Anti-MAP Triple Therapy Supports Immunomodulatory Therapeutic Response in Crohn’s Disease through Downregulation of NF-κB Activation in the Absence of MAP Detection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Infection and Treatment of Monocyte-Derived Macrophages
2.2. Measurement of MAP Viability in Infected Macrophages
2.3. Measurement of NF-κB p65 Transcription Factor Activation in Macrophages
2.4. Measurement of iNOS, CD206, TNF-α, IL-6, and IL-10 Expression in Treated Macrophages
2.5. Measurement of TNF-α, IL-6, and Il-10 Protein Expression in Treated Macrophages
2.6. Caco-2 Cell Culture and Treatment
2.7. Trypan Blue Exclusion Assay in Caco-2 Monolayers
2.8. Measuring LDH Activity in Caco-2 Monolayers
2.9. Measuring Reduced GSH Levels in Caco-2 Monolayers
2.10. DHE Fluorescence Staining Assay
2.11. T-Cell Proliferation Assay Following Antibiotic Treatment
3. Statistical Analysis
4. Results
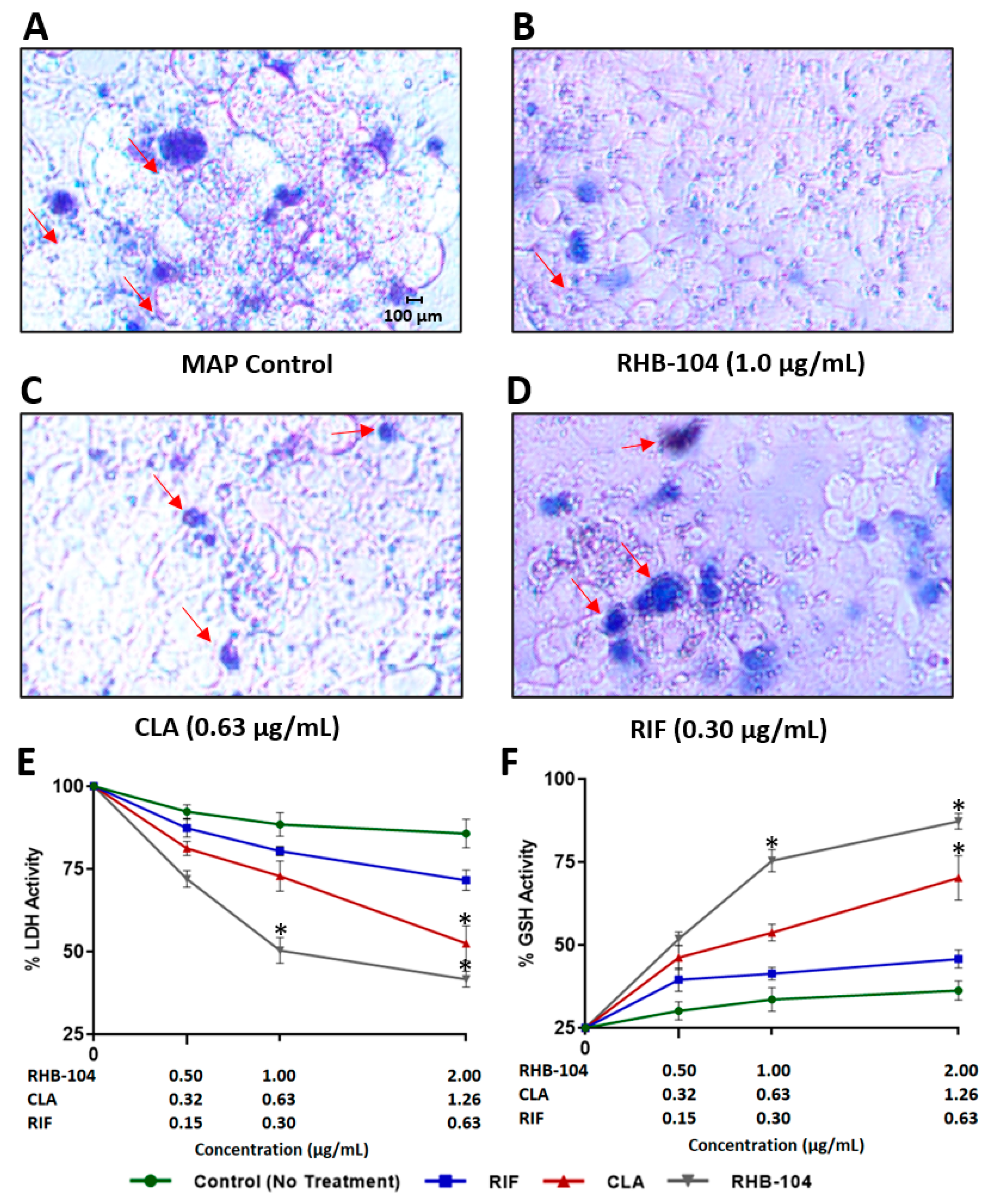
4.1. RHB-104 Decreases MAP Survival in Infected Macrophages at Lower Concentrations than CLA and RIF
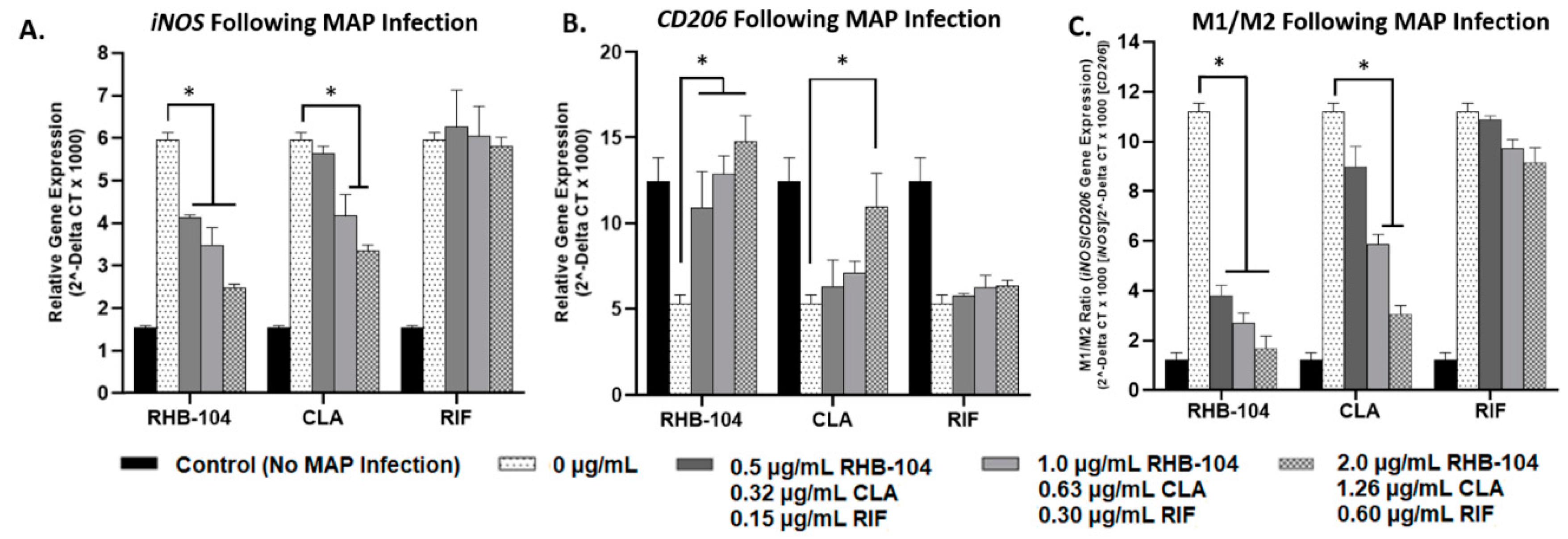
