Secreted Factors from Keloid Keratinocytes Modulate Collagen Deposition by Fibroblasts from Normal and Fibrotic Tissue: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Isolation and Culture of Fibroblasts
2.3. Isolation and Culture of Keratinocytes
2.4. qPCR for COL1A
2.5. Fluorescent Resonance Energy Transfer (FRET) Assay to Quantitate Procollagen I
2.6. Fibroblast and Keratinocyte Co-Culture
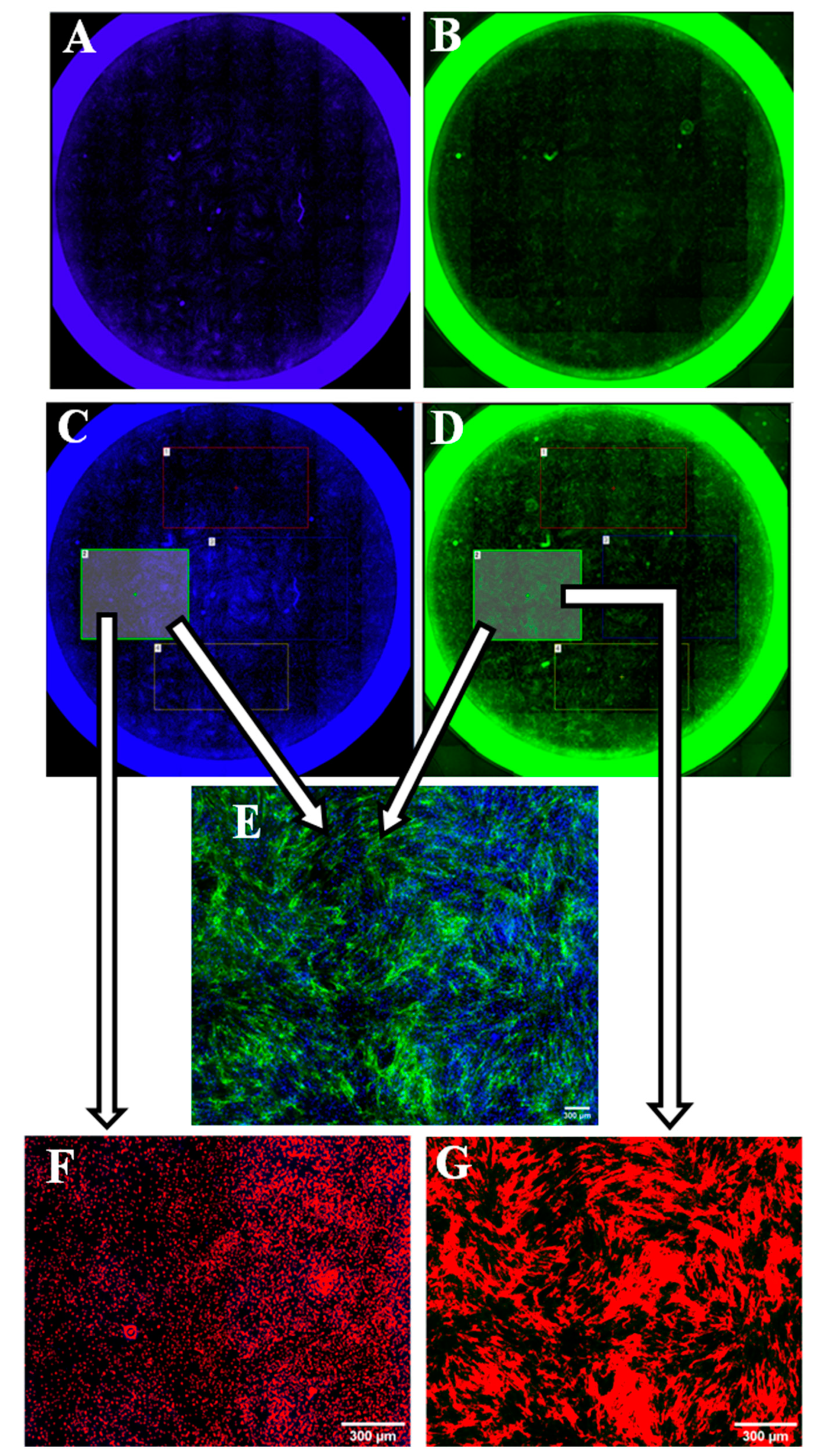
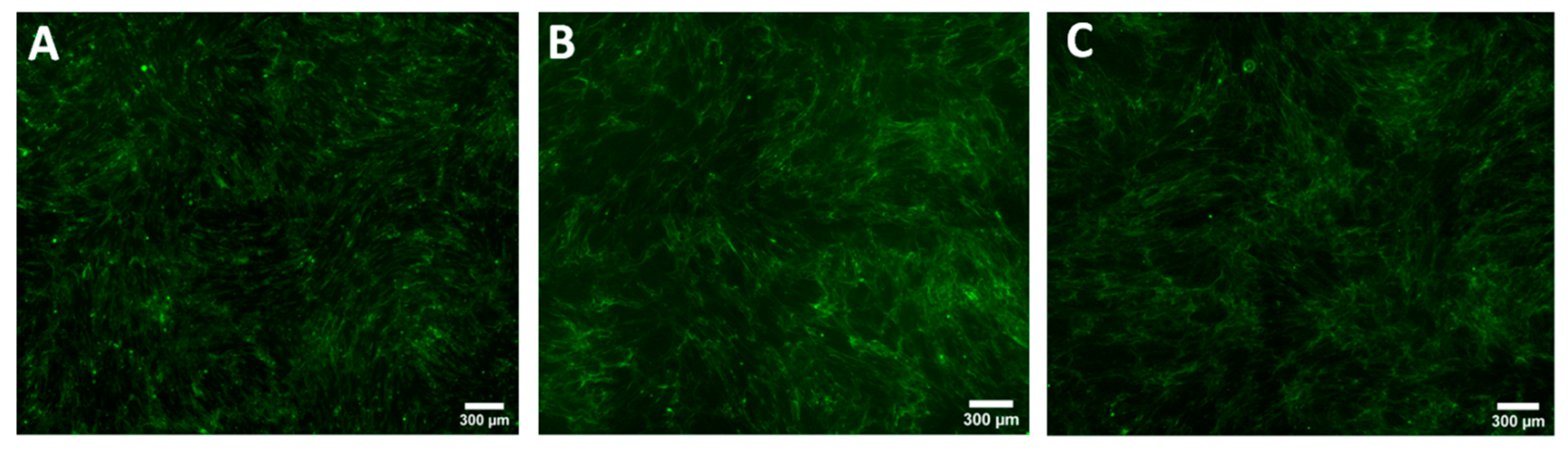
2.7. Staining for Collagen Type I
2.8. Blind Assessment
2.9. Whole Well Imaging for Collagen Quantitation
2.10. Statistical Analysis
3. Results
3.1. Collagen Production of Keloid Fibroblasts Is Significantly Higher Than Normal Skin Fibroblasts in Monoculture
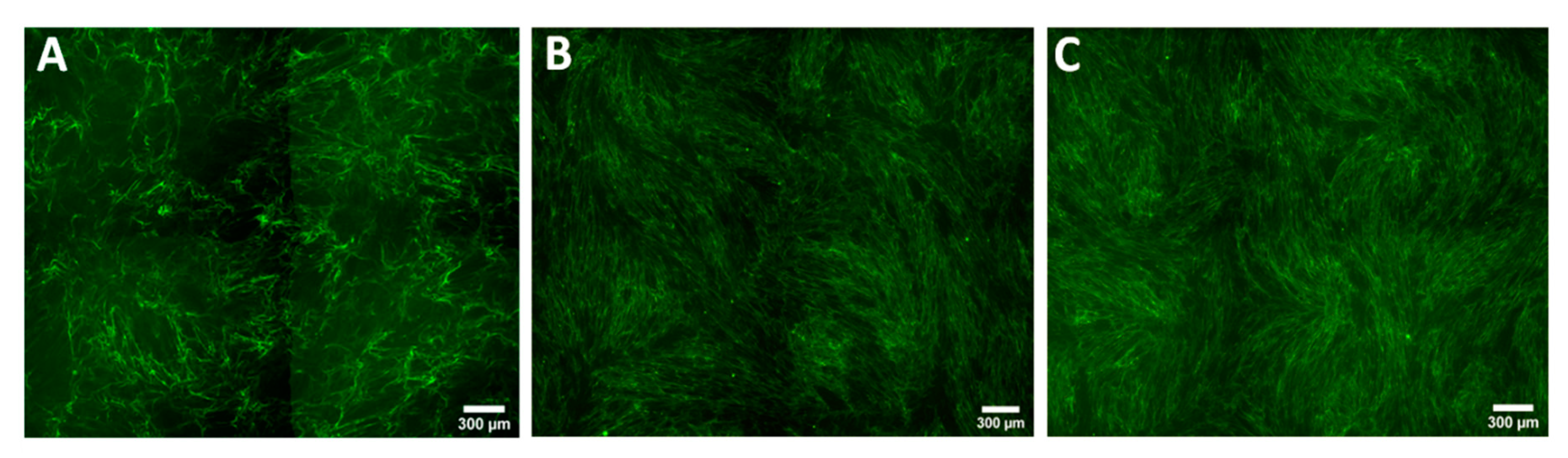
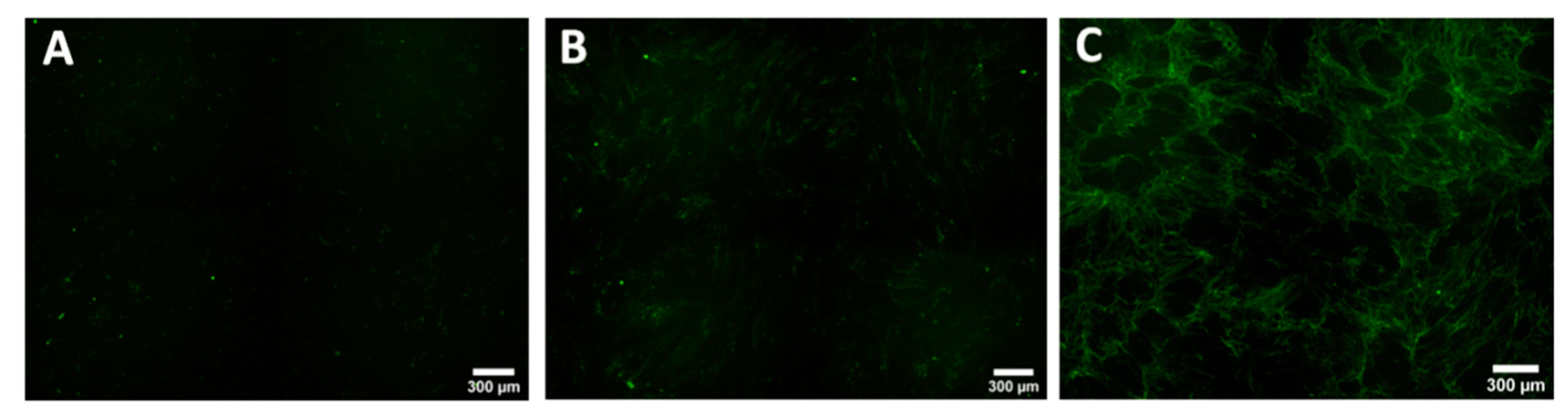
3.2. Keloid Keratinocytes Significantly Increase Collagen Production by Normal Skin Fibroblasts
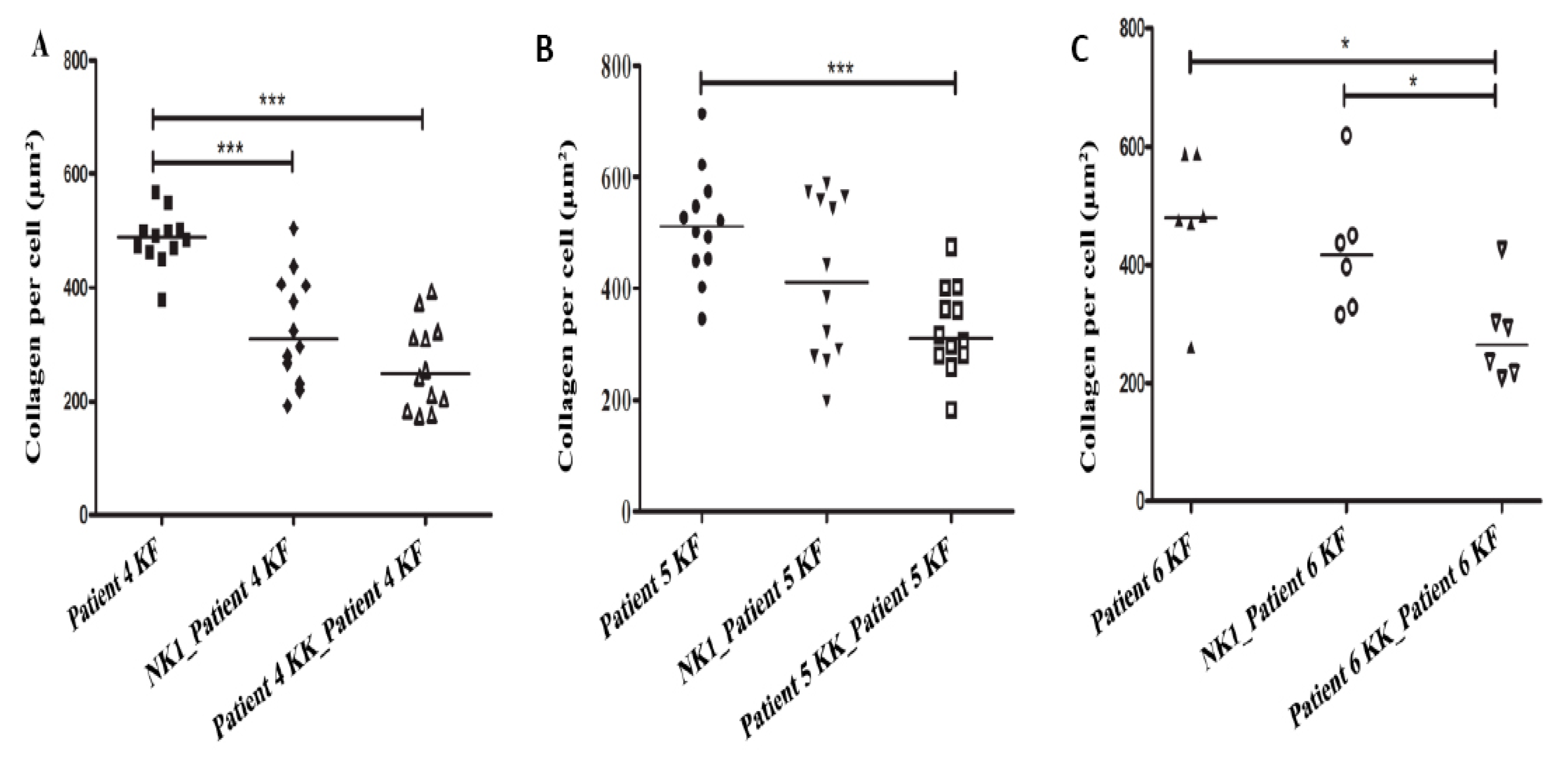
3.3. Collagen Production by Keloid Fibroblasts Is Reduced in the Presence of Keloid Keratinocytes
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| KFs | Keloid fibroblasts |
| KKs | keloid keratinocytes |
| NFs | Normal fibroblasts |
| NKs | Normal keratinocytes |
| KGF | Keratinocyte growth factor |
| COL1A | Collagen type I |
| KF | Keloid fibroblasts |
| KK | Keloid keratinocytes |
| P/S | Penicillin/Streptomycin |
| DMSO | Dimethylsulfoxide |
| DMEM | Dulbecco’s Modified Eagle’s Medium |
| KGM | keratinocytes growth media |
References
- Slemp, A.E.; Kirschner, R.E. Keloids and scars: A review of keloids and scars, their pathogenesis, risk factors, and management. Curr. Opin. Pediatr. 2006, 18, 396–402. [Google Scholar] [CrossRef]
