The Potential Effect of Fucoidan on Inhibiting Epithelial-to-Mesenchymal Transition, Proliferation, and Increase in Apoptosis for Endometriosis Treatment: In Vivo and In Vitro Study
Abstract
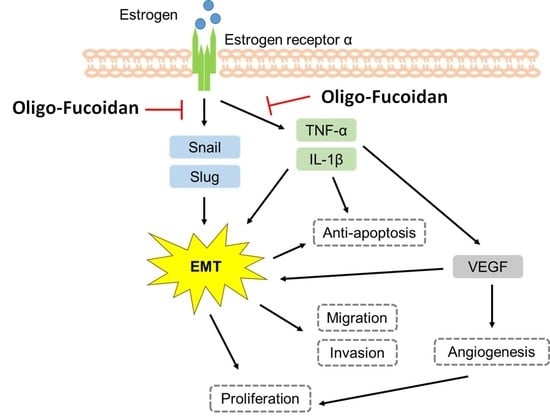
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Culture
2.3. Cell Viability Assay
2.4. Cell Counting Assay
2.5. Wound Healing Assay
2.6. Western Blot Analysis
2.7. Animals
2.8. Mouse Model for Endometriosis
2.9. Quantification of Cytokines
2.10. Statistical Analysis
3. Results
3.1. Effects of FC on β-Estradiol (E2)-Induced Proliferation of Endometriosis Cells
3.2. Effects of FC on β-Estradiol-Induced Migration and EMT-Related Protein Expression of Endometriotic Cells
3.3. Effects of FC on Endometriotic Lesions Growth in In Vivo Study
3.4. Effects of FC on Apoptosis, Proliferation-Related Protein, and ER-α Expression of endometriotic Lesions in Mice
3.5. Effects of FC on EMT-Related Protein of Endometriotic Lesions in Mice
3.6. Effects of FC on VEGF, TNF-α, and IL-1β in Endometriotic Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bulun, S.E. Endometriosis. N. Engl. J. Med. 2009, 360, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, S.; Pouly, J.L.; Canis, M. In vitro and in vivo effects of MK2206 and chloroquine combination therapy on endometriosis: Autophagy may be required for regrowth of endometriosis. Br. J. Pharmacol. 2018, 175, 1637–1653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giudice, L.C.; Kao, L.C. Endometriosis. Lancet (Lond. Engl.) 2004, 364, 1789–1799. [Google Scholar] [CrossRef]
- Bulletti, C.; Coccia, M.E.; Battistoni, S.; Borini, A. Endometriosis and infertility. J. Assist. Reprod. Genet. 2010, 27, 441–447. [Google Scholar] [CrossRef]
- Wu, R.; Zhou, W.; Chen, S.; Shi, Y.; Su, L.; Zhu, M.; Chen, Q.; Chen, Q. Lipoxin A 4 suppresses the development of endometriosis in an ALX receptor-dependent manner via the p38 MAPK pathway. Br. J. Pharmacol. 2014, 171, 4927–4940. [Google Scholar] [CrossRef] [Green Version]
- Mogensen, J.B.; Kjar, S.K.; Mellemkjær, L.; Jensen, A. Endometriosis and risks for ovarian, endometrial and breast cancers: A nationwide cohort study. Gynecol. Oncol. 2016, 143, 87–92. [Google Scholar] [CrossRef]
- Sampson, J.A. Peritoneal endometriosis due to the menstrual dissemination of endometrial tissue into the peritoneal cavity. Am. J. Obstet. Gynecol. 1927, 14, 422–469. [Google Scholar] [CrossRef]
- Van Langendonckt., A.; Francoise Casanas., R.; Jacques., D. Oxidative stress and peritoneal endometriosis. Fertil. Steril. 2002, 77, 861–870. [Google Scholar] [CrossRef]
- Nenicu, A.; Gu, Y.; Körbel, C.; Menger, M.D.; Laschke, M.W. Combination therapy with telmisartan and parecoxib induces regression of endometriotic lesions. Br. J. Pharmacol. 2017, 174, 2623–2635. [Google Scholar] [CrossRef]
- Mosher, A.; Tsoulis, M.W.; Lim, J.; Tan, C.; Agarwal, S.; Leyland, N.; Foster, W. Melatonin activity and receptor expression in endometrial tissue and endometriosis. Hum. Reprod. 2019, 34, 1215–1224. [Google Scholar] [CrossRef]
- El-Shennawy, L.; Dubrovskyi, O.; Kastrati, I.; Danes, J.M.; Zhang, Y.; Whiteley, H.E.; Creighton, C.J.; Frasor, J. Coactivation of Estrogen Receptor and IKKβ Induces a Dormant Metastatic Phenotype in ER-Positive Breast Cancer. Cancer Res. 2018, 78, 974–984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Malentacchi, F.; Fambrini, M.; Harrath, A.H.; Huang, H.; Petraglia, F. Epigenetics of Estrogen and Progesterone Receptors in Endometriosis. Reprod. Sci. (Thousand Oaks Calif.) 2020, 27, 1967–1974. [Google Scholar] [CrossRef] [PubMed]
