Higher Epoxyeicosatrienoic Acids in Cardiomyocytes-Specific CYP2J2 Transgenic Mice Are Associated with Improved Myocardial Remodeling
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Solvents
2.2. Standard Curve
2.3. Animal Model
2.4. Echocardiography and MI Surgery
2.5. Histological Analysis, Cell Surface Area Measurement, and Terminal Deoxynucleotidyl Transferase dUTP Nick End Labelling (TUNEL)
2.6. Detection of ROS
2.7. Extraction of EETs from Erythrocyte Membrane and Heart Tissue
2.8. Liquid Chromatography and MS Assay to Quantify EETs
2.9. Human Cardiomyocytes and Cobalt (II) Chloride (CoCl2) Treatment
2.10. Data Analysis
3. Results
3.1. EET Levels and Correlation in Erythrocyte Membrane and Cardiac Tissue from WT and Tr Mice
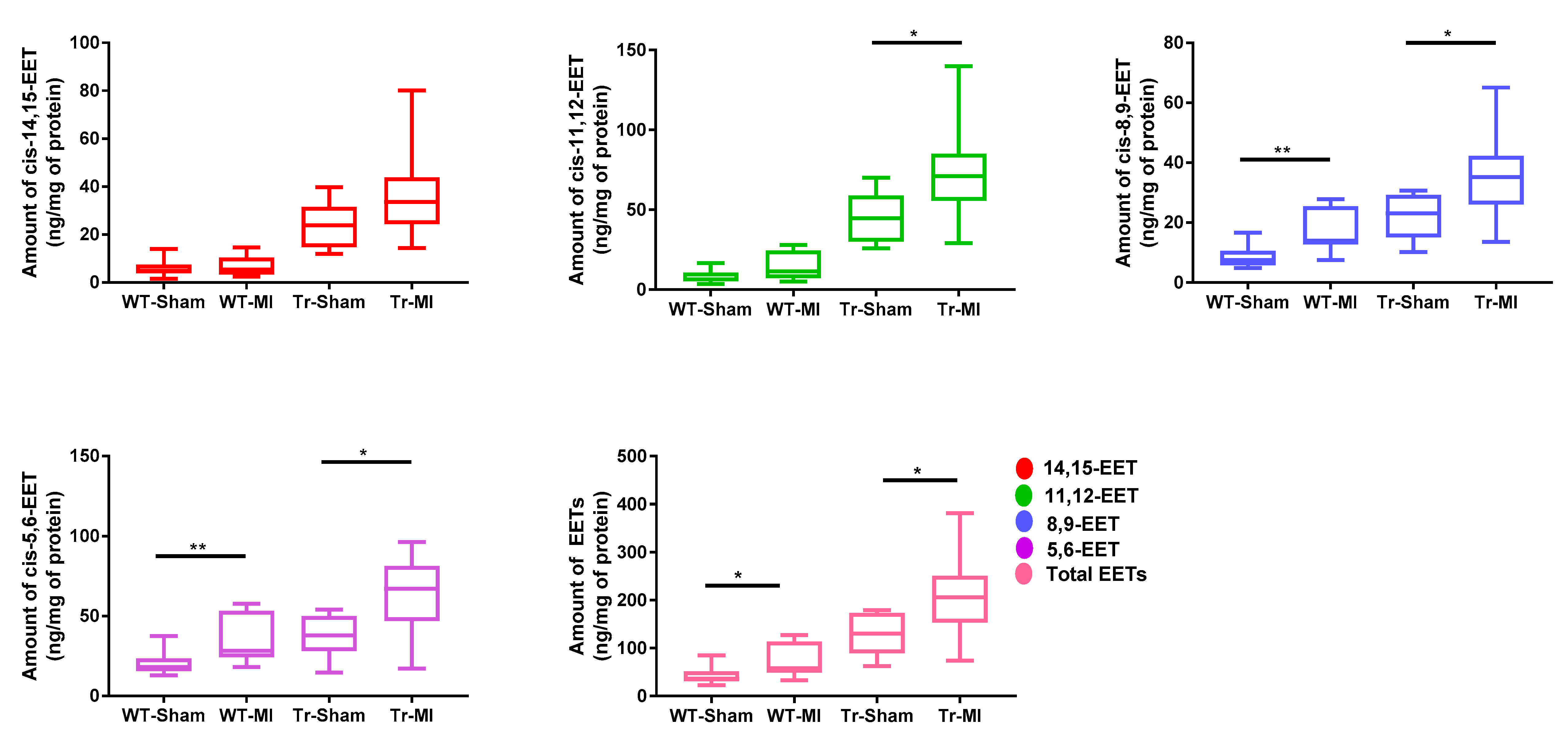
3.2. Cis- and Trans-EET Levels, Cardiac Morphology, and Function Following MI
3.3. Effect of Hypoxia on Cardiac Cells in Human Cardiomyocytes with Altered CYP2J2 Expression
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bellien, J.; Joannides, R.; Richard, V.; Thuillez, C. Modulation of cytochrome-derived epoxyeicosatrienoic acids pathway: A promising pharmacological approach to prevent endothelial dysfunction in cardiovascular diseases? Pharm. Ther. 2011, 131, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Capdevila, J.H.; Falck, J.R.; Harris, R.C. Cytochrome P450 and arachidonic acid bioactivation. Molecular and functional properties of the arachidonate monooxygenase. J. Lipid Res. 2000, 41, 163–181. [Google Scholar] [PubMed]
- Yu, Z.; Xu, F.; Huse, L.M.; Morisseau, C.; Draper, A.J.; Newman, J.W.; Parker, C.; Graham, L.; Engler, M.M.; Hammock, B.D.; et al. Soluble epoxide hydrolase regulates hydrolysis of vasoactive epoxyeicosatrienoic acids. Circ. Res. 2000, 87, 992–998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weintraub, N.L.; Fang, X.; Kaduce, T.L.; VanRollins, M.; Chatterjee, P.; Spector, A.A. Potentiation of endothelium-dependent relaxation by epoxyeicosatrienoic acids. Circ. Res. 1997, 81, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Quilley, J.; Reddy, L.M.; Falck, J.R.; Wong, P.Y.; McGiff, J.C. Red blood cells: Reservoirs of cis-and trans-epoxyeicosatrienoic acids. Prostaglandins Other Lipid Mediat. 2005, 75, 65–78. [Google Scholar] [CrossRef]
- Jiang, H.; Anderson, G.D.; McGiff, J.C. Red blood cells (RBCs), epoxyeicosatrienoic acids (EETs) and adenosine triphosphate (ATP). Pharmacol. Rep. 2010, 62, 468–474. [Google Scholar] [CrossRef] [Green Version]
- Karara, A.; Wei, S.; Spady, D.; Swift, L.; Capdevila, J.H.; Falck, J.R. Arachidonic acid epoxygenase: Structural characterization and quantification of epoxyeicosatrienoates in plasma. Biochem. Biophys. Res. Commun. 1992, 182, 1320–1325. [Google Scholar] [CrossRef]
