Anthocyanins, Vibrant Color Pigments, and Their Role in Skin Cancer Prevention
Abstract
:1. Introduction
2. Anthocyanins’ Chemistry
3. Anthocyanins, as Part of the Daily Human Diet
4. Rich Sources of Anthocyanins
4.1. Flowers
| Source | Major Anthocyanin Reported | Total Anthocyanins Content mg/100 g FW * | Total Anthocyanins Content mg/100 g DW ** | Ref. |
|---|---|---|---|---|
| Purple basil (Ocimum basilicum L.) | Cyanidin-3-O-glucoside | [70] | ||
| Purple basil Ocimum basilicum L. Purple Ruffles | Cyanidin-based, p-coumaryl acid | 0.0127 | [64] | |
| Butterfly peas (Clitoria ternatea L.) | Delphinidin derivates | NR *** | NR | [74] |
| Butterfly peas (Clitoria ternatea L.) | Delphinidin-3-O-malonyl-glucoside | NR | NR | [74] |
| Butterfly peas (Clitoria ternatea L.) | Delphinidin-3-O-(6′′-O-malonyl)-β-glucoside-3′,5′-di-O-β-glucoside | NR | NR | [68] |
| Camelia (Camelia cv Dalicha) | Cyanidin-3-O-(2-O-β-xylo- pyranosyl)-β-galactopyranoside Cyanidin-3-O-(2-O-β-xylopyranosyl-6-O-(z)-p-coumaroyl)-β-galactopyranoside | NR | NR | [75] |
| Camelia (Camelia cv
| Cyanidin-3-O-(6-O-(e)-p-coumaroyl)-β-glucopyranoside Cyanidin-3-O-(6-O-(e)-p-coumaroyl)-β-galactopyranoside | 5.6± 1.6 5.6± 2.2 48.5± 24.2 26.9± 6.0 20.5± 1.5 | NR | [76] |
| Saffron (Crocus sativus) | Delphinidin-3,7-O-diglucoside | 480 ± 2.33 | [66] | |
| Saffron (Crocus antalyensis) | 3,7-di-O-β-d-glucoside of delphinidin Petunidin-3,7-di-O-(β-d-glucopyranoside) Delphinidin-3-O-(β-d-glucopyranoside)-5-O-(6-O-malonyl-β-d-glucopyranoside | NR | NR | [67] |
| Saffron (Crocus etruscus) | Delphinidin-3,5-di-O-β-glucoside Petunidin-3,5-di-O-β-glucoside | [77] | ||
| Chrysanths (Chrysanthemum Dendranthema grandiflorum Ramat. cv Angel) | Cyanidin-3-(3ʹʹ-malonoyl)glucoside | NR | 1386± 3.9 | [78] |
| Chrysanths (Chrysanthemum grandiflorum
| Cyanidin-3-O-(6″-O-malonylglucoside) Cyanidin-3-O-(3″,6″-O-dimalonylglucoside) | NR | NR | [79] |
| Clematis (Clematis
| Cyanidin-3-O-β-(2′′-e-caffeoylglucopyranosyl)-(1 --> 2)-O-β-galactopyranoside-3′′-O-β-glucuronopyranoside Cyanidin-3-O-β-(2′′-e-caffeoylglucopyranosyl)-(1 --> 2)-O-β-(6′′-malonylgalactopyranoside)-3′-O-β-glucuronopyranoside Cyanidin-3-O-β-(2′-e-feruloylglucopyranosyl)-(1 --> 2)-O-β-(6′′-malonylgalactoside)-3′-O-beta-glucuronopyranoside | NR | NR | [71] |
| Clematis (Clematis cv. Jackmanii Superba Fujimusume) | Delphinidin-3-O-β-[(2”-trans-caffeoylglucopyranosyl)-(1 --> 2)-(6”-succinylgalactopyranoside)]-7-O-β-glucopyranoside Delphinidin-3-O-β-[(2”-trans-caffeoylglucopyranosyl)-(1 --> 2)-(6”-trans-caffeoyl-tartaroyl-malonylgalactopyranoside)]-7-O-β-glucopyranoside Delphinidin-3-O-β-[(2”-trans-caffeoylglucopyranosyl)-(1 --> 2)-(6”-trans-caffeoyl-tartaroyl-malonylgalactopyranoside)]-3′-O-β-glucuronopyranoside | NR | NR | [80] |
| Carnation (Dianthus caryophyllus) | 3, 5-di-O-(β-glucopyranosyl) pelargonidin-6”-O-4, 6”-O-l-cyclic 3, 5-di-O-(β-glucopyranosyl) cyanidin-6”-O-4, 6”-O-l-cyclic malate | NR | NR | [81] |
| Carnation (Dianthus caryophyllus cv.
