Extracellular Vesicles Derived from Kefir Grain Lactobacillus Ameliorate Intestinal Inflammation via Regulation of Proinflammatory Pathway and Tight Junction Integrity
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of PRCC-1301 EVs
2.2. Cell Culture
2.3. Quantitative Real-Time Reverse Transcription PCR
2.4. In Vitro Epithelial Monolayer Permeability Assay
2.5. Induction and Treatment of Colitis
2.6. Immunofluorescence Analysis
2.7. Immunohistochemical Analysis
2.8. Statistical Analysis
2.9. Ethical Considerations
3. Results
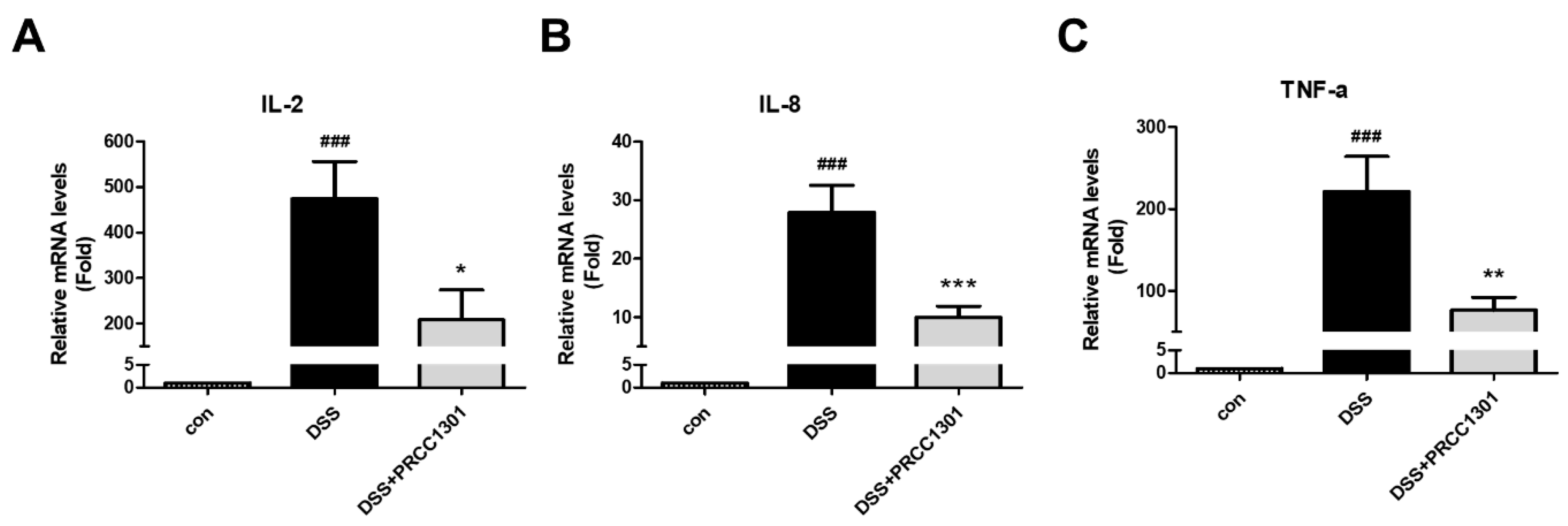
3.1. PRCC-1301 EVs Inhibit Pro-Inflammatory Cytokine Gene Expression in Caco-2 Cells
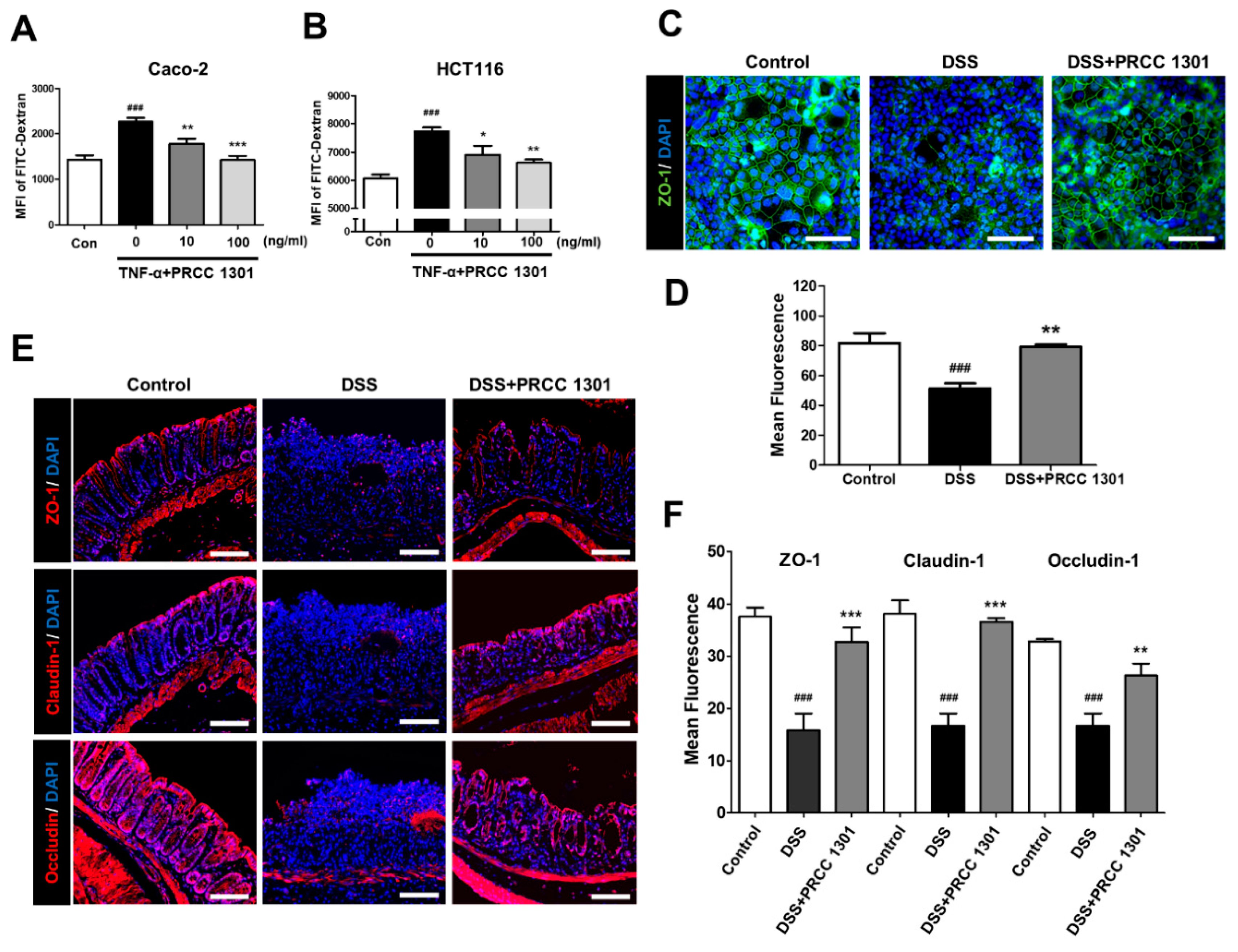
3.2. PRCC-1301 EVs Recover Increase in Intestinal Permeability and Disruption of Tight Junction Complexes
3.3. PRCC-1301 EVs Prevent DSS-Induced Acute Colitis in Mice
3.4. PRCC-1301 EVs Attenuate Chronic Colitis in IL-10-/- Mice
3.5. PRCC-1301 EVs Inhibit the NF-κB Pathway in the Colitis Mucosa
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lees, C.; Barrett, J.; Parkes, M.; Satsangi, J. New IBD genetics: Common pathways with other diseases. Gut 2011, 60, 1739–1753. [Google Scholar] [CrossRef] [PubMed]
- Clough, J.N.; Omer, O.S.; Tasker, S.; Lord, G.M.; Irving, P.M. Regulatory T-cell therapy in Crohn’s disease: Challenges and advances. Gut 2020, 69, 942–952. [Google Scholar] [CrossRef] [PubMed]
- De Souza, H.S.; Fiocchi, C. Immunopathogenesis of IBD: Current state of the art. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 13. [Google Scholar] [CrossRef] [PubMed]
- Martini, E.; Krug, S.M.; Siegmund, B.; Neurath, M.F.; Becker, C. Mend your fences: The epithelial barrier and its relationship with mucosal immunity in inflammatory bowel disease. Cell. Mol. Gastroenterol. Hepatol. 2017, 4, 33–46. [Google Scholar] [CrossRef]
- Miner-Williams, W.M.; Moughan, P.J. Intestinal barrier dysfunction: Implications for chronic inflammatory conditions of the bowel. Nutr. Res. Rev. 2016, 29, 40–59. [Google Scholar] [CrossRef]
- Schmitz, H.; Barmeyer, C.; Fromm, M.; Runkel, N.; Foss, H.-D.; Bentzel, C.J.; Riecken, E.-O.; Schulzke, J.-D. Altered tight junction structure contributes to the impaired epithelial barrier function in ulcerative colitis. Gastroenterology 1999, 116, 301–309. [Google Scholar] [CrossRef]
- Merga, Y.; Campbell, B.J.; Rhodes, J.M. Mucosal barrier, bacteria and inflammatory bowel disease: Possibilities for therapy. Dig. Dis. 2014, 32, 475–483. [Google Scholar] [CrossRef]
