Photo-Based Nanomedicines Using Polymeric Systems in the Field of Cancer Imaging and Therapy
Abstract
:1. Introduction
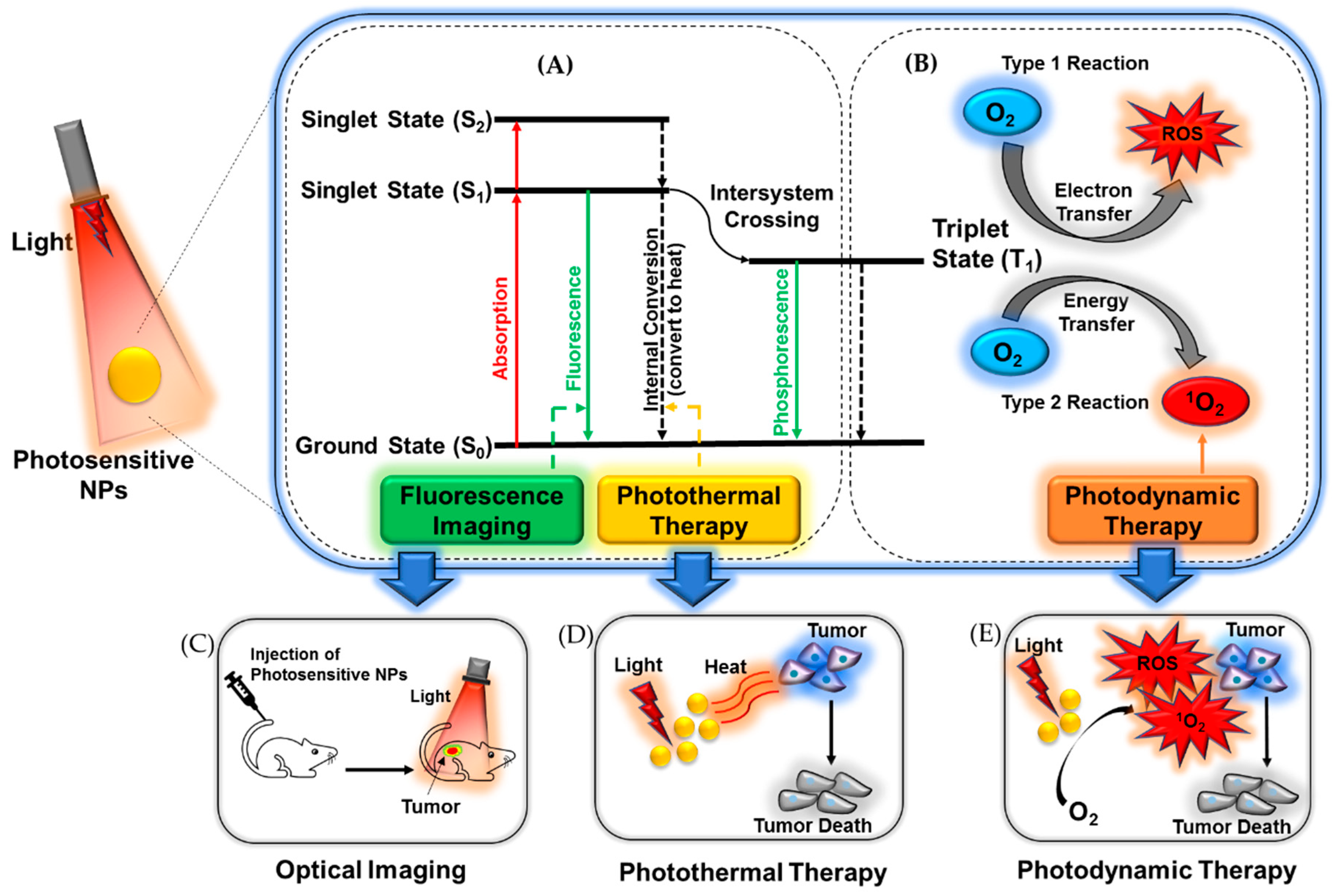
2. Benefits and Risks of Photo-Based Imaging and Therapy
3. Optical Imaging
4. Phototherapy
5. Photosensitive Nanomedicines
6. Polymer-Based Photosensitive Nanomedicine
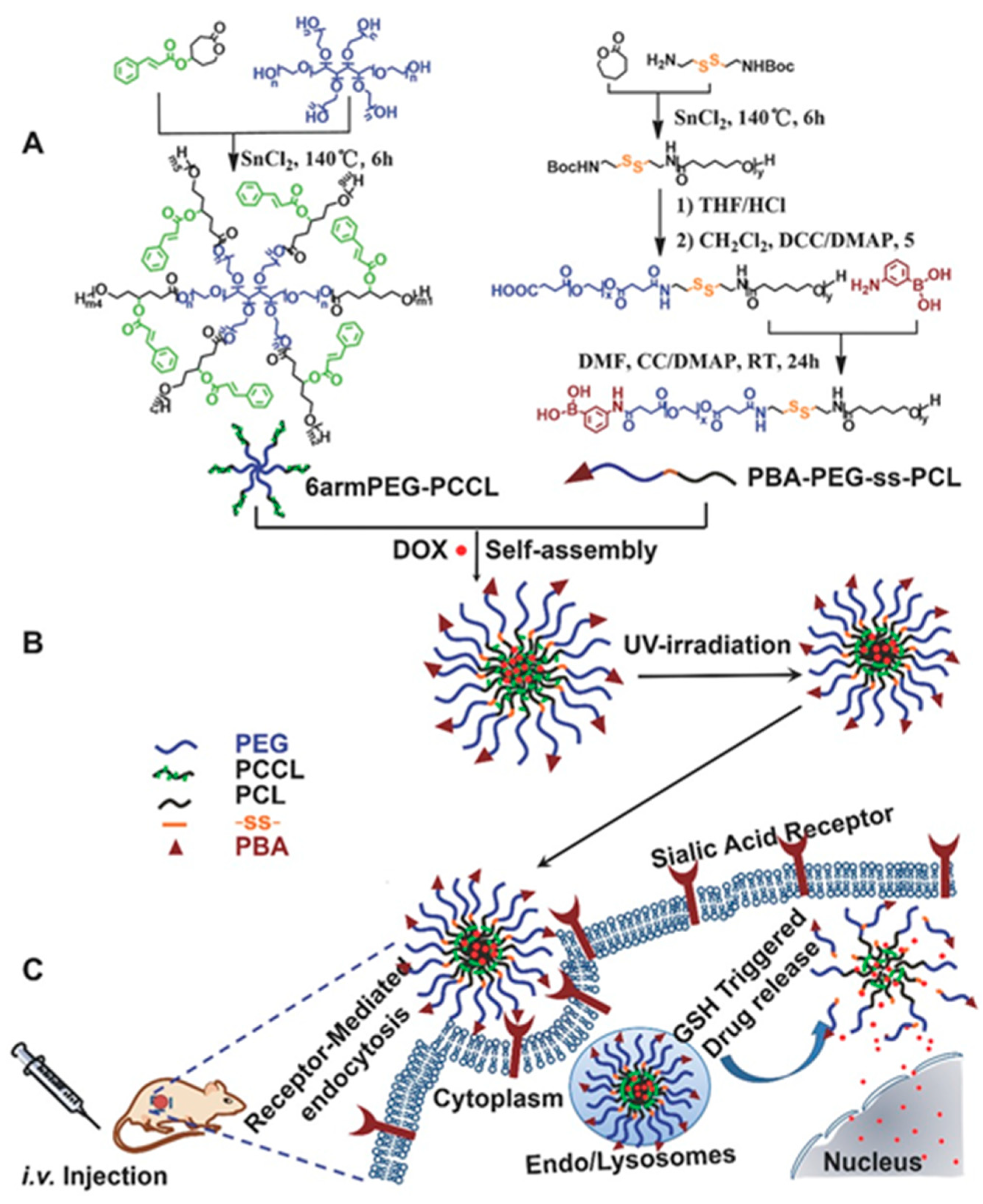
6.1. Photosensitive Nanomedicine Using Linear Polymers
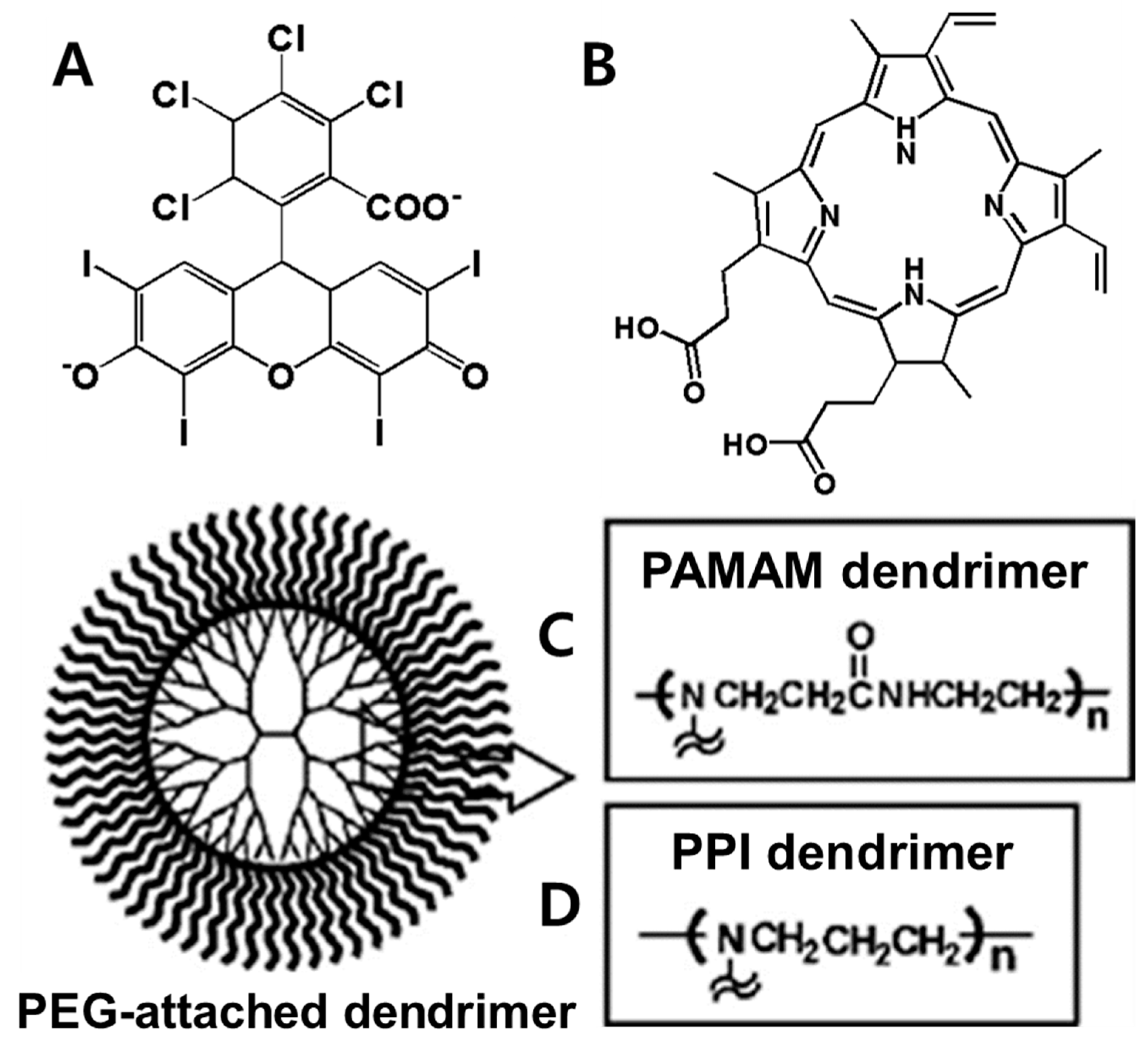
6.2. Photosensitive Nanomedicine Using Branched Polymers
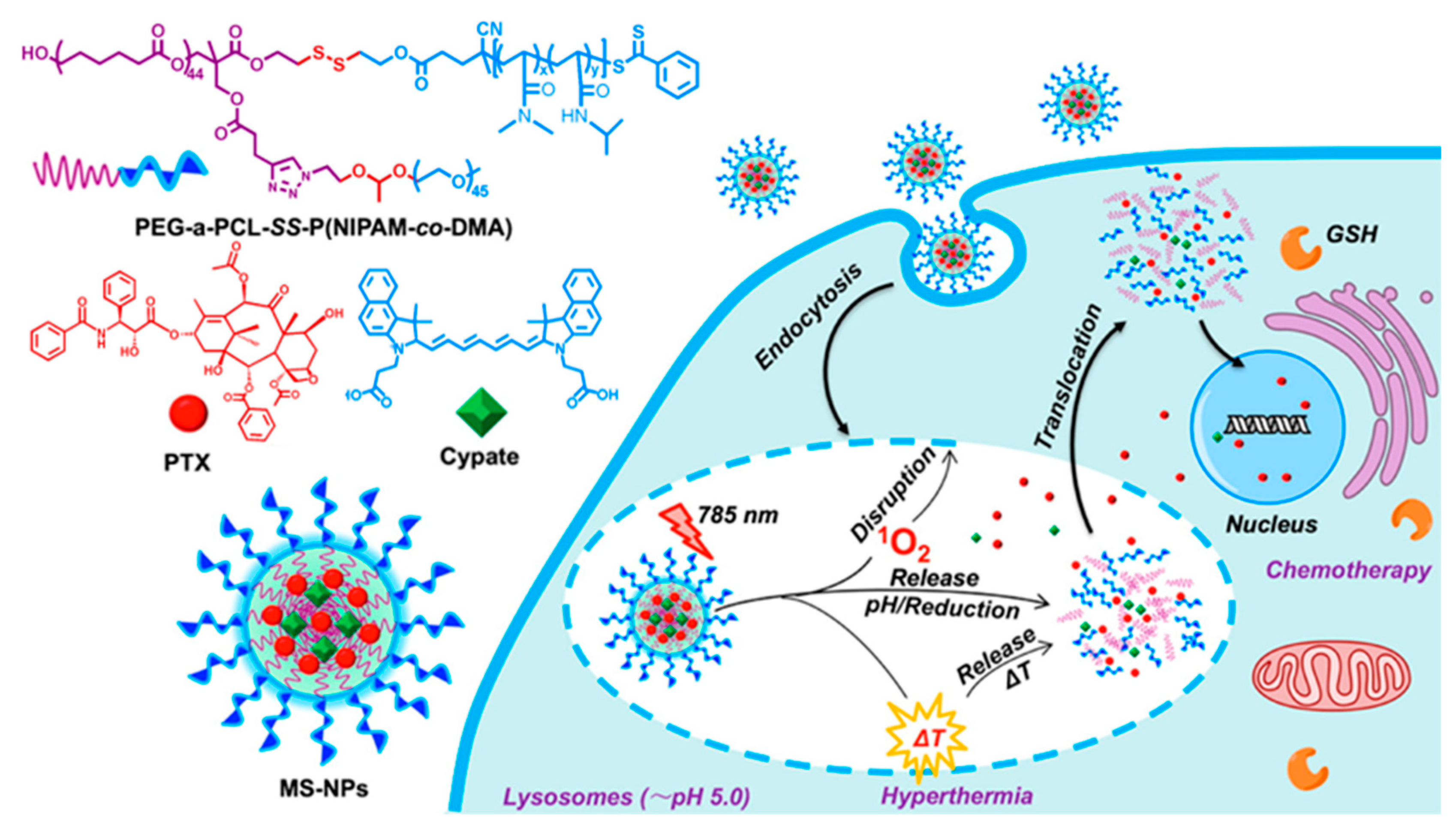
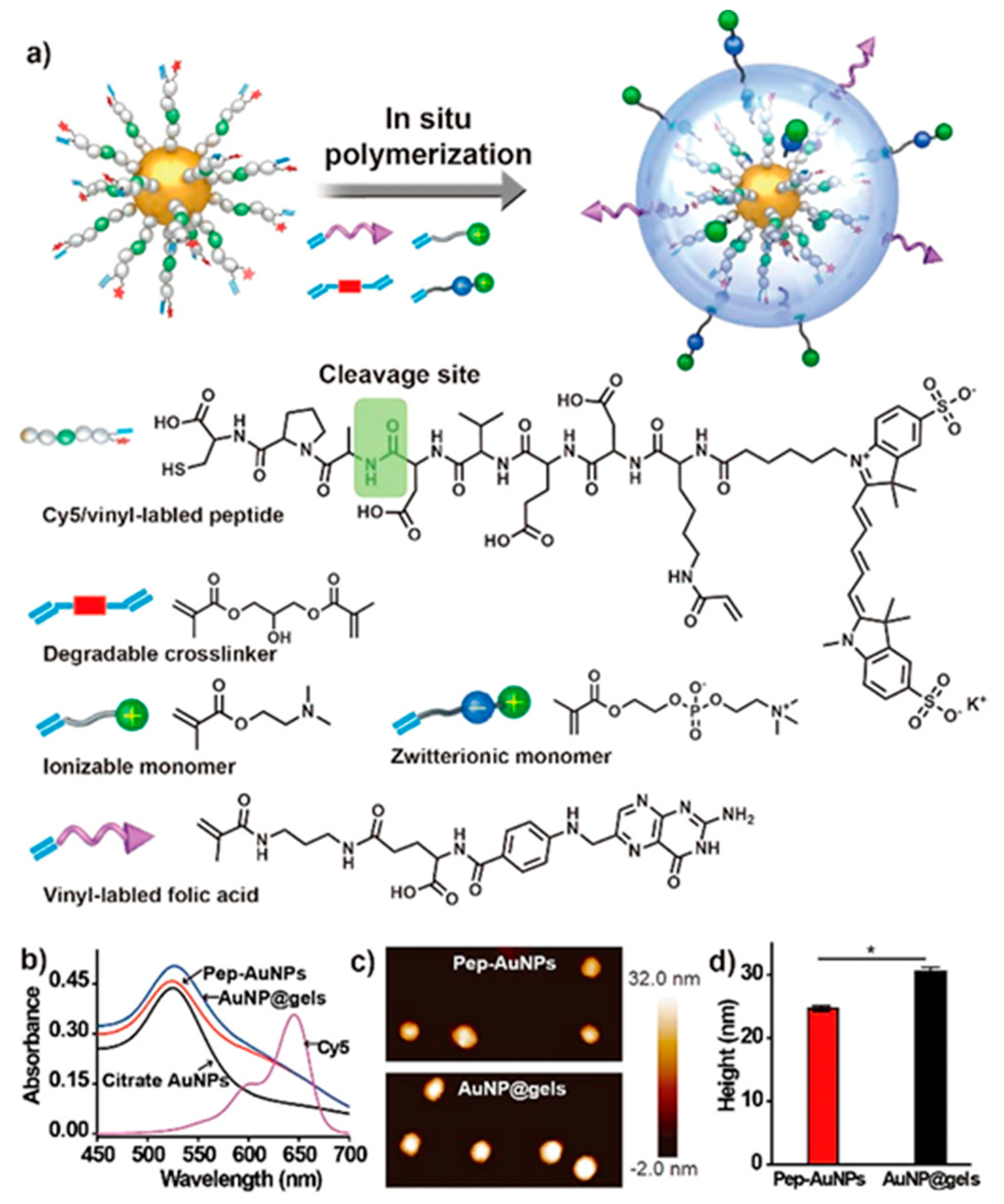
6.3. Photosensitive Nanomedicine Using Crosslinked Polymers
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lim, C.; Won, W.R.; Moon, J.; Sim, T.; Shin, Y.; Kim, J.C.; Lee, E.S.; Youn, Y.S.; Oh, K.T. Co-delivery of d-(KLAKLAK) 2 peptide and doxorubicin using a pH-sensitive nanocarrier for synergistic anticancer treatment. J. Mater. Chem. B 2019, 7, 4299–4308. [Google Scholar] [CrossRef]
- Pedrosa, P.; Vinhas, R.; Fernandes, A.; Baptista, P.V. Gold nanotheranostics: Proof-of-concept or clinical tool? Nanomaterials 2015, 5, 1853–1879. [Google Scholar] [CrossRef] [Green Version]
- Lim, C.; Moon, J.; Sim, T.; Won, W.R.; Lee, E.S.; Youn, Y.S.; Oh, K.T. A nano-complex system to overcome antagonistic photo-chemo combination cancer therapy. J. Control. Release 2019, 295, 164–173. [Google Scholar] [CrossRef]
- Gao, D.; Guo, X.; Zhang, X.; Chen, S.; Wang, Y.; Chen, T.; Huang, G.; Gao, Y.; Tian, Z.; Yang, Z. Multifunctional phototheranostic nanomedicine for cancer imaging and treatment. Mater. Today Bio 2020, 5, 100035. [Google Scholar] [CrossRef]
- Cheng, S.-H.; Lee, C.-H.; Chen, M.-C.; Souris, J.S.; Tseng, F.-G.; Yang, C.-S.; Mou, C.-Y.; Chen, C.-T.; Lo, L.-W. Tri-functionalization of mesoporous silica nanoparticles for comprehensive cancer theranostics—The trio of imaging, targeting and therapy. J. Mater. Chem. 2010, 20, 6149–6157. [Google Scholar] [CrossRef]
- Bagalkot, V.; Zhang, L.; Levy-Nissenbaum, E.; Jon, S.; Kantoff, P.W.; Langer, R.; Farokhzad, O.C. Quantum dot− aptamer conjugates for synchronous cancer imaging, therapy, and sensing of drug delivery based on bi-fluorescence resonance energy transfer. Nano Lett. 2007, 7, 3065–3070. [Google Scholar] [CrossRef] [PubMed]
- Galanzha, E.I.; Kim, J.W.; Zharov, V.P. Nanotechnology-based molecular photoacoustic and photothermal flow cytometry platform for in-vivo detection and killing of circulating cancer stem cells. J. Biophotonics 2009, 2, 725–735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szaciłowski, K.; Macyk, W.; Drzewiecka-Matuszek, A.; Brindell, M.; Stochel, G. Bioinorganic photochemistry: Frontiers and mechanisms. Chem. Rev. 2005, 105, 2647–2694. [Google Scholar] [CrossRef] [PubMed]
- Ponsonby, A.-L.; McMichael, A.; Van Der Mei, I. Ultraviolet radiation and autoimmune disease: Insights from epidemiological research. Toxicology 2002, 181, 71–78. [Google Scholar] [CrossRef]
- Jori, G.; Spikes, J.D. Photothermal sensitizers: Possible use in tumor therapy. J. Photochem. Photobiol. B 1990, 6, 93–101. [Google Scholar] [CrossRef]
- Rosencwaig, A. Photoacoustic spectroscopy of biological materials. Science 1973, 181, 657–658. [Google Scholar] [CrossRef]
- Denk, W.; Strickler, J.H.; Webb, W.W. Two-photon laser scanning fluorescence microscopy. Science 1990, 248, 73–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tong, R.; Kohane, D.S. Shedding light on nanomedicine. WIREs Nanomed. Nanobiotechnol. 2012, 4, 638–662. [Google Scholar] [CrossRef] [PubMed]
- Allémann, E.; Rousseau, J.; Brasseur, N.; Kudrevich, S.V.; Lewis, K.; Van Lier, J.E. Photodynamic therapy of tumours with hexadecafluoro zinc phthalocyanine formulated in PEG-coated poly (lactic acid) nanoparticles. Int. J. Cancer 1996, 66, 821–824. [Google Scholar] [CrossRef]
- Ideta, R.; Tasaka, F.; Jang, W.-D.; Nishiyama, N.; Zhang, G.-D.; Harada, A.; Yanagi, Y.; Tamaki, Y.; Aida, T.; Kataoka, K. Nanotechnology-based photodynamic therapy for neovascular disease using a supramolecular nanocarrier loaded with a dendritic photosensitizer. Nano Lett. 2005, 5, 2426–2431. [Google Scholar] [CrossRef] [PubMed]
- Man, F.; Lammers, T.; de Rosales, R.T.M. Imaging nanomedicine-based drug delivery: A review of clinical studies. Mol. Imaging Biol. 2018, 20, 683–695. [Google Scholar] [CrossRef] [Green Version]
- Germain, M.; Caputo, F.; Metcalfe, S.; Tosi, G.; Spring, K.; Åslund, A.K.O.; Pottier, A.; Schiffelers, R.; Ceccaldi, A.; Schmid, R. Delivering the power of nanomedicine to patients today. J. Control. Release 2020, 326, 164–171. [Google Scholar] [CrossRef]
- Moghimi, S.M.; Hunter, A.C.; Murray, J.C. Nanomedicine: Current status and future prospects. FASEB J. 2005, 19, 311–330. [Google Scholar] [CrossRef] [Green Version]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941. [Google Scholar] [CrossRef]
- Li, J. Nanotechnology-based platform for early diagnosis of cancer. Sci. Bull. 2015, 60, 488–490. [Google Scholar] [CrossRef]
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016, 1, 1–12. [Google Scholar] [CrossRef]
- Kiio, T.M.; Park, S. Physical properties of nanoparticles do matter. J. Pharm. Investig. 2020. [Google Scholar] [CrossRef]
- van Nostrum, C.F. Polymeric micelles to deliver photosensitizers for photodynamic therapy. Adv. Drug Deliv. Rev. 2004, 56, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, D.K.; Fong, L.S.; Zhang, Y. Nanoparticles in photodynamic therapy: An emerging paradigm. Adv. Drug Deliv. Rev. 2008, 60, 1627–1637. [Google Scholar] [CrossRef] [PubMed]
