Fluorescent Labeling of Helminth Extracellular Vesicles Using an In Vivo Whole Organism Approach
Abstract
1. Introduction
2. Experimental Section
2.1. T. suis Hatching and Culture
2.2. Anisakis Spp. Harvest and Culture
2.3. Larval Uptake of Fluorescent Lipid Analogues in Vitro
2.4. Anisakis spp. in Vivo EV Labelling
2.5. Anisakis spp. Extracellular Vesicle Isolation
2.6. Nanoparticle Tracking Analysis
2.7. Cryo-Transmission Electron Microscopy
2.8. Proteomic Analysis of Extracellular Vesicles
2.9. Extracellular Vesicle Uptake in Human Macrophage-like THP-1 Cells
3. Results
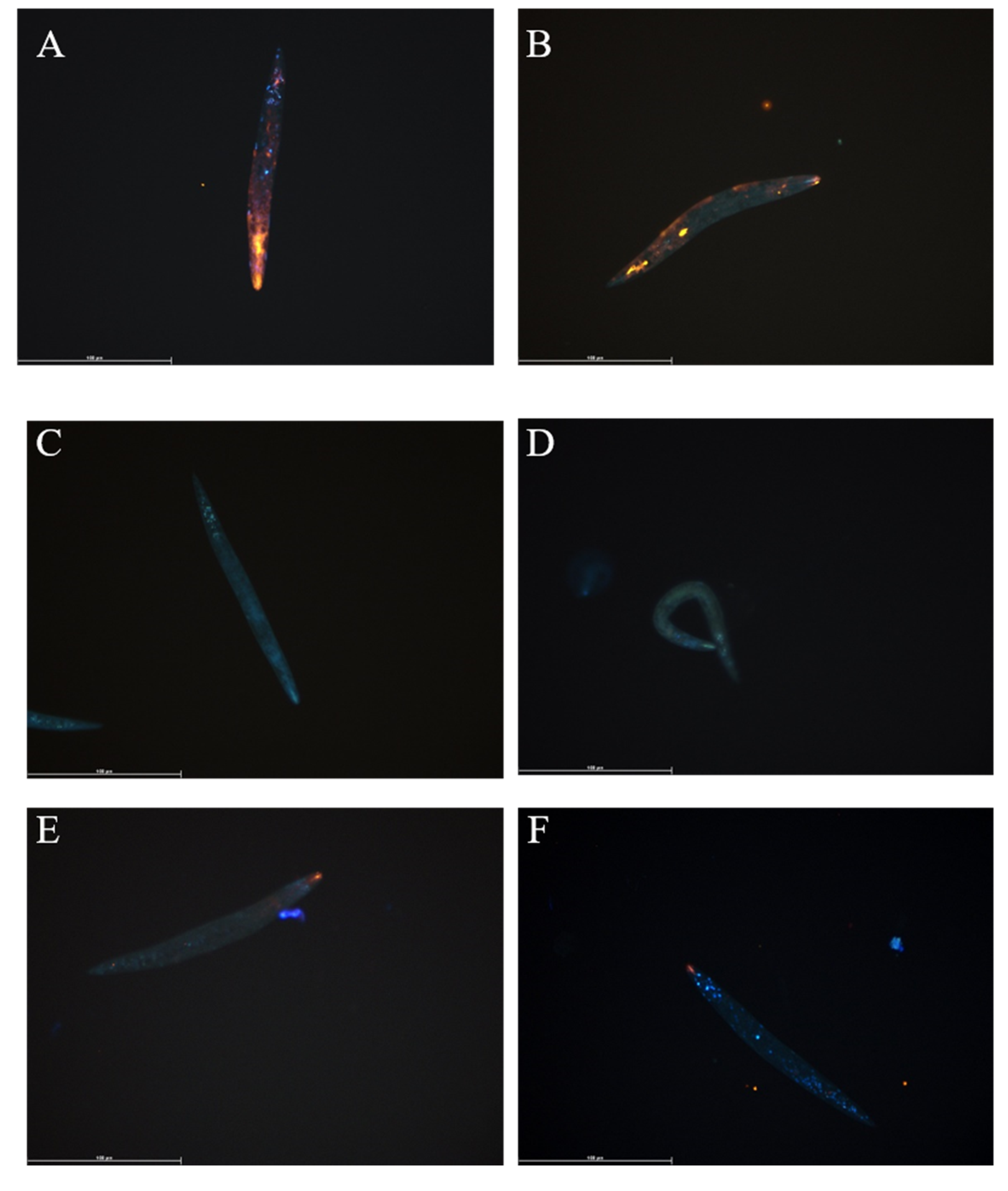
3.1. Fluorescent Lipid Analogue Uptake by T. suis L1 Larvae
3.2. Fluorescent Lipid Analogue Uptake in Anisakis spp.
3.3. EVs from Anisakis spp. Characterization and Assessment of Labelling
3.4. Cryo-TEM of Anisakis spp.
3.5. EVs from Anisakis spp. Assessed by Quantitative Proteomics to Identify EV Candidate EV Markers
3.6. Uptake of DOPE-Rho Labelled EVs in THP-1 Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Woith, E.; Fuhrmann, G.; Melzig, M.F. Molecular Sciences Extracellular Vesicles-Connecting Kingdoms. Int. J. Mol. Sci. 2019, 20, 1–26. [Google Scholar] [CrossRef]
- Claridge, B.; Kastaniegaard, K.; Stensballe, A.; Greening, D.W. Post-translational and transcriptional dynamics–regulating extracellular vesicle biology. Expert Rev. Proteomics 2019, 16, 17–31. [Google Scholar] [CrossRef]
- Zakeri, A.; Hansen, E.P.; Andersen, S.D.; Williams, A.R.; Nejsum, P. Immunomodulation by Helminths: Intracellular Pathways and Extracellular Vesicles. Front. Immunol. 2018, 9, 2349. [Google Scholar] [CrossRef]
- Mardahl, M.; Borup, A.; Nejsum, P. A new level of complexity in parasite-host interaction: The role of extracellular vesicles. Adv. Parasitol. 2019, 104, 39–112. [Google Scholar] [CrossRef]
- Buck, A.H.; Coakley, G.; Simbari, F.; McSorley, H.J.; Quintana, J.F.; Le Bihan, T.; Kumar, S.; Abreu-Goodger, C.; Lear, M.; Harcus, Y.; et al. Exosomes secreted by nematode parasites transfer small RNAs to mammalian cells and modulate innate immunity. Nat. Commun. 2014, 5, 1–11. [Google Scholar] [CrossRef]
- Hansen, E.P.; Fromm, B.; Andersen, S.D.; Marcilla, A.; Andersen, K.L.; Borup, A.; Williams, A.R.; Jex, A.R.; Gasser, R.B.; Young, N.D.; et al. Exploration of extracellular vesicles from Ascaris suum provides evidence of parasite–host cross talk. J. Extracell. Vesicles 2019, 8, 1578116. [Google Scholar] [CrossRef] [PubMed]
- Coakley, G.; McCaskill, J.L.; Borger, J.; Simbari, F.; Robertson, E.; Millar, M.; Harcus, Y.; McSorley, H.J.; Maizels, R.M.; Buck, A.H. Extracellular Vesicles from a Helminth Parasite Suppress Macrophage Activation and Constitute an Effective Vaccine for Protective Immunity. Cell Rep. 2017, 19, 1545–1557. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, B.; Boysen, A.T.; Mardahl, M.; Nejsum, P. Unique glycan and lipid composition of helminth-derived extracellular vesicles may reveal novel roles in host-parasite interactions. Int. J. Parasitol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Eichenberger, R.M.; Sotillo, J.; Loukas, A. Immunobiology of parasitic worm extracellular vesicles. Immunol. Cell Boil. 2018, 96, 704–713. [Google Scholar] [CrossRef] [PubMed]