- Tambuwala, M.M. Natural nuclear factor kappa beta inhibitors: Safe therapeutic options for inflammatory bowel disease. Inflamm. Bowel Dis. 2016, 22, 719–723. [Google Scholar] [CrossRef]
- Brown, L.; Wolf, J.M.; Prados-Rosales, R.; Casadevall, A. Through the wall: Extracellular vesicles in Gram-positive bacteria, mycobacteria and fungi. Nat. Rev. Microbiol. 2015, 13, 620–630. [Google Scholar] [CrossRef]
- Liu, Y.; Defourny, K.A.; Smid, E.J.; Abee, T. Gram-positive bacterial extracellular vesicles and their impact on health and disease. Front. Microbiol. 2018, 9, 1502. [Google Scholar] [CrossRef]
- Kim, J.-H.; Jeun, E.-J.; Hong, C.-P.; Kim, S.-H.; Jang, M.S.; Lee, E.-J.; Moon, S.J.; Yun, C.H.; Im, S.-H.; Jeong, S.-G. Extracellular vesicle–derived protein from Bifidobacterium longum alleviates food allergy through mast cell suppression. J. Allergy Clin. Immunol. 2016, 137, 507–516. e8. [Google Scholar] [CrossRef] [PubMed]
- Anselmo, R.J.; Viora, S.S.; Ojeda, P.A.; Lausada, L.I. Efecto antagónico del kefir sobre endosporas y células vegetativas de Bacillus cereus y Clostridium perfringens. Inf. Tecnológica 2010, 21, 131–138. [Google Scholar] [CrossRef][Green Version]
- Hong, W.-S.; Chen, H.-C.; Chen, Y.-P.; Chen, M.-J. Effects of kefir supernatant and lactic acid bacteria isolated from kefir grain on cytokine production by macrophage. Int. Dairy J. 2009, 19, 244–251. [Google Scholar] [CrossRef]
- Diniz, R.; Garla, L.; Schneedorf, J.M.; Carvalho, J. Study of anti-inflammatory activity of Tibetan mushroom, a symbiotic culture of bacteria and fungi encapsulated into a polysaccharide matrix. Pharmacol. Res. 2003, 47, 49–52. [Google Scholar] [CrossRef]
- Seo, M.; Park, E.; Ko, S.; Choi, E.; Kim, S. Therapeutic effects of kefir grain Lactobacillus-derived extracellular vesicles in mice with 2, 4, 6-trinitrobenzene sulfonic acid-induced inflammatory bowel disease. J. Dairy Sci. 2018, 101, 8662–8671. [Google Scholar] [CrossRef] [PubMed]
- Konoshenko, M.Y.; Lekchnov, E.A.; Vlassov, A.V.; Laktionov, P.P. Isolation of extracellular vesicles: General methodologies and latest trends. Biomed Res. Int. 2018, 2018, 27. [Google Scholar] [CrossRef]
- Altermann, E.; Russell, W.M.; Azcarate-Peril, M.A.; Barrangou, R.; Buck, B.L.; McAuliffe, O.; Souther, N.; Dobson, A.; Duong, T.; Callanan, M. Complete genome sequence of the probiotic lactic acid bacterium Lactobacillus acidophilus NCFM. Proc. Natl. Acad. Sci. USA 2005, 102, 3906–3912. [Google Scholar] [CrossRef]
- Rees, V. Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin. Exp. Immunol. 1998, 114, 385–391. [Google Scholar]