- Segalla, A.; Borsarelli, C.D.; Braslavsky, S.E.; Spikes, J.D.; Roncucci, G.; Dei, D.; Chiti, G.; Jori, G.; Reddi, E. Photophysical, photochemical and antibacterial photosensitizing properties of a novel octacationic Zn (II)-phthalocyanine. Photochem. Photobiol. Sci. 2002, 1, 641–648. [Google Scholar] [CrossRef]
- Jeon, G.; Ko, Y.T. Enhanced photodyamic therapy via photosensitizer-loaded nanoparticles for cancer treatment. J. Pharm. Investig. 2019, 49, 1–8. [Google Scholar] [CrossRef]
- Lepeltier, E.; Rijo, P.; Rizzolio, F.; Popovtzer, R.; Petrikaite, V.; Assaraf, Y.G.; Passirani, C. Nanomedicine to target multidrug resistant tumors. Drug Resist. Update 2020. [Google Scholar] [CrossRef]
- Barui, A.K.; Oh, J.Y.; Jana, B.; Kim, C.; Ryu, J. Cancer-Targeted Nanomedicine: Overcoming the Barrier of the Protein Corona. Adv. Therap. 2020, 3, 1900124. [Google Scholar] [CrossRef]
- Nakamura, Y.; Mochida, A.; Choyke, P.L.; Kobayashi, H. Nanodrug delivery: Is the enhanced permeability and retention effect sufficient for curing cancer? Bioconjug. Chem. 2016, 27, 2225–2238. [Google Scholar] [CrossRef]
- Tao, W.; Kong, N.; Ji, X.; Zhang, Y.; Sharma, A.; Ouyang, J.; Qi, B.; Wang, J.; Xie, N.; Kang, C.; et al. Emerging two-dimensional monoelemental materials (Xenes) for biomedical applications. Chem. Soc. Rev. 2019, 48, 2891–2912. [Google Scholar] [CrossRef]
- Xie, Z.; Fan, T.; An, J.; Choi, W.; Duo, Y.; Ge, Y.; Zhang, B.; Nie, G.; Xie, N.; Zheng, T.; et al. Emerging combination strategies with phototherapy in cancer nanomedicine. Chem. Soc. Rev. 2020. [Google Scholar] [CrossRef] [PubMed]
- Qiu, M.; Ren, W.X.; Jeong, T.; Won, M.; Park, G.Y.; Sang, D.K.; Liu, L.P.; Zhang, H.; Kim, J.S. Omnipotent phosphorene: A next-generation, two-dimensional nanoplatform for multidisciplinary biomedical applications. Chem. Soc. Rev. 2018, 47, 5588–5601. [Google Scholar] [CrossRef] [PubMed]
- Youssef, P.N.; Sheibani, N.; Albert, D.M. Retinal light toxicity. Eye (Lond.) 2011, 25, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Legat, F.J. The antipruritic effect of phototherapy. Front. Med. (Lausanne) 2018, 5, 333. [Google Scholar] [CrossRef] [Green Version]
- Narayanan, D.L.; Saladi, R.N.; Fox, J.L. Ultraviolet radiation and skin cancer. Int. J. Dermatol. 2010, 49, 978–986. [Google Scholar] [CrossRef]
- Watson, M.; Holman, D.M.; Maguire-Eisen, M. Ultraviolet radiation exposure and its impact on skin cancer risk. Semin. Oncol Nurs. 2016, 32, 241–254. [Google Scholar] [CrossRef] [Green Version]
- Cheng, L.; Wang, C.; Feng, L.; Yang, K.; Liu, Z. Functional nanomaterials for phototherapies of cancer. Chem. Rev. 2014, 114, 10869–10939. [Google Scholar] [CrossRef]
- Yun, S.H.; Kwok, S.J. Light in diagnosis, therapy and surgery. Nat. Biomed. Eng. 2017, 1, 1–16. [Google Scholar] [CrossRef]
- Lan, G.; Ni, K.; Lin, W. Nanoscale metal–organic frameworks for phototherapy of cancer. Coord. Chem. Rev. 2019, 379, 65–81. [Google Scholar] [CrossRef]
- Perry, S.W.; Burke, R.M.; Brown, E.B. Two-photon and second harmonic microscopy in clinical and translational cancer research. Ann. Biomed. Eng. 2012, 40, 277–291. [Google Scholar] [CrossRef] [Green Version]
- Yelin, D.; Rizvi, I.; White, W.; Motz, J.; Hasan, T.; Bouma, B.; Tearney, G. Three-dimensional miniature endoscopy. Nature 2006, 443, 765. [Google Scholar] [CrossRef] [PubMed]
- Flusberg, B.A.; Cocker, E.D.; Piyawattanametha, W.; Jung, J.C.; Cheung, E.L.; Schnitzer, M.J. Fiber-optic fluorescence imaging. Nat. Methods 2005, 2, 941–950. [Google Scholar] [CrossRef] [PubMed]
- Brancaleon, L.; Moseley, H. Laser and non-laser light sources for photodynamic therapy. Lasers Med. Sci. 2002, 17, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Ntziachristos, V.; Ripoll, J.; Wang, L.V.; Weissleder, R. Looking and listening to light: The evolution of whole-body photonic imaging. Nat. Biotechnol. 2005, 23, 313–320. [Google Scholar] [CrossRef]
- Weissleder, R. A clearer vision for in vivo imaging. Nat. Biotechnol. 2001, 19, 316–317. [Google Scholar] [CrossRef]
- Srinivasan, S.; Pogue, B.W.; Jiang, S.; Dehghani, H.; Kogel, C.; Soho, S.; Gibson, J.J.; Tosteson, T.D.; Poplack, S.P.; Paulsen, K.D. Interpreting hemoglobin and water concentration, oxygen saturation, and scattering measured in vivo by near-infrared breast tomography. Proc. Natl. Acad. Sci. USA 2003, 100, 12349–12354. [Google Scholar] [CrossRef] [Green Version]
- Ai, X.; Mu, J.; Xing, B. Recent advances of light-mediated theranostics. Theranostics 2016, 6, 2439. [Google Scholar] [CrossRef]
- Gai, S.; Yang, G.; Yang, P.; He, F.; Lin, J.; Jin, D.; Xing, B. Recent advances in functional nanomaterials for light–triggered cancer therapy. Nano today 2018, 19, 146–187. [Google Scholar] [CrossRef]
- Lee, E.; Koo, J.; Berger, T. UVB phototherapy and skin cancer risk: A review of the literature. Int. J. Dermatol. 2005, 44, 355–360. [Google Scholar] [CrossRef]
- Wang, E.; Sasaki, J.; Nakamura, M.; Koo, J. Cutaneous carcinogenic risk of phototherapy: An updated comprehensive review. J. Psoriasis Psoriatic Arthritis 2015, 1, 44–51. [Google Scholar] [CrossRef]
- Blakely, K.M.; Drucker, A.M.; Rosen, C.F. Drug-induced photosensitivity—An update: Culprit drugs, prevention and management. Drug Saf. 2019, 42, 827–847. [Google Scholar] [CrossRef] [PubMed]