- Gauglitz, G.G.; Korting, H.C.; Pavicic, T.; Ruzicka, T.; Jeschke, M.G. Hypertrophic scarring and keloids: Pathomechanisms and current and emerging treatment strategies. Mol. Med. 2011, 17, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Podobed, O.V.; Prozorovskiĭ, N.N.; Kozlov, E.A.; Tsvetkova, T.A.; Vozdvidzhensky, C.I.; Delvig, A.A. Comparative study of collagen in hypertrophic and keloid cicatrix. Vopr. Med. Khim. 1996, 42, 240–245. [Google Scholar] [PubMed]
- Uitto, J.; Perejda, A.J.; Abergel, R.P.; Chu, M.L.; Ramirez, F. Altered steady-state ratio of type I/III procollagen mRNAs correlates with selectively increased type I procollagen biosynthesis in cultured keloid fibroblasts. Proc. Natl. Acad. Sci. USA 1985, 82, 5935–5939. [Google Scholar] [CrossRef] [PubMed]
- Syed, F.; Ahmadi, E.; Iqbal, S.A.; Singh, S.; McGrouther, D.A.; Bayat, A. Fibroblasts from the growing margin of keloid scars produce higher levels of collagen I and III compared with intralesional and extralesional sites: Clinical implications for lesional site-directed therapy. Br. J. Dermatol. 2011, 164, 83–96. [Google Scholar] [CrossRef]
- El-Ghalbzouri, A.; Gibbs, S.; Lamme, E.; Van Blitterswijk, C.A.; Ponec, M. Effect of fibroblasts on epidermal regeneration. Br. J. Dermatol. 2002, 147, 230–243. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y.; Farhangfar, F.; Zimmer, M.; Zhang, Y. Enhanced keratinocyte proliferation and migration in co-culture with fibroblasts. PLoS ONE 2012, 7, e40951. [Google Scholar] [CrossRef]