4.2. RHB-104 Significantly Reduces M1/M2 Macrophage Polarization at Lower Concentrations than Its Major Components
4.3. RHB-104 Decreases NF-κB p65 Activation in Macrophages More Effectively than Its Major Components
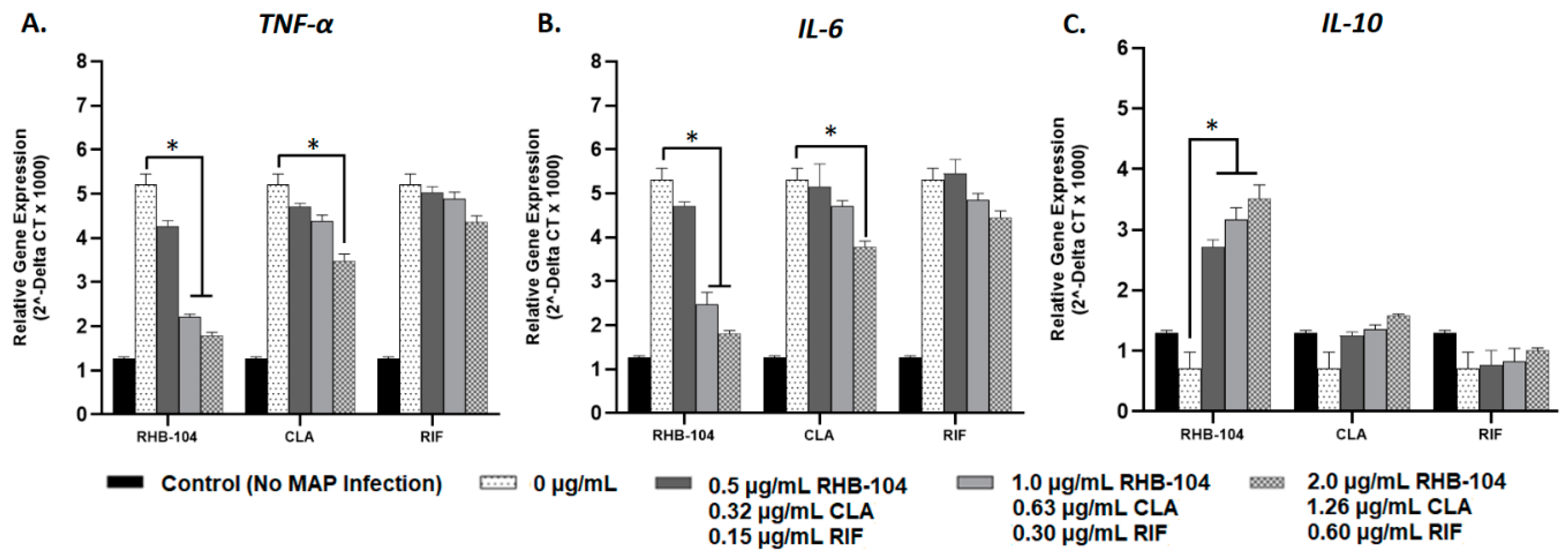
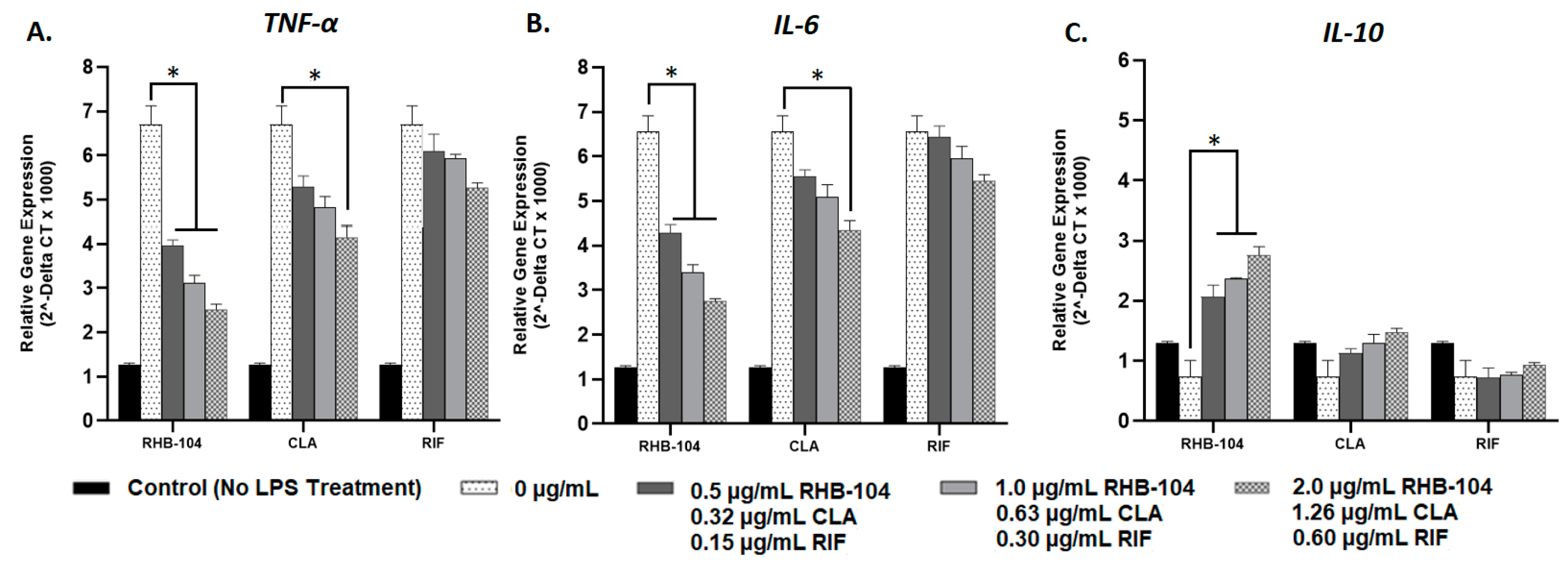
4.4. RHB-104 Is Superior to Clarithromycin and Rifabutin at Modulating Cytokine Gene Expression
4.5. RHB-104 Is Superior to Clarithromycin and Rifabutin at Modulating Cytokine Protein Levels
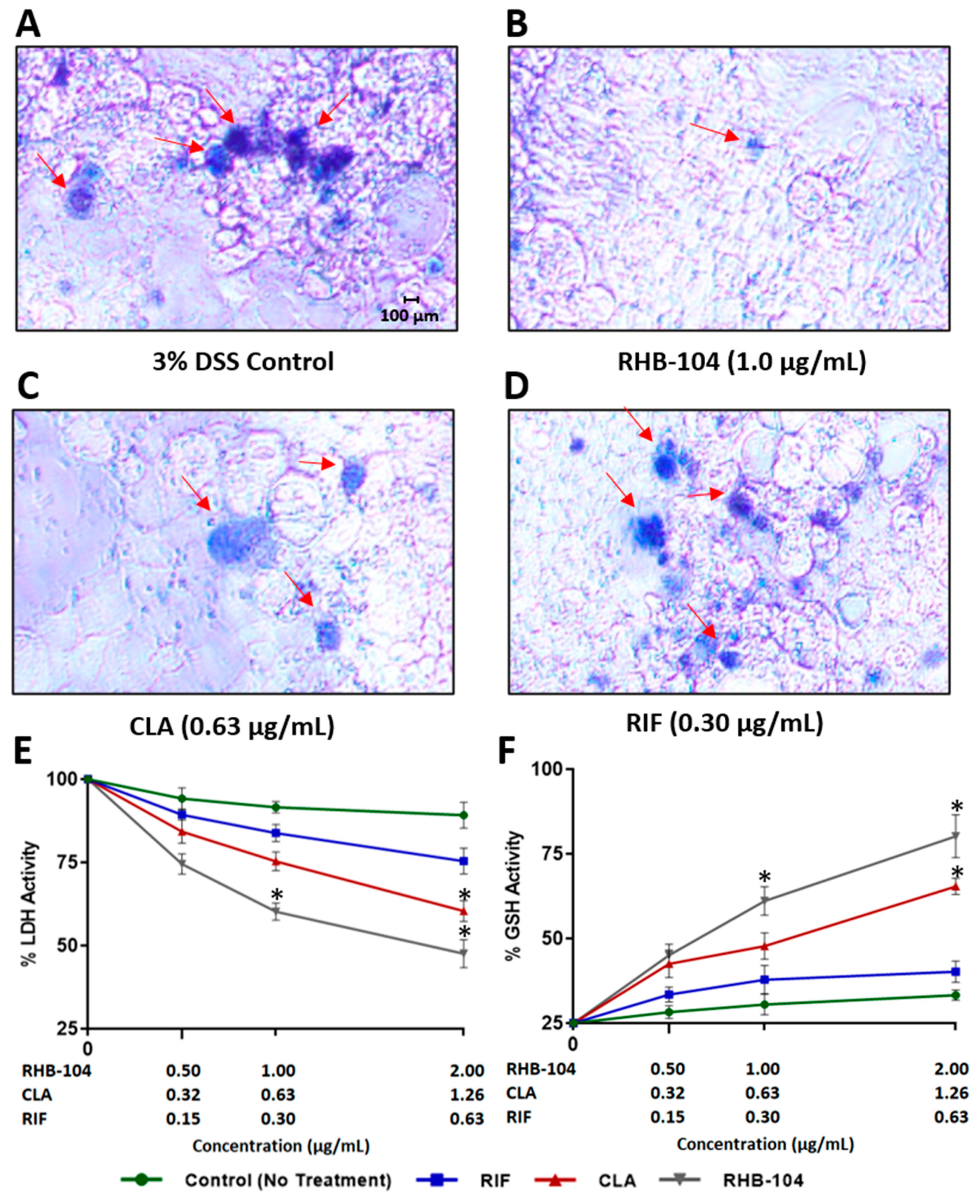
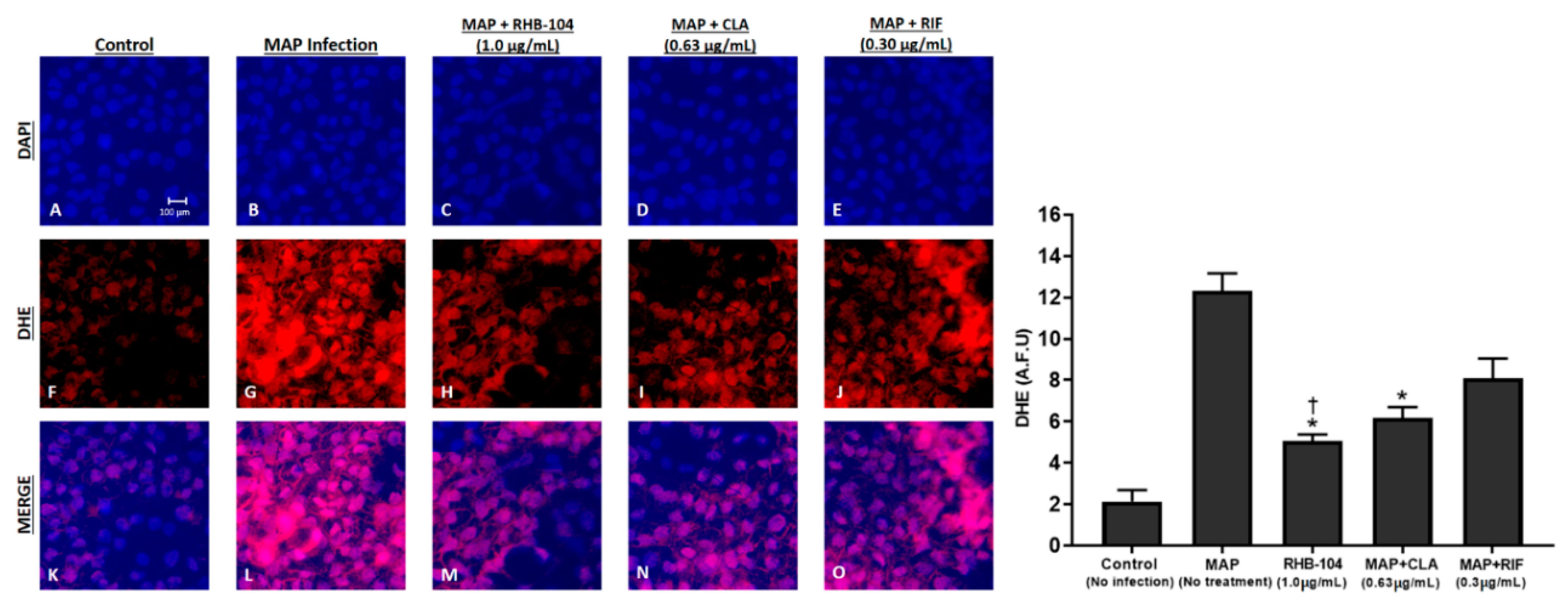
4.6. RHB-104 Synergistically Reduces Tissue Damage and Oxidative Stress in Caco-2 Monolayers
4.7. RHB-104 Most Effectively Reduces T-cell Proliferation in Phytohemagglutinin (PHA) and MAP PPD-Treated Cells
5. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Buret, A.G. Immuno-modulation and anti-inflammatory benefits of antibiotics: The example of tilmicosin. Can. J. Vet. Res. 2010, 74, 1–10. [Google Scholar] [PubMed]
- Pradhan, S.; Madke, B.; Kabra, P.; Singh, A.L. Anti-inflammatory and Immunomodulatory Effects of Antibiotics and Their Use in Dermatology. Indian J. Dermatol. 2016, 61, 469–481. [Google Scholar] [CrossRef] [PubMed]
