Trained Immunity Based-Vaccines as a Prophylactic Strategy in Common Variable Immunodeficiency. A Proof of Concept Study
Abstract
:1. Introduction
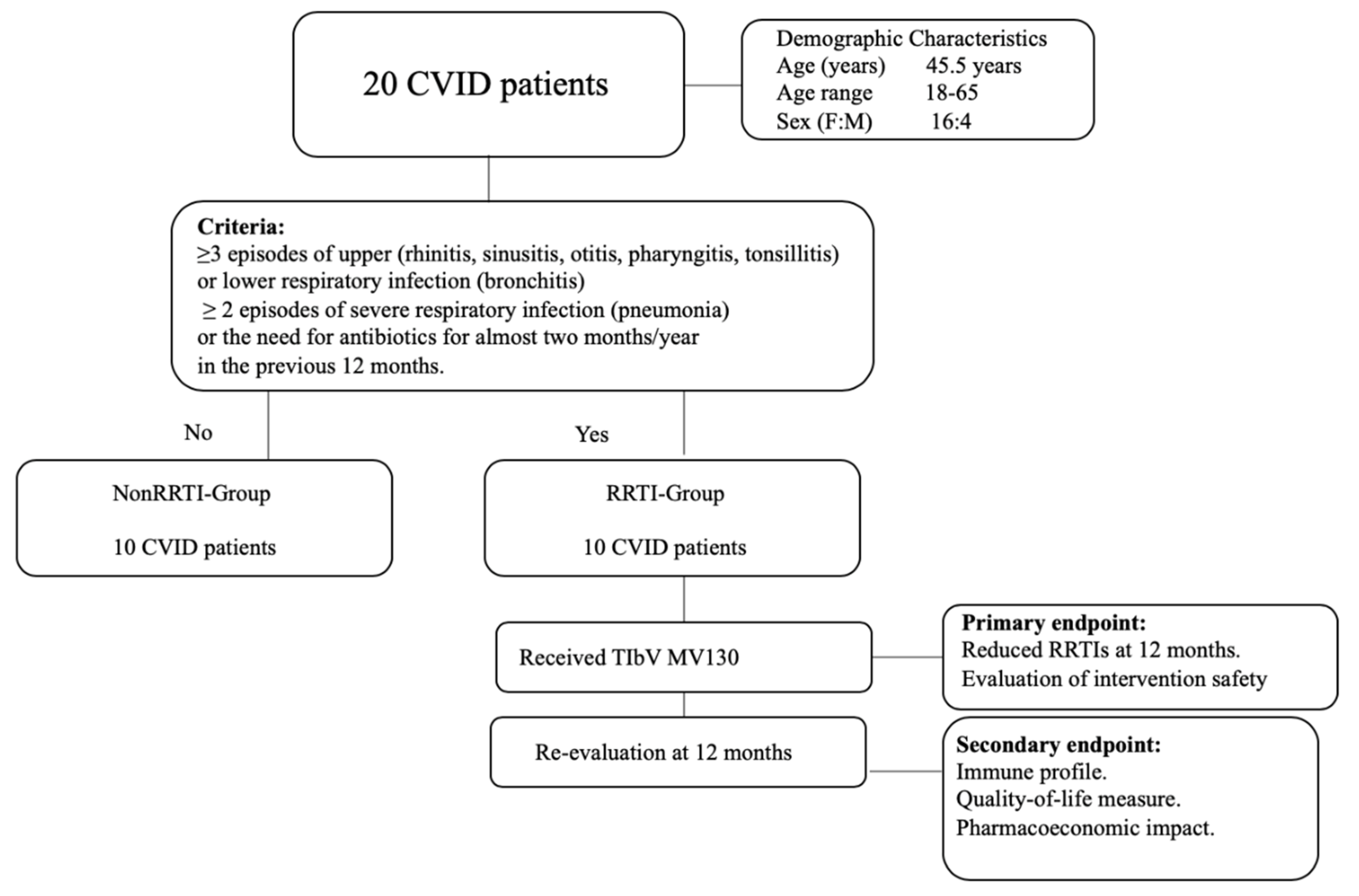
2. Methods
2.1. Population
2.2. Immunological Assessment
2.3. Endpoints
2.4. Statistical Analysis
3. Results
3.1. MV130 Significantly Decreased Respiratory Infection Rate, Antibiotic Use and Unscheduled Outpatients’ Visits in CVID Patients
3.2. Immune Profile
3.3. Perceived QoL Improved in CVID Patients after MV130
3.4. Pharmacoeconomic Impact
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CVID | Common variable immunodeficiency. |
| RRTI | Recurrent respiratory tract infections, defined by ≥3 episodes of upper (rhinitis, sinusitis, otitis, pharyngitis, tonsillitis) or lower respiratory infection (bronchitis), ≥2 episode of severe respiratory infection (pneumonia), or the need for antibiotics for almost two months/year in the previous 12 months [9,10]. |
| IVIg | Intravenous immunoglobulin. |
| SCIg | Subcutaneous immunoglobulin. |
| QoL | Quality of life. |
| IP-10 | Interferon gamma-induced protein 10. |
| VEGF | Vascular endothelial growth factor. |
| TIbV | Trained immunity-based vaccines. |
| DCs | Dendritic cells |
References
- Fried, A.J.; Bonilla, F.A. Pathogenesis, Diagnosis, and Management of Primary Antibody Deficiencies and Infections. Clin. Microbiol. Rev. 2009, 22, 396–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez-Ramón, S.; Radigan, L.; Yu, J.E.; Bard, S.; Cunningham-Rundles, C. Memory B cells in common variable immunodeficiency: Clinical associations and sex differences. Clin. Immunol. 2008, 128, 314–321. [Google Scholar] [CrossRef] [Green Version]
- Cunningham-Rundles, C. The many faces of common variable immunodeficiency. Hematol. Am. Soc. Hematol. Educ. Program 2012, 2012, 301–305. [Google Scholar] [CrossRef] [Green Version]