- Lim, I.J.; Phan, T.-T.; Song, C.; Tan, W.T.; Longaker, M.T. Investigation of the influence of keloid- derived keratinocytes on fibroblast growth and proliferation in vitro. Plast. Reconstr. Surg. 2001, 107, 797–808. [Google Scholar] [CrossRef] [PubMed]
- Bellemare, J.; Roberge, C.J.; Bergeron, D.; Lopez-Vallé, C.A.; Roy, M.; Moulin, V.J. Epidermis promotes dermal fibrosis: Role in the pathogenesis of hypertrophic scars. J. Pathol. 2005, 206, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Funayama, E.; Chodon, T.; Oyama, A.; Sugihara, T. Keratinocytes promote proliferation and inhibit apoptosis of the underlying fibroblasts: An important role in the pathogenesis of keloid. J. Invest. Dermatol. 2003, 121, 1326–1331. [Google Scholar] [CrossRef] [PubMed]
- Garner, W.L. Epidermal regulation of dermal fibroblast activity. Plast. Reconstr. Surg. 1998, 102, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Ghaffari, A.; Kilani, R.T.; Ghahary, A. Keratinocyte-conditioned media regulate collagen expression in dermal fibroblasts. J. Invest. Dermatol. 2009, 129, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Harrison, C.A.; Gossiel, F.; Bullock, A.J.; Sun, T.; Blumsohn, A.; Mac Neil, S. Investigation of keratinocyte regulation of collagen I synthesis by dermal fibroblasts in a simple in vitro model. Br. J. Dermatol. 2006, 154, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Ghahary, A.; Ghaffari, A. Role of keratinocyte–fibroblast crosstalk in development of hypertrophic scar. Wound Repair Regen. 2007, 15, S46–S53. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.P.; Phan, T.T.; Lim, I.J.; Cao, X. Cytokine profiling and Stat3 phosphorylation in epithelial–mesenchymal interactions between keloid keratinocytes and fibroblasts. J. Invest. Dermatol. 2009, 129, 851–861. [Google Scholar] [CrossRef] [PubMed]