- Kaspera, R.; Totah, R.A. Epoxyeicosatrienoic acids: Formation, metabolism and potential role in tissue physiology and pathophysiology. Expert Opin. Drug Metab. Toxicol. 2009, 5, 757–771. [Google Scholar] [CrossRef]
- Shahabi, P.; Siest, G.; Meyer, U.A.; Visvikis-Siest, S. Human cytochrome P450 epoxygenases: Variability in expression and role in inflammation-related disorders. Pharmacol. Ther. 2014, 144, 134–161. [Google Scholar] [CrossRef]
- Behm, D.J.; Ogbonna, A.; Wu, C.; Burns-Kurtis, C.L.; Douglas, S.A. Epoxyeicosatrienoic acids function as selective, endogenous antagonists of native thromboxane receptors: Identification of a novel mechanism of vasodilation. J. Pharmacol. Exp. Ther. 2009, 328, 231–239. [Google Scholar] [CrossRef] [Green Version]
- Webler, A.C.; Michaelis, U.R.; Popp, R.; Barbosa-Sicard, E.; Murugan, A.; Falck, J.R.; Fisslthaler, B.; Fleming, I. Epoxyeicosatrienoic acids are part of the VEGF-activated signaling cascade leading to angiogenesis. Am. J. Physiol. Cell Physiol. 2008, 295, C1292–C1301. [Google Scholar] [CrossRef] [Green Version]
- Lu, T.; Ye, D.; Wang, X.; Seubert, J.M.; Graves, J.P.; Bradbury, J.A.; Zeldin, D.C.; Lee, H.C. Cardiac and vascular KATP channels in rats are activated by endogenous epoxyeicosatrienoic acids through different mechanisms. J. Physiol. 2006, 575, 627–644. [Google Scholar] [CrossRef] [PubMed]
- Theken, K.N.; Schuck, R.N.; Edin, M.L.; Tran, B.; Ellis, K.; Bass, A.; Lih, F.B.; Tomer, K.B.; Poloyac, S.M.; Wu, M.C.; et al. Evaluation of cytochrome P450-derived eicosanoids in humans with stable atherosclerotic cardiovascular disease. Atherosclerosis 2012, 222, 530–536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schuck, R.N.; Theken, K.N.; Edin, M.L.; Caughey, M.; Bass, A.; Ellis, K.; Tran, B.; Steele, S.; Simmons, B.P.; Lih, F.B.; et al. Cytochrome P450-derived eicosanoids and vascular dysfunction in coronary artery disease patients. Atherosclerosis 2013, 227, 442–448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oni-Orisan, A.; Edin, M.L.; Lee, J.A.; Wells, M.A.; Christensen, E.S.; Vendrov, K.C.; Lih, F.B.; Tomer, K.B.; Bai, X.; Taylor, J.M.; et al. Cytochrome P450-derived epoxyeicosatrienoic acids and coronary artery disease in humans: A targeted metabolomics study. J. Lipid Res. 2016, 57, 109–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, S.; Chen, W.; Murphy, E.; Gabel, S.; Tomer, K.B.; Foley, J.; Steenbergen, C.; Falck, J.R.; Moomaw, C.R.; Zeldin, D.C. Molecular cloning, expression, and functional significance of a cytochrome P450 highly expressed in rat heart myocytes. J. Biol. Chem. 1997, 272, 12551–12559. [Google Scholar] [CrossRef] [Green Version]