- Takov, K.; Yellon, D.M.; Davidson, S.M. Confounding factors in vesicle uptake studies using fluorescent lipophilic membrane dyes. J. Extracell. Vesicles 2017, 6, 1388731. [Google Scholar] [CrossRef]
- Simonsen, J.B. Pitfalls associated with lipophilic fluorophore staining of extracellular vesicles for uptake studies. J. Extracell. Vesicles 2019, 8, 1582237. [Google Scholar] [CrossRef] [PubMed]
- Gangadaran, P.; Li, X.J.; Lee, H.W.; Oh, J.M.; Kalimuthu, S.; Rajendran, R.L.; Son, S.H.; Baek, S.H.; Singh, T.D.; Zhu, L.; et al. A new bioluminescent reporter system to study the biodistribution of systematically injected tumor-derived bioluminescent extracellular vesicles in mice. Oncotarget 2017, 8, 109894–109914. [Google Scholar] [CrossRef] [PubMed]
- Mittelbrunn, M.; Gutiérrez-Vázquez, C.; Villarroya-Beltri, C.; Gonzalez, S.; Sánchez-Cabo, F.; Ángel; González, M.; Bernad, A.; Sánchez-Madrid, F. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat. Commun. 2011, 2, 282. [Google Scholar] [CrossRef]
- Gangadaran, P.; Hong, C.M.; Ahn, B.-C. An Update on in Vivo Imaging of Extracellular Vesicles as Drug Delivery Vehicles. Front. Pharmacol. 2018, 9, 169. [Google Scholar] [CrossRef]
- Whitehead, B.; Wu, L.-P.; Hvam, M.L.; Aslan, H.; Dong, M.; Dyrskjøt, L.; Ostenfeld, M.S.; Moghimi, S.M.; Howard, K.A. Tumour exosomes display differential mechanical and complement activation properties dependent on malignant state: Implications in endothelial leakiness. J. Extracell. Vesicles 2015, 4, 29685. [Google Scholar] [CrossRef]
- Brouwers, J.F.; Smeenk, I.M.; Van Golde, L.M.; Tielens, A.G.M. The incorporation, modification and turnover of fatty acids in adult Schistosoma mansoni. Mol. Biochem. Parasitol. 1997, 88, 175–185. [Google Scholar] [CrossRef]
- McDermott, L.C.; Kennedy, M.W.; McManus, N.P.; Bradley, J.E.; Cooper, A.; Storch, J.; Bradley, J.E. How Helminth Lipid-Binding Proteins Offload Their Ligands to Membranes: Differential Mechanisms of Fatty Acid Transfer by the ABA-1 Polyprotein Allergen and Ov-FAR-1 Proteins of Nematodes and Sj-FABPc of Schistosomes. Biochemistry 2002, 41, 6706–6713. [Google Scholar] [CrossRef]
- Alvite, G.; Esteves, A. Lipid binding proteins from parasitic platyhelminthes. Front. Physiol. 2012, 3, 363. [Google Scholar] [CrossRef]
- Franchini, G.R.; Porfido, J.L.; Shimabukuro, M.I.; Burusco, M.F.R.; Bélgamo, J.A.; Smith, B.O.; Kennedy, M.W.; Córsico, B. The unusual lipid binding proteins of parasitic helminths and their potential roles in parasitism and as therapeutic targets. Prostaglandins Leukot. Essent. Fat. Acids 2015, 93, 31–36. [Google Scholar] [CrossRef]