- Tenson, T.; Lovmar, M.; Ehrenberg, M. The mechanism of action of macrolides, lincosamides and streptogramin B reveals the nascent peptide exit path in the ribosome. J. Mol. Biol. 2003, 330, 1005–1014. [Google Scholar] [CrossRef]
- Ichiyama, T.; Nishikawa, M.; Yoshitomi, T.; Hasegawa, S.; Matsubara, T.; Hayashi, T.; Furukawa, S. Clarithromycin inhibits NF-kappaB activation in human peripheral blood mononuclear cells and pulmonary epithelial cells. Antimicrob. Agents Chemother. 2001, 45, 44–47. [Google Scholar] [CrossRef] [Green Version]
- Oeckinghaus, A.; Ghosh, S. The NF-kappaB family of transcription factors and its regulation. Cold Spring Harb. Perspect. Biol. 2009, 1, a000034. [Google Scholar] [CrossRef]
- Inoue, S.; Nakase, H.; Matsuura, M.; Ueno, S.; Uza, N.; Kitamura, H.; Mikami, S.; Tamaki, H.; Kasahara, K.; Chiba, T. Open label trial of clarithromycin therapy in Japanese patients with Crohn’s disease. J. Gastroenterol. Hepatol. 2007, 22, 984–988. [Google Scholar] [CrossRef]
