?-Caryophyllene Reduces the Inflammatory Phenotype of Periodontal Cells by Targeting CB2 Receptors
Abstract
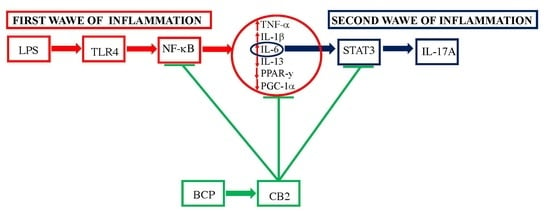
:1. Introduction
2. Materials and Methods
2.1. Cell Cultures
2.2. Treatments of Cells
2.3. MTT Assay
2.4. Measurements of Cytokines by Enzyme-Linked Immunosorbent Assay (ELISA)
2.5. Real Time Quantitative PCR Amplification (RTqPCR)
2.6. Statistical Analysis
3. Results
3.1. BCP Reverts the Inflammatory Phenotype Induced by LPS in Gingival Fibroblasts and Oral Mucosa Epithelial Cells
3.2. BCP Modulates Upstream Signals that Trigger the First Phase of the Inflammatory Response
3.3. BCP Halts the Second Phase of the Inflammatory Response in Gingival Fibroblasts and Oral Mucosa Epithelial Cells
3.4. BCP Does Not Affect Cell Viability
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Elting, L.S.; Cooksley, C.D.; Chambers, M.S.; Garden, A.S. Risk, Outcomes, and Costs of Radiation-Induced Oral Mucositis Among Patients With Head-and-Neck Malignancies. Int. J. Radiat. Oncol. Biol. Phys. 2007, 68, 1110–1120. [Google Scholar] [CrossRef] [PubMed]
- Elting, L.S.; Keefe, D.M.; Sonis, S.T.; Garden, A.S.; Spijkervet, F.K.; Barasch, A.; Tishler, R.B.; Canty, T.P.; Kudrimoti, M.K.; Vera-Llonch, M. Patient-reported measurements of oral mucositis in head and neck cancer patients treated with radiotherapy with or without chemotherapy. Cancer 2008, 113, 2704–2713. [Google Scholar] [CrossRef] [PubMed]
- Murphy, B.A.; Beaumont, J.L.; Isitt, J.; Garden, A.S.; Gwede, C.K.; Trotti, A.M.; Meredith, R.F.; Epstein, J.B.; Le, Q.T.; Brizel, D.M. Mucositis-Related Morbidity and Resource Utilization in Head and Neck Cancer Patients Receiving Radiation Therapy With or Without Chemotherapy. J. Pain Symptom Manag. 2009, 38, 522–532. [Google Scholar] [CrossRef] [PubMed]
- Lalla, R.V.; Sonis, S.T.; Peterson, D.E. Management of oral mucositis in patients who have cancer. Dent. Clin. N. Am. 2008, 52, 61–77. [Google Scholar] [CrossRef] [Green Version]
- Pjetursson, B.E.; Asgeirsson, A.G.; Zwahlen, M.; Sailer, I. Improvements in implant dentistry over the last decade: Comparison of survival and complication rates in older and newer publications. Int. J. Oral Maxillofac. Implants 2014, 29, 308–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albrektsson, T.; Donos, N. Implant survival and complications. The third EAO consensus conference 2012. Clin. Oral Implants Res. 2012, 6, 63–65. [Google Scholar] [CrossRef]
- Atieh, M.A.; Alsabeeha, N.H.M.; Faggion, C.M.; Druncan, W.J. The frequency of peri-implant diseases: A systematic review and metaanalysis. J. Periodontol. 2013, 84, 1586–1598. [Google Scholar]
- Abrahamsson, I.; Berglundh, T. Effects of different implant surfaces and designs on marginal bone-level alterations: A review. Clin. Oral Implants Res. 2009, 4, 207–215. [Google Scholar] [CrossRef]
- Sonis, S.T. New thoughts on the initiation of mucositis. Oral Dis. 2010, 16, 597–600. [Google Scholar] [CrossRef]
- Mombelli, A.; Oosten, M.A.C.; Schürch, E., Jr.; Land, N.P. The microbiota associated with successful or failing osseointegrated titanium implants. Oral Microbiol. Immunol. 1987, 2, 145–151. [Google Scholar] [CrossRef]
