Microbiota in Clostridioides difficile-Associated Diarrhea: Comparison in Recurrent and Non-Recurrent Infections
Abstract
:1. Introduction
2. Results
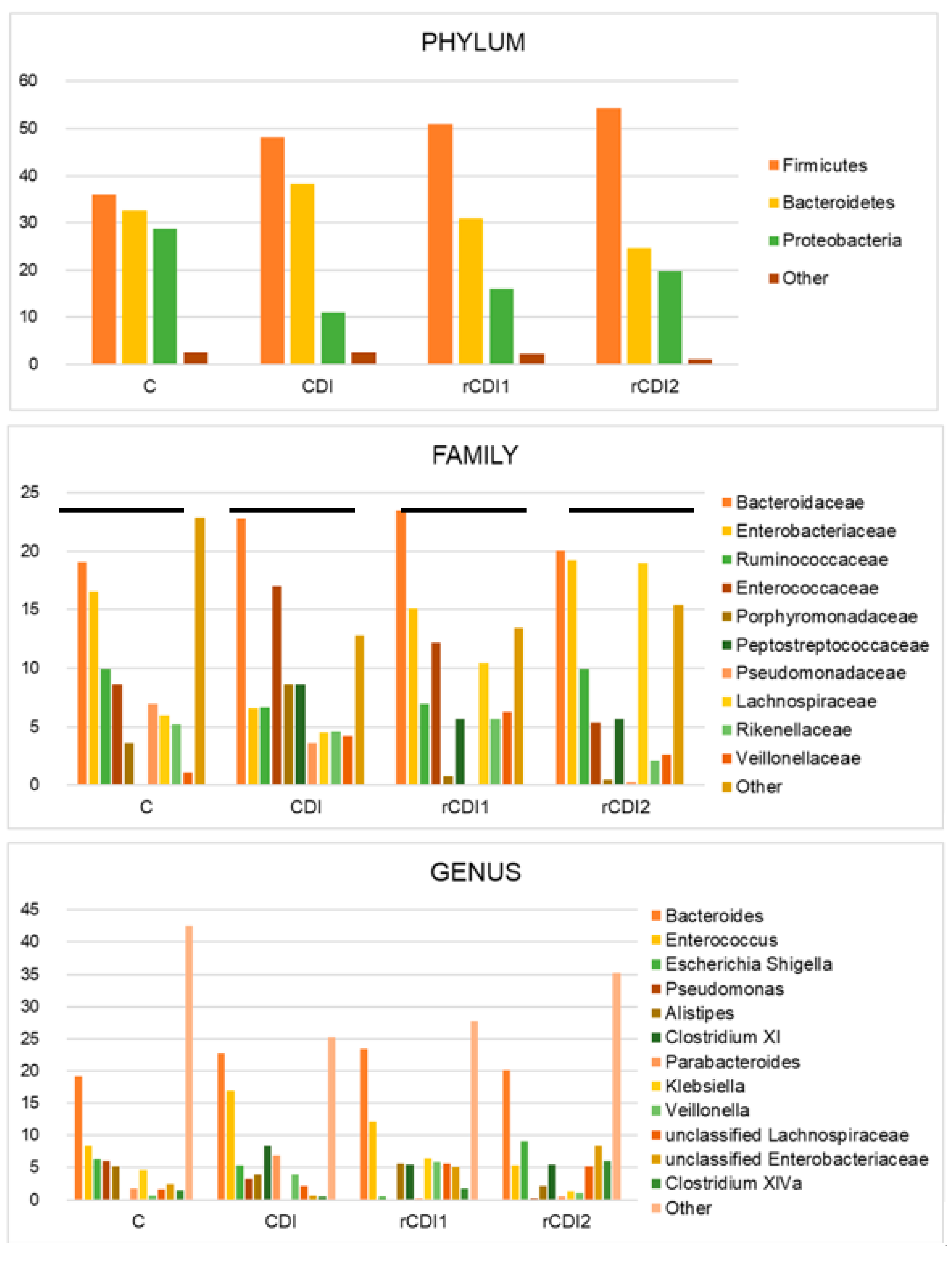
2.1. Taxonomic Structure of Fecal Bacterial Communities in CDI and rCDI Patients Compared to Non-Infected Controls
2.2. Ecological Analyses of Fecal Communities in Infected and Non-Infected Patients
2.3. Taxonomic Composition and Diversity of the Gut Microbiota in Relation to the Severity of C. difficile Infection
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Biological Samples
4.3. Extraction of DNA and Production of 16S rRNA Amplicons (V1–V3 Regions)
4.4. Bioinformatics and Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vincent, C.; Miller, M.A.; Edens, T.J.; Mehrotra, S.; Dewar, K.; Manges, A.R. Bloom and bust: Intestinal microbiota dynamics in response to hospital exposures and Clostridium difficile colonization or infection. Microbiome 2016, 4, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samarkos, M.; Mastrogianni, E.; Olga, K. The role of gut microbiota in Clostridium difficile infection. Eur. J. Intern. Med. 2018, 50, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, E.J.; Bhalodia, S.; Jacob, S.; Patel, H.; Trinh, K.V.; Varghese, B.; Yang, J.; Young, S.R.; Raffa, R.B. Clostridium difficile infection: A brief update in emerging therapies. Am. J. Health Syst. Pharm. 2015, 72, 1007–1012. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Joyee, A.G.; Nandagopal, S.; Lopez, M.; Ma, X.; Berry, J.; Lin, F. Effects of Clostridium difficile toxin A and B on human T lymphocyte migration. Toxins 2013, 5, 926–938. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez, C.; Romero, E.; Garrido-Sanchez, L.; Alcaín-Martínez, G.; Andrade, R.J.; Taminiau, B.; Daube, G.; García-Fuentes, E. Microbiota insights in Clostridium difficile infection and inflammatory bowel disease. Gut Microbes 2020, 4, 1–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuntz, J.L.; Chrischilles, E.A.; Pendergast, J.F.; Herwaldt, L.A.; Polgreen, P.M. Incidence of and risk factors for community-associated Clostridium difficile infection: A nested case-control study. BMC Infect. Dis. 2011, 11, 194. [Google Scholar] [CrossRef] [Green Version]
- Deshpande, A.; Pasupuleti, V.; Thota, P.; Pant, C.; Rolston, D.D.; Sferra, T.J.; Hernandez, A.V.; Donskey, C.J. Community-associated Clostridium difficile infection and antibiotics: A meta-analysis. J. Antimicrob. Chemother. 2013, 68, 1951–1961. [Google Scholar] [CrossRef] [Green Version]
- Seekatz, A.M.; Rao, K.; Santhosh, K.; Young, V.B. Dynamics of the fecal microbiome in patients with recurrent and nonrecurrent Clostridium difficile infection. Genome Med. 2016, 8, 47. [Google Scholar] [CrossRef] [Green Version]
- Drekonja, D.; Reich, J.; Gezahegn, S.; Greer, N.; Shaukat, A.; MacDonald, R.; Rutks, I.; Wilt, T.J. Fecal Microbiota Transplantation for Clostridium difficile Infection: A Systematic Review. Ann. Intern. Med. 2015, 162, 630–638. [Google Scholar] [CrossRef]