- Jolles, S. The variable in common variable immunodeficiency: A disease of complex phenotypes. J. Allergy Clin. Immunol. Pract. 2013, 1, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Warnatz, K.; Denz, A.; Dräger, R.; Braun, M.; Groth, C.; Wolff-Vorbeck, G.; Eibel, H.; Schlesier, M.; Peter, H.H. Severe deficiency of switched memory B cells (CD27(+)IgM(-)IgD(-)) in subgroups of patients with common variable immunodeficiency: A new approach to classify a heterogeneous disease. Blood 2002, 99, 1544–1551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arandi, N.; Mirshafiey, A.; Jeddi-Tehrani, M.; Abolhassani, H.; Sadeghi, B.; Mirminachi, B.; Shaghaghi, M.; Aghamohammadi, A. Evaluation of CD4+CD25+FOXP3+ regulatory T cells function in patients with common variable immunodeficiency. Cell. Immunol. 2013, 281, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Siachoque, H.; Satisteban, N.; Iglesias-Gamarra, A. T regulatory lymphocytes: Subpopulations, mechanism of action and importance in the control of autoimmunity. Rev. Colomb. Reumatol. 2011, 18, 203–220. [Google Scholar]
- Cunningham-Rundles, C.; Bodian, C. Common variable immunodeficiency: Clinical and immunological features of 248 patients. Clin. Immunol. 1999, 92, 34–48. [Google Scholar] [CrossRef]
- Ballow, M. Approach to the patient with recurrent infections. Clin. Rev. Allergy Immunol. 2008, 34, 129–140. [Google Scholar] [CrossRef]
- Kainulainen, L.; Vuorinen, T.; Rantakokko-Jalava, K.; Osterback, R.; Ruuskanen, O. Recurrent and persistent respiratory tract viral infections in patients with primary hypogammaglobulinemia. J. Allergy Clin. Immunol. 2010, 126, 120–126. [Google Scholar] [CrossRef]
- Bazregari, S.; Azizi, G.; Tavakol, M.; Asgardoon, M.H.; Kiaee, F.; Tavakolinia, N.; Valizadeh, A.; Abolhassani, H.; Aghamohammadi, A. Evaluation of infectious and non-infectious complications in patients with primary immunodeficiency. Cent. Eur. J. Immunol. 2017, 42, 336–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cunningham-Rundles, C. Common variable immune deficiency: Dissection of the variable. Immunol. Rev. 2019, 287, 145–161. [Google Scholar] [CrossRef]
- Chapel, H.; Lucas, M.; Lee, M.; Bjorkander, J.; Webster, D.; Grimbacher, B.; Fieschi, C.; Thon, V.; Abedi, M.R.; Hammarstrom, L. Common variable immunodeficiency disorders: Division into distinct clinical phenotypes. Blood 2008, 112, 277–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geha, R.S.; Notarangelo, L.D.; Casanova, J.