- Sardaro, N.; Della Vella, F.; Incalza, M.A.; Di Stasio, D.; Lucchese, A.; Contaldo, M.; Laudadio, C.; Petruzzi, M. Oxidative Stress and Oral Mucosal Diseases: An Overview. In Vivo 2019, 33, 289–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Curra, M.; Pellicioli, A.C.; Filho, N.A.; Ochs, G.; Matte, U.; Filho, M.S.; Martins, M.A.; Martins, M.D. Photobiomodulation reduces oral mucositis by modulating NF-kB. J. Biomed. Opt. 2015, 20, 125008. [Google Scholar] [CrossRef] [PubMed]
- Mangoni, M.; Sottili, M.; Gerini, C.; Desideri, I.; Bastida, C.; Pallotta, S.; Castiglione, F.; Bonomo, P.; Meattini, I.; Greto, D. A PPAR gamma agonist protects against oral mucositis induced by irradiation in a murine model. Oral Oncol. 2017, 64, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Sottili, M.; Mangoni, M.; Gerini, C.; Salvatore, G.; Castiglione, F.; Desideri, I.; Bonomo, P.; Meattini, I.; Greto, D.; Loi, M. Peroxisome proliferator activated receptor gamma stimulation for prevention of 5-fluorouracil-induced oral mucositis in mice. Head Neck 2018, 40, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Panahipour, L.; Nasserzare, S.; Amer, Z.; Brücke, F.; Stähli, A.; Kreissl, A.; Haiden, N.; Gruber, R. The anti-inflammatory effect of milk and dairy products on periodontal cells: An in vitro approach. Clin. Oral Investig. 2019, 23, 1959–1966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russo, E.B. Beyond Cannabis: Plants and the Endocannabinoid System. Trends Pharmacol. Sci. 2016, 37, 594–605. [Google Scholar] [CrossRef]
- Sharma, C.; Al Kaabi, J.M.; Nurulain, S.M.; Goyal, S.N.; Kamal, M.A.; Ojha, S. Polypharmacological Properties and Therapeutic Potential of β-Caryophyllene: A Dietary Phytocannabinoid of Pharmaceutical Promise. Curr. Pharm. Des. 2016, 22, 3237–3264. [Google Scholar] [CrossRef]
- D’Ascola, A.; Irrera, N.; Ettari, R.; Bitto, A.; Pallio, G.; Mannino, F.; Atteritano, M.; Campo, G.M.; Minutoli, L.; Arcoraci, V. Exploiting curcumin synergy with natural products using quantitative analysis of doe-effect relationships in an in vitro model of osteoarthritis. Front. Pharmacol. 2019, 10, 1347. [Google Scholar] [CrossRef] [Green Version]
- Marini, H.; Polito, F.; Altavilla, D.; Irrera, N.; Minutoli, L.; Calò, M.; Adamo, E.B.; Vaccaro, M.; Squadrito, F.; Bitto, A. Genistein aglycone improves skin repair in an incisional model of wound healing: A comparison with raloxifene and oestradiol in ovariectomized rats. Br. J. Pharmacol. 2010, 160, 1185–1194. [Google Scholar] [CrossRef] [Green Version]
- Minutoli, L.; Marini, H.; Rinaldi, M.; Bitto, A.; Irrera, N.; Pizzino, G.; Pallio, G.; Calò, M.; Adamo, E.B.; Trichilo, V. A dual inhibitor of cyclooxygenase and 5-lipoxygenase protects against kainic acid-induced brain injury. Neuromolecular Med. 2015, 17, 192–201. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Bitto, A.; Pallio, G.; Mannino, F.; Arcoraci, V.; Aliquò, F.; Minutoli, L.; De Ponte, C.; D’Andrea, P. Cadmium-Induced Oxidative Stress Impairs Glycemic Control in Adolescents. Oxid. Med. Cell Longev. 2017, 2017, 6341671. [Google Scholar] [CrossRef] [PubMed]
