C.; Cheong, J.H.; Lee, K.T.; et al. Sinapic acid attenuates kainic acid-induced hippocampal neuronal damage in mice. Neuropharmacology 2010, 59, 20–30. [Google Scholar] [CrossRef]
- Kim, S.; Jung, U.J.; Oh, Y.S.; Jeon, M.T.; Kim, H.J.; Shin, W.H.; Hong, J.; Kim, S.R. Beneficial effects of silibinin against kainic acid-induced neurotoxicity in the hippocampus in vivo. Exp. Neurobiol. 2017, 26, 266–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, E.J.; Koh, Y.H.; Kim, A.Y.; Nah, S.Y.; Jeong, J.H.; Chae, J.S.; Kim, S.C.; Yen, T.P.; Yoon, H.J.; Kim, W.K.; et al. Ginsenosides attenuate kainic acid-induced synaptosomal oxidative stress via stimulation of adenosine A(2A) receptors in rat hippocampus. Behav. Brain Res. 2009, 197, 239–245. [Google Scholar] [CrossRef]
- Haddadi, R.; Mohajjel Nayebi, A.; Brooshghalan, S.E. Pre-treatment with silymarin reduces brain myeloperoxidase activity and inflammatory cytokines in 6-OHDA hemi-parkinsonian rats. Neurosci. Lett. 2013, 555, 106–111. [Google Scholar] [CrossRef]
- Aboelwafa, H.R.; El-Kott, A.F.; Abd-Ella, E.M.; Yousef, H.N. The possible neuroprotective effect of silymarin against aluminum chloride-prompted Alzheimer’s-like disease in Rats. Brain Sci. 2020, 10, 68. [Google Scholar] [CrossRef]
- Yang, T.; Hsu, C.; Liao, W.; Chuang, J.S. Heat shock protein 70 expression in epilepsy suggests stress rather than protection. Acta Neuropathol. 2008, 115, 219–230. [Google Scholar] [CrossRef]
- Hu, F.; Zhou, J.; Lu, Y.; Guan, L.; Wei, N.N.; Tang, Y.Q.; Wang, K. Inhibition of hsp70 suppresses neuronal hyperexcitability and attenuates epilepsy by enhancing A-Type potassium current. Cell Rep. 2019, 26, 168–181. [Google Scholar] [CrossRef] [Green Version]
- Tsuchiya, D.; Hong, S.; Matsumori, Y.; Kayama, T.; Swanson, R.A.; Dillman, W.H.; Liu, J.; Panter, S.S.; Weinstein, P.R. Overexpression of rat heat shock protein 70 reduces neuronal injury after transient focal ischemia, transient global ischemia, or kainic acid-induced seizures. Neurosurgery 2003, 53, 1179–1187. [Google Scholar] [CrossRef]
- Chang, C.C.; Chen, S.D.; Lin, T.K.; Chang, W.N.; Liou, C.W.; Chang, A.Y.; Chan, S.H.; Chuang, Y.C. Heat shock protein 70 protects against seizure-induced neuronal cell death in the hippocampus following experimental status epilepticus via inhibition of nuclear factor-κB activation-induced nitric oxide synthase II expression. Neurobiol. Dis. 2014, 62, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Qu, Y.; Tang, J.; Chen, D.; Fu, X.; Mao, M.; Mu, D. PI3K/Akt signaling pathway is required for neuroprotection of thalidomide on hypoxic-ischemic cortical neurons in vitro. Brain Res. 2010, 1357, 157–165. [Google Scholar] [CrossRef]
- Zhang, Q.; Shen, M.; Ding, M.; Shen, D.; Ding, F. The neuroprotective action of pyrroloquinoline quinone against glutamate-induced apoptosis in hippocampal neurons is mediated through the activation of PI3K/Akt pathway. Toxicol. Appl. Pharmacol. 2011, 252, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Chun, W.; Kong, P.J.; Han, J.A.; Cho, B.P.; Kwon, O.Y.; Lee, H.J.; Kim, S.S. Sustained activation of Akt by melatonin contributes to the protection against kainic acid-induced neuronal death in hippocampus. J. Pineal Res. 2006, 40, 79–85. [Google Scholar] [CrossRef]