-L.; Chapel, H.; Conley, M.E.; Fischer, A.; Hammarström, L.; Nonoyama, S.; Ochs, H.D.; Puck, J.; et al. The International Union of Immunological Societies (IUIS) Primary Immunodeficiency Diseases (PID) Classification Committee. J. Allergy Clin. Immunol. 2007, 120, 776–794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vivas-Rosales, I.J.; Hernández-Ojeda, M.; O’Farrill-Romanillos, P.M.; Herrera-Sánchez, D.A.; Maciel-Fierro, A.E.; Núñez-Enríquez, J.C. Bronchiectasis severity in adult patients with common variable immunodeficiency. Rev. Alerg. Mex. 2018, 65, 242–249. [Google Scholar] [CrossRef] [Green Version]
- Sherani, K.; Upadhyay, H.; Vakil, A.; Cervellione, K.; Thurm, C. Common Variable Immunodeficiency and Bronchiectasis: An Easily Missed Common Association. CHEST 2014, 145, 123A. [Google Scholar] [CrossRef]
- Milito, C.; Pulvirenti, F.; Cinetto, F.; Lougaris, V.; Soresina, A.; Pecoraro, A.; Vultaggio, A.; Carrabba, M.; Lassandro, G.; Plebani, A.; et al. Double-blind, placebo-controlled, randomized trial on low-dose azithromycin prophylaxis in patients with primary antibody deficiencies. J. Allergy Clin. Immunol. 2019, 144, 584–593.e7. [Google Scholar] [CrossRef] [Green Version]
- Kuruvilla, M.; de la Morena, M.T. Antibiotic prophylaxis in primary immune deficiency disorders. J. Allergy Clin. Immunol. Pract. 2013, 1, 573–582. [Google Scholar] [CrossRef]
- Sperlich, J.M.; Grimbacher, B.; Workman, S.; Haque, T.; Seneviratne, S.L.; Burns, S.O.; Reiser, V.; Vach, W.; Hurst, J.R.; Lowe, D.M. Respiratory Infections and Antibiotic Usage in Common Variable Immunodeficiency. J. Allergy Clin. Immunol. Pract. 2018, 6, 159–168.e3. [Google Scholar] [CrossRef]
- Mohammadinejad, P.; Ataeinia, B.; Kaynejad, K.; Zeinoddini, A.; Sadeghi, B.; Hosseini, M.; Rezaei, N.; Aghamohammadi, A. Antibiotic resistance in patients with primary immunodeficiency disorders versus immunocompetent patients. Expert Rev. Clin. Immunol. 2015, 11, 1163–1172. [Google Scholar] [CrossRef]
- Antimicrobial Resistance Spotlight—Emerging Infectious Diseases Journal—CDC. Available online: https://wwwnc.cdc.gov/eid/spotlight/antimicrobial-resistance (accessed on 25 May 2020).