- Lagier, J.-C. Gut microbiota and Clostridium difficile. Hum. Microbiome J. 2016, 2, 10–14. [Google Scholar] [CrossRef] [Green Version]
- Chang, J.Y.; Antonopoulos, D.A.; Kalra, A.; Tonelli, A.; Khalife, W.T.; Schmidt, T.M.; Young, V.B. Decreased diversity of the fecal microbiome in recurrent Clostridium difficile-associated diarrhea. J. Infect. Dis. 2008, 197, 435–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jernberg, C.; Löfmark, S.; Edlund, C.; Jansson, J.K. Long-term ecological impacts of antibiotic administration on the human intestinal microbiota. ISME J. 2007, 1, 56–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weingarden, A.; González, A.; Vázquez-Baeza, Y.; Weiss, S.; Humphry, G.; Berg-Lyons, D.; Knights, D.; Unno, T.; Bobr, A.; Kang, J.; et al. Dynamic changes in short- and long-term bacterial composition following fecal microbiota transplantation for recurrent Clostridium difficile infection. Microbiome 2015, 3, 10. [Google Scholar] [CrossRef] [Green Version]
- Zar, F.A.; Bakkanagari, S.R.; Moorthi, K.M.; Davis, M.B. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin. Infect. Dis. 2007, 45, 302–307. [Google Scholar] [CrossRef]
- Almeida, R.; Gerbaba, T.; Petrof, E.O. Recurrent Clostridium difficile infection and the microbiome. J. Gastroenterol. 2016, 51, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Britton, R.A.; Young, V.B. Interactions between the intestinal microbiota and host in Clostridium difficile colonization resistance. Trends Microbiol. 2012, 20, 313–319. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Cobas, A.E.; Artacho, A.; Ott, S.J.; Moya, A.; Gosalbes, M.J.; Latorre, A. Structural and functional changes in the gut microbiota associated to Clostridium difficile infection. Front. Microbiol. 2014, 5, 335. [Google Scholar]
- Schubert, A.M.; Rogers, M.A.M.; Ring, C.; Mogle, J.; Petrosino, J.P.; Young, V.B.; Aronoff, D.M.; Schloss, P.D. Microbiome data distinguish patients with Clostridium difficile infection and non-C. difficile-associated diarrhea from healthy controls. mBio 2014, 5, e01021-14. [Google Scholar] [CrossRef] [Green Version]
- Vincent, C.; Manges, A.R. Antimicrobial use, human gut microbiota and Clostridium difficile colonization and infection. Antibiotics 2015, 4, 230–253. [Google Scholar] [CrossRef]
- Strati, F.; Cavalieri, D.; Albanese, D.; De Felice, C.; Donati, C.; Hayek, J.; Jousson, O.; Leoncini, S.; Renzi, D.; Calabrò, A.; et al. New evidences on the altered gut microbiota in autism spectrum disorders. Microbiome 2017, 5, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, W.; Yang, Y.; Wang, H.; Wang, H.; Yu, X.; Lu, Y.; Shen, S.; Teng, L. Gut microbiota shifts in patients with gastric cancer in perioperative period. Medicine 2019, 98, e16626. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Zhang, C. High Fat High Calories Diet (HFD) Increase Gut Susceptibility to Carcinogens by Altering the Gut Microbial Community. J. Cancer 2020, 11, 4091–4098. [Google Scholar] [CrossRef] [Green Version]