- Snyder, M.; Turrentine, J.E.; Cruz, P.D. Photocontact dermatitis and its clinical mimics: An overview for the allergist. Clin. Rev. Allergy Immunol. 2019, 56, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Ibbotson, S. Drug and chemical induced photosensitivity from a clinical perspective. Photochem. Photobiol. Sci. 2018, 17, 1885–1903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González Maglio, D.; Paz, M.; Leoni, J. Sunlight effects on immune system: Is there something else in addition to UV-induced immunosuppression? BioMed Res. Int. 2016. [Google Scholar] [CrossRef] [Green Version]
- Kunjachan, S.; Gremse, F.; Theek, B.; Koczera, P.; Pola, R.; Pechar, M.; Etrych, T.; Ulbrich, K.; Storm, G.; Kiessling, F. Noninvasive optical imaging of nanomedicine biodistribution. ACS Nano 2013, 7, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.K.; Huang, X.; El-Sayed, I.H.; El-Sayed, M.A. Noble metals on the nanoscale: Optical and photothermal properties and some applications in imaging, sensing, biology, and medicine. Acc. Chem. Res. 2008, 41, 1578–1586. [Google Scholar] [CrossRef] [PubMed]
- Zepeda, A.; Arias, C.; Sengpiel, F. Optical imaging of intrinsic signals: Recent developments in the methodology and its applications. J. Neurosci. Methods 2004, 136, 1–21. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, J.; Chen, C. Near-infrared light-mediated nanoplatforms for cancer thermo-chemotherapy and optical imaging. Adv. Mater. 2013, 25, 3869–3880. [Google Scholar] [CrossRef]
- Pansare, V.J.; Hejazi, S.; Faenza, W.J.; Prud’homme, R.K. Review of long-wavelength optical and NIR imaging materials: Contrast agents, fluorophores, and multifunctional nano carriers. Chem. Mater. 2012, 24, 812–827. [Google Scholar] [CrossRef] [Green Version]
- Gobin, A.M.; Lee, M.H.; Halas, N.J.; James, W.D.; Drezek, R.A.; West, J.L. Near-infrared resonant nanoshells for combined optical imaging and photothermal cancer therapy. Nano Lett. 2007, 7, 1929–1934. [Google Scholar] [CrossRef]
- Gibson, A.; Hebden, J.; Arridge, S.R. Recent advances in diffuse optical imaging. Phys. Med. Biol. 2005, 50, R1. [Google Scholar] [CrossRef] [PubMed]
- Balas, C. Review of biomedical optical imaging—A powerful, non-invasive, non-ionizing technology for improving in vivo diagnosis. Meas. Sci. Technol. 2009, 20, 104020. [Google Scholar] [CrossRef]
- Jung, Y.; Jung, J.; Huh, Y.; Kim, D. Benzo[g]coumarin-based fluorescent probes for bioimaging applications. J. Anal. Methods Chem. 2018, 2018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonçalves, M.S. Fluorescent labeling of biomolecules with organic probes. Chem. Rev. 2009, 109, 190–212. [Google Scholar] [CrossRef]
- Hilderbrand, S.A.; Weissleder, R. Near-infrared fluorescence: Application to in vivo molecular imaging. Curr. Opin. Chem. Biol. 2010, 14, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Mujumdar, R.B.; Ernst, L.A.; Mujumdar, S.R.; Waggoner, A.S. Cyanine dye labeling reagents containing isothiocyanate groups. Cytometry 1989, 10, 11–19. [Google Scholar] [CrossRef]
- Pinaud, F.; Michalet, X.; Bentolila, L.A.; Tsay, J.M.; Doose, S.; Li, J.J.; Iyer, G.; Weiss, S. Advances in fluorescence imaging with quantum dot bio-probes. Biomaterials 2006, 27, 1679–1687. [Google Scholar] [CrossRef] [Green Version]
- Xue, J.; Shan, L.; Chen, H.; Li, Y.; Zhu, H.; Deng, D.; Qian, Z.; Achilefu, S.; Gu, Y. Visual detection of STAT5B gene expression in living cell using the hairpin DNA modified gold nanoparticle beacon. Biosens. Bioelectron. 2013, 41, 71–77. [Google Scholar] [CrossRef]
- Huang, X.; El-Sayed, I.H.; Qian, W.; El-Sayed, M.A. Cancer cell imaging and photothermal therapy in the near-infrared region by using gold nanorods. J. Am. Chem. Soc. 2006, 128, 2115–2120. [Google Scholar] [CrossRef]
- Yamamoto, N.; Jiang, P.; Yang, M.; Xu, M.; Yamauchi, K.; Tsuchiya, H.; Tomita, K.; Wahl, G.M.; Moossa, A.R.; Hoffman, R.M. Cellular dynamics visualized in live cells in vitro and in vivo by differential dual-color nuclear-cytoplasmic fluorescent-protein expression. Cancer Res. 2004, 64, 4251–4256. [Google Scholar] [CrossRef] [Green Version]
- Luker, G.D.; Luker, K.E. Optical imaging: Current applications and future directions. J. Nucl. Med. 2008, 49, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Tsolekile, N.; Nelana, S.; Oluwafemi, O.S. Porphyrin as diagnostic and therapeutic agent. Molecules 2019, 24, 2669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, J.; Dragulescu-Andrasi, A.; Yao, H. Fluorescence imaging in vivo: Recent advances. Curr. Opin. Biotechnol. 2007, 18, 17–25. [Google Scholar] [CrossRef]
- Lippincott-Schwartz, J.; Patterson, G.H. Development and use of fluorescent protein markers in living cells. Science 2003, 300, 87–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Etrych, T.; Lucas, H.; Janoušková, O.; Chytil, P.; Mueller, T.; Mäder, K. Fluorescence optical imaging in anticancer drug delivery. J. Control. Release 2016, 226, 168–181. [Google Scholar] [CrossRef] [PubMed]
- Owais, A.H.; Setapar, S.H.M.; Ashraf, G.M.; Aliev, G. Molecular mechanisms of drug photodegradation and photosensitization. Curr. Pharm. Des. 2016, 22, 768–782. [Google Scholar]
- Castano, A.P.; Demidova, T.N.; Hamblin, M.R. Mechanisms in photodynamic therapy: Part one—Photosensitizers, photochemistry and cellular localization. Photodiagnosis Photodyn. Ther. 2004, 1, 279–293. [Google Scholar] [CrossRef] [Green Version]