- Bellanti, J.A.; Settipane, R.A. Bacterial vaccines and the innate immune system: A journey of rediscovery for the allergist-immunologist and all health care providers. Allergy Asthma Proc. 2009, 30 (Suppl. 1), S3–S4. [Google Scholar] [CrossRef] [PubMed]
- García González, L.-A.; Arrutia Díez, F. Mucosal bacterial immunotherapy with MV130 highly reduces the need of tonsillectomy in adults with recurrent tonsillitis. Hum. Vaccines Immunother. 2019, 15, 2150–2153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez-Ramón, S.; Conejero, L.; Netea, M.G.; Sancho, D.; Palomares, Ó.; Subiza, J.L. Trained Immunity-Based Vaccines: A New Paradigm for the Development of Broad-Spectrum Anti-infectious Formulations. Front. Immunol. 2018, 9. [Google Scholar] [CrossRef]
- Cirauqui, C.; Benito-Villalvilla, C.; Sánchez-Ramón, S.; Sirvent, S.; Diez-Rivero, C.M.; Conejero, L.; Brandi, P.; Hernández-Cillero, L.; Ochoa, J.L.; Pérez-Villamil, B.; et al. Human dendritic cells activated with MV130 induce Th1, Th17 and IL-10 responses via RIPK2 and MyD88 signalling pathways. Eur. J. Immunol. 2018, 48, 180–193. [Google Scholar] [CrossRef] [PubMed]
- Alecsandru, D.; Valor, L.; Sánchez-Ramón, S.; Gil, J.; Carbone, J.; Navarro, J.; Rodríguez, J.J.; Rodríguez-Sainz, C.; Fernández-Cruz, E. Sublingual therapeutic immunization with a polyvalent bacterial preparation in patients with recurrent respiratory infections: Immunomodulatory effect on antigen-specific memory CD4+ T cells and impact on clinical outcome. Clin. Exp. Immunol. 2011, 164, 100–107. [Google Scholar] [CrossRef]
- Tejera-Alhambra, M.; Palomares, O.; Perez de Diego, R.; Diaz-Lezcano, I.; Sanchez-Ramon, S. New Biological Insights in the Immunomodulatory Effects of Mucosal Polybacterial Vaccines in Clinical Practice. Curr. Pharm. Des. 2016, 22, 6283–6293. [Google Scholar] [CrossRef] [PubMed]
- Collet, J.P.; Shapiro, S.; Ernst, P.; Renzi, P.; Ducruet, T.; Robinson, A. Effects of an Immunostimulating Agent on Acute Exacerbations and Hospitalizations in Patients with Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 1997, 156, 1719–1724. [Google Scholar] [CrossRef]
- Roży, A.; Chorostowska-Wynimko, J. Bacterial immunostimulants-mechanism of action and clinical application in respiratory diseases. Adv. Respir. Med. 2008, 76, 353–359. [Google Scholar]
- Lusuardi, M. Challenging mucosal immunity with bacterial extracts to prevent respiratory infections: An old therapy revisited. Monaldi Arch. Chest Dis. 2004, 61, 4–5. [Google Scholar]
- Quinti, I.; Pulvirenti, F.; Giannantoni, P.; Hajjar, J.; Canter, D.L.; Milito, C.; Abeni, D.; Orange, J.S.; Tabolli, S. Development and Initial Validation of a Questionnaire to Measure Health-Related Quality of Life of Adults with Common Variable Immune Deficiency: The CVID_QoL Questionnaire. J. Allergy Clin. Immunol. Pract. 2016, 4, 1169–1179.e4. [Google Scholar] [CrossRef] [Green Version]
- Gasto farmacéutico por unidades. Available online: https://www.comunidad.madrid/servicios/salud/gasto-farmaceutico-unidades (accessed on 28 June 2020).
- Coste salarial por hora efectiva, tipo de jornada, sectores de actividad (6040). Available online: https://www.ine.es/jaxiT3/Tabla.htm?t=6040 (accessed on 28 June 2020).
- Boletín Estadístico del Personal al Servicio de la Comunidad de Madrid. Available online: https://www.comunidad.madrid/gobierno/transparencia/boletin-estadistico-personal-servicio-comunidad-madrid (accessed on 2 May 2020).
