1. Introduction
The adult human heart has a minimal ability to regenerate, and thus the loss of viable cardiomyocytes in coronary heart disease, accelerated by myocardial infarction (MI), frequently leads to heart failure (HF). Bergmann et al. reported that cardiomyocyte renewal in physiological conditions in humans is as little as 1% of cells renewed per year at the age of 20 and further drops to 0.4% at the age of 75 [
1]. This rate can increase upon myocardial damage; however, no more than 3% of cardiomyocytes located near the injury region activate cell division, which is far too little for meaningful regeneration [
2]. Thus, a fibrotic scar produced by fibroblasts and myofibroblasts replaces lost cardiomyocytes, which sustains organ architecture but concurrently impairs the proper electromechanical activity of the heart [
3]. Importantly, no available treatment targeting MI enables restoration of functional cardiomyocytes in place of fibrous tissue, and in consequence, heart transplant remains the only therapeutic approach for the most severe cases. Thus, there is an immense need for novel therapies, which would allow for the recovery of viable myocardium in the injured site.
Mesenchymal stromal cells (MSCs), isolated from different tissues including bone marrow, fat and umbilical cord have been extensively investigated in recent years as a novel therapeutic approach for heart regeneration [
4]. None of these MSCs, however, demonstrate the ability to differentiate into cardiomyocytes and replace cells lost during MI. Hence, the putative beneficial effect of their administration in animal models has been ascribed to the paracrine activity of these cells [
4]. Additionally, as many experiments revealed, most MSCs injected intravenously are trapped in the lungs and rapidly die as a result of complement activation [
5,
6]. Thus, there is no compelling evidence for their ability to migrate and home into injured tissues upon systemic administration. Similarly, the limited survival of MSCs after local delivery into infarcted myocardium has so far precluded the development of effective therapy for heart damage [
7].
In contrast, human induced pluripotent stem cells (hiPSCs), generated from easily accessible somatic cells such as fibroblasts and peripheral blood mononuclear cells (PBMCs) demonstrate the capacity to efficiently differentiate into cardiomyocytes (hiPSC-CMs). Due to such properties, hiPSCs have provided novel opportunities to obtain cells applicable in regenerative medicine [
8]. Indeed, preclinical studies confirmed that administration of hiPSC-CMs into the infarcted myocardium improves heart function; however, further investigation is needed to develop the most effective approach for myocardial regeneration upon MI [
9]. Importantly, the limited survival of administered cells in the unfavorable environment of damaged tissue represents a major hindrance for successful cell therapy. Thus, novel strategies providing cytoprotection and increased angiogenesis in the site of injection may augment the therapeutic potential of any tested cell types.
Heme oxygenase-1 (HO-1, encoded by
HMOX1), is the enzyme that catalyzes the reaction of heme degradation leading to the generation of carbon monoxide (CO), iron ions and biliverdin [
10]. Studies of our group and many others reported a potent antioxidative, antiapoptotic and anti-inflammatory activity of HO-1 mediated by the products of its activity [
11], underlining the cytoprotective role of HO-1 in the maintenance of cellular homeostasis in various stress conditions including oxidative stress and hypoxia. Of note, it was demonstrated that HO-1 promotes cardiac mitochondrial biogenesis and its overexpression protects from doxorubicin-mediated induction of dilated cardiomyopathy through the regulation of mitochondrial quality control in the heart [
12]. Our recent study further indicated that lack of HO-1 negatively affects cardiac healing as we observed higher macrophage infiltration, prolonged postischemic inflammatory response and adverse late left ventricle remodeling in HO-1-deficient mice upon induction of MI [
13]. Similarly, stromal cell-derived factor 1α (SDF-1α, encoded by
CXCL12), a potent proangiogenic chemokine, has emerged as an important cardioprotective factor [
14]. Particularly, it protects cardiomyocytes from cell death during the first 72 h after left anterior descending artery (LAD) ligation in a murine model of MI and stimulates angiogenesis in hypoxic regions through upregulation of vascular endothelial growth factor (VEGF). Additionally, it was demonstrated that SDF-1α activates STAT3-dependent signaling pathways providing protection of heart function in the murine model of hypoxia/reoxygenation cardiac injury [
14].