- Maeda, M. Determination of biological substances using bioluminescent reaction based on luciferin-luciferase. Rinsho Byori 2004, 52, 595–603. [Google Scholar]
- Fleiss, A.; Sarkisyan, K.S. A brief review of bioluminescent systems (2019). Curr. Genet. 2019, 65, 877–882. [Google Scholar] [CrossRef] [Green Version]
- Hastings, J.W. Chemistries and colors of bioluminescent reactions: A review. Gene 1996, 173, 5–11. [Google Scholar] [CrossRef]
- Jenkins, D.E.; Oei, Y.; Hornig, Y.S.; Yu, S.-F.; Dusich, J.; Purchio, T.; Contag, P.R. Bioluminescent imaging (BLI) to improve and refine traditional murine models of tumor growth and metastasis. Clin. Exp. Metastasis 2003, 20, 733–744. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Wang, Y.; Wu, Y.; Zhang, X.; Zhang, X.; Liu, J.; Wang, T.; Fan, J.; Sun, J.; Yang, A. EZH2-mediated Epigenetic Silencing of miR-29/miR-30 targets LOXL4 and contributes to Tumorigenesis, Metastasis, and Immune Microenvironment Remodeling in Breast Cancer. Theranostics 2020, 10, 8494. [Google Scholar] [CrossRef] [PubMed]
- Chinn, S.B.; Darr, O.A.; Owen, J.H.; Bellile, E.; McHugh, J.B.; Spector, M.E.; Papagerakis, S.M.; Chepeha, D.B.; Bradford, C.R.; Carey, T.E. Cancer stem cells: Mediators of tumorigenesis and metastasis in head and neck squamous cell carcinoma. Head Neck 2015, 37, 317–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stollfuss, J.; Landvogt, N.; Abenstein, M.; Ziegler, S.; Schwaiger, M.; Senekowitsch-Schmidtke, R.; Wieder, H. Non-invasive imaging of implanted peritoneal carcinomatosis in mice using PET and bioluminescence imaging. EJNMMI Res. 2015, 5, 125. [Google Scholar] [CrossRef] [Green Version]
- Stowe, C.L.; Burley, T.A.; Allan, H.; Vinci, M.; Kramer-Marek, G.; Ciobota, D.M.; Parkinson, G.N.; Southworth, T.L.; Agliardi, G.; Hotblack, A.; et al. Near-infrared dual bioluminescence imaging in mouse models of cancer using infraluciferin. eLife 2019, 8. [Google Scholar] [CrossRef]
- Hu, Y.; Huang, J. The chimeric antigen receptor detection toolkit. Front. Immunol. 2020, 11, 1770. [Google Scholar] [CrossRef] [PubMed]
- Luker, K.E.; Luker, G.D. Applications of bioluminescence imaging to antiviral research and therapy: Multiple luciferase enzymes and quantitation. Antivir. Res. 2008, 78, 179–187. [Google Scholar] [CrossRef] [Green Version]
- Chitgupi, U.; Qin, Y.; Lovell, J.F. Targeted nanomaterials for phototherapy. Nanotheranostics 2017, 1, 38–58. [Google Scholar] [CrossRef]
- Wang, C.; Xu, L.; Liang, C.; Xiang, J.; Peng, R.; Liu, Z. Immunological responses triggered by photothermal therapy with carbon nanotubes in combination with anti-CTLA-4 therapy to inhibit cancer metastasis. Adv. Mater. 2014, 26, 8154–8162. [Google Scholar] [CrossRef]
- Nam, J.; Son, S.; Ochyl, L.J.; Kuai, R.; Schwendeman, A.; Moon, J.J. Chemo-photothermal therapy combination elicits anti-tumor immunity against advanced metastatic cancer. Nat Commun 2018, 9, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Zhang, C.-C.; Wang, X.-Y.; Li, L.; Chen, Q.-Q.; Liu, W.-W.; Cao, Y.; Ran, H.-T. Light-responsive core–shell nanoplatform for bimodal imaging-guided photothermal therapy-primed cancer immunotherapy. ACS Appl. Mater. Interfaces 2020. [Google Scholar] [CrossRef]
- Li, X.; Lovell, J.F.; Yoon, J.; Chen, X. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat. Rev. Clin. Oncol. 2020, 17, 657–674. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Jain, P.K.; El-Sayed, I.H.; El-Sayed, M.A. Plasmonic photothermal therapy (PPTT) using gold nanoparticles. Lasers Med. Sci. 2008, 23, 217. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.T.; Tran, T.T.P.; Jin, S.G.; Yong, C.S.; Truong, D.H.; Tran, T.H.; Kim, J.O. Combined hyperthermia and chemotherapy as a synergistic anticancer treatment. J. Pharm. Investig. 2019, 49, 519–526. [Google Scholar]
- Celli, J.P.; Spring, B.Q.; Rizvi, I.; Evans, C.L.; Samkoe, K.S.; Verma, S.; Pogue, B.W.; Hasan, T. Imaging and photodynamic therapy: Mechanisms, monitoring, and optimization. Chem. Rev. 2010, 110, 2795–2838. [Google Scholar] [CrossRef] [Green Version]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D. Photodynamic therapy of cancer: An update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef]
- Juarranz, Á.; Jaén, P.; Sanz-Rodríguez, F.; Cuevas, J.; González, S. Photodynamic therapy of cancer. Basic principles and applications. Clin. Transl. Oncol. 2008, 10, 148–154. [Google Scholar] [CrossRef]
- Lovell, J.F.; Liu, T.W.; Chen, J.; Zheng, G. Activatable photosensitizers for imaging and therapy. Chem. Rev. 2010, 110, 2839–2857. [Google Scholar] [CrossRef] [PubMed]
- Grabinski, C.; Schaeublin, N.; Wijaya, A.; D’Couto, H.; Baxamusa, S.H.; Hamad-Schifferli, K.; Hussain, S.M. Effect of gold nanorod surface chemistry on cellular response. ACS Nano 2011, 5, 2870–2879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheng, G.; Chen, Y.; Han, L.; Huang, Y.; Liu, X.; Li, L.; Mao, Z. Encapsulation of indocyanine green into cell membrane capsules for photothermal cancer therapy. J. Acta Biomater. 2016, 43, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Mirhadi, E.; Nassirli, H.; Malaekeh-Nikouei, B. An updated review on therapeutic effects of nanoparticle-based formulations of saffron components (safranal, crocin, and crocetin). J. Pharm. Investig. 2020. [Google Scholar] [CrossRef]