- Bexkens, M.L.; Mebius, M.M.; Houweling, M.; Brouwers, J.F.; Tielens, A.G.; Van Hellemond, J.J. Schistosoma mansoni does not and cannot oxidise fatty acids, but these are used for biosynthetic purposes instead. Int. J. Parasitol. 2019, 49, 647–656. [Google Scholar] [CrossRef]
- Keeney, D.B.; Lagrue, C.; Bryan-Walker, K.; Khan, N.; Leung, T.L.; Poulin, R. The use of fluorescent fatty acid analogs as labels in trematode experimental infections. Exp. Parasitol. 2008, 120, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Furlong, S.T.; Thibault, K.S.; A Rogers, R. Fluorescent phospholipids preferentially accumulate in sub-tegumental cells of schistosomula of Schistosoma mansoni. J. Cell Sci. 1992, 103, 823–830. [Google Scholar] [PubMed]
- Furlong, S.T.; Thibault, K.S.; Morbelli, L.M.; Quinn, J.J.; A Rogers, R. Uptake and compartmentalization of fluorescent lipid analogs in larval Schistosoma mansoni. J. Lipid Res. 1995, 36, 1–12. [Google Scholar] [PubMed]
- James, S.L.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef]
- Jeon, C.-H.; Kim, J.-H. Pathogenic Potential of Two Sibling Species, Anisakis simplex (s.s.) and Anisakis pegreffii (Nematoda: Anisakidae): In Vitro and In Vivo Studies. BioMed Res. Int. 2015, 2015, 1–9. [Google Scholar] [CrossRef]
- Audicana, M.T.; Kennedy, M.W. Anisakis simplex: From Obscure Infectious Worm to Inducer of Immune Hypersensitivity. Clin. Microbiol. Rev. 2008, 21, 360–379. [Google Scholar] [CrossRef]
- Herrador, Z.; Daschner, Á.; Perteguer, M.J.; Benito, A. Epidemiological Scenario of Anisakidosis in Spain Based on Associated Hospitalizations: The Tip of the Iceberg. Clin. Infect. Dis. 2019, 69, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.; Mann, M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 2008, 26, 1367–1372. [Google Scholar] [CrossRef] [PubMed]
- Götz, S.; García-Gómez, J.M.; Terol, J.; Williams, T.; Nagaraj, S.H.; Nueda, M.J.; Robles, M.; Talón, M.; Dopazo, J.; Conesa, A. High-throughput functional annotation and data mining with the Blast2GO suite. Nucleic Acids Res. 2008, 36, 3420–3435. [Google Scholar] [CrossRef]
- Cwiklinski, K.; De La Torre-Escudero, E.; Trelis, M.; Bernal, D.; Dufresne, P.J.; Brennan, G.P.; O’Neill, S.; Tort, J.F.; Paterson, S.; Marcilla, A.; et al. The Extracellular Vesicles of the Helminth Pathogen, Fasciola hepatica: Biogenesis Pathways and Cargo Molecules Involved in Parasite Pathogenesis. Mol. Cell. Proteomics. 2015, 14, 3258–3273. [Google Scholar] [CrossRef]
- De La Torre-Escudero, E.; Gerlach, J.Q.; Bennett, A.P.S.; Cwiklinski, K.; Jewhurst, H.L.; Huson, K.M.; Joshi, L.; Kilcoyne, M.; O’Neill, S.; Dalton, J.P.; et al. Surface molecules of extracellular vesicles secreted by the helminth pathogen Fasciola hepatica direct their internalisation by host cells. PLOS Negl. Trop. Dis. 2019, 13, e0007087. [Google Scholar] [CrossRef]