- Kang, E.A.; Soh, H.; Park, S.; Lee, H.J.; Im, J.P.; Kim, J.S. Soluble Siglec-9 alleviates intestinal inflammation through inhibition of the NF-κB pathway. Int. Immunopharmacol. 2020, 86, 106695. [Google Scholar] [CrossRef]
- Berg, D.J.; Zhang, J.; Weinstock, J.V.; Ismail, H.F.; Earle, K.A.; Alila, H.; Pamukcu, R.; Moore, S.; Lynch, R.G. Rapid development of colitis in NSAID-treated Il-10–deficient mice. Gastroenterology 2002, 123, 1527–1542. [Google Scholar] [CrossRef]
- Choi, J.H.; Moon, C.M.; Shin, T.-S.; Kim, E.K.; McDowell, A.; Jo, M.-K.; Joo, Y.H.; Kim, S.-E.; Jung, H.-K.; Shim, K.-N. Lactobacillus paracasei-derived extracellular vesicles attenuate the intestinal inflammatory response by augmenting the endoplasmic reticulum stress pathway. Exp. Mol. Med. 2020, 52, 423–437. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-H.; Choi, S.J.; Choi, H.-I.; Choi, J.-P.; Park, H.-K.; Kim, E.K.; Kim, M.-J.; Moon, B.S.; Min, T.-k.; Rho, M. Lactobacillus plantarum-derived extracellular vesicles protect atopic dermatitis induced by Staphylococcus aureus-derived extracellular vesicles. Allergyasthma Immunol. Res. 2018, 10, 516–532. [Google Scholar] [CrossRef] [PubMed]
- Aoki-Yoshida, A.; Saito, S.; Tsuruta, T.; Ohsumi, A.; Tsunoda, H.; Sonoyama, K. Exosomes isolated from sera of mice fed Lactobacillus strains affect inflammatory cytokine production in macrophages in vitro. Biochem. Biophys. Res. Commun. 2017, 489, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Krishna Rao, R.; Samak, G. Protection and restitution of gut barrier by probiotics: Nutritional and clinical implications. Curr. Nutr. Food Sci. 2013, 9, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Casini-Raggi, V.; Kam, L.; Chong, Y.; Fiocchi, C.; Pizarro, T.T.; Cominelli, F. Mucosal imbalance of IL-1 and IL-1 receptor antagonist in inflammatory bowel disease. A novel mechanism of chronic intestinal inflammation. J. Immunol. 1995, 154, 2434–2440. [Google Scholar] [PubMed]
- Xavier, R.; Podolsky, D. Unravelling the pathogenesis of inflammatory bowel disease. Nature 2007, 448, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Kucharzik, T.; Maaser, C.; Lügering, A.; Kagnoff, M.; Mayer, L.; Targan, S.; Domschke, W. Recent understanding of IBD pathogenesis: Implications for future therapies. Inflamm. Bowel Dis. 2006, 12, 1068–1083. [Google Scholar] [CrossRef]
- Rovedatti, L.; Kudo, T.; Biancheri, P.; Sarra, M.; Knowles, C.; Rampton, D.S.; Corazza, G.R.; Monteleone, G.; Di Sabatino, A.; MacDonald, T.T. Differential regulation of interleukin 17 and interferon γ production in inflammatory bowel disease. Gut 2009, 58, 1629–1636. [Google Scholar] [CrossRef]