- Paterni, I.; Granchi, C.; Katzenellenbogen, J.A.; Minutolo, F. Estrogen receptors alpha (ERα) and beta (ERβ): Subtype-selective ligands and clinical potential. Steroids 2014, 90, 13–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kulak, J., Jr.; Fischer, C.; Komm, B.; Taylor, H.S. Treatment with bazedoxifene, a selective estrogen receptor modulator, causes regression of endometriosis in a mouse model. Endocrinology 2011, 152, 3226–3232. [Google Scholar] [CrossRef] [PubMed]
- Pluchino, N.; Mamillapalli, R.; Wenger, J.M.; Ramyead, L.; Drakopoulos, P.; Tille, J.C.; Taylor, H.S. Estrogen receptor-α immunoreactivity predicts symptom severity and pain recurrence in deep endometriosis. Fertil. Steril. 2020, 113, 1224–1231.e1221. [Google Scholar] [CrossRef] [PubMed]
- Symons, L.K.; Miller, J.E.; Kay, V.R.; Marks, R.M.; Liblik, K.; Koti, M.; Tayade, C. The Immunopathophysiology of Endometriosis. Trends Mol. Med. 2018, 24, 748–762. [Google Scholar] [CrossRef]
- Berkkanoglu, M.; Arici, A. Immunology and endometriosis. Am. J. Reprod. Immunol. 2003, 50, 48–59. [Google Scholar] [CrossRef] [Green Version]
- Kaur, K.K.; Allahbadia, G. An Update on Pathophysiology and Medical Management of Endometriosis. Adv. Reprod. Sci. 2016, 04, 53–73. [Google Scholar] [CrossRef] [Green Version]
- Kiriakidis, S.; Andreakos, E.; Monaco, C.; Foxwell, B.; Feldmann, M.; Paleolog, E. VEGF expression in human macrophages is NF-κB-dependent: Studies using adenoviruses expressing the endogenous NF-κB inhibitor IκBα and a kinase-defective form of the IκB kinase 2. J. Cell Sci. 2003, 116, 665–674. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Cao, Z.; Yu, B.; Chai, C. An in vivo mouse model of primary dysmenorrhea. Exp. Anim. 2015, 64, 295–303. [Google Scholar] [CrossRef] [Green Version]
- Albertsen, H.M.; Ward, K. Genes linked to endometriosis by GWAS are integral to cytoskeleton regulation and suggests that mesothelial barrier homeostasis is a factor in the pathogenesis of endometriosis. Reprod. Sci. 2017, 24, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-H.; Cheung, L.W.T.; Wong, A.S.T.; Leung, P.C.K. Estrogen regulates Snail and Slug in the down-regulation of E-cadherin and induces metastatic potential of ovarian cancer cells through estrogen receptor alpha. Mol. Endocrinol. 2008, 22, 2085–2098. [Google Scholar] [CrossRef] [PubMed]
- Thiery, J.P. Epithelial–mesenchymal transitions in tumour progression. Nat. Rev. Cancer 2002, 2, 442–454. [Google Scholar] [CrossRef] [PubMed]
- Bartley, J.; Julicher, A.; Hotz, B.; Mechsner, S.; Hotz, H. Epithelial to mesenchymal transition (EMT) seems to be regulated differently in endometriosis and the endometrium. Arch. Gynecol. Obs. 2014, 289, 871–881. [Google Scholar] [CrossRef]
- Nieto, M.A. The snail superfamily of zinc-finger transcription factors. Nat. Rev. Mol. Cell Biol. 2002, 3, 155–166. [Google Scholar] [CrossRef]
- Garzon, S.; Laganà, A.S.; Barra, F.; Casarin, J.; Cromi, A.; Raffaelli, R.; Uccella, S.; Franchi, M.; Ghezzi, F.; Ferrero, S. Novel drug delivery methods for improving efficacy of endometriosis treatments. Expert Opin. Drug Deliv. 2020, 1–13. [Google Scholar] [CrossRef]
- Machairiotis, N.; Vasilakaki, S.; Kouroutou, P. Natural products: Potential lead compounds for the treatment of endometriosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 245, 7–12. [Google Scholar] [CrossRef]