- Khanna, S.; Montassier, E.; Schmidt, B.; Patel, R.; Knights, D.; Pardi, D.S.; Kashyap, P. Gut microbiome predictors of treatment response and recurrence in primary Clostridium difficile infection. Aliment. Pharmacol. Ther. 2016, 44, 715–727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daquigan, N.; Seekatz, A.M.; Greathouse, K.L.; Young, V.B.; White, J.R. High-resolution profiling of the gut microbiome reveals the extent of Clostridium difficile burden. NPJ Biofilms Microbiomes 2017, 3, 35. [Google Scholar] [CrossRef]
- Horvat, S.; Rupnik, M. Interactions between Clostridoides difficile and fecal microbiota in in vitro batch model: Growth, sporulation and microbiota changes. Front. Microbiol. 2018, 9, 1633. [Google Scholar] [CrossRef]
- Crobach, M.J.T.; Ducarmon, Q.R.; Terveer, E.M.; Harmanus, C.; Sanders, I.M.J.G.; Verduin, K.M.; Kuijper, E.J.; Zwittink, R.D. The Bacterial Gut Microbiota of Adult Patients Infected, Colonized or Noncolonized by Clostridioides difficile. Microorganisms 2020, 8, 677–690. [Google Scholar] [CrossRef]
- Belmares, J.; Gerding, D.N.; Parada, J.P.; Miskevics, S.; Weaver, F.; Johnson, S. Outcome of metronidazole therapy for Clostridium difficile disease and correlation with a scoring system. J. Infect. 2007, 55, 495–501. [Google Scholar] [CrossRef]
- Kelly, C.P.; Kyne, L. The host immune response to Clostridium difficile. J. Med. Microbiol. 2011, 60, 1070–1079. [Google Scholar] [CrossRef] [Green Version]
- Ananthakrishnan, A.N.; Oxford, E.C.; Nguyen, D.D.; Sauk, J.; Yajnik, V.; Xavier, R.J. Genetic risk factors for Clostridium difficile infection in ulcerative colitis. Aliment. Pharmacol. Ther. 2013, 38, 522–530. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.Y.; Kim, H.; Cha, M.Y.; Park, H.G.; Kim, Y.J.; Kim, I.Y.; Kim, J.M. Clostridium difficile toxin A promotes dendritic cell maturation and chemokine CXCL2 expression through p38, IKK, and the NF-kappaB signaling pathway. J. Mol. Med. (Berl.) 2009, 87, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Panpetch, W.; Somboonna, N.; Palasuk, M.; Hiengrach, P.; Finkelman, M.; Tumwasorn, S.; Leelahavanichkul, A. Oral Candida administration in a Clostridium difficile mouse model worsens disease severity but is attenuated by Bifidobacterium. PLoS ONE 2019, 14, e0210798. [Google Scholar] [CrossRef] [PubMed]
- McDonald, L.C.; Coignard, B.; Dubberke, E.; Song, X.; Horan, T.; Kutty, P.K. Recommendations for surveillance of Clostridium difficile-associated disease. Infect. Control Hosp. Epidemiol. 2007, 28, 140–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Infected Patients | Controls | |||
|---|---|---|---|---|
| CDI (10 Cases) | rCDI (10 Cases) | C (20 Cases) | ||
| Number of Cases | 10 | 10 | 20 | |
| Male: Female Ratio | 6:4 | 6:4 | 7:13 | |
| Mean Age in Years (+/− standard deviation) | 67 +/− 18 | 66 +/− 16 | 66 +/− 17 | |
| Co-Morbidities: | ||||
| No Comorbidities° | 1 | 0 | 7 | |
| Digestive | 2 | 2 | 6 | |
| Cardio-Vascular | 7 | 10 | 8 | |
| Respiratory | 1 | 4 | 4 | |