- Hampson, F.A.; Chandra, A.; Screaton, N.J.; Condliffe, A.; Kumararatne, D.S.; Exley, A.R.; Babar, J.L. Respiratory disease in common variable immunodeficiency and other primary immunodeficiency disorders. Clin. Radiol. 2012, 67, 587–595. [Google Scholar] [CrossRef] [PubMed]
- do Amor Divino, P.H.; de Carvalho Basilio, J.H.; Fabbri, R.M.A.; Bastos, I.P.; Forte, W.C.N. Bronchiectasis caused by common variable immunodeficiency. J. Bras. Pneumol. 2015, 41, 482–483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erdem, S.B.; Gulez, N.; Genel, F.; Karaman, S.; Nacaroglu, H.T. Characteristics of the patients followed with the diagnosis of common variable immunodeficiency and the complications. Cent. Eur. J. Immunol. 2019, 44, 119–126. [Google Scholar] [CrossRef]
- Lucas, M.; Lee, M.; Lortan, J.; Lopez-Granados, E.; Misbah, S.; Chapel, H. Infection outcomes in patients with common variable immunodeficiency disorders: Relationship to immunoglobulin therapy over 22 years. J. Allergy Clin. Immunol. 2010, 125, 1354–1360.e4. [Google Scholar] [CrossRef] [PubMed]
- Orange, J.S.; Grossman, W.J.; Navickis, R.J.; Wilkes, M.M. Impact of trough IgG on pneumonia incidence in primary immunodeficiency: A meta-analysis of clinical studies. Clin. Immunol. 2010, 137, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Orange, J.S.; Belohradsky, B.H.; Berger, M.; Borte, M.; Hagan, J.; Jolles, S.; Wasserman, R.L.; Baggish, J.S.; Saunders, R.; Grimbacher, B. Evaluation of correlation between dose and clinical outcomes in subcutaneous immunoglobulin replacement therapy. Clin. Exp. Immunol. 2012, 169, 172–181. [Google Scholar] [CrossRef] [Green Version]
- Phillips, A.C.; Carroll, D.; Drayson, M.T.; Der, G. Salivary Immunoglobulin A Secretion Rate Is Negatively Associated with Cancer Mortality: The West of Scotland Twenty-07 Study. PLoS ONE 2015, 10, e0145083. [Google Scholar] [CrossRef]
- Rodríguez, A.; Tjärnlund, A.; Ivanji, J.; Singh, M.; García, I.; Williams, A.; Marsh, P.D.; Troye-Blomberg, M.; Fernández, C. Role of IgA in the defense against respiratory infections IgA deficient mice exhibited increased susceptibility to intranasal infection with Mycobacterium bovis BCG. Vaccine 2005, 23, 2565–2572. [Google Scholar] [CrossRef]
- Boyaka, P.N. Inducing mucosal IgA: A challenge for vaccine adjuvants and delivery systems. J. Immunol. 2017, 199, 9–16. [Google Scholar] [CrossRef]
- Diana, J.; Moura, I.C.; Vaugier, C.; Gestin, A.; Tissandie, E.; Beaudoin, L.; Corthésy, B.; Hocini, H.; Lehuen, A.; Monteiro, R.C. Secretory IgA induces tolerogenic dendritic cells through SIGNR1 dampening autoimmunity in mice. J. Immunol. 2013, 191, 2335–2343. [Google Scholar] [CrossRef] [Green Version]
- Gutzeit, C.; Magri, G.; Cerutti, A. Intestinal IgA production and its role in host-microbe interaction. Immunol. Rev. 2014, 260, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Tezuka, H.; Ohteki, T. Regulation of IgA Production by Intestinal Dendritic Cells and Related Cells. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Eickhoff, C.S.; Blazevic, A.; Killoran, E.A.; Morris, M.S.; Hoft, D.F. Induction of mycobacterial protective immunity by sublingual BCG vaccination. Vaccine 2019, 37, 5364–5370. [Google Scholar] [CrossRef]
- Gallorini, S.; Taccone, M.; Bonci, A.; Nardelli, F.; Casini, D.; Bonificio, A.; Kommareddy, S.; Bertholet, S.; O’Hagan, D.T.; Baudner, B.C. Sublingual immunization with a subunit influenza vaccine elicits comparable systemic immune response as intramuscular immunization, but also induces local IgA and TH17 responses. Vaccine 2014, 32, 2382–2388. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Ramón, S.; Pérez de Diego, R.; Dieli-Crimi, R.; Subiza, J.-L. Extending the clinical horizons of mucosal bacterial vaccines: Current evidence and future prospects. Curr. Drug Targets 2014, 15, 1132–1143. [Google Scholar] [CrossRef]
- Varkey, J.B.; Varkey, A.B.; Varkey, B. Prophylactic vaccinations in chronic obstructive pulmonary disease: Current status. Curr. Opin. Pulm. Med. 2009, 15, 90–99. [Google Scholar] [CrossRef]
- Selva, B.; Nieto, M.; Bartoll, E.; Mazon, A.; Calaforra, S.; Calderón, R.; Palau, M.J.; Nieto, A.; Caballero, R.; Guzmán-Fulgencio, M.; et al. Sublingual therapeutic immunotherapy with a polyvalent bacterial preparation in preschool children with recurrent respiratory tract infections [abstract]. Allergy 2017, 72, 196. [Google Scholar]