- Wehrli, W.; Staehelin, M. Actions of the rifamycins. Baceteriol. Rev. 1971, 35, 290–309. [Google Scholar] [CrossRef]
- Savarino, E.; Bertani, L.; Ceccarelli, L.; Bodini, G.; Zingone, F.; Buda, A.; Facchin, S.; Lorenzon, G.; Marchi, S.; Marabotto, E.; et al. Antimicrobial treatment with the fixed-dose antibiotic combination RHB-104 for Mycobacterium avium subspecies paratuberculosis in Crohn’s disease: Pharmacological and clinical implications. Expert Opin. Biol. Ther. 2019, 19, 79–88. [Google Scholar] [CrossRef]
- Rosette, C.; Agan, F.J.; Rosette, N.; Moro, L.; Mazzetti, A.; Hassan, C.; Gerloni, M. Rifamycin SV exhibits strong anti-inflammatory in vitro activity through pregnane X receptor stimulation and NFκB inhibition. Drug Metab. Pharmacokinet. 2019, 34, 172–180. [Google Scholar] [CrossRef]
- Alcedo, K.P.; Thanigachalam, S.; Naser, S.A. RHB-104 triple antibiotics combination in culture is bactericidal and should be effective for treatment of Crohn’s disease associated with Mycobacterium paratuberculosis. Gut Pathog. 2016, 8, 32. [Google Scholar] [CrossRef] [Green Version]
- Naser, S.A.; Ghobrial, G.; Romero, C.; Valentine, J.F. Culture of Mycobacterium avium subspecies paratuberculosis from the blood of patients with Crohn’s disease. The Lancet. 2004, 364, 1039–1044. [Google Scholar] [CrossRef]
- Qasem, A.; Ramesh, S.; Naser, S.A. Genetic polymorphisms in tumour necrosis factor receptors (TNFRSF1A/1B) illustrate differential treatment response to TNF-α inhibitors in patients with Crohn’s disease. BMJ Open Gastroenterol. 2019, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Di Sabatino, A.; Paccagnini, D.; Vidali, F.; Rosu, V.; Biancheri, P.; Cossu, A.; Sechi, L.A. Detection of Mycobacterium avium subsp. paratuberculosis (MAP)-specific IS900 DNA and antibodies against MAP peptides and lysate in the blood of Crohn’s disease patients. Inflamm. Bowel Dis. 2011, 17, 1254–1255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- RedHill Biopharma Limited. Efficacy and Safety of Anti-MAP Therapy in Adult Crohn’s Disease (MAPUS). Identification No. NCT01951326; 2019. Available online: https://clinicaltrials.gov/ct2/show/NCT01951326 (accessed on 20 October 2020).