- Lim, I.J.; Phan, T.T.; Bay, B.H.; Qi, R.; Huynh, H.; Tan, W.T.; Lee, S.T.; Longaker, M.T. Fibroblasts cocultured with keloid keratinocytes: Normal fibroblasts secrete collagen in a keloid like manner. Am. J. Physiol. Cell Physiol. 2002, 283, C212–C222. [Google Scholar] [CrossRef]
- Butler, P.D.; Ly, D.P.; Longaker, M.T.; Yang, G.P. Use of organotypic coculture to study keloid biology. Am. J. Surg. 2008, 195, 144–148. [Google Scholar] [CrossRef]
- Phan, T.T.; Lim, I.J.; Bay, B.H.; Qi, R.; Huynh, H.T.; Lee, S.T.; Longaker, M.T. Differences in collagen production between normal and keloid-derived fibroblasts in serum-media co-culture with keloid-derived keratinocytes. J. Dermatol. Sci. 2002, 29, 26–34. [Google Scholar] [CrossRef]
- Isaac, C.; Paggiaro, A.O.; Aldunate, J.L.C.B. Role of keratinocytes in wound contraction: An impact assessment using a model of collagen matrix populated with fibroblasts. Rev. Bras. Cir. Plást. 2011, 26, 402–406. [Google Scholar] [CrossRef]
- Chen, C.Z.; Peng, Y.X.; Wang, Z.B.; Fish, P.V.; Kaar, J.L.; Koepsel, R.R.; Russell, A.J.; Lareu, R.R.; Raghunath, M. The scar-in-a-jar: Studying potential antifibrotic compounds from the epigenetic to extracellular level in a single well. Br. J. Pharmacol. 2009, 158, 1196–1209. [Google Scholar] [CrossRef]
- Keira, S.M.; Ferreira, L.M.; Gragnani, A.; Duarte, I.D.; Santos, I.A. Experimental model for fibroblast culture. Acta Cir. Bras. 2004, 19, 11–16. [Google Scholar] [CrossRef]