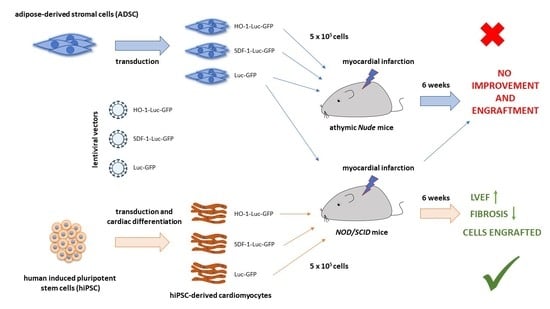
Accordingly, the aim of this study was to investigate and compare the therapeutic potential of genetically modified, either HO-1- or SDF-1α-overexpressing, MSCs and hiPSC-CMs in a murine model of acute MI. We utilized adipose-derived stromal cells (ADSCs) in the study, as adipose tissue represents one of the most convenient cell sources for autologous cell therapy due to its accessibility and ease of isolation [
4]. Nevertheless, our results demonstrate no effect of ADSC administration on heart function in sharp contrast to hiPSC-CMs, which provided significant recovery of left ventricle ejection fraction (LVEF) 6 weeks after MI induction. Additionally, our data indicate the importance of long-term evaluation of cell therapy effects, as the most pronounced changes in LVEF occurred between days 28 and 48 of cardiac function analysis, performed after cell administration.
2. Methods
2.1. ADSC Isolation and Culture
ADSCs were isolated from liposuction aspirate from the Hospital for Minimally Invasive and Reconstructive Surgery in Bielsko-Biała, Poland, upon obtaining informed consent from donors (approval of the Institutional Review Board and Bioethical Committee No. KB/430-62/13) as previously described [
15]. The experiments conformed to the principles outlined in the Declaration of Helsinki. Cells were cultured in αMEM medium (Macopharma, Tourcoing, France) supplemented with 10% human platelet lysate (Macopharma), heparin (2 U/mL, Polfa, Warsaw, Poland) and 1% penicillin–streptomycin solution (Sigma-Aldrich, St. Louis, MO, USA) under standard culture conditions (37 °C, 5% CO
2) and passaged upon reaching full confluency using 0.25% trypsin/EDTA solution (ThermoFisher Scientific, Waltham, MA, USA). Immunophenotyping of these cells confirming expression of CD105, CD29, CD73, CD90 and CD44 surface markers was previously described by Czapla et al. [
15], and the same ADSCs were used for transduction and further in vivo administration.
2.2. Generation and Culture of hiPSCs
The hiPSC line used in this study was generated from peripheral blood mononuclear cells (PBMCs) isolated from a healthy volunteer upon obtaining informed consent (approval of Jagiellonian University Bioethical Committee No. 122.6120.303.2016; the experiments conformed to the principles outlined in the Declaration of Helsinki). For that purpose, 10 mL of blood was diluted in 30 mL of phosphate-buffered saline (PBS) (Lonza, Basel, Switzerland) with 2 mM EDTA, layered onto 15 mL of Ficoll-Paque (GE Healthcare, Chicago, IL, USA) and centrifuged at 400 ×
g for 40 min at room temperature (RT). The layer of mononuclear cells was then transferred into a new conical tube, washed twice with PBS and resuspended in StemPro-34 medium supplemented with StemPro-34 Nutrient Supplement (ThermoFisher Scientific) and cytokines: 100 ng/mL SCF (Peprotech, Cranbury, NJ, USA), 100 ng/mL FLT3-Ligand (Peprotech), 20 ng/mL IL-3 (Peprotech) and 20 ng/mL IL-6 (PBMC medium). Cells were plated on a 24-well plate and cultured for an additional 6 days with medium changed every other day. Subsequently, 10
5 isolated PBMCs were reprogrammed using Cytotune-iPS 2.0 Sendai Reprogramming Kit (ThermoFisher Scientific) according to the manufacturer’s protocol. Briefly, cells were suspended in PBMC medium containing KOS (encoding KLF4, OCT4 and SOX2), hc-MYC and hKLF4 Sendai vectors (multiplicity of infection (MOI) = 5, 5 and 3, respectively), centrifuged at 1000 ×
g for 30 min at RT and seeded on a 24-well plate. After 24 h, medium was replaced with fresh PBMC medium for an additional 2 days, after which cells were collected and seeded in 2 wells of a 6-well plate covered with Geltrex (ThermoFisher Scientific, diluted according to manufacturer’s protocol) in StemPro-34 medium supplemented with StemPro-34 Nutrient Supplement. On day 7, half of the medium was replaced with Essential 8 medium (E8, ThermoFisher Scientific), and cells were further cultured in E8 for additional 2 weeks. Then, the hiPSC colonies were picked and expanded. The hiPSC line used in this study was cultured in E8 on Geltrex-coated plates in standard culture conditions (37 °C, 5% CO
2) and passaged upon reaching 80–90% confluency using 0.5 mM EDTA solution. Pluripotency of hiPSCs was confirmed with immunofluorescent analysis of OCT4, NANOG, SSEA4, TRA-1-60 and TRA-1-81 expression as well as in vitro spontaneous differentiation assay (as described in the
Supplementary Methods).