| Pelargonidin-3,5-cyclicmalyldiglucoside Cyanidin-3-O-malylglucoside Pelargonidin-3,5-diglucoside | NR | NR | [82] |
| Carnation (Dianthus caryophyllus cv.
| Delphinidin-3,5-diglucoside-6”-O-4, 6”-O-1-cyclic-malyl diester | NR | NR | [83] |
| Carnation (Dianthus caryophyllus cv.
| Cyanidin-3,5-d-O-glucosides Cyanidin-3-O-(6-O-malyl glucoside)-5-O-glucoside | NR | NR | [84] |
| Edible roses (An ning) | Cyanidin-3,5-di-O-glucoside | 353.56 ± 2.50 | [2] | |
| Edible violet (Viola tricolor L.) | Delphinidin-3-(4”-p-coumaroyl)-rutinoside-5-glucoside | [85] | ||
| Freesias (Freesia hybrida) | Malvidin-3-O-glucoside | NR | [86] | |
| Roselle (Hibiscus sabdariffa) | Delphinidin-3-O-sambubioside Cyanidin-3-O-sambubioside | NR | NR | [87] |
| Delphinidin-3-O-sambubioside Cyanidin-3-O-sambubioside | NR | [68] | ||
| Morning glory (Ipomoea tricolor Cav.) | Peonidin-3-O-sophoroside-5-O-glucoside Peonidin-3-O-(2-O-(6-O-(trans-caffeoyl)-β -glucopyranosyl)-6-O-(trans-caffeoyl)-β-glucopyranoside)-5-O-(β glucopyranoside) Peonidin-3-O-(2-O-(β-glucopyranosyl)-6-O-(trans-caffeoyl)-β- glucopyranoside)-5-O-(β-glucopyranoside) | NR | NR | [68] |
| Leopard lily (Iris cv.
| Delphinidin-3-O-(cis-p-coumaroyl)rutinoside-5-O-glucoside Delphinidin-3-O-(trans-p-coumaroyl)-rutinoside-5-O-glucoside Delphinidin-3-O-(feruloyl)rutinoside-5-O-glucoside Pelargonidin-3-O-(cis-p-coumaroyl)rutinoside-5-O-glucoside Pelargonidin-3-O-(feruloyl)rutinoside-5-O-glucoside | 5.82-258.6 | [88] | |
| Japanese water iris (Iris ensata) | Malvidin-3-O-(p coumaroyl)rhamnosylglucoside-5-O-glucosides Petunidin-3-O-(p-coumaroyl)rhamnosylglucoside-5-O-glucosides | NR | NR | [89] |
| Dutch iris (Iris hollandica) | Delphinidin-3-O-(p-coumaroyl)rhammnosylglucoside-5-O-glucoside | NR | NR | [90] |
| Crimean iris (Iris lutescens) | Delphinidin-3-O-(p-coumaroylrutinoside)-5-O-glucoside | NR | NR | [1] |
| Edging lobelia (Lobelia erinus cv Rosamond) | Cyanidin-3-O-(6-O-(4-O-trans-p-coumaryl-α-l-rhamnopyranosyl)-β-d-glucopyranoside)-5- O-(6-O-malonyl-β-d glucopyranoside)-3′-O-(6-O-trans-caffeyl-β-d-glucopyranoside) Cyanidin-3-O-rutinoside-5,3′-diglucoside | NR | NR | [91] |
| Edging lobelia (Lobelia erinus cv.
| Delphinidin-3-O-p-coumaroylrutinoside-5-O-malonylglucoside-3′5′-O-dihydroxycinnamoylglucoside Delphinidin-3-O-glucoside | NR | NR | [26] |
| Meadow/bloody crane’s-bill (Geranium
| Malvidin-3-O-β-d-glucopyranoside-5-O-β-d-[6-O-acetylglucopyranoside] | NR | NR | [92] |
| Peony (Paeonia
| Peonidin-3,5-di-O-glucoside Cyanidin-3-O-glucosid | NR | NR | [93] |
Peony
| Peonidin-3,5-di-O-glucoside Cyanidin-3-O-glucoside | NR | NR | [94] |
| Petunia (