- Kang, J.K.; Kim, J.C.; Shin, Y.; Han, S.M.; Won, W.R.; Her, J.; Park, J.Y.; Oh, K.T. Principles and applications of nanomaterial-based hyperthermia in cancer therapy. Arch. Pharm. Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Lee, D.Y. Photothermal therapy with gold nanoparticles as an anticancer medication. J. Pharm. Investig. 2017, 47, 19–26. [Google Scholar] [CrossRef]
- Riley, R.S.; Day, E.S. Gold nanoparticle-mediated photothermal therapy: Applications and opportunities for multimodal cancer treatment. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9, e1449. [Google Scholar] [CrossRef]
- Wu, X.; Ming, T.; Wang, X.; Wang, P.; Wang, J.; Chen, J. High-photoluminescence-yield gold nanocubes: For cell imaging and photothermal therapy. ACS Nano 2010, 4, 113–120. [Google Scholar] [CrossRef]
- Zhou, B.; Li, Y.; Niu, G.; Lan, M.; Jia, Q.; Liang, Q. Near-infrared organic dye-based nanoagent for the photothermal therapy of cancer. ACS Appl. Mater. Interfaces 2016, 8, 29899–29905. [Google Scholar] [CrossRef] [PubMed]
- Kamkaew, A.; Lim, S.H.; Lee, H.B.; Kiew, L.V.; Chung, L.Y.; Burgess, K. BODIPY dyes in photodynamic therapy. Chem. Soc. Rev. 2013, 42, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Abrahamse, H.; Hamblin, M.R. New photosensitizers for photodynamic therapy. Biochem. J. 2016, 473, 347–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kou, J.; Dou, D.; Yang, L. Porphyrin photosensitizers in photodynamic therapy and its applications. Oncotarget 2017, 8, 81591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, K.K.; Takada, M.; Jin, C.C.; Zheng, G. Self-sensing porphysomes for fluorescence-guided photothermal therapy. Bioconjug. Chem. 2015, 26, 345–351. [Google Scholar] [CrossRef]
- Lim, C.; Sim, T.; Hoang, N.H.; Jung, C.E.; Lee, E.S.; Youn, Y.S.; Oh, K.T. A charge-reversible nanocarrier using PEG-PLL (-g-Ce6, DMA)-PLA for photodynamic therapy. Int. J. Nanomedicine 2017, 12, 6185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Peng, X.H.; Zheng, B.D.; Tang, J.; Zhao, Y.; Zheng, B.Y.; Ke, M.R.; Huang, J.D. New application of phthalocyanine molecules: From photodynamic therapy to photothermal therapy by means of structural regulation rather than formation of aggregates. Chem. Sci. 2018, 9, 2098–2104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pucelik, B.; Sułek, A.; Dąbrowski, J.M. Bacteriochlorins and their metal complexes as NIR-absorbing photosensitizers: Properties, mechanisms, and applications. Coord. Chem. Rev. 2020, 416, 213340. [Google Scholar] [CrossRef]
- Barras, A.; Boussekey, L.; Courtade, E.; Boukherroub, R. Hypericin-loaded lipid nanocapsules for photodynamic cancer therapy in vitro. Nanoscale 2013, 5, 10562–10572. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Yin, R.; Chen, D.; Ren, J.; Wang, Y.; Zhanga, J.; Deng, H.; Wang, Y.; Qiu, H.; Huang, N.; et al. In vitro and in vivo antitumor activity of a novel hypocrellin B derivative for photodynamic therapy. Photodiagnosis Photodyn. Ther. 2014, 11, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Pogorelaya, M.; Martynov, A.; Romanova, E. Riboflavin in photodynamic inactivation of pathogens and photodynamic therapy. Ann. Mechnikov Inst. 2020. [Google Scholar] [CrossRef]
- Ellerkamp, V.; Bortel, N.; Schmid, E.; Kirchner, B.; Armeanu-Ebinger, S.; Fuchs, J. Photodynamic therapy potentiates the effects of curcumin on pediatric epithelial liver tumor cells. Anticancer Res. 2016, 36, 3363–3372. [Google Scholar]
- Tardivo, J.P.; Del Giglio, A.; de Oliveira, C.S.; Gabrielli, D.S.; Junqueira, H.C.; Tada, D.B.; Severino, D.; de Fátima Turchiello, R.; Baptista, M.S. Methylene blue in photodynamic therapy: From basic mechanisms to clinical applications. Photodiagnosis Photodyn. Ther. 2005, 2, 175–191. [Google Scholar] [CrossRef]
- Di Stasio, D.; Romano, A.; Russo, D.; Fiori, F.; Laino, L.; Caponio, V.C.A.; Troiano, G.; Muzio, L.L.; Serpico, R.; Lucchese, A. Photodynamic therapy using topical toluidine blue for the treatment of oral leukoplakia: A prospective case series. Photodiagnosis Photodyn. Ther. 2020, 31, 101888. [Google Scholar] [CrossRef]
- Gianotti, E.; Martins Estevão, B.; Cucinotta, F.; Hioka, N.; Rizzi, M.; Renò, F.; Marchese, L. An efficient rose bengal based nanoplatform for photodynamic therapy. Chemistry 2014, 20, 10921–10925. [Google Scholar] [CrossRef]
- Ferreira, D.P.; Conceição, D.S.; Fernandes, F.; Sousa, T.; Calhelha, R.C.; Ferreira, I.C.; Santos, P.F.; Vieira Ferreira, L.F. Characterization of a squaraine/chitosan system for photodynamic therapy of cancer. J. Phys. Chem. B 2016, 120, 1212–1220. [Google Scholar] [CrossRef] [PubMed]
- Awuah, S.G.; You, Y. Boron dipyrromethene (BODIPY)-based photosensitizers for photodynamic therapy. RSC Adv. 2012, 2, 11169–11183. [Google Scholar] [CrossRef]
- Salmerón, M.L.; Quintana-Aguiar, J.; De La Rosa, J.V.; López-Blanco, F.; Castrillo, A.; Gallardo, G.; Tabraue, C. Phenalenone-photodynamic therapy induces apoptosis on human tumor cells mediated by caspase-8 and p38-MAPK activation. Mol. Carcinog. 2018, 57, 1525–1539. [Google Scholar] [CrossRef] [PubMed]