- Tucci-Viegas, V.M.; Hochman, B.; França, J.P.; Ferreira, L.M. Keloid explant culture: A model for keloid fibroblasts isolation and cultivation based on the biological differences of its specific regions. Int. Wound J. 2010, 7, 339–348. [Google Scholar] [CrossRef] [PubMed]
- CELLnTEC Advanced Cell Systems AG (Bern, Switzerland). Epidermal Keratinocyte Isolation. Available online: https://cellntec.com/wp-content/uploads/pdf/Isolation_Keratinocyte_2.pdf (accessed on 19 October 2012).
- Aasen, T.; Belmonte, J.C. Isolation and cultivation of human keratinocytes from skin or plucked hair for the generation of induced pluripotent stem cells. Nat. Protoc. 2010, 5, 371. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Phan, T.T.; Lim, I.J.; Longaker, M.T.; Yang, G.P. Complex epithelial–mesenchymal interactions modulate transforming growth factor-β expression in keloid-derived cells. Wound Repair Regen. 2004, 12, 546–556. [Google Scholar] [CrossRef] [PubMed]
- Varani, J.; Dame, M.K.; Rittie, L.; Fligiel, S.E.; Kang, S.; Fisher, G.J.; Voorhees, J.J. Decreased collagen production in chronologically aged skin: Roles of age-dependent alteration in fibroblast function and defective mechanical stimulation. Am. J. Pathol. 2006, 168, 1861–1868. [Google Scholar] [CrossRef] [PubMed]
- Cancela, M.L.; Hu, B.; Price, P.A. Effect of cell density and growth factors on matrix GLA protein expression by normal rat kidney cells. J. Cell. Physiol. 1997, 171, 125–134. [Google Scholar] [CrossRef]
- Natoli, M.; Leoni, B.D.; D’Agnano, I.; D’Onofrio, M.; Brandi, R.; Arisi, I.; Zucco, F.; Felsani, A. Cell growing density affects the structural and functional properties of Caco-2 differentiated monolayer. J. Cell. Physiol. 2011, 226, 1531–1543. [Google Scholar] [CrossRef]