- van der Meer, J.W.M.; Joosten, L.A.B.; Riksen, N.; Netea, M.G. Trained immunity: A smart way to enhance innate immune defence. Mol. Immunol. 2015, 68, 40–44. [Google Scholar] [CrossRef]
- Netea, M.G.; Domínguez-Andrés, J.; Barreiro, L.B.; Chavakis, T.; Divangahi, M.; Fuchs, E.; Joosten, L.A.B.; van der Meer, J.W.M.; Mhlanga, M.M.; Mulder, W.J.M.; et al. Defining trained immunity and its role in health and disease. Nat. Rev. Immunol. 2020, 1–14. [Google Scholar] [CrossRef] [Green Version]
- van der Heijden, C.D.C.C.; Noz, M.P.; Joosten, L.A.B.; Netea, M.G.; Riksen, N.P.; Keating, S.T. Epigenetics and Trained Immunity. Antioxid. Redox Signal. 2018, 29, 1023–1040. [Google Scholar] [CrossRef]
- Mourits, V.P.; Wijkmans, J.C.; Joosten, L.A.; Netea, M.G. Trained immunity as a novel therapeutic strategy. Curr. Opin. Pharmacol. 2018, 41, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Brandi, P.; Conejero, L.; Cueto, F.J.; Martínez-Cano, S.; Saz-Leal, P.; Enamorado, M.; Amores-Iniesta, J.; Subiza, J.L.; Sancho, D. MV130, a polybacterial mucosal preparation, protects mice against experimental viral infections inducing trained immunity [abstract]. Allergy 2019, 74, 111. [Google Scholar]
- Rezaei, N.; Amirzargar, A.A.; Shakiba, Y.; Mahmoudi, M.; Moradi, B.; Aghamohammadi, A. Proinflammatory cytokine gene single nucleotide polymorphisms in common variable immunodeficiency. Clin. Exp. Immunol. 2009, 155, 21–27. [Google Scholar] [CrossRef]
- Holm, A.M.; Aukrust, P.; Damås, J.K.; Müller, F.; Halvorsen, B.; Frøland, S.S. Abnormal interleukin-7 function in common variable immunodeficiency. Blood 2005, 105, 2887–2890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holm, A.M.; Aukrust, P.; Aandahl, E.M.; Müller, F.; Taskén, K.; Frøland, S.S. Impaired secretion of IL-10 by T cells from patients with common variable immunodeficiency—involvement of protein kinase A type I. J. Immunol. Baltim. Md 1950 2003, 170, 5772–5777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischer, M.B.; Hauber, I.; Vogel, E.; Wolf, H.M.; Mannhalter, J.W.; Eibl, M.M. Defective interleukin-2 and interferon-gamma gene expression in response to antigen in a subgroup of patients with common variable immunodeficiency. J. Allergy Clin. Immunol. 1993, 92, 340–352. [Google Scholar] [CrossRef]
- North, M.E.; Webster, A.D.; Farrant, J. Role of interleukin-2 and interleukin-6 in the mitogen responsiveness of T cells from patients with “common-variable” hypogammaglobulinaemia. Clin. Exp. Immunol. 1990, 81, 412–416. [Google Scholar] [CrossRef]
- Del Vecchio, G.C.; Martire, B.; Lassandro, G.; Cecinati, V.; De Mattia, D.; Ciccarelli, M.; Piacente, L.; Giordano, P. Reduced interleukin-5 production by peripheral CD4+ T cells in common variable immunodeficiency patients. Immunopharmacol. Immunotoxicol. 2008, 30, 679–686. [Google Scholar] [CrossRef]
- Zhou, Z.; Huang, R.; Danon, M.; Mayer, L.; Cunningham-Rundles, C. IL-10 production in common variable immunodeficiency. Clin. Immunol. Immunopathol. 1998, 86, 298–304. [Google Scholar] [CrossRef]
- Kasztalska, K.; Ciebiada, M.; Cebula-Obrzut, B.; Górski, P. Intravenous immunoglobulin replacement therapy in the treatment of patients with common variable immunodeficiency disease: An open-label prospective study. Clin. Drug Investig. 2011, 31, 299–307. [Google Scholar] [CrossRef]
- Isgrò, A.; Marziali, M.; Mezzaroma, I.; Luzi, G.; Mazzone, A.M.; Guazzi, V.; Andolfi, G.; Cassani, B.; Aiuti, A.; Aiuti, F. Bone marrow clonogenic capability, cytokine production, and thymic output in patients with common variable immunodeficiency. J. Immunol. 2005, 174, 5074–5081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pons, J.; Ferrer, J.M.; Martínez-Pomar, N.; Iglesias-Alzueta, J.; Matamoros, N. Costimulatory molecules and cytokine production by T lymphocytes in common variable immunodeficiency disease. Scand. J. Immunol. 2006, 63, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Andersen, J.B.; Midttun, K.; Feragen, K.J.B. Measuring quality of life of primary antibody deficiency patients using a disease-specific health-related quality of life questionnaire for common variable immunodeficiency (CVID_QoL). J. Patient-Rep. Outcomes 2019, 3, 15. [Google Scholar] [CrossRef] [PubMed]
- EconPapers: Financial Integration, International Portfolio Choice and the European Monetary Union. Available online: https://econpapers.repec.org/paper/ecbecbwps/2006626.htm (accessed on 28 June 2020).