- Urbanska, K.; Romanowska-Dixon, B.; Matuszak, Z.; Oszajca, J.; Nowak-Sliwinska, P.; Stochel, G. Indocyanine green as a prospective sensitizer for photodynamic therapy of melanomas. Acta Biochim. Pol. 2002, 49, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Wu, H.; Liu, H.; Deng, Z.; Liu, H.; Duan, W.; Liu, X.; Zheng, H. Molecular imaging-guided photothermal/photodynamic therapy against tumor by iRGD-modified indocyanine green nanoparticles. J. Control. Release 2016, 224, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Youssef, Z.; Vanderesse, R.; Colombeau, L.; Baros, F.; Roques-Carmes, T.; Frochot, C.; Wahab, H.; Toufaily, J.; Hamieh, T.; Acherar, S.; et al. The application of titanium dioxide, zinc oxide, fullerene, and graphene nanoparticles in photodynamic therapy. Cancer Nanotechnol. 2017, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraghavan, P.; Liu, C.H.; Vankayala, R.; Chiang, C.S.; Hwang, K.C. Designing multi-branched gold nanoechinus for NIR light activated dual modal photodynamic and photothermal therapy in the second biological window. Adv Mater. 2014, 26, 6689–6695. [Google Scholar] [CrossRef]
- Jung, H.S.; Kong, W.H.; Sung, D.K.; Lee, M.Y.; Beack, S.E.; Keum, D.H.; Kim, K.S.; Yun, S.H.; Hahn, S.K. Nanographene oxide–hyaluronic acid conjugate for photothermal ablation therapy of skin cancer. ACS Nano 2014, 8, 260–268. [Google Scholar] [CrossRef]
- Samia, A.C.; Chen, X.; Burda, C. Semiconductor quantum dots for photodynamic therapy. J. Am. Chem. Soc. 2003, 125, 15736–15737. [Google Scholar] [CrossRef]
- Yoo, E.; Choi, J.H.; Hoang, N.H.; Lee, J.S.; Vuong, S.; Hur, B.; Han, P.; Oh, K.T.; Fahmy, T.; Kim, D. Particle-in-particle platform for nanoconfinement-induced oncothermia. ACS Appl. Bio Mater. 2018, 1, 1927–1941. [Google Scholar] [CrossRef]
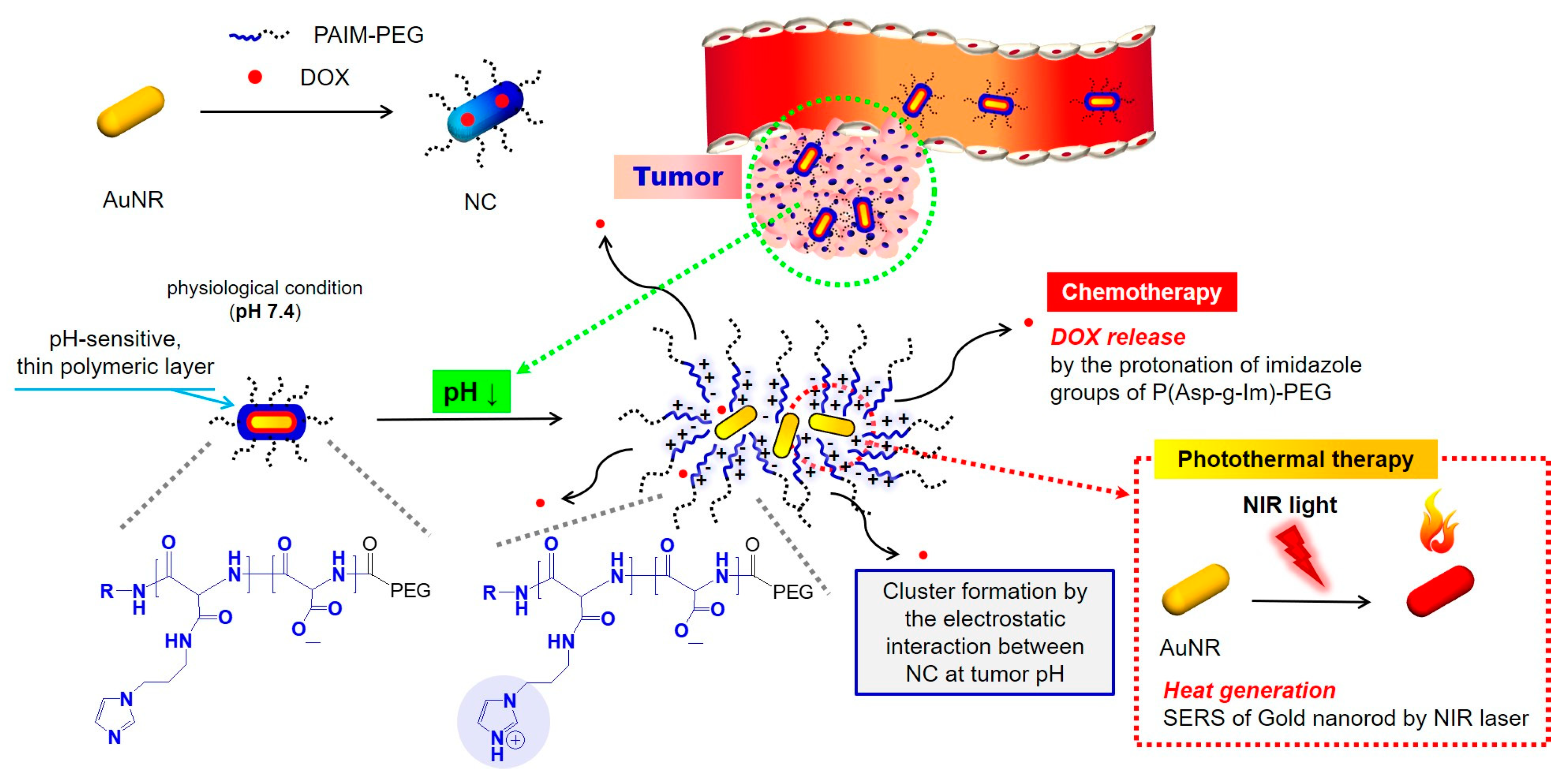
- Sim, T.; Lim, C.; Hoang, N.H.; Shin, Y.; Kim, J.C.; Park, J.Y.; Her, J.; Lee, E.S.; Youn, Y.S.; Oh, K.T. An on-demand pH-sensitive nanocluster for cancer treatment by combining photothermal therapy and chemotherapy. J. Pharm. 2020, 12, 839. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Jiang, Q.; Feng, D.; Mao, L.; Zhou, H.-C. Size-controlled synthesis of porphyrinic metal–organic framework and functionalization for targeted photodynamic therapy. J. Am. Chem. Soc. 2016, 138, 3518–3525. [Google Scholar] [CrossRef]
- Nasseri, B.; Turk, M.; Kosemehmetoglu, K.; Kaya, M.; Piskin, E.; Rabiee, N.; Webster, T.J. The pimpled gold nanosphere: A superior candidate for plasmonic photothermal therapy. Int. J. Nanomedicine 2020, 15, 2903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, Q.; Mitschang, F.; Agarwal, S. Biocompatible drug delivery system for photo-triggered controlled release of 5-fluorouracil. Biomacromolecules 2011, 12, 3684–3691. [Google Scholar] [CrossRef] [PubMed]
- Beauté, L.; McClenaghan, N.; Lecommandoux, S. Photo-triggered polymer nanomedicines: From molecular mechanisms to therapeutic applications. Adv. Drug Deliv. Rev. 2019, 138, 148–166. [Google Scholar] [CrossRef] [PubMed]
- Mi, P. Stimuli-responsive nanocarriers for drug delivery, tumor imaging, therapy and theranostics. Theranostics 2020, 10, 4557–4588. [Google Scholar] [CrossRef]
- Sun, S.; Liang, S.; Xu, W.; Xu, G.; Wu, S. Photoresponsive polymers with multi-azobenzene groups. Polym. Chem. 2019, 10, 4389–4401. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Dong, R.; Zhu, X.; Yan, D. Photo-responsive polymeric micelles. Soft Matter 2014, 10, 6121–6138. [Google Scholar] [CrossRef]
- Sun, L.; Yang, Y.; Dong, C.M.; Wei, Y. Two-photon-sensitive and sugar-targeted nanocarriers from degradable and dendritic amphiphiles. Small 2011, 7, 401–406. [Google Scholar] [CrossRef]
- Liu, G.; Dong, C.-M. Photoresponsive poly (S-(o-nitrobenzyl)-l-cysteine)-b-PEO from a l-cysteine N-carboxyanhydride monomer: Synthesis, self-assembly, and phototriggered drug release. Biomacromolecules 2012, 13, 1573–1583. [Google Scholar] [CrossRef]
- Kumar, S.; Allard, J.-F.; Morris, D.; Dory, Y.L.; Lepage, M.; Zhao, Y. Near-infrared light sensitive polypeptide block copolymer micelles for drug delivery. J. Mater. Chem. 2012, 22, 7252–7257. [Google Scholar] [CrossRef]
- Jiang, J.; Tong, X.; Zhao, Y. A new design for light-breakable polymer micelles. J. Am. Chem. Soc. 2005, 127, 8290–8291. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Chen, W.H.; Xu, X.D.; Li, C.; Zhang, J.; Zhuo, R.X.; Zhang, X.Z. Design of a cellular-uptake-shielding “plug and play” template for photo controllable drug release. Adv. Mater. 2011, 23, 3526–3530. [Google Scholar] [CrossRef] [PubMed]
- Sortino, S. Photoactivated nanomaterials for biomedical release applications. J. Mater. Chem. 2012, 22, 301–318. [Google Scholar] [CrossRef]
- Sortino, S. Nanostructured molecular films and nanoparticles with photoactivable functionalities. Photochem. Photobiol. Sci. 2008, 7, 911–924. [Google Scholar] [CrossRef]
- Rai, P.; Mallidi, S.; Zheng, X.; Rahmanzadeh, R.; Mir, Y.; Elrington, S.; Khurshid, A.; Hasan, T. Development and applications of photo-triggered theranostic agents. Adv. Drug Deliv. Rev. 2010, 62, 1094–1124. [Google Scholar] [CrossRef] [Green Version]
- McCarthy, J.R.; Perez, J.M.; Brückner, C.; Weissleder, R. Polymeric nanoparticle preparation that eradicates tumors. Nano Lett. 2005, 5, 2552–2556. [Google Scholar] [CrossRef]
- Elsadek, B.; Kratz, F. Impact of albumin on drug delivery—New applications on the horizon. J. Control. Release 2012, 157, 4–28. [Google Scholar] [CrossRef]
- Zhang, Y.; Chan, H.F.; Leong, K.W. Advanced materials and processing for drug delivery: The past and the future. Adv. Drug Deliv. Rev. 2013, 65, 104–120. [Google Scholar] [CrossRef] [Green Version]
- Bae, Y.H.; Park, K. Targeted drug delivery to tumors: Myths, reality and possibility. J. Control. Release 2011, 153, 198. [Google Scholar] [CrossRef] [Green Version]