- Juste, R.A.; Elguezabal, N.; Garrido, J.M.; Pavon, A.; Geijo, M.V.; Sevilla, I.; Ochotorena, I. On the prevalence of M. avium subspecies paratuberculosis DNA in the blood of healthy individuals and patients with inflammatory bowel disease. PLoS ONE 2008, 3, e2537. [Google Scholar] [CrossRef]
- Sharp, R.C.; Naser, E.S.; Alcedo, K.P.; Qasem, A.; Abdelli, L.S.; Naser, S.A. Development of multiplex PCR and multi-color fluorescent in situ hybridization (m-FISH) coupled protocol for detection and imaging of multi-pathogens involved in inflammatory bowel disease. Gut Pathog. 2018, 10, 1–12. [Google Scholar] [CrossRef]
- Graham, D.Y.; Naser, S.A.; Offman, E.; Kassir, N.; Hardi, R.; Welton, T.; Fehrmann, C. RHB-104, a Fixed-Dose, Oral Antibiotic Combination Against Mycobacterium Avium Paratuberculosis (MAP) Infection, Is Effective in Moderately to Severely Active Crohn’s Disease: 643. Am. J. Gastroenterol. 2019, 114, S376–S377. [Google Scholar] [CrossRef]
- Qasem, A.; Naser, S.A. TNF-α inhibitors exacerbate Mycobacterium paratuberculosis infection in tissue culture: A rationale for poor response of patients with Crohn’s disease to current approved therapy. BMJ Open Gastroenterol. 2018, 5, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Rosette, C.; Buendia-Laysa, F.; Patkar, S.; Moro, L.; Celasco, G.; Bozzella, R.; Ajani, M.; Gerloni, M. Anti-inflammatory and immunomodulatory activities of rifamycin SV. Int. J. Antimicrob. Agents. 2013, 42, 182–186. [Google Scholar] [CrossRef]
- Han, Y.M.; Koh, J.; Kim, J.W.; Lee, C.; Koh, S.J.; Kim, B.; Lee, K.L.; Im, J.P.; Kim, J.S. NF-kappa B activation correlates with disease phenotype in Crohn’s disease. PLoS ONE 2017, 12, e0182071. [Google Scholar] [CrossRef] [Green Version]
- Peppelenbosch, M.P.; van Deventer, S.J.H. T cell apoptosis and inflammatory bowel disease. Gut 2004, 53, 1556–1558. [Google Scholar] [CrossRef]
- Sabatino, A.D.; Ciccocioppo, R.; Luinetti, O.; Ricevuti, L.; Morera, R.; Cifone, M.G.; Solcia, E.; Corazza, G.R. Increased enterocyte apoptosis in inflamed areas of Crohn’s disease. Dis. Colon Rectum. 2003, 46, 1498–1507. [Google Scholar] [CrossRef] [PubMed]
- Qasem, A.; Safavikhasraghi, M.; Naser, S.A. A single capsule formulation of RHB-104 demonstrates higher anti-microbial growth potency for effective treatment of Crohn’s disease associated with Mycobacterium avium subspecies paratuberculosis. Gut Pathog. 2016, 8, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadrai, Z.; Hajrasouliha, A.R.; Chauhan, S.; Saban, D.R.; Dastjerdi, M.H.; Dana, R. Effect of topical azithromycin on corneal innate immune responses. Investig. Ophthalmol. Vis. Sci. 2011, 52, 2525–2531. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, K.; Shirai, R.; Mukae, H.; Ishimoto, H.; Nagata, T.; Sakamoto, N.; Kohno, S. Differing effects of clarithromycin and azithromycin on cytokine production by murine dendritic cells. Clin. Exp. Immunol. 2007, 147, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Yatsunami, J.; Fukuno, Y.; Nagata, M.; Tominaga, M.; Aoki, S.; Tsuruta, N.; Hayashi, S.I. Antiangiogenic and antitumor effects of 14-membered ring macrolides on mouse B16 melanoma cells. Clin. Exp. Metastasis 1999, 17, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Yatsunami, J.; Turuta, N.; Wakamatsu, K.; Hara, N.; Hayashi, S.I. Clarithromycin is a potent inhibitor of tumor-induced angiogenesis. Res. Exp. Med. 1997, 197, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Aoki, D.; Ueno, S.; Kubo, F.; Oyama, T.; Sakuta, T.; Matsushita, K.; Aikou, T. Roxithromycin inhibits angiogenesis of human hepatoma cells in vivo by suppressing VEGF production. Anticancer Res. 2005, 25, 133–138. [Google Scholar]
- Bearden, D.T.; Rodvold, K.A. Penetration of macrolides into pulmonary sites of infection. Infect. Med. 1999, 16, 480. [Google Scholar]