- Werner, S.; Krieg, T.; Smola, H. Keratinocyte–fibroblast interactions in wound healing. J. Invest. Dermatol. 2007, 127, 998–1008. [Google Scholar] [CrossRef]
- Karrer, S.; Bosserhoff, A.K.; Weiderer, P.; Landthaler, M.; Szeimies, R.M. Keratinocyte-derived cytokines after photodynamic therapy and their paracrine induction of matrix metalloproteinases in fibroblasts. Br. J. Dermatol. 2004, 151, 776–783. [Google Scholar] [CrossRef]
- Huang, P.; Bi, J.; Owen, G.R.; Chen, W.; Rokka, A.; Koivisto, L.; Heino, J.; Häkkinen, L.; Larjava, H. Keratinocyte macrovesicles regulate the expression of multiple genes in dermal fibroblasts. J. Invest. Dermatol. 2015, 135, 3051–3059. [Google Scholar] [CrossRef]
- Than, U.T.; Guanzon, D.; Broadbent, J.; Leavesley, D.I.; Salomon, C.; Parker, T. Differential expression of keratinocyte-derived extracellular vesicle miRNAs discriminate exosomes from apoptotic bodies and microvesicles. Front. Endocrinol. 2018, 9, 535. [Google Scholar] [CrossRef] [PubMed]




| Patient ID. | Age | Gender 1 | Phenotype | Site of Excision or Biopsy | Ethnicity |
|---|---|---|---|---|---|
| (Scare Age/Year) | |||||
| Patient 1 | 53 | M | Keloid (25) | Sterunm | Northwest European |
| Patient 2 | 42 | M | Keloid (5) | Ear | Northwest European |
| Patient 3 | 30 | M | Keloid (15) | Sterunm | East Asian |
| Patient 4 | 27 | F | Keloid (10) | Shoulders | East Asian |
| Patient 5 | 47 | F | Keloid (3) | Sterunm | Northwest European |
| Patient 6 | 29 | M | Keloid (6) | Sterunm | East Asian |