2.3. Cardiac Differentiation of hiPSCs
To obtain genetically modified cardiomyocytes, lentiviral vector-transduced and sorted hiPSCs were subjected to cardiac differentiation according to the protocol described by Lian et al. [
16]. Briefly, 3 × 10
4 cells were seeded on Geltrex-coated 24-well plate and cultured in E8 for additional 4 days until they reached 100% confluency. Then, the medium was changed to RPMI1640 (Lonza) supplemented with 2% B27 without insulin (ThermoFisher Scientific, RMPI/B27-ins medium) and 12 µM CHIR99021 (Sigma-Aldrich). After 24 h, the medium was replaced with RMPI/B27-ins for an additional 2 days. On day 3, cells were stimulated with 5 µM IWR-1 (Sigma-Aldrich) in RPMI/B27-ins (collected from the cells and fresh mixed 1:1). After 48 h, the medium was replaced with fresh RPMI/B27-ins; starting from day 7, cells were cultured in RPMI1640 supplemented with 2% B27 (ThermoFisher Scientific, RMPI/B27), which was changed every third day. On day 20, the cells were collected and passaged using Multi Tissue Dissociation Kit 3 (Miltenyi Biotec, Bergisch Gladbach, Germany), collected in RPMI1640 supplemented with 20% FBS (EURx, Gdańsk, Poland), centrifuged (200×
g, 5 min, RT), resuspended in fresh RPMI/B27 and seeded on Geltrex-coated 10 cm plates.
2.4. Experimental Animals
Female, 6–8-week-old athymic Foxn1nu mice (Nude, purchased from Harlan Laboratories, Füllinsdorf, Switzerland) and 6–8-week-old female NOD.CB-17-Prkdc scid/Rj (NOD/SCID, Janvier Labs, Le Genest-Saint-Isle, France) mice were used in this study. All animal procedures were performed according to guidelines from Directive 2010/63/EU of the European Parliament on the protection of animals used for scientific purposes and were accepted by the Second Local Ethical Committee for Animal Research in Krakow, Poland (approval numbers: 21/2014, 136/2016 and 58/2018). Mice were maintained under controlled specific pathogen-free (SPF) environmental conditions with standard laboratory food and water provided ad libitum.
2.5. Induction of Myocardial Infarction and Administration of Cells
MI was induced in both murine strains as previously described [
13]. Briefly, mice were anesthetized with 2,2,2-tribromoethanol (400 mg/kg of body weight), intubated and mechanically ventilated (respiration rate: 220 breaths/min, stroke volume 280 µL). Upon surgical exposition of the heart, LAD was permanently ligated with a silk suture placed 1–2 mm below the left auricle. Instant change of the myocardium color from bright red to white exposed the area of myocardium affected by sudden occlusion of the blood vessel supplying this region. Cells were injected into 4 sites at the border of the ischemic area (as shown in Figure 2a; 2.5 µL containing 1.25 × 10
5 cells suspended in saline per site; 5 × 10
5 cells in the volume of 10 µL in total), immediately after LAD ligation. Cells were injected with a 25 μL Hamilton syringe with a 33-gauge needle. MI control group was injected with saline only (without the cells suspended). Sham control mice were subjected to the same procedure; however, upon placing the suture underneath LAD, the vessel was not ligated, and the suture was removed. All animals were subjected to analgesia twice daily for 3 consecutive days after the surgery by subcutaneous injection of buprenorphine at the dose of 0.08 mg/kg of body weight.