- Gladue, R.P.; Bright, G.M.; Isaacson, R.E.; Newborg, M.F. In vitro and in vivo uptake of azithromycin (CP-62,993) by phagocytic cells: Possible mechanism of delivery and release at sites of infection. Antimicrob. Agents Chemother. 1989, 33, 277–282. [Google Scholar] [CrossRef] [Green Version]
- Caruso, J. Twenty years of experience with intra-articular rifamycin for chronic arthritides. J. Int. Med. Res. 1997, 25, 307–317. [Google Scholar] [CrossRef]
- Spisani, S.; Traniello, S.; Martuccio, C.; Rizzuti, O.; Cellai, L. Rifamycins inhibit human neutrophil functions: New derivatives with potential antiinflammatory activity. Inflammation 1997, 21, 391–400. [Google Scholar] [CrossRef]
- Pimentel, M.; Park, S.; Mirocha, J.; Kane, S.V.; Kong, Y. The effect of a nonabsorbed oral antibiotic (rifaximin) on the symptoms of the irritable bowel syndrome: A randomized trial. Ann. Intern. Med. 2006, 145, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Quattrocchi, C.; Mangano, K.; Aiello, C.; Milasi, A.; Celasco, G.; Moro, L.; Di Marco, R. P308-Rifamycin SV: Evaluation of the activity in DNB-induced colitis in rats and on IFNγ production in ConA-stimulated rat splenocytes. J. Crohn’s Colitis 2009, 3, S131–S132. [Google Scholar] [CrossRef] [Green Version]
- Qasem, A.; Abdel-Aty, A.; Abu-Suwa, H.; Naser, S.A. Oxidative stress due to Mycobacterium avium subspecies paratuberculosis (MAP) infection upregulates selenium-dependent GPx activity. Gut Pathog. 2016, 8, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sturm, A.; de Souza, H.S.; Fiocchi, C. Mucosal T cell proliferation and apoptosis in inflammatory bowel disease. Curr. Drug Targets 2008, 9, 1–7. [Google Scholar] [CrossRef]
- Torres, J.; Bonovas, S.; Doherty, G.; Kucharzik, T.; Gisbert, J.P.; Raine, T.; Adamina, M.; Armuzzi, A.; Bachmann, O.; Bager, P.; et al. ECCO Guidelines on Therapeutics in Crohn’s Disease: Medical treatment. J. Crohn’s and Colitis 2020, 14, 4–22. [Google Scholar] [CrossRef]
- Leal, R.F.; Planell, N.; Kajekar, R.; Lozano, J.J.; Ordás, I.; Dotti, I.; Panés, J. Identification of inflammatory mediators in patients with Crohn’s disease unresponsive to anti-TNFα therapy. Gut 2015, 64, 233–242. [Google Scholar] [CrossRef]
- Cao, B.L.; Qasem, A.; Sharp, R.C.; Abdelli, L.S.; Naser, S.A. Systematic review and meta-analysis on the association of tuberculosis in Crohn’s disease patients treated with tumor necrosis factor-α inhibitors (Anti-TNF-α). World J. Gastroenterol. 2018, 24, 2764. [Google Scholar] [CrossRef]
- Qasem, A.; Naser, A.E.; Naser, S.A. The alternate effects of anti-TNF-α therapeutics and their role in mycobacterial granulomatous infection in Crohn’s disease. Expert Rev. Anti-Infect. Ther. 2017, 15, 637–643. [Google Scholar] [CrossRef]
- Honap, S.; Johnston, E.; Agrawal, G.; Al-Hakim, B.; Hermon-Taylor, J.; Sanderson, J. Anti-Mycobacterium paratuberculosis (MAP) therapy for Crohn’s disease: An overview and update. Frontline Gastroenterol. 2020, 0, 1–7. [Google Scholar] [CrossRef]
- Beran, V.; Havelkova, M.; Kaustova, J.; Dvorska, L.; Pavlik, I. Cell wall deficient forms of mycobacteria: A review. Vet. Med. -Praha-. 2006, 51, 365. [Google Scholar] [CrossRef] [Green Version]
- AlQasrawi, D.; Qasem, A.; Naser, S.A. Divergent Effect of Cigarette Smoke on Innate Immunity in Inflammatory Bowel Disease: A Nicotine-Infection Interaction. Int. J. Mol. Sci. 2020, 21, 5801. [Google Scholar] [CrossRef] [PubMed]
- Coussens, P.M. Mycobacterium paratuberculosis and the bovine immune system. Anim. Health Res. Rev. 2001, 2, 141. [Google Scholar] [CrossRef] [PubMed]
- Houben, R.M.; Dodd, P.J. The global burden of latent tuberculosis infection: A re-estimation using mathematical modelling. PLoS Med. 2016, 13, e1002152. [Google Scholar] [CrossRef] [Green Version]



| Gene | Forward Primer Sequence (5′→3′) | Reverse Primer Sequence (5′→3′) |
|---|---|---|
| GAPDH | 5′-CTTTTGCAGACCACAGTCCATG-3′ (22 bases) | 5′-TTTTCTAGACGGCAGGTCAGG-3′ (21 bases) |