- Irrera, N.; D’Ascola, A.; Pallio, G.; Bitto, A.; Mazzon, E.; Mannino, F.; Squadrito, V.; Arcoraci, V.; Minutoli, L.; Campo, G.M. β-Caryophyllene Mitigates Collagen Antibody Induced Arthritis (CAIA) in Mice Through a Cross-Talk between CB2 and PPAR-γ Receptors. Biomolecules 2019, 9, 326. [Google Scholar] [CrossRef] [Green Version]
- Tang, D.; Kang, R.; Coyne, C.B.; Zeh, H.J.; Lotze, M.T. PAMPs and DAMPs: Signal 0s that spur autophagy and immunity. Immunol. Rev. 2012, 249, 158–175. [Google Scholar] [CrossRef]
- Hou, J.; Zheng, H.; Li, P.; Liu, H.; Zhou, H.; Yang, X. Distinct shifts in the oral microbiota are associated with the progression and aggravation of mucositis during radiotherapy. Radiother. Oncol. 2018, 129, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Lalla, R.V.; Bowen, J.; Barasch, A.; Elting, L.; Epstein, J.; Keefe, D.M.; McGuire, D.B.; Migliorati, C.; Nicolatou-Galitis, O.; Peterson, D.E. Mucositis Guidelines Leadership Group of the Multinational Association of Supportive Care in Cancer and International Society of Oral Oncology (MASCC/ISOO). MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer 2014, 120, 1453–1461. [Google Scholar] [CrossRef] [Green Version]
- Li, C.L.; Huang, H.L.; Wang, W.C.; Hua, H. Efficacy and safety of topical herbal medicine treatment on recurrent aphthous stomatitis: A systemic review. Drug Des. Devel. Ther. 2016, 10, 107–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Ng, K.H.; Kuo, C.Y.; Wu, D.J. Chinese herbal medicine for recurrent aphthous stomatitis: A protocol for systematic review and meta-analysis. Medicine 2018, 97, e13681. [Google Scholar] [CrossRef] [PubMed]
- Baharvand, M.; Jafari, S.; Mortazavi, H. Herbs in Oral Mucositis. J. Clin. Diagn. Res. 2017, 11, ZE05–ZE11. [Google Scholar] [CrossRef]
- La Porta, C.; Bura, S.A.; Llorente-Onaindia, J.; Pastor, A.; Navarrete, F.; García-Gutiérrez, M.S.; De la Torre, R.; Manzanares, J.; Monfort, J.; Maldonado, R. Role of the endocannabinoid system in the emotional manifestations of osteoarthritis pain. Pain 2015, 156, 2001–2012. [Google Scholar] [CrossRef] [Green Version]
- Machado, K.D.C.; Islam, M.T.; Ali, E.S.; Rouf, R.; Uddin, S.J.; Dev, S.; Shilpi, J.A.; Shill, M.C.; Reza, H.M.; Das, A.K. A systematic review on the neuroprotective perspectives of beta-caryophyllene. Phytother. Res. 2018, 32, 2376–2388. [Google Scholar] [CrossRef]
- Sonis, S.T. Pathobiology of oral mucositis: Novel insights and opportunities. J. Support. Oncol. 2007, 5 (Suppl. S4), 3–11. [Google Scholar]
- Youssef, D.A.; El-Fayoumi, H.M.; Mahmoud, M.F. Beta-caryophyllene protects against diet-induced dyslipidemia and vascular inflammation in rats: Involvement of CB2 and PPAR-γ receptors. Chem. Biol. Interact. 2019, 297, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Caldera, A.; Vernal, R.; Paredes, R.; Veloso-Matta, P.; Astorga, J.; Hernández, M. Human periodontal ligament fibroblasts synthesize C-reactive protein and Th-related cytokines in response to interleukin (IL)-6 trans-signalling. Int. Endod. J. 2018, 51, 632–640. [Google Scholar] [CrossRef]