- Goulitquer, S.; Dréano, Y.; Berthou, F.; Corcos, L.; Lucas, D. Determination of epoxyeicosatrienoic acids in human red blood cells and plasma by GC/MS in the NICI mode. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2008, 876, 83–88. [Google Scholar] [CrossRef]
- Wu, S.; Moomaw, C.R.; Tomer, K.B.; Falck, J.R.; Zeldin, D.C. Molecular cloning and expression of CYP2J2, a human cytochrome P450 arachidonic acid epoxygenase highly expressed in heart. J. Biol. Chem. 1996, 271, 3460–3468. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.; Bradbury, J.A.; King, L.; Maronpot, R.; Davis, L.S.; Breyer, M.D.; Zeldin, D.C. Molecular cloning and characterization of mouse CYP2J6, an unstable cytochrome P450 isoform. Biochem. Pharmacol. 2002, 64, 1447–1460. [Google Scholar] [CrossRef]
- Graves, J.P.; Edin, M.L.; Bradbury, J.A.; Gruzdev, A.; Cheng, J.; Lih, F.B.; Masinde, T.A.; Qu, W.; Clayton, N.P.; Morrison, J.P.; et al. Characterization of four new mouse cytochrome P450 enzymes of the CYP2J subfamily. Drug Metab. Dispos. 2013, 41, 763–773. [Google Scholar] [CrossRef] [Green Version]
- Aliwarga, T.; Raccor, B.S.; Lemaitre, R.N.; Sotoodehnia, N.; Gharib, S.A.; Xu, L.; Totah, R.A. Enzymatic and free radical formation of cis-and trans-epoxyeicosatrienoic acids in vitro and in vivo. Free Radic. Biol. Med. 2017, 112, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Seubert, J.; Yang, B.; Bradbury, J.A.; Graves, J.; Degraff, L.M.; Gabel, S.; Gooch, R.; Foley, J.; Newman, J.; Mao, L.; et al. Enhanced postischemic functional recovery in CYP2J2 transgenic hearts involves mitochondrial ATP-sensitive K+ channels and p42/p44 MAPK pathway. Circ. Res. 2004, 95, 506–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, C.R.; Imig, J.D.; Edin, M.L.; Foley, J.; DeGraff, L.M.; Bradbury, J.A.; Graves, J.P.; Lih, F.B.; Clark, J.; Myers, P.; et al. Endothelial expression of human cytochrome P450 epoxygenases lowers blood pressure and attenuates hypertension-induced renal injury in mice. FASEB J. 2010, 24, 3770–3781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Guo, X.; Chen, Y.; Yin, H.; Li, J.; Doan, J.; Liu, Q. Assessment of cardiac morphological and functional changes in mouse model of transverse aortic constriction by echocardiographic imaging. J. Vis. Exp. 2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, X.; Yin, H.; Li, L.; Chen, Y.; Li, J.; Doan, J.; Steinmetz, R.; Liu, Q. Cardioprotective role of tumor necrosis factor receptor-associated factor 2 by suppressing apoptosis and necroptosis. Circulation 2017, 136, 729–742. [Google Scholar] [CrossRef]