| Control subject 1 | 35 | F | Normal | Inner upper arm | Unknown |
| Control subject 2 | 25 | M | Normal | Forearm | European |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alghamdi, M.A.; AL-Eitan, L.N.; Stevenson, A.; Chaudhari, N.; Hortin, N.; Wallace, H.J.; Danielsen, P.L.; Manzur, M.; Wood, F.M.; Fear, M.W. Secreted Factors from Keloid Keratinocytes Modulate Collagen Deposition by Fibroblasts from Normal and Fibrotic Tissue: A Pilot Study. Biomedicines 2020, 8, 200. https://doi.org/10.3390/biomedicines8070200
Alghamdi MA, AL-Eitan LN, Stevenson A, Chaudhari N, Hortin N, Wallace HJ, Danielsen PL, Manzur M, Wood FM, Fear MW. Secreted Factors from Keloid Keratinocytes Modulate Collagen Deposition by Fibroblasts from Normal and Fibrotic Tissue: A Pilot Study. Biomedicines. 2020; 8(7):200. https://doi.org/10.3390/biomedicines8070200
Chicago/Turabian StyleAlghamdi, Mansour A., Laith N. AL-Eitan, Andrew Stevenson, Nutan Chaudhari, Nicole Hortin, Hilary J. Wallace, Patricia L. Danielsen, Mitali Manzur, Fiona M. Wood, and Mark W. Fear. 2020. "Secreted Factors from Keloid Keratinocytes Modulate Collagen Deposition by Fibroblasts from Normal and Fibrotic Tissue: A Pilot Study" Biomedicines 8, no. 7: 200. https://doi.org/10.3390/biomedicines8070200
APA StyleAlghamdi, M. A., AL-Eitan, L. N., Stevenson, A., Chaudhari, N., Hortin, N., Wallace, H. J., Danielsen, P. L., Manzur, M., Wood, F. M., & Fear, M. W. (2020). Secreted Factors from Keloid Keratinocytes Modulate Collagen Deposition by Fibroblasts from Normal and Fibrotic Tissue: A Pilot Study. Biomedicines, 8(7), 200. https://doi.org/10.3390/biomedicines8070200