Of note, in our research, we have previously used 2,2,2-tribromoethanol to anesthetize mice for MI surgery, and it was very well tolerated by the mice of
C57BL/6xFVB strain [
13], which on the other hand did not tolerate ketamine–xylazine well (unpublished). In that case, all animals anesthetized with 2,2,2-tribromoethanol survived the MI, and any observed demise occurred mainly in wild-type
C57BL/6xFVB mice between the 3rd and 5th days after LAD ligation due to left ventricular free wall rupture [
13]. It is known that ketamine–xylazine produces profound bradycardia [
17], and our pilot experiments in
Nude and
NOD/SCID mice also revealed that these drugs did not provide satisfactory depth of anesthesia (based on the limb withdrawal response to toe pinch) and their increased doses were lethal (not shown). Therefore, taking into account our previous good experience with 2,2,2-tribromoethanol [
13], we used this compound also for anesthesia of
Nude and
NOD/SCID mice.
To confirm successful MI induction, 24 h after surgery, facial vein puncture was performed intravitally and peripheral blood (PB) was collected, anticoagulated with heparin (5 U per 1 mL blood) and centrifuged (800 × g, 10 min, 4 °C), after which plasma was transferred to a new tube and stored frozen at −80 °C. Cardiac troponin I (cTnI) was assessed in 100 μL of plasma with ELISA (DRG MedTek, Warsaw, Poland) according to the manufacturer’s protocol.
2.6. Transthoracic Echocardiography
Transthoracic echocardiography was performed on days 7, 14, 28 and 42 after MI using a Vevo 2100 system (Visual Sonics, Toronto, ON, Canada) with a 30-MHz transducer and inhalation anesthesia (2% isoflurane in air, Aerrane, Baxter, Deerfield, IL, USA) as previously described [
13]. The heart was imaged in the bidimensional (2-D) mode, in the parasternal long-axis (PSLAX) view. For analysis of left ventricular end-diastolic volume (LVVd) and left ventricular end-systolic volume (LVVs), the endocardium of the left ventricle was traced at both diastole and systole. An integrated software tool (LV-Trace) was used for single-plane PSLAX analysis.
2.7. Bioluminescence Imaging
To assess the presence of ADSCs-Luc-GFP in Nude mice, animals were subjected to intracardiac injection of 5 × 105 cells. After 72 h, luciferin solution was administered intraperitoneally (150 mg/kg of body weight in volume of 100 µL) and after 10 min mice were anesthetized by isoflurane inhalation (5% v/v isoflurane/air mixture for induction and 1.5–2% v/v isoflurane/air for maintenance of anesthesia) and transferred onto a temperature-controlled, heated table (37 °C) in the detecting chamber of an IVIS Lumina II detector. Bioluminescence from the heart was recorded for 5 min.
To assess the presence of injected genetically modified hiPSC-CMs and ADSCs-Luc-GFP, the NOD/SCID mice were subjected to bioluminescence detection using an IVIS Lumina II detector on days 7, 14, 28 and 42 after surgery. For that purpose, luciferin solution was administered intraperitoneally (150 mg/kg of body weight in volume of 100 µL), and after 10 min mice were anesthetized by isoflurane inhalation (5% v/v isoflurane/air mixture for induction and 1.5–2% v/v isoflurane/air for maintenance of anesthesia), chest-shaved and transferred onto a temperature-controlled, heated table (37 °C) in the detecting chamber. Bioluminescence from the heart was recorded for 10 min. On day 42, the bioluminescence was recorded as described intravitally and directly in hearts isolated from sacrificed animals.