- Evangelista, E.A.; Lemaitre, R.N.; Sotoodehnia, N.; Gharib, S.A.; Totah, R.A. CYP2J2 Expression in adult ventricular myocytes protects against reactive oxygen species toxicity. Drug Metab. Dispos. 2018, 46, 380–386. [Google Scholar] [CrossRef]
- Wang, X.; Ni, L.; Yang, L.; Duan, Q.; Chen, C.; Edin, M.L.; Zeldin, D.C.; Wang, D.W. CYP2J2-derived epoxyeicosatrienoic acids suppress endoplasmic reticulum stress in heart failure. Mol. Pharmacol. 2014, 85, 105–115. [Google Scholar] [CrossRef] [Green Version]
- Westphal, C.; Spallek, B.; Konkel, A.; Marko, L.; Qadri, F.; DeGraff, L.M.; Schubert, C.; Bradbury, J.A.; Regitz-Zagrosek, V.; Falck, J.R.; et al. CYP2J2 overexpression protects against arrhythmia susceptibility in cardiac hypertrophy. PLoS ONE 2013, 8, e73490. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; El-Sikhry, H.; Chaudhary, K.R.; Batchu, S.N.; Shayeganpour, A.; Jukar, T.O.; Bradbury, J.A.; Graves, J.P.; DeGraff, L.M.; Myers, P.; et al. Overexpression of CYP2J2 provides protection against doxorubicin-induced cardiotoxicity. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H37–H46. [Google Scholar] [CrossRef] [Green Version]
- Marin-Garcia, J.; Goldenthal, M.J.; Moe, G.W. Mitochondrial pathology in cardiac failure. Cardiovasc. Res. 2001, 49, 17–26. [Google Scholar] [CrossRef] [Green Version]
- Kinugawa, S.; Tsutsui, H.; Hayashidani, S.; Ide, T.; Suematsu, N.; Satoh, S.; Utsumi, H.; Takeshita, A. Treatment with dimethylthiourea prevents left ventricular remodeling and failure after experimental myocardial infarction in mice: Role of oxidative stress. Circ. Res. 2000, 87, 392–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaudhary, K.R.; Zordoky, B.N.; Edin, M.L.; Alsaleh, N.; El-Kadi, A.O.; Zeldin, D.C.; Seubert, J.M. Differential effects of soluble epoxide hydrolase inhibition and CYP2J2 overexpression on postischemic cardiac function in aged mice. Prostaglandins Other Lipid Mediat. 2013, 104, 8–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salehi, B.; Berkay-Yılmaz, Y.; Antika, G.; Boyunegmez-Tumer, T.; Fawzi-Mahomoodally, M.; Lobine, D.; Akram, M.; Riaz, M.; Capanoglu, E.; Sharopov, F.; et al. Insights on the use of α-lipoic acid for therapeutic purposes. Biomolecules 2019, 9, 356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.J.; Bird, K.E.; McMillen, T.S.; LeBoeuf, R.C.; Hagen, T.M.; Frei, B. Dietary alpha-lipoic acid supplementation inhibits atherosclerotic lesion development in apolipoprotein E-deficient and apolipoprotein E/low-density lipoprotein receptor-deficient mice. Circulation 2008, 117, 421–428. [Google Scholar] [CrossRef] [Green Version]