- Bhowmik, M.; Khanam, R.; Saini, N.; Vohora, D. Activation of AKT/GSK3β pathway by TDZD-8 attenuates kainic acid induced neurodegeneration but not seizures in mice. Neurotoxicology 2015, 46, 44–52. [Google Scholar] [CrossRef]
- Lu, C.W.; Hsieh, H.L.; Lin, T.Y.; Hsieh, T.Y.; Huang, S.K.; Wang, S.J. Echinacoside, an active constituent of Cistanche Herba, exerts a neuroprotective effect in a kainic acid rat model by inhibiting inflammatory processes and activating the Akt/GSK3β pathway. Biol. Pharm. Bull. 2018, 41, 1685–1693. [Google Scholar] [CrossRef] [Green Version]
- Borah, A.; Paul, R.; Choudhury, S.; Choudhury, A.; Bhuyan, B.; Das Talukdar, A.; Dutta Choudhury, M.; Mohanakumar, K.P. Neuroprotective potential of silymarin against CNS disorders: Insight into the pathways and molecular mechanisms of action. CNS Neurosci. Ther. 2013, 19, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Ullah, H.; Khan, H. Anti-Parkinson Potential of Silymarin: Mechanistic Insight and Therapeutic Standing. Front. Pharmacol. 2018, 9, 422. [Google Scholar] [CrossRef] [Green Version]
- Velussi, M.; Cernigoi, A.M.; De Monte, A.; Dapas, F.; Caffau, C.; Zilli, M. Long-term (12 months) treatment with an anti-oxidant drug (silymarin) is effective on hyperinsulinemia, exogenous insulin need and malondialdehyde levels in cirrhotic diabetic patients. J. Hepatol. 1997, 26, 871–879. [Google Scholar] [CrossRef]
- Valentová, K.; Vidlář, A.; Zatloukalová, M.; Stuchlík, M.; Vacek, J.; Šimánek, V.; Ulrichová, J. Biosafety and antioxidant effects of a beverage containing silymarin and arginine. A pilot, human intervention cross-over trial. Food Chem. Toxicol. 2013, 56, 178–183. [Google Scholar] [CrossRef]
- Yuan, R.; Fan, H.; Cheng, S.; Gao, W.; Xu, X.; Lv, S.; Ye, M.; Wu, M.; Zhu, X.; Zhang, Y. Silymarin prevents NLRP3 inflammasome activation and protects against intracerebral hemorrhage. Biomed. Pharmacother. 2017, 93, 308–315. [Google Scholar] [CrossRef]
- Gupta, S.; Gupta, Y.K. Combination of Zizyphus jujuba and silymarin showed better neuroprotective effect as compared to single agent in MCAo-induced focal cerebral ischemia in rats. J. Ethnopharmacol. 2017, 197, 118–127. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, C.-W.; Lin, T.-Y.; Chiu, K.-M.; Lee, M.-Y.; Huang, J.-H.; Wang, S.-J. Silymarin Inhibits Glutamate Release and Prevents against Kainic Acid-Induced Excitotoxic Injury in Rats. Biomedicines 2020, 8, 486. https://doi.org/10.3390/biomedicines8110486
Lu C-W, Lin T-Y, Chiu K-M, Lee M-Y, Huang J-H, Wang S-J. Silymarin Inhibits Glutamate Release and Prevents against Kainic Acid-Induced Excitotoxic Injury in Rats. Biomedicines. 2020; 8(11):486. https://doi.org/10.3390/biomedicines8110486
Chicago/Turabian StyleLu, Cheng-Wei, Tzu-Yu Lin, Kuan-Ming Chiu, Ming-Yi Lee, Jih-Hsin Huang, and Su-Jane Wang. 2020. "Silymarin Inhibits Glutamate Release and Prevents against Kainic Acid-Induced Excitotoxic Injury in Rats" Biomedicines 8, no. 11: 486. https://doi.org/10.3390/biomedicines8110486