- Fujino, S.; Andoh, A.; Bamba, S.; Ogawa, A.; Hata, K.; Araki, Y.; Bamba, T.; Fujiyama, Y. Increased expression of interleukin 17 in inflammatory bowel disease. Gut 2003, 52, 65–70. [Google Scholar] [CrossRef]
- Anderson, R.C.; Cookson, A.L.; McNabb, W.C.; Park, Z.; McCann, M.J.; Kelly, W.J.; Roy, N.C. Lactobacillus plantarum MB452 enhances the function of the intestinal barrier by increasing the expression levels of genes involved in tight junction formation. BMC Microbiol. 2010, 10, 316. [Google Scholar] [CrossRef]
- Miyauchi, E.; O’Callaghan, J.; Buttó, L.F.; Hurley, G.; Melgar, S.; Tanabe, S.; Shanahan, F.; Nally, K.; O’Toole, P.W. Mechanism of protection of transepithelial barrier function by Lactobacillus salivarius: Strain dependence and attenuation by bacteriocin production. Am. J. Physiol. Gastrointest Liver Physiol. 2012, 303, G1029–G1041. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.M.; Myers, L.S.; Kurundkar, A.R.; Maheshwari, A.; Nusrat, A.; Lin, P.W. Probiotic bacteria induce maturation of intestinal claudin 3 expression and barrier function. Am. J. Pathol. 2012, 180, 626–635. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.Y.; Osaka, T.; Moriyama, E.; Date, Y.; Kikuchi, J.; Tsuneda, S. Strengthening of the intestinal epithelial tight junction by Bifidobacterium bifidum. Physiol. Rep. 2015, 3, e12327. [Google Scholar] [CrossRef] [PubMed]
- Lippai, D.; Bala, S.; Catalano, D.; Kodys, K.; Szabo, G. Micro-RNA-155 deficiency prevents alcohol-induced serum endotoxin increase and small bowel inflammation in mice. Alcohol. Clin. Exp. Res. 2014, 38, 2217–2224. [Google Scholar] [CrossRef]
- Laukoetter, M.G.; Nava, P.; Nusrat, A. Role of the intestinal barrier in inflammatory bowel disease. World J. Gastroenterol. Wjg 2008, 14, 401. [Google Scholar] [CrossRef]
- MacDonald, T.; Hutchings, P.; Choy, M.Y.; Murch, S.; Cooke, A. Tumour necrosis factor-alpha and interferon-gamma production measured at the single cell level in normal and inflamed human intestine. Clin. Exp. Immunol. 1990, 81, 301–305. [Google Scholar] [CrossRef]
- Ye, D.; Ma, I.; Ma, T.Y. Molecular mechanism of tumor necrosis factor-α modulation of intestinal epithelial tight junction barrier. Am. J. Physiol. -Gastrointest. Liver Physiol. 2006, 290, G496–G504. [Google Scholar] [CrossRef]
- Mankertz, J.; Tavalali, S.; Schmitz, H.; Mankertz, A.; Riecken, E.; Fromm, M.; Schulzke, J. Expression from the human occludin promoter is affected by tumor necrosis factor alpha and interferon gamma. J. Cell Sci. 2000, 113, 2085–2090. [Google Scholar]