- Sardu, C.; Santulli, G.; Santamaria, M.; Barbieri, M.; Sacra, C.; Paolisso, P.; D’Amico, F.; Testa, N.; Caporaso, I.; Paolisso, G.; et al. Effects of alpha lipoic acid on multiple cytokines and biomarkers and recurrence of atrial fibrillation within 1 year of catheter ablation. Am. J. Cardiol. 2017, 119, 1382–1386. [Google Scholar] [CrossRef] [Green Version]
- Jiang, S.; Zhu, W.; Li, C.; Zhang, X.; Lu, T.; Ding, Z.; Cao, K.; Liu, L. α-Lipoic acid attenuates LPS-induced cardiac dysfunction through a PI3K/Akt-dependent mechanism. Int. Immunopharmacol. 2013, 16, 100–107. [Google Scholar] [CrossRef]
- Dhanasekaran, A.; Gruenloh, S.K.; Buonaccorsi, J.N.; Zhang, R.; Gross, G.J.; Falck, J.R.; Patel, P.K.; Jacobs, E.R.; Medhora, M. Multiple antiapoptotic targets of the PI3K/Akt survival pathway are activated by epoxyeicosatrienoic acids to protect cardiomyocytes from hypoxia/anoxia. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H724–H735. [Google Scholar] [CrossRef]
- Moss, A.J.; Hall, W.J.; Cannom, D.S.; Klein, H.; Brown, M.W.; Daubert, J.P.; Estes, N.A.; Foster, E.; Greenberg, H.; Higgins, S.L.; et al. Cardiac-resynchronization therapy for the prevention of heart-failure events. N. Engl. J. Med. 2009, 361, 1329–1338. [Google Scholar] [CrossRef] [Green Version]
- Vanderheyden, M.; Mullens, W.; Delrue, L.; Goethals, M.; de Bruyne, B.; Wijns, W.; Geelen, P.; Verstreken, S.; Wellens, F.; Bartunek, J. Myocardial gene expression in heart failure patients treated with cardiac resynchronization therapy responders versus nonresponders. J. Am. Coll. Cardiol. 2008, 51, 129–136. [Google Scholar] [CrossRef] [Green Version]
- Marfella, R.; Di Filippo, C.; Potenza, N.; Sardu, C.; Rizzo, M.R.; Siniscalchi, M.; Musacchio, E.; Barbieri, M.; Mauro, C.; Mosca, N.; et al. Circulating microRNA changes in heart failure patients treated with cardiac resynchronization therapy: Responders vs. non-responders. Eur. J. Heart Fail. 2013, 15, 1277–1288. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.; Chen, C.; Yang, S.; Gong, W.; Wang, Y.; Cianflone, K.; Tang, J.; Wang, D.W. Let-7b inhibits human cancer phenotype by targeting cytochrome P450 epoxygenase 2J2. PLoS ONE 2012, 7, e39197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, R.; Lee, S.; Lee, J.; Kim, M.; Kim, W.J.; Lee, H.W.; Lee, M.Y.; Kim, J.; Chang, W. Exosomes derived from microRNA-584 transfected mesenchymal stem cells: Novel alternative therapeutic vehicles for cancer therapy. BMB Rep. 2018, 51, 406–411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tolonen, A.M.; Magga, J.; Szabó, Z.; Viitala, P.; Gao, E.; Moilanen, A.M.; Ohukainen, P.; Vainio, L.; Koch, W.J.; Kerkelä, R.; et al. Inhibition of Let-7 microRNA attenuates myocardial remodeling and improves cardiac function postinfarction in mice. Pharmacol. Res. Perspect. 2014, 2, e00056. [Google Scholar] [CrossRef] [PubMed]