2.8. Tissue Collection
On day 42 after MI induction and cell administration, mice were euthanized by intraperitoneal injection of ketamine (200 mg/kg of body weight) and xylazine (40 mg/kg of body weight); their chests were opened and the hearts arrested in diastole by intraventricular injection of 30 mM KCl in saline containing 0.5 U/mL of heparin, followed by right atrium removal and heart perfusion with 5 mL of saline supplemented with 0.5 U/mL of heparin as previously described [
13]. Organs were then excised, embedded in OCT compound (Tissue-Tek, Sakura Finetek, Torrance, CA, USA) and snap-frozen on dry ice.
2.9. Histological Analysis
To assess the level of fibrosis within the infarct zone, Masson’s trichrome stain was performed on 8 µm frozen sections of the heart. For that purpose, sections were washed with PBS and fixed for 1 h at RT in 10% buffered formalin solution and then incubated in Bouin’s solution (Sigma-Aldrich) for 30 min at 37 °C. All further steps were performed using the trichrome stain (Masson) kit (Sigma-Aldrich) according to the manufacturer’s protocol. The level of fibrosis was calculated based on histological analysis of heart sections. Each dot represents a single section from 2–4 animals. Scans of each stained section were collected using an LMD7000 Leica Microsystem microscope. The level of fibrosis was assessed using QuPath software [
18] and calculated as the ratio of the area of blue-stained tissue to the entire surface area of the analyzed section. For ADSC-treated hearts, samples from 2 surviving animals were analyzed, whereas, for saline and hiPSC-CM-treated hearts, samples from 3–4 animals were assessed. In each group, from 3 to 23 sections were analyzed.
2.10. Immunohistochemical Analysis
To assess the presence of human cells within murine myocardium, the proliferation of injected cells and the number of alpha smooth muscle actin (αSMA)-positive vessels, the frozen 8 µm sections were washed in PBS, fixed in cold (−20 °C) acetone for 10 min and incubated for 30 min at RT in 0.2% Sudan Black B solution prepared as described previously [
19]. Specimens were washed 5 times in water, blocked in Mouse on Mouse (MOM, Vector Laboratories, Burlingame, CA, USA) solution for 30 min at RT and then in 10% goat or donkey serum (Sigma-Aldrich) diluted in PBS for 1 h at RT and incubated overnight at 4 °C with primary antibodies 1:200 rabbit anti-human Ku80 (Abcam, Cambridge, UK, clone EPR3468), 1:200 rabbit anti-αSMA (Abcam, polyclonal), 1:100 FITC-conjugated mouse anti-Ki67 (BD Biosciences, San Jose, CA, USA, clone: B56), 1:100 anti-connexin 43 (Cx43, Abcam, polyclonal), 1:100 anti-α-actinin (Sigma-Aldrich, clone EA-53), anti-NKX2.5 (Santa Cruz Biotechnology, Dallas, TX, USA, polyclonal), 1:200 anti-GATA4 (Santa Cruz Biotechnology, polyclonal) and 1:200 mouse anti-cardiac troponin T (ThermoFisher Scientific, clone: 13-11) diluted in 1% goat or donkey serum. After washing 5 times with PBS, samples were incubated for 1 h at RT with secondary antibodies AlexaFluor568 goat anti-rabbit (for human Ku80 detection), AlexaFluor488 goat anti-rabbit (for αSMA detection), AlexaFluor568 goat anti-mouse, AlexaFluor647 donkey anti-mouse, AlexaFluor568 donkey anti-rabbit, AlexaFluor488 donkey anti-mouse (all from ThermoFisher Scientific) diluted in 1% goat or donkey serum with 0.2 µg/mL DAPI. Donkey serum and donkey-originated secondary antibodies were used for high-resolution images. Of note, AlexaFluor488-conjugated secondary antibody used as GFP in hiPSC-CMs was quenched after fixation of sections (data not shown). Sections were eventually mounted (DAKO Fluorescence mounting medium) and visualized under a fluorescent microscope (Nikon Eclipse TS100). The number of αSMA-positive vessels per mm
2 of tissue was calculated using ImageJ software [
20]. In each group (saline- and hiPSC-CM-treated hearts) samples from 2–3 animals were analyzed (5–12 sections per animal).