- Kan, J.; Hood, M.; Burns, C.; Scholten, J.; Chuang, J.; Tian, F.; Pan, X.; Du, J.; Gui, M. A Novel Combination of Wheat Peptides and Fucoidan Attenuates Ethanol-Induced Gastric Mucosal Damage through Anti-Oxidant, Anti-Inflammatory, and Pro-Survival Mechanisms. Nutrients 2017, 9, 978. [Google Scholar] [CrossRef] [Green Version]
- van Weelden, G.; Bobiński, M.; Okła, K.; van Weelden, W.J.; Romano, A.; Pijnenborg, J.M.A. Fucoidan Structure and Activity in Relation to Anti-Cancer Mechanisms. Mar. Drugs 2019, 17, 32. [Google Scholar] [CrossRef] [Green Version]
- Rui, X.; Pan, H.F.; Shao, S.L.; Xu, X.M. Anti-tumor and anti-angiogenic effects of Fucoidan on prostate cancer: Possible JAK-STAT3 pathway. BMC Complement. Altern. Med. 2017, 17, 378. [Google Scholar] [CrossRef] [Green Version]
- Fernando, I.P.S.; Dias, M.; Madusanka, D.M.D.; Han, E.J.; Kim, M.J.; Jeon, Y.J.; Lee, K.; Cheong, S.H.; Han, Y.S.; Park, S.R.; et al. Human Keratinocyte UVB-Protective Effects of a Low Molecular Weight Fucoidan from Sargassum horneri Purified by Step Gradient Ethanol Precipitation. Antioxidants 2020, 9, 340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.; Lim, W.; Bazer, F.W.; Whang, K.Y.; Song, G. Quercetin inhibits proliferation of endometriosis regulating cyclin D1 and its target microRNAs in vitro and in vivo. J. Nutr. Biochem. 2019, 63, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.W.; Chen, H.Y.; Chiang, Y.F.; Chang, L.C.; Lin, P.H.; Hsia, S.M. The effects of isoliquiritigenin on endometriosis in vivo and in vitro study. Phytomedicine 2020, 77, 153214. [Google Scholar] [CrossRef] [PubMed]
- Machairiotis, N.; Stylianaki, A.; Dryllis, G.; Zarogoulidis, P.; Kouroutou, P.; Tsiamis, N.; Katsikogiannis, N.; Sarika, E.; Courcoutsakis, N.; Tsiouda, T.; et al. Extrapelvic endometriosis: A rare entity or an under diagnosed condition? Diagn. Pathol. 2013, 8, 194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laganà, A.S.; Garzon, S.; Götte, M.; Viganò, P.; Franchi, M.; Ghezzi, F.; Martin, D.C. The Pathogenesis of Endometriosis: Molecular and Cell Biology Insights. Int. J. Mol. Sci. 2019, 20, 5615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, W.; Zhang, L.; Liu, H.; Li, N.; Du, Y.; He, H.; Zhang, Z.; Liu, Y. E(2) -mediated EMT by activation of β-catenin/Snail signalling during the development of ovarian endometriosis. J. Cell. Mol. Med. 2019, 23, 8035–8045. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.H.; Kao, A.P.; Lin, T.C.; Chang, C.C.; Kuo, T.C. Promotion of epithelial-mesenchymal transition and tumor growth by 17β-estradiol in an ER(+)/HER2(+) cell line derived from human breast epithelial stem cells. Biotechnol. Appl. Biochem. 2012, 59, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Bilyk, O.; Coatham, M.; Jewer, M.; Postovit, L.M. Epithelial-to-Mesenchymal Transition in the Female Reproductive Tract: From Normal Functioning to Disease Pathology. Front. Oncol. 2017, 7, 145. [Google Scholar] [CrossRef] [Green Version]
- Fadin, M.; Nicoletti, M.C.; Pellizzato, M.; Accardi, M.; Baiett, M.G. Effectiveness of the integration of quercetin, turmeric, and N-acetylcysteine in reducing inflammation and pain associated with endometriosis. In vitro and in vivo studies. Minerva Ginecol. 2020. [Google Scholar] [CrossRef]
- Della Corte, L.; Noventa, M.; Ciebiera, M.; Magliarditi, M.; Sleiman, Z.; Karaman, E.; Catena, U.; Salvaggio, C.; Falzone, G.; Garzon, S. Phytotherapy in endometriosis: An up-to-date review. J. Complement. Integr. Med. 2020, 17. [Google Scholar] [CrossRef]