- Sardu, C.; Paolisso, G.; Marfella, R. Molecular mechanisms and therapeutic targets of inflammatory-related cardiovascular diseases: From molecular mechanisms to therapeutic targets. Curr. Pharm. Des. 2020. [Google Scholar] [CrossRef]








| Heart | Erythrocyte | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| cis-/trans- ratio | cis-/trans- ratio | |||||||||
| 14,15-EET | 11,12-EET | 8,9-EET | 5,6-EET | Total | 14,15-EET | 11,12-EET | 8,9-EET | 5,6-EET | Total | |
| WT-Sham | 0.59 ± 0.1 | 0.66 ± 0.1 | 0.47 ± 0.1 | 0.18 ± 0.08 | 0.30 ± 0.06 | 2.2 ± 0.3 | 1.9 ± 0.2 | 2.4 ± 0.4 | 1.4 ± 0.2 | 1.8 ± 0.3 |
| Tr-Sham | 0.53 ± 0.05 | 0.78 ± 0.09 | 0.27 ± 0.04 | 0.11 ± 0.005 | 0.28 ± 0.02 | 2.2 ± 0.4 | 1.9 ± 0.2 | 1.8 ± 0.6 | 1.6 ± 0.3 | 1.8 ± 0.1 |
| WT-MI | 0.50 ± 0.1 | 0.60 ± 0.1 | 0.56 ± 0.1 | 0.38 ± 0.09 | 0.47 ± 0.1 | 2.0 ± 0.2 | 1.8 ± 0.2 | 2.6 ± 0.3 | 1.9 ± 0.2 | 2.0 ± 0.2 |
| Tr-MI | 0.49 ± 0.07 | 0.68 ± 0.1 | 0.25 ± 0.04 | 0.13 ± 0.01 | 0.28 ± 0.03 | 2.4 ± 0.2 | 1.8 ± 0.3 | 1.3 ± 0.4 | 1.5 ± 0.3 | 1.7 ± 0.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aliwarga, T.; Guo, X.; Evangelista, E.A.; Lemaitre, R.N.; Sotoodehnia, N.; Gharib, S.A.; Zeldin, D.C.; Liu, Q.; Totah, R.A. Higher Epoxyeicosatrienoic Acids in Cardiomyocytes-Specific CYP2J2 Transgenic Mice Are Associated with Improved Myocardial Remodeling. Biomedicines 2020, 8, 144. https://doi.org/10.3390/biomedicines8060144
Aliwarga T, Guo X, Evangelista EA, Lemaitre RN, Sotoodehnia N, Gharib SA, Zeldin DC, Liu Q, Totah RA. Higher Epoxyeicosatrienoic Acids in Cardiomyocytes-Specific CYP2J2 Transgenic Mice Are Associated with Improved Myocardial Remodeling. Biomedicines. 2020; 8(6):144. https://doi.org/10.3390/biomedicines8060144
Chicago/Turabian StyleAliwarga, Theresa, Xiaoyun Guo, Eric A. Evangelista, Rozenn N. Lemaitre, Nona Sotoodehnia, Sina A. Gharib, Darryl C. Zeldin, Qinghang Liu, and Rheem A. Totah. 2020. "Higher Epoxyeicosatrienoic Acids in Cardiomyocytes-Specific CYP2J2 Transgenic Mice Are Associated with Improved Myocardial Remodeling" Biomedicines 8, no. 6: 144. https://doi.org/10.3390/biomedicines8060144
APA StyleAliwarga, T., Guo, X., Evangelista, E. A., Lemaitre, R. N., Sotoodehnia, N., Gharib, S. A., Zeldin, D. C., Liu, Q., & Totah, R. A. (2020). Higher Epoxyeicosatrienoic Acids in Cardiomyocytes-Specific CYP2J2 Transgenic Mice Are Associated with Improved Myocardial Remodeling. Biomedicines, 8(6), 144. https://doi.org/10.3390/biomedicines8060144