- Shao, L.; Serrano, D.; Mayer, L. The Role of Epithelial Cells in Immune Regulation in the Gut; Seminars in immunology; Elsevier: Amsterdam, The Netherlands, 2001; pp. 163–175. [Google Scholar]
- Araki, Y.; Sugihara, H.; Hattori, T. In vitro effects of dextran sulfate sodium on a Caco-2 cell line and plausible mechanisms for dextran sulfate sodium-induced colitis. Oncol. Rep. 2006, 16, 1357–1362. [Google Scholar] [CrossRef]
- Neurath, M.F. Cytokines in inflammatory bowel disease. Nat. Rev. Immunol. 2014, 14, 329–342. [Google Scholar] [CrossRef]
- Bedirli, A.; Salman, B.; Pasaoglu, H.; Ofluoglu, E.; Sakrak, O. Effects of nuclear factor-κB inhibitors on colon anastomotic healing in rats. J. Surg. Res. 2011, 171, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Xiang, J.; Lin, Y.; Ma, J.; Zhang, J.; Zhang, H.; Sun, J.; Danial, N.N.; Liu, J.; Lin, A. Inactivation of BAD by IKK inhibits TNFα-induced apoptosis independently of NF-κB activation. Cell 2013, 152, 304–315. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, E.A.; Choi, H.-I.; Hong, S.W.; Kang, S.; Jegal, H.-Y.; Choi, E.W.; Park, B.-S.; Kim, J.S. Extracellular Vesicles Derived from Kefir Grain Lactobacillus Ameliorate Intestinal Inflammation via Regulation of Proinflammatory Pathway and Tight Junction Integrity. Biomedicines 2020, 8, 522. https://doi.org/10.3390/biomedicines8110522
Kang EA, Choi H-I, Hong SW, Kang S, Jegal H-Y, Choi EW, Park B-S, Kim JS. Extracellular Vesicles Derived from Kefir Grain Lactobacillus Ameliorate Intestinal Inflammation via Regulation of Proinflammatory Pathway and Tight Junction Integrity. Biomedicines. 2020; 8(11):522. https://doi.org/10.3390/biomedicines8110522
Chicago/Turabian StyleKang, Eun Ae, Hye-In Choi, Seung Wook Hong, Seokwoo Kang, Hyeon-Young Jegal, Eun Wook Choi, Byung-Soon Park, and Joo Sung Kim. 2020. "Extracellular Vesicles Derived from Kefir Grain Lactobacillus Ameliorate Intestinal Inflammation via Regulation of Proinflammatory Pathway and Tight Junction Integrity" Biomedicines 8, no. 11: 522. https://doi.org/10.3390/biomedicines8110522
APA StyleKang, E. A., Choi, H.-I., Hong, S. W., Kang, S., Jegal, H.-Y., Choi, E. W., Park, B.-S., & Kim, J. S. (2020). Extracellular Vesicles Derived from Kefir Grain Lactobacillus Ameliorate Intestinal Inflammation via Regulation of Proinflammatory Pathway and Tight Junction Integrity. Biomedicines, 8(11), 522. https://doi.org/10.3390/biomedicines8110522