| iNOS | 5′-GGAGCAACGTTGAGGAAATAAGACT-3′ (25 bases) | 5′-AAGAGCCAGAAGCGCTATCAC-3′ (21 bases) |
| CD206 | 5′-GGAGGATTCCATGTATTTGTGAGC-3′ (24 bases) | 5′-AAATGAGTGAAGTGAAATCAGTTACCT-3′ (27 bases) |
| TNF-α | 5′-TCTCCCTCAAGGACTCAGCTTTCTG-3′ (25 bases) | 5′-TGAGAGGAAGAGAACCTGCCTGG-3′ (23 bases) |
| IL-6 | 5′-CAGGAGAAGATTCCAAAGATGTAGCC-3′ (26 bases) | 5′-TGCTCTAGAACCCAGCAAAGAC-3′ (22 bases) |
| IL-10 | 5′-ATGTCTAGTTCAGGCAGTCCCA-3′ (22 bases) | 5′-GGGCTTGCTCTTGCAAAACC-3′ (20 bases) |
| Treatment | Antibiotic Concentration (µg/mL) | NF-κB p65 Activation Score (%) |
|---|---|---|
| Positive Control | - | 100.0 |
| Negative Control | - | 6.9 |
| MAP + RHB-104 | 0.50 | 48.4 |
| 1.00 | 21.5 | |
| 2.00 | 13.4 | |
| MAP + CLA | 0.32 | 58.3 |
| 0.63 | 49.6 | |
| 1.26 | 24.1 | |
| MAP + RIF | 0.15 | 83.8 |
| 0.30 | 73.1 | |
| 0.60 | 57.5 | |
| LPS + RHB-104 | 0.5 | 67.2 |
| 1.0 | 37.8 | |
| 2.0 | 23.3 | |
| LPS + CLA | 0.32 | 68.2 |
| 0.63 | 57.2 | |
| 1.26 | 33.6 | |
| LPS + RIF | 0.15 | 89.1 |
| 0.30 | 83.4 | |
| 0.60 | 68.7 |
| Treatment | Antibiotic Concentration (µg/mL) | TNF-α ± SD (pg/mL) | IL-6 ± SD (pg/mL) | IL-10 ± SD (pg/mL) |
|---|---|---|---|---|
| MAP Control | - | 191.2 ± 8.6 | 201.8 ± 11.7 | 61.9 ± 9.8 |
| LPS Control | - | 217.4 ± 5.1 | 224.3 ± 9.6 | 52.2 ± 7.4 |
| No Treatment | - | 75.7 ± 6.5 | 82.9 ± 5.1 | 100.4 ± 6.5 |
| MAP + RHB-104 | 0.50 | 175.3 ± 8.4 | 182.2 ± 8.1 | 79.2 ± 2.9 |
| 1.00 | 123.7 ± 6.7 * | 139.4 ± 3.2 * | 88.6 ± 3.5 * | |
| 2.00 | 95.8 ± 1.8 * | 117.5 ± 2.5 * | 95.1 ± 5.8 * | |
| MAP + CLA | 0.32 | 184.7 ± 3.6 | 191.5 ± 8.3 | 68.3 ± 3.1 |
| 0.63 | 165.8 ± 5.1 | 173.6 ± 3.1 | 75.4 ± 2.7 | |
| 1.26 | 146.1 ± 6.2 * | 153.2 ± 4.6 * | 80.3 ± 8.7 | |
| MAP + RIF | 0.15 | 187.2 ± 5.3 | 197.3 ± 7.2 | 63.6 ± 4.1 |
| 0.30 | 179.1 ± 1.9 | 184.9 ± 5.9 | 70.2 ± 2.1 | |
| 0.60 | 166.4 ± 8.3 | 172.8 ± 3.9 | 77.3 ± 3.4 | |
| LPS + RHB-104 | 0.5 | 179.3 ± 2.0 | 182.6 ± 5.1 | 76.4 ± 6.3 |
| 1.0 | 139.7 ± 4.7 * | 151.1 ± 2.5 * | 84.3 ± 6.9 * | |
| 2.0 | 110.9 ± 4.0 * | 137.2 ± 2.7 * | 91.1 ± 5.2 * | |
| LPS + CLA | 0.32 | 185.8 ± 1.6 | 193.3 ± 4.4 | 61.2 ± 4.2 |
| 0.63 | 162.4 ± 6.2 | 174.8 ± 3.6 | 69.7 ± 7.8 | |
| 1.26 | 158.2 ± 7.3 * | 169.7 ± 1.1 * | 71.9 ± 6.9 | |
| LPS + RIF | 0.15 | 188.3 ± 3.1 | 206.8 ± 8.0 | 54.6 ± 2.3 |
| 0.30 | 179.7 ± 8.9 | 192.4 ± 1.3 | 62.4 ± 7.2 | |
| 0.60 | 166.1 ± 5.6 | 182.1 ± 1.6 | 67.5 ± 4.9 |
| Treatment | T-cell Proliferation (OD at 450 nm) | Relative T-cell Proliferation |
|---|---|---|
| Control (no PHA or MAP PPD) | 0.061 | 1.00 ± 0.003 |
| PHA Control | 0.219 | 3.60 ± 0.059 |
| PHA + 1.0 µg/mL RHB-104 | 0.046 | 0.75 ± 0.002 *,† |
| PHA + 0.63 µg/mL CLA | 0.066 | 1.09 ± 0.021 * |
| PHA + 0.30 µg/mL RIF | 0.060 | 0.99 ± 0.013 * |
| MAP PPD Control | 0.189 | 3.12 ± 0.087 |
| MAP PPD + 1.0 µg/mL RHB-104 | 0.066 | 1.09 ± 0.010 *,† |
| MAP PPD + 0.63 µg/mL CLA | 0.107 | 1.76 ± 0.060 * |
| MAP PPD + 0.30 µg/mL RIF | 0.113 | 1.86 ± 0.034 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qasem, A.; Elkamel, E.; Naser, S.A. Anti-MAP Triple Therapy Supports Immunomodulatory Therapeutic Response in Crohn’s Disease through Downregulation of NF-κB Activation in the Absence of MAP Detection. Biomedicines 2020, 8, 513. https://doi.org/10.3390/biomedicines8110513
Qasem A, Elkamel E, Naser SA. Anti-MAP Triple Therapy Supports Immunomodulatory Therapeutic Response in Crohn’s Disease through Downregulation of NF-κB Activation in the Absence of MAP Detection. Biomedicines. 2020; 8(11):513. https://doi.org/10.3390/biomedicines8110513
Chicago/Turabian StyleQasem, Ahmad, Erij Elkamel, and Saleh A. Naser. 2020. "Anti-MAP Triple Therapy Supports Immunomodulatory Therapeutic Response in Crohn’s Disease through Downregulation of NF-κB Activation in the Absence of MAP Detection" Biomedicines 8, no. 11: 513. https://doi.org/10.3390/biomedicines8110513