- Bharadwaj, U.; Kasembeli, M.M.; Robinson, P.; Tweardy, D.J. Targeting Janus Kinases and Signal Transducer and Activator of Transcription 3 to Treat Inflammation, Fibrosis, and Cancer: Rationale, Progress, and Caution. Pharmacol. Rev. 2020, 72, 486–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vernal, R.; Dutzan, N.; Chaparro, A.; Puente, J.; Antonieta Valenzuela, M.; Gamonal, J. Levels of interleukin-17 in gingival crevicular fluid and in supernatants of cellular cultures of gingival tissue from patients with chronic periodontitis. J. Clin. Periodontol. 2005, 32, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Mitani, A.; Niedbala, W.; Fujimura, T.; Mogi, M.; Miyamae, S.; Higuchi, N.; Abe, A.; Hishikawa, T.; Mizutani, M.; Ishihara, Y. Increased expression of interleukin (IL)-35 and IL-17, but not IL-27, in gingival tissues with chronic periodontitis. J. Periodontol. 2015, 86, 301–309. [Google Scholar] [CrossRef]
- Irrera, N.; D’Ascola, A.; Pallio, G.; Bitto, A.; Mannino, F.; Arcoraci, V.; Rottura, M.; Ieni, A.; Minutoli, L.; Metro, D. β-Caryophyllene Inhibits Cell Proliferation through a Direct Modulation of CB2 Receptors in Glioblastoma Cells. Cancers 2020, 23, 12. [Google Scholar]






| Gene | Sequence |
|---|---|
| β-actin | Fw:5′AGAGCTACGAGCTGCCTGAC3′ |
| Rw:5′AGCACTGTGTTGGCGTACAG3′ | |
| TNF-α | Fw:5′CAGAGGGCCTGTACCTCATC3′ |
| Rw:5′GGAAGACCCCTCCCAGATAG3′ | |
| IL-1β | Fw:5′TGAGCTCGCCAGTGAAATGA3′ |
| Rw:5′AGATTCGTAGCTGGATGCCG3′ | |
| IL-13 | Fw:5′CATGGCGCTTTTGTTGACCA 3′ |
| Rw:5′AGCTGTCAGGTTGATGCTCC3′ | |
| NF-κB | Fw:5′CCTGGATGACTCTTGGGAAA3′ |
| Rw:5′TCAGCCAGCTGTTTCATGTC3′ | |
| PPAR-γ | Fw:5′TCGACCAGCTGAATCCAGAG3′ |
| Rw:5′GGGGGTGATGTGTTTGAACTTG3′ | |
| PGC-1α | Fw:5′CATGTGCAACCAGGACTCTGA3′ |
| Rw:5 GCGCATCAAATGAGGGCAAT3′ | |
| STAT3 | Fw:5′GAGCTGCACCTGATCACCTT3′ |
| Rw:5′CCCAGAAGGAGAAGCCCTTG3′ | |
| IL-6 | Fw:5′TTCGGTCCAGTTGCCTTCTC3′ |
| Rw:5′CAGCTCTGGCTTGTTCCTCA3′ | |
| IL-17A | Fw:5′CTGTCCCCATCCAGCAAGAG3′ |
| Rw:5′AGGCCACATGGTGGACAATC3′ |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Picciolo, G.; Pallio, G.; Altavilla, D.; Vaccaro, M.; Oteri, G.; Irrera, N.; Squadrito, F. ?-Caryophyllene Reduces the Inflammatory Phenotype of Periodontal Cells by Targeting CB2 Receptors. Biomedicines 2020, 8, 164. https://doi.org/10.3390/biomedicines8060164
Picciolo G, Pallio G, Altavilla D, Vaccaro M, Oteri G, Irrera N, Squadrito F. ?-Caryophyllene Reduces the Inflammatory Phenotype of Periodontal Cells by Targeting CB2 Receptors. Biomedicines. 2020; 8(6):164. https://doi.org/10.3390/biomedicines8060164
Chicago/Turabian StylePicciolo, Giacomo, Giovanni Pallio, Domenica Altavilla, Mario Vaccaro, Giacomo Oteri, Natasha Irrera, and Francesco Squadrito. 2020. "?-Caryophyllene Reduces the Inflammatory Phenotype of Periodontal Cells by Targeting CB2 Receptors" Biomedicines 8, no. 6: 164. https://doi.org/10.3390/biomedicines8060164
APA StylePicciolo, G., Pallio, G., Altavilla, D., Vaccaro, M., Oteri, G., Irrera, N., & Squadrito, F. (2020). ?-Caryophyllene Reduces the Inflammatory Phenotype of Periodontal Cells by Targeting CB2 Receptors. Biomedicines, 8(6), 164. https://doi.org/10.3390/biomedicines8060164