- Nicolas, J.; Mura, S.; Brambilla, D.; Mackiewicz, N.; Couvreur, P. Design, functionalization strategies and biomedical applications of targeted biodegradable/biocompatible polymer-based nanocarriers for drug delivery. Chem. Soc. Rev. 2013, 42, 1147–1235. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Nakamura, H.; Fang, J. The EPR effect for macromolecular drug delivery to solid tumors: Improvement of tumor uptake, lowering of systemic toxicity, and distinct tumor imaging in vivo. Adv. Drug Deliv. Rev. 2013, 65, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Rodríguez, R.; Arias, F.J.; Santos, M.; Testera, A.M.; Rodríguez-Cabello, J.C. Gold Tailored Photosensitive Elastin-like Polymer: Synthesis of Temperature, pH and UV-vis Sensitive Probes. Macromol. Rapid Commun. 2010, 31, 568–573. [Google Scholar] [CrossRef] [PubMed]
- Tran, P.; Lee, S.-E.; Kim, D.-H.; Pyo, Y.-C.; Park, J.-S. Recent advances of nanotechnology for the delivery of anticancer drugs for breast cancer treatment. J. Pharm. Investig. 2020, 50, 261–270. [Google Scholar] [CrossRef]
- Lee, D.J.; Ahn, Y.S.; Youn, Y.S.; Lee, E.S. Poly (ethylene glycol)-crosslinked fullerenes for high efficient phototherapy. Polym. Adv. Technol. 2013, 24, 220–227. [Google Scholar] [CrossRef]
- Qiu, L.Y.; Bae, Y.H. Polymer architecture and drug delivery. Pharm Res. 2006, 23, 1–30. [Google Scholar] [CrossRef]
- Atlihan-Gundogdu, E.; Ilem-Ozdemir, D.; Ekinci, M.; Ozgenc, E.; Demir, E.S.; Sánchez-Dengra, B.; González-Alvárez, I. Recent developments in cancer therapy and diagnosis. J. Pharm. Investig. 2020. [Google Scholar] [CrossRef]
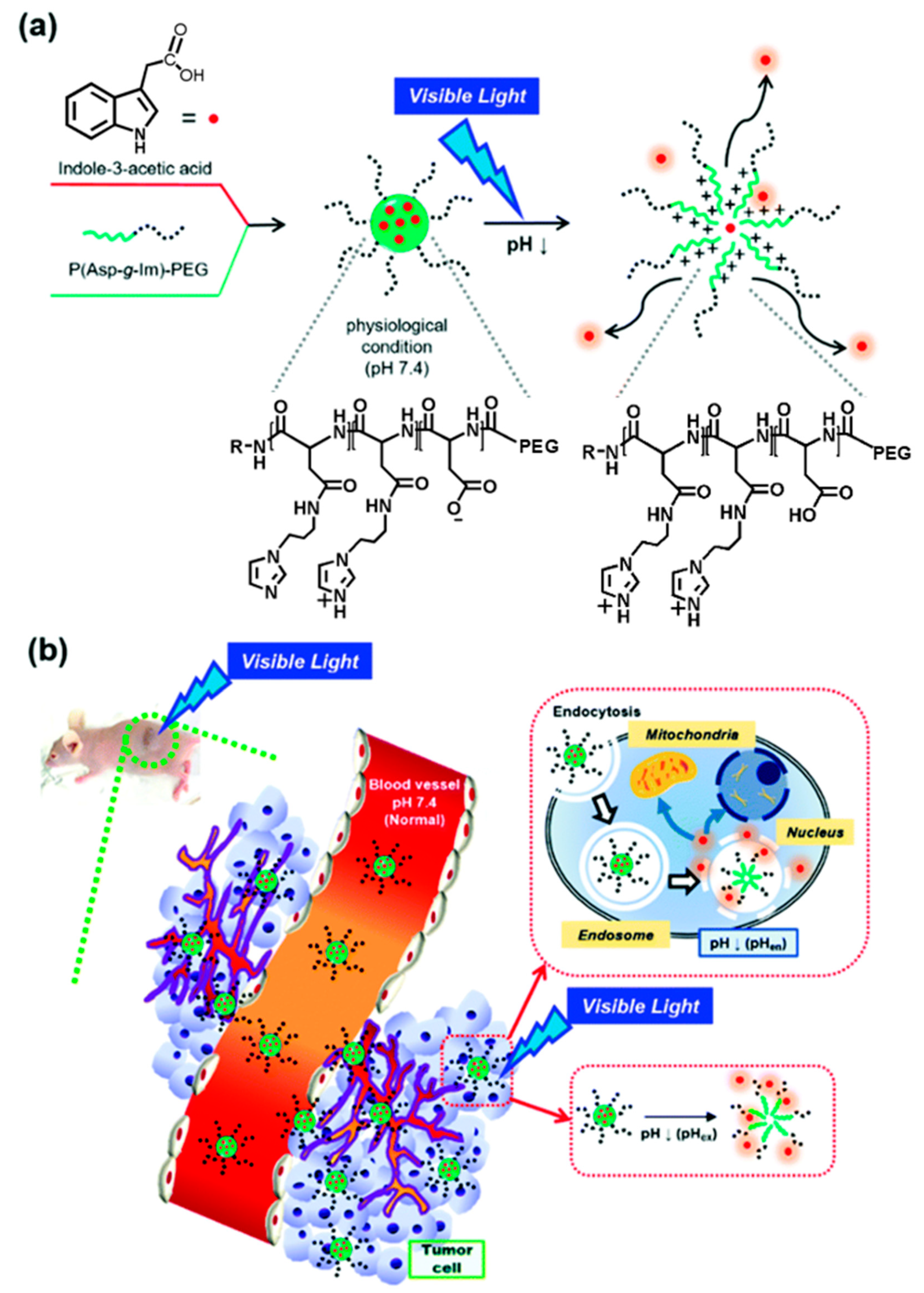
- Sim, T.; Lim, C.; Hoang, N.H.; Kim, J.E.; Lee, E.S.; Youn, Y.S.; Oh, K.T. Synergistic photodynamic therapeutic effect of indole-3-acetic acid using a pH sensitive nano-carrier based on poly(aspartic acid-graft-imidazole)-poly(ethylene glycol). J. Mater. Chem. B 2017, 5, 8498–8505. [Google Scholar] [CrossRef]
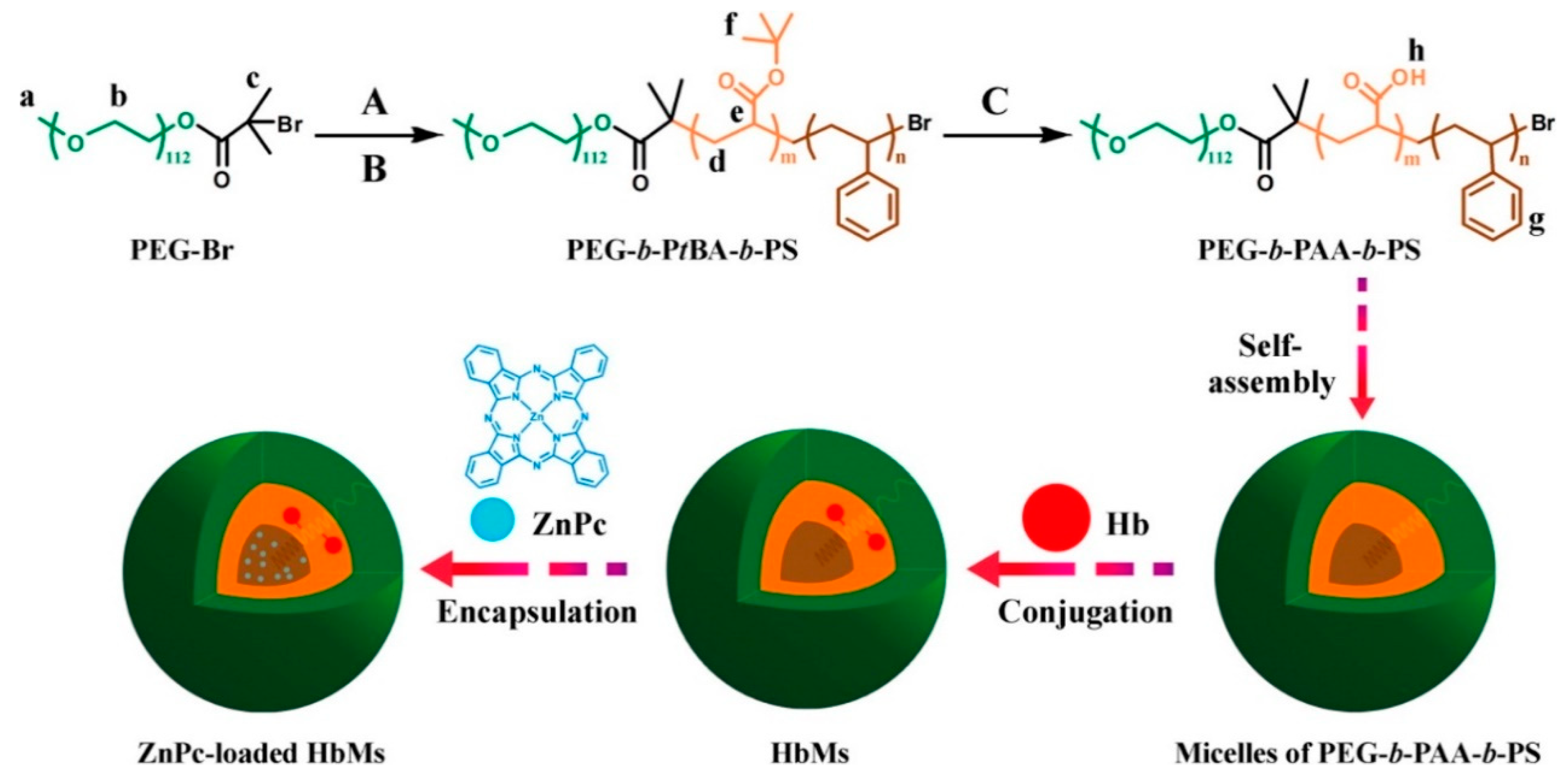
- Wang, S.; Yuan, F.; Chen, K.; Chen, G.; Tu, K.; Wang, H.; Wang, L. Synthesis of hemoglobin conjugated polymeric micelle: A ZnPc carrier with oxygen self-compensating ability for photodynamic therapy. Biomacromolecules 2015, 16, 2693–2700. [Google Scholar] [CrossRef]
- Li, Y.; Lv, S.; Song, Z.; Dang, J.; Li, X.; He, H.; Xu, X.; Zhou, Z.; Yin, L. Photodynamic therapy-mediated remote control of chemotherapy toward synergistic anticancer treatment. Nanoscale 2018, 10, 14554–14562. [Google Scholar] [CrossRef]
- an, G.; Jia, H.; Zhu, Y.; Wu, F. Turning double hydrophilic into amphiphilic: IR825-conjugated polymeric nanomicelles for near-infrared fluorescence imaging-guided photothermal cancer therapy. Nanoscale 2018, 10, 2115–2127. [Google Scholar]
- Guo, B.; Feng, G.; Manghnani, P.N.; Cai, X.; Liu, J.; Wu, W.; Xu, S.; Cheng, X.; Teh, C.; Liu, B. A Porphyrin-Based Conjugated Polymer for Highly Efficient In Vitro and In Vivo Photothermal Therapy. Small 2016, 12, 6243–6254. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Xu, H.; Cheng, L.; Sun, C.; Wang, J.; Liu, Z. In vitro and in vivo near-infrared photothermal therapy of cancer using polypyrrole organic nanoparticles. Adv. Mater. 2012, 24, 5586–5592. [Google Scholar] [CrossRef] [PubMed]
- Qu, Q.; Wang, Y.; Zhang, L.; Zhang, X.; Zhou, S. A nanoplatform with precise control over release of cargo for enhanced cancer therapy. Small 2016, 12, 1378–1390. [Google Scholar] [CrossRef]
- Dai, X.; Wang, Z.; Liu, W.; Dong, C.; Pan, J.; Yuan, S.; Yan, Y.; Liu, D.; Sun, L. Biomimetic star-shaped porphyrin-cored poly (l-lactide)-b-glycopolymer block copolymers for targeted photodynamic therapy. Colloid Polym. Sci. 2014, 292, 2111–2122. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Q.; Sun, X.; Zhang, B.; Kang, H.; Zhang, F.; Jin, Y. Doxorubicin-loaded photosensitizer-core pH-responsive copolymer nanocarriers for combining photodynamic therapy and chemotherapy. ACS Biomater. Sci. Eng. 2017, 3, 1008–1016. [Google Scholar] [CrossRef]
- Gangopadhyay, M.; Singh, T.; Behara, K.K.; Karwa, S.; Ghosh, S.K.; Singh, N.D. Coumarin-containing-star-shaped 4-arm-polyethylene glycol: Targeted fluorescent organic nanoparticles for dual treatment of photodynamic therapy and chemotherapy. Photochem. Photobiol. Sci. 2015, 14, 1329–1336. [Google Scholar] [CrossRef]
- An, X.; Zhu, A.; Luo, H.; Ke, H.; Chen, H.; Zhao, Y. Rational design of multi-stimuli-responsive nanoparticles for precise cancer therapy. ACS Nano 2016, 10, 5947–5958. [Google Scholar] [CrossRef]
- Kojima, C.; Toi, Y.; Harada, A.; Kono, K. Preparation of poly (ethylene glycol)-attached dendrimers encapsulating photosensitizers for application to photodynamic therapy. Bioconjug. Chem. 2007, 18, 663–670. [Google Scholar] [CrossRef]
- Taratula, O.; Schumann, C.; Duong, T.; Taylor, K.L.; Taratula, O. Dendrimer-encapsulated naphthalocyanine as a single agent-based theranostic nanoplatform for near-infrared fluorescence imaging and combinatorial anticancer phototherapy. Nanoscale 2015, 7, 3888–3902. [Google Scholar] [CrossRef]
- Yuan, W.; Gao, X.; Pei, E.; Li, Z. Light-and pH-dually responsive dendrimer-star copolymer containing spiropyran groups: Synthesis, self-assembly and controlled drug release. Polym. Chem. 2018, 9, 3651–3661. [Google Scholar] [CrossRef]