- Eichenberger, R.M.; Talukder, H.; Field, M.; Wangchuk, P.; Giacomin, P.; Loukas, A.; Sotillo, J. Characterization of Trichuris muris secreted proteins and extracellular vesicles provides new insights into host–parasite communication. J. Extracell. Vesicles 2018, 7, 1428004. [Google Scholar] [CrossRef] [PubMed]
- Sotillo, J.; Pearson, M.S.; Potriquet, J.; Becker, L.; Pickering, D.; Mulvenna, J.; Loukas, A. Extracellular vesicles secreted by Schistosoma mansoni contain protein vaccine candidates. Int. J. Parasitol. 2016, 46, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Tzelos, T.; Matthews, J.; Buck, A.H.; Simbari, F.; Frew, D.; Inglis, N.F.; McLean, K.; Nisbet, A.J.; Whitelaw, C.B.A.; Knox, D.P.; et al. A preliminary proteomic characterisation of extracellular vesicles released by the ovine parasitic nematode, Teladorsagia circumcincta. Vet. Parasitol. 2016, 221, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Redman, C.A.; Kusel, J.R. Distribution and biophysical properties of fluorescent lipids on the surface of adult Schistosoma mansoni. Parasitology 1996, 113, 137–143. [Google Scholar] [CrossRef]
- Jeppesen, D.; Hvam, M.L.; Primdahl-Bengtson, B.; Boysen, A.T.; Whitehead, B.; Dyrskjøt, L.; Ørntoft, T.F.; Howard, K.A.; Ostenfeld, M.S. Comparative analysis of discrete exosome fractions obtained by differential centrifugation. J. Extracell. Vesicles 2014, 3, 25011. [Google Scholar] [CrossRef]
- Simbari, F.; McCaskill, J.; Coakley, G.; Millar, M.; Maizels, R.M.; Fabriás, G.; Casas, J.; Buck, A.H. Plasmalogen enrichment in exosomes secreted by a nematode parasite versus those derived from its mouse host: Implications for exosome stability and biology. J. Extracell. Vesicles 2016, 5, 373. [Google Scholar] [CrossRef]
- Hansen, E.P.; Kringel, H.; Williams, A.R.; Nejsum, P. Secretion of RNA-containing extracellular vesicles by the porcine whipworm, Trichuris suis. J. Parasitol. 2015, 101, 336–340. [Google Scholar] [CrossRef]
- Zamanian, M.; Fraser, L.M.; Agbedanu, P.N.; Harischandra, H.; Moorhead, A.R.; Day, T.; Bartholomay, L.C.; Kimber, M.J. Release of Small RNA-containing Exosome-like Vesicles from the Human Filarial Parasite Brugia malayi. PLoS. Negl. Trop. Dis. 2015, 9, e0004069. [Google Scholar] [CrossRef]
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Boil. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Jeppesen, D.; Nawrocki, A.; Jensen, S.G.; Thorsen, K.; Whitehead, B.; Howard, K.A.; Dyrskjøt, L.; Ørntoft, T.F.; Larsen, M.R.; Ostenfeld, M.S. Quantitative proteomics of fractionated membrane and lumen exosome proteins from isogenic metastatic and nonmetastatic bladder cancer cells reveal differential expression of EMT factors. Proteomics 2014, 14, 699–712. [Google Scholar] [CrossRef]
- Gomis, A.M.; Trelis, M.; Cortés, A.; Sotillo, J.; Cantalapiedra, F.; Minguez, M.T.; Valero, M.L.; Del Pino, M.M.S.; Muñoz-Antoli, C.; Toledo, R.; et al. Extracellular Vesicles from Parasitic Helminths Contain Specific Excretory/Secretory Proteins and Are Internalized in Intestinal Host Cells. PLoS ONE 2012, 7, e45974. [Google Scholar] [CrossRef]