2.11. Statistical Analysis
Data are presented as mean ± SEM. To analyze statistical significance, a one-way ANOVA followed by Bonferroni’s post hoc test for multiple comparisons was used. Survival curves were analyzed by log-rank test. Statistical analyses were performed using GraphPad Prism software. p < 0.05 was considered as statistically significant.
4. Discussion
The salient finding of the present study is the demonstration that hiPSC-CMs can ameliorate the development of heart failure after myocardial infarction while the ADSCs are ineffective. The work adds to elucidating the potential of different cell types tested for the treatment of heart injury.
Numerous studies have focused so far on the utilization of so-called mesenchymal stromal cells, improperly named generally as “stem cells”; however, due to their inability to differentiate into cardiomyocytes and limited survival upon in vivo administration, no effective treatment targeting myocardial infarction has been developed so far [
4]. Importantly, the results from the current study further corroborate these findings, as neither native ADSCs nor ADSCs overexpressing cardioprotective, proangiogenic and immunomodulatory factors provided improved heart function in the model of acute MI. A similar lack of therapeutic potential in two immunocompromised murine strains, namely
Nude and
NOD/SCID, indicates that the reported failure to provide beneficial effects did not depend on the receptiveness of mice to human cells.
In contrast to ADSCs, cardiomyocytes generated from pluripotent stem cells represent biologically justified candidates for cell therapy, as these cells are lost and do not regenerate after heart injury. Previous studies have also demonstrated that genetic or electromechanical conditioning of human embryonic stem cell (hESC)- or hiPSC-derived cardiomyocytes enhances their therapeutic potential in animal MI models. For instance, genetically modified hiPSC-CMs expressing N-cadherin enhanced LVEF and decreased infarct size more profoundly than nonmodified cells [
24].
In this study, we hypothesized that overexpression of HO-1 and SDF-1α would further enhance the regenerative potential of hiPSC-CMs. The rationale for overexpression of
HMOX-1, encoding HO-1, was based on our previous studies demonstrating that lack of HO-1 resulted in adverse late LV remodeling due to overactive and prolonged postischemic inflammatory response [
13]. Reversely, when Yet et al. subjected transgenic mice with cardiac-specific overexpression of human HO-1 to ischemic/reperfusion injury of the heart, the improved contractile activity in the reperfusion phase and reduced infarct size in these animals in comparison to the control counterparts was observed [
25]. Of note, this phenotypic effect was underlain by decreased inflammatory cell infiltration and oxidative damage. Other reports also revealed an important role of HO-1 in the regulation of cardiomyocyte metabolism [
12,
26]. On the other hand, SDF-1α has been also described as potent cardioprotective chemokine acting mainly through binding to its cognate receptor CXCR4 and downstream activation of STAT3 transcription factor in the ischemia/reperfusion conditions [
14].
Nonetheless, we did not observe that HO-1 or SDF-1α overexpression additionally augments the already substantial therapeutic potential of control hiPSC-CMs in the experimental settings used in the study. Interestingly, the most profound restoration of cardiac function in all groups receiving hiPSC-CMs was detected between day 28 and day 42 post-MI, highlighting the need for long-term evaluation of cell therapy effects. This appears to be an important observation as many studies terminate cardiac function measurements on day 28 after MI. Van Laake et al. raised a similar issue after describing that the therapeutic effect of hESC-CM administration in a murine model of acute MI is transient and can be observed 4 weeks after injection but not after 3 months [
27]. Nevertheless, the improvement in cardiac function described in that study was much lower (LVEF in hESC-CM-treated animals was less than 30% on week 4) than in our experiments. Longer follow-up of our analysis may be essential to assess whether overexpression of either HO-1 or SDF-1α in hiPSC-CMs further augments LVEF in time points later than 6 weeks post-MI. To this end, however, we cannot exclude that the level of HO-1 and SDF-1α locally in murine myocardium was too low to further enhance heart function and that, within the time period tested, both factors do not provide a significant therapeutic effect in the applied experimental model.