- Sun, H.; Erdman III, W.; Yuan, Y.; Mohamed, M.A.; Xie, R.; Wang, Y.; Gong, S.; Cheng, C. Crosslinked polymer nanocapsules for therapeutic, diagnostic, and theranostic applications. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2020, 12, e1653. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Pourgholami, M.H.; Morris, D.L.; Stenzel, M.H. Effect of cross-linking on the performance of micelles as drug delivery carriers: A cell uptake study. Biomacromolecules 2012, 13, 814–825. [Google Scholar] [CrossRef]
- Shuai, X.; Merdan, T.; Schaper, A.K.; Xi, F.; Kissel, T. Core-cross-linked polymeric micelles as paclitaxel carriers. J. Bioconjugate Chem. 2004, 15, 441–448. [Google Scholar] [CrossRef]
- Tang, Y.; Chen, H.; Chang, K.; Liu, Z.; Wang, Y.; Qu, S.; Xu, H.; Wu, C. Photo-cross-linkable polymer dots with stable sensitizer loading and amplified singlet oxygen generation for photodynamic therapy. ACS Appl. Mater. Interfaces 2017, 9, 3419–3431. [Google Scholar] [CrossRef]
- Li, Q.; Qiao, X.; Wang, F.; Li, X.; Yang, J.; Liu, Y.; Shi, L.; Liu, D. Encapsulating a single nanoprobe in a multifunctional nanogel for high-fidelity imaging of caspase activity in vivo. Anal. Chem. 2019, 91, 13633–13638. [Google Scholar] [CrossRef] [PubMed]
- Chambre, L.; Saw, W.S.; Ekineker, G.; Kiew, L.V.; Chong, W.Y.; Lee, H.B.; Chung, L.Y.; Bretonnière, Y.; Dumoulin, F.; Sanyal, A. Surfactant-free direct access to porphyrin-cross-linked nanogels for photodynamic and photothermal therapy. Bioconjug. Chem. 2018, 29, 4149–4159. [Google Scholar] [CrossRef] [PubMed]
- Ji, P.; Zhang, W.; Ai, S.; Zhang, Y.; Liu, J.; Liu, J.; He, P.; Li, Y. Hybridization of graphene oxide into nanogels to acquire higher photothermal effects for therapeutic delivery. Nanotechnology 2019, 30, 115701. [Google Scholar] [CrossRef]



| Benefits | Ref. |
|---|---|
| High selectivity | [4,30] |
| High efficacy and low/no systemic toxicity | [4,30] |
| Light irradiation in the location of lesions can be controlled well | [4,13] |
| Minimally or non-invasive and effective modality | [13,30,31,32] |
| Convenient method | [13,30,31,32] |
| Risks | |
| Photic injury, photochemical injury, and photomechanical damage | [13,33] |
| Phototoxicity | [13,33] |
| Frequent PT treatments can lead to immunosuppression | [34] |
| Increased risk of developing skin cancer | [35,36] |
| Type of PSAs | PSAs | Structure | Imaging Modality | Ref. |
|---|---|---|---|---|
| Fluorescent dye | Coumarin | 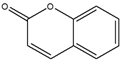 | Fluorescence | [63] |
| Fluorescent dye | Fluorescein |  | Fluorescence | [64] |
| Fluorescent dye | Alexa Fluor | 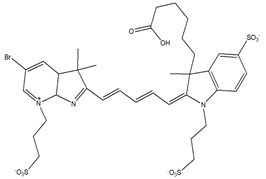 | Fluorescence | [65] |
| Fluorescent dye | Cyanine |  | Fluorescence | [64,66] |
| Quantum dot | CdSe/Zns | 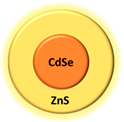 | Fluorescence | [67] |
| Quantum dot | CdSxSe1-x/ZnS | 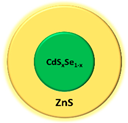 | Fluorescence | [67] |
| Metallic NPs | Gold NPs and gold nanorods | 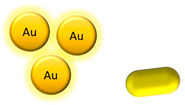  | Fluorescence | [68,69] |
| Fluorescent protein | Green fluorescent protein (GFP) |  | Fluorescence | [70] |
| Fluorescent protein | Red fluorescent protein (RFP) |  | Fluorescence | [70] |
| Bioluminescent probe | Luciferin | 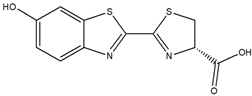 | Bioluminescence | [71] |
| Organic compound | Porphyrin | 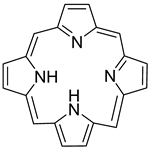 | Fluorescence | [72] |
| Type of PSAs | PSAs | Modality | Ref. |
|---|---|---|---|
| Tetrapyrrole | Porphyrin | PDT, PTT | [109,110] |
| Chlorin | PDT | [111] | |
| Phthalocyanine | PDT, PTT | [112] | |
| Bacteriochlorin | PDT | [113] | |
| Natural compound | Hypericin | PDT | [114] |
| Hypocrellin | PDT | [115] | |
| Riboflavin | PDT | [116] | |
| Curcumin | PDT | [117] | |
| Other photoactive dye | Methylene blue | PDT | [118] |
| Toluidine blue | PDT | [119] | |
| Rose Bengal (RB) | PDT | [120] | |
| Squaraine | PDT | [121] | |
| Boron dipyrromethene | PDT | [122] | |
| Phenalenones | PDT | [123] | |
| Indocyanine green (ICG) | PDT, PTT | [124,125] | |
| Inorganic NPs | Titanium dioxide (TiO2) | PDT | [126] |
| Zinc oxide (ZnO) | PDT | [126] | |
| Metallic NPs | Gold NPs | PDT, PTT | [102,127] |
| Carbon-based NPs | Fullerene | PDT | [126] |
| Graphene | PDT, PTT | [126,128] | |
| Quantum dots (QDs) | Ge-QDs, Ag2S QDs, CdS, CdSe, PbSe, InP, CdTe, and tungsten sulfide (WS2) QDs | PDT, PTT | [129] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Husni, P.; Shin, Y.; Kim, J.C.; Kang, K.; Lee, E.S.; Youn, Y.S.; Rusdiana, T.; Oh, K.T. Photo-Based Nanomedicines Using Polymeric Systems in the Field of Cancer Imaging and Therapy. Biomedicines 2020, 8, 618. https://doi.org/10.3390/biomedicines8120618
Husni P, Shin Y, Kim JC, Kang K, Lee ES, Youn YS, Rusdiana T, Oh KT. Photo-Based Nanomedicines Using Polymeric Systems in the Field of Cancer Imaging and Therapy. Biomedicines. 2020; 8(12):618. https://doi.org/10.3390/biomedicines8120618
Chicago/Turabian StyleHusni, Patihul, Yuseon Shin, Jae Chang Kim, Kioh Kang, Eun Seong Lee, Yu Seok Youn, Taofik Rusdiana, and Kyung Taek Oh. 2020. "Photo-Based Nanomedicines Using Polymeric Systems in the Field of Cancer Imaging and Therapy" Biomedicines 8, no. 12: 618. https://doi.org/10.3390/biomedicines8120618
APA StyleHusni, P., Shin, Y., Kim, J. C., Kang, K., Lee, E. S., Youn, Y. S., Rusdiana, T., & Oh, K. T. (2020). Photo-Based Nanomedicines Using Polymeric Systems in the Field of Cancer Imaging and Therapy. Biomedicines, 8(12), 618. https://doi.org/10.3390/biomedicines8120618