| Endocrine-Metabolic | 2 | 6 | 8 | |
| Neoplasia | 1 | 1 | 3 | |
| Other | 5 | 8 | 9 | 8 |
| Drugs Other than Antibiotics: | Ep 1 | Ep 2 | ||
| Protonic Pump Inhibitors | 8 | 9 | 8 | 12/15 |
| Nonsteroidal Anti-Inflammatory Drugs | 0 | 0 | 1 | 1/14 |
| Enteral Nutrition | 0/9 | 2 | 2 | 0/12 |
| Antibiotics in the Last 6 Months | 6/8 | 7/10 | 10/10 | 6/15 |
| Penicillins | 3 | 5 | 4 | |
| Cephalosporins/Carbapenems | 3 | 1 | ||
| Fluoroquinolones | 2 | 2 | 3 | |
| Aminoglycosides | ||||
| Glycopeptides | 1 | 1 | ||
| Tetracyclines | 1 | |||
| Macrolides | 1 | 2 | ||
| Metronidazole | 2 | |||
| Others | 4 | |||
| NA | 5 | 3 | 13 | |
| Antibiotics Other than Antibiotics for Treating C. difficile | 7/10 | 5/10 | 8/14 | |
| Penicillins | 4 | 2 | 3 | |
| Cephalosporins/Carbapenems | 5 | 3 | 1 | |
| Fluoroquinolones | 3 | 1 | ||
| Aminoglycosides | 1 | 1 | ||
| Glycopeptides | 1 | 1 | ||
| Tetracyclines | ||||
| Macrolides | 1 | |||
| Metronidazole | 1 | |||
| Others | 1 | 1 | 2 | |
| NA | 3 | 6 | 13 | |
| Previous hospitalization (6 months) | 8/10 | 7/10 | 10/10 | 5/15 |
| CDI (Cases 10) | rCDI1 (Cases 10) | rCDI2 (Cases 10) | |
|---|---|---|---|
| Type of Infection: | |||
| Nosocomial 1 | 5 | 5 | 3 |
| Community Associated 2 | 5 | 5 | 7 |
| Antibiotics for Treating C. difficile (duration in days) | |||
| Metronidazole | 1 (9) | 0 | 0 |
| Vancomycin | 6 (10) | 8 (12) | 7 (13) |
| Vancomycin + Metronidazole | 1 (7) | 1 (NA) | 0 |
| Fidaxomicin | 0 | 1 (9) | 0 |
| Not known | 2 (NA) | 1 (NA) | 3 (NA) |
| Zar-score: | |||
| 0–1 | 7 | 4 | 6 |
| ≥2 | 3 | 6 | 4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gazzola, A.; Panelli, S.; Corbella, M.; Merla, C.; Comandatore, F.; De Silvestri, A.; Piralla, A.; Zuccaro, V.; Bandi, C.; Marone, P.; et al. Microbiota in Clostridioides difficile-Associated Diarrhea: Comparison in Recurrent and Non-Recurrent Infections. Biomedicines 2020, 8, 335. https://doi.org/10.3390/biomedicines8090335
Gazzola A, Panelli S, Corbella M, Merla C, Comandatore F, De Silvestri A, Piralla A, Zuccaro V, Bandi C, Marone P, et al. Microbiota in Clostridioides difficile-Associated Diarrhea: Comparison in Recurrent and Non-Recurrent Infections. Biomedicines. 2020; 8(9):335. https://doi.org/10.3390/biomedicines8090335
Chicago/Turabian StyleGazzola, Alessandra, Simona Panelli, Marta Corbella, Cristina Merla, Francesco Comandatore, Annalisa De Silvestri, Antonio Piralla, Valentina Zuccaro, Claudio Bandi, Piero Marone, and et al. 2020. "Microbiota in Clostridioides difficile-Associated Diarrhea: Comparison in Recurrent and Non-Recurrent Infections" Biomedicines 8, no. 9: 335. https://doi.org/10.3390/biomedicines8090335
APA StyleGazzola, A., Panelli, S., Corbella, M., Merla, C., Comandatore, F., De Silvestri, A., Piralla, A., Zuccaro, V., Bandi, C., Marone, P., & Cambieri, P. (2020). Microbiota in Clostridioides difficile-Associated Diarrhea: Comparison in Recurrent and Non-Recurrent Infections. Biomedicines, 8(9), 335. https://doi.org/10.3390/biomedicines8090335