Including longer time-points is also particularly important, as vector-based genetic modifications provide permanent expression of transgenes and thus generate a risk of detrimental impact of delivered factors on the administered cell population and/or surrounding tissue. Gabisonia et al., for instance, reported that AAV-based expression of human microRNA-199a (miR-199a) in pig myocardium subjected to MI resulted initially in significant improvement of heart function and decreased infarct size up to week 7 post-surgery, when sudden death of most of the animals occurred [
28]. As miR-199a induces proliferation of cardiomyocytes, the initial therapeutic effect depended on the restoration of the proliferative capacity of swine nondamaged myocardium. Further histological analyses revealed that the same mechanism accounted for the formation of dividing cells, which displayed poorly differentiated myoblastic phenotype in the long term [
28]. Similarly, in our previous study, we demonstrated that high persistent overexpression of HO-1 in murine myoblasts augmented their proliferation and upon in vivo administration led to the development of fast-growing, hyperplastic tumors infiltrating the surrounding tissues [
29]. However, in the current study, we noticed only single Ki67-positive hiPSC-CMs that survived in murine myocardium up to day 42 post-MI, with no signs of tumor growth in any of the tested groups. Interestingly, we detected the highest signal of bioluminescence originating from luciferase expression in mice treated with HO-1-overexpressing hiPSC-CMs, which may indicate better survival of these cells in murine myocardium. This observation goes in line with those of Luo et al., who demonstrated that pretreatment of hESC-CMs with cobalt protoporphyrin IX (CoPPIX), a potent inducer of HO-1 expression, resulted in significantly larger graft size upon intramyocardial administration in the rat model of acute myocardial infarction in comparison to PBS-pretreated cells [
30]. Interestingly, in another report, Kirby et al. searched for small molecules that can protect hiPSC-CMs from peroxide-induced cell death. Evaluation of 48,640 compounds revealed that the hit molecule strongly upregulated HO-1 expression [
31], indicating that the regulation of HO-1 level in cardiomyocytes may increase their survival in oxidative stress conditions. Nevertheless, long-term studies in larger animal models are necessary to demonstrate the sustained beneficial effect and to exclude any detrimental influence of persistent HO-1 overexpression.
Vagnozzi et al. recently documented that the beneficial effect of cell therapy using fractionated bone marrow mononuclear cells (often used in human clinical trials) and cardiac mesenchymal cells in a murine model of ischemia/reperfusion heart injury does not depend on the delivered cells, which were cleared from the host tissue within two weeks after administration [
32]. Particularly, the same effect was achieved with zymosan, a potent inducer of innate immune response, and with freeze/thawed killed cells, whereas further analyses confirmed that the improvement of cardiac function was dependent on the acute inflammation-based wound healing. However, our histological examinations revealed the presence of human cardiomyocytes in the murine hearts treated with control and either HO-1- or SDF-1α-overexpressing hiPSC-CMs. This strongly suggests that the beneficial effect of hiPSC-CM administration observed in our study was related to the activity of these cells, either paracrine and/or via direct effect on murine myocardium. Additionally, as we used immunocompromised animals, we may anticipate that the acute immune response was blunted and could not play such an important role. This would also explain the lack of the therapeutic effect upon injection of ADSCs, which were not detected in the murine myocardium based on the luciferase activity at any tested time points. We cannot, however, completely exclude the effect of macrophages at the site of injury and cell injection, as our study did not address the evaluation of the early innate immune response. Thus, further studies are needed to address the role of immune cells in hiPSC-CM- and ADSC-based cell therapy outcomes.
Detection of hiPSC-CMs in close proximity to murine myocardium surviving in the left ventricle after MI described in this study emphasizes possible direct interactions between human and mouse cardiomyocytes and thus a risk of pro-arrhythmogenic activity of delivered cells. Indeed, immunohistochemical analysis revealed the expression of connexin 43 in engrafted hiPSC-CMs with direct coupling of human cells with murine myocardium. An arrhythmogenic effect was already described for hESC-derived CMs administrated in large animal models, including pigs [
33] and macaques [
34], and therefore telemetric monitoring of heart rate would be of high importance for the future studies to comprehensively assess the effect of hiPSC-CMs and their genetic modifications on the electromechanical activity of host myocardium.