- Eichenberger, R.M.; Ryan, S.; Jones, L.; Buitrago, G.; Polster, R.; De Oca, M.M.; Zuvelek, J.; Giacomin, P.; Dent, L.A.; Engwerda, C.R.; et al. Hookworm Secreted Extracellular Vesicles Interact With Host Cells and Prevent Inducible Colitis in Mice. Front. Immunol. 2018, 9, 1–14. [Google Scholar] [CrossRef]
- Stevens, T.L.; Gibson, G.R.; Adam, R.; Maier, J.; Allison-Ennis, M.; Das, S. Uptake and Cellular Localization of Exogenous Lipids by Giardia lamblia, a Primitive Eukaryote. Exp. Parasitol. 1997, 86, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Ramirez, D.; Maier, J.; Castillo, C.; Das, S. Giardia lamblia: Incorporation of Free and Conjugated Fatty Acids into Glycerol-Based Phospholipids. Exp. Parasitol. 1999, 92, 1–11. [Google Scholar] [CrossRef]
- Charron, A.J.; Sibley, L.D. Host cells: Mobilizable lipid resources for the intracellular parasite Toxoplasma gondii. J. Cell Sci. 2002, 115, 3049–3059. [Google Scholar]




| Accession | Protein | Reference 1 |
|---|---|---|
| A0A0M3K8U5 | 14-3-3 | [30,31,32,33] |
| A0A0M3K9V2 | HSP70 | [5,6,30,32,33] |
| A0A0M3IZK3 | Tubulin beta | [6,30,31,34] |
| A0A0M3K9P2 | CBN-exc 4 | [6,30,31,32] 2 |
| A0A0M3KB40; A0A0M3J0M4 | Actin | [5,6,33,34] |
| A0A0M3KFX3 | Ras like protein 3 | [31] |
| A0A0M3J8F3; A0A0M3JD57; A0A0M3K4N2; A0A0M3KAB8; A0A0M3KCN6; A0A158PMY7 | Maltase glucoamylase | [6] |
| A0A0M3J727; A0A0M3KA60 | Histidine acid phosphatase | [5,6,30,32] 3 |
| A0A0M3JAF9 | Prostatic acid phosphatase | [5,30,32] 3 |
| A0A0M3K4H2 | Glutamate dehydrogenase | [6,30,32] |
| A0A0M3JY91; A0A0M3K219 | ATP synthase F1 (alpha + beta subunit) | [6] |
| A0A0M3JYW4 | RAS-like GTP-binding protein RhoA | [6,30] |
| A0A0M3J718; A0A0M3JZV6 | Superoxide dismutase | [32] |
| A0A0M3JVA0 | ADP ribosylation factor 1 | [34] |
| A0A0M3JAH0 | Pepsin inhibitor | [32] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boysen, A.T.; Whitehead, B.; Stensballe, A.; Carnerup, A.; Nylander, T.; Nejsum, P. Fluorescent Labeling of Helminth Extracellular Vesicles Using an In Vivo Whole Organism Approach. Biomedicines 2020, 8, 213. https://doi.org/10.3390/biomedicines8070213
Boysen AT, Whitehead B, Stensballe A, Carnerup A, Nylander T, Nejsum P. Fluorescent Labeling of Helminth Extracellular Vesicles Using an In Vivo Whole Organism Approach. Biomedicines. 2020; 8(7):213. https://doi.org/10.3390/biomedicines8070213
Chicago/Turabian StyleBoysen, Anders T., Bradley Whitehead, Allan Stensballe, Anna Carnerup, Tommy Nylander, and Peter Nejsum. 2020. "Fluorescent Labeling of Helminth Extracellular Vesicles Using an In Vivo Whole Organism Approach" Biomedicines 8, no. 7: 213. https://doi.org/10.3390/biomedicines8070213
APA StyleBoysen, A. T., Whitehead, B., Stensballe, A., Carnerup, A., Nylander, T., & Nejsum, P. (2020). Fluorescent Labeling of Helminth Extracellular Vesicles Using an In Vivo Whole Organism Approach. Biomedicines, 8(7), 213. https://doi.org/10.3390/biomedicines8070213