- Modell, V.; Orange, J.S.; Quinn, J.; Modell, F. Global report on primary immunodeficiencies: 2018 update from the Jeffrey Modell Centers Network on disease classification, regional trends, treatment modalities, and physician reported outcomes. Immunol. Res. 2018, 66, 367–380. [Google Scholar] [CrossRef]




| Condition | Average # of Episodes before TIbV MV130 | Average # of Episodes after TIbV MV130 | Cost per Patient per Episode/Day € | Annual Cost per Patient before TIbV MV130 € | Annual Cost per Patient after TIbV MV130 € | Annual Savings per Patient with TIbV MV130 € |
|---|---|---|---|---|---|---|
| # of RRTIs | 3.7 | 0.4 | 1656 | 6127 | 662 | 5464 |
| # of physician/hospital/ER visits | 4.4 | 1.1 | 1288 | 5667 | 1416 | 4250 |
| # Days Hospitalizations for RRTIs | 7 | 3 | 792 | 5546 | 2377 | 3169 |
| Cycles of antibiotics | 4.8 | 1 | 259 | 1243 | 259 | 984 |
| School/work days missed (Absenteeism) | 1.6 | 0.5 | 14 | 22.4 | 7 | 15 |
| Total per patient | 18,606 | 4722 | 13,884 | |||
| Annual cost TibV MV130 prophylaxis | 190 | 13,694 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guevara-Hoyer, K.; Saz-Leal, P.; Diez-Rivero, C.M.; Ochoa-Grullón, J.; Fernández-Arquero, M.; Pérez de Diego, R.; Sánchez-Ramón, S. Trained Immunity Based-Vaccines as a Prophylactic Strategy in Common Variable Immunodeficiency. A Proof of Concept Study. Biomedicines 2020, 8, 203. https://doi.org/10.3390/biomedicines8070203
Guevara-Hoyer K, Saz-Leal P, Diez-Rivero CM, Ochoa-Grullón J, Fernández-Arquero M, Pérez de Diego R, Sánchez-Ramón S. Trained Immunity Based-Vaccines as a Prophylactic Strategy in Common Variable Immunodeficiency. A Proof of Concept Study. Biomedicines. 2020; 8(7):203. https://doi.org/10.3390/biomedicines8070203
Chicago/Turabian StyleGuevara-Hoyer, Kissy, Paula Saz-Leal, Carmen M. Diez-Rivero, Juliana Ochoa-Grullón, Miguel Fernández-Arquero, Rebeca Pérez de Diego, and Silvia Sánchez-Ramón. 2020. "Trained Immunity Based-Vaccines as a Prophylactic Strategy in Common Variable Immunodeficiency. A Proof of Concept Study" Biomedicines 8, no. 7: 203. https://doi.org/10.3390/biomedicines8070203
APA StyleGuevara-Hoyer, K., Saz-Leal, P., Diez-Rivero, C. M., Ochoa-Grullón, J., Fernández-Arquero, M., Pérez de Diego, R., & Sánchez-Ramón, S. (2020). Trained Immunity Based-Vaccines as a Prophylactic Strategy in Common Variable Immunodeficiency. A Proof of Concept Study. Biomedicines, 8(7), 203. https://doi.org/10.3390/biomedicines8070203