- Sutrisno, S.; Aprina, H.; Simanungkalit, H.M.; Andriyani, A.; Barlianto, W.; Sujuti, H.; Santoso, S.; Dwijayasa, P.M.; Wahyuni, E.S.; Mustofa, E. Genistein modulates the estrogen receptor and suppresses angiogenesis and inflammation in the murine model of peritoneal endometriosis. J. Tradit. Complement. Med. 2018, 8, 278–281. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.C.; Huang, R.Y.; Chou, T.C. Oligo-Fucoidan Improves Diabetes-Induced Renal Fibrosis via Activation of Sirt-1, GLP-1R, and Nrf2/HO-1: An In Vitro and In Vivo Study. Nutrients 2020, 12, 3068. [Google Scholar] [CrossRef]
- Chau, Y.T.; Chen, H.Y.; Lin, P.H.; Hsia, S.M. Preventive Effects of Fucoidan and Fucoxanthin on Hyperuricemic Rats Induced by Potassium Oxonate. Mar. Drugs 2019, 17, 343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, W.J.; Lin, M.H.; Kuo, T.C.; Chou, C.M.; Mi, F.L.; Cheng, C.H.; Lin, C.W. Fucoidan from Laminaria japonica exerts antitumor effects on angiogenesis and micrometastasis in triple-negative breast cancer cells. Int. J. Biol. Macromol. 2020, 149, 600–608. [Google Scholar] [CrossRef]
- Chen, H.Y.; Huang, T.C.; Lin, L.C.; Shieh, T.M.; Wu, C.H.; Wang, K.L.; Hong, Y.H.; Hsia, S.M. Fucoidan Inhibits the Proliferation of Leiomyoma Cells and Decreases Extracellular Matrix-Associated Protein Expression. Cell. Physiol. Biochem. 2018, 49, 1970–1986. [Google Scholar] [CrossRef]
- P, A.; K, A.; L, S.; M, M.; K, M. Anticancer effect of fucoidan on cell proliferation, cell cycle progression, genetic damage and apoptotic cell death in HepG2 cancer cells. Toxicol. Rep. 2019, 6, 556–563. [Google Scholar] [CrossRef]
- Di Zazzo, E.; Galasso, G.; Giovannelli, P.; Di Donato, M.; Bilancio, A.; Perillo, B.; Sinisi, A.A.; Migliaccio, A.; Castoria, G. Estrogen Receptors in Epithelial-Mesenchymal Transition of Prostate Cancer. Cancers 2019, 11, 1418. [Google Scholar] [CrossRef] [Green Version]
- Xue, M.; Ji, X.; Xue, C.; Liang, H.; Ge, Y.; He, X.; Zhang, L.; Bian, K.; Zhang, L. Caspase-dependent and caspase-independent induction of apoptosis in breast cancer by fucoidan via the PI3K/AKT/GSK3β pathway in vivo and in vitro. Biomed. Pharmacother. 2017, 94, 898–908. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, L.-C.; Chiang, Y.-F.; Chen, H.-Y.; Huang, Y.-J.; Liu, A.-C.; Hsia, S.-M. The Potential Effect of Fucoidan on Inhibiting Epithelial-to-Mesenchymal Transition, Proliferation, and Increase in Apoptosis for Endometriosis Treatment: In Vivo and In Vitro Study. Biomedicines 2020, 8, 528. https://doi.org/10.3390/biomedicines8110528
Chang L-C, Chiang Y-F, Chen H-Y, Huang Y-J, Liu A-C, Hsia S-M. The Potential Effect of Fucoidan on Inhibiting Epithelial-to-Mesenchymal Transition, Proliferation, and Increase in Apoptosis for Endometriosis Treatment: In Vivo and In Vitro Study. Biomedicines. 2020; 8(11):528. https://doi.org/10.3390/biomedicines8110528
Chicago/Turabian StyleChang, Li-Chun, Yi-Fen Chiang, Hsin-Yuan Chen, Yun-Ju Huang, An-Chieh Liu, and Shih-Min Hsia. 2020. "The Potential Effect of Fucoidan on Inhibiting Epithelial-to-Mesenchymal Transition, Proliferation, and Increase in Apoptosis for Endometriosis Treatment: In Vivo and In Vitro Study" Biomedicines 8, no. 11: 528. https://doi.org/10.3390/biomedicines8110528
APA StyleChang, L.-C., Chiang, Y.-F., Chen, H.-Y., Huang, Y.-J., Liu, A.-C., & Hsia, S.-M. (2020). The Potential Effect of Fucoidan on Inhibiting Epithelial-to-Mesenchymal Transition, Proliferation, and Increase in Apoptosis for Endometriosis Treatment: In Vivo and In Vitro Study. Biomedicines, 8(11), 528. https://doi.org/10.3390/biomedicines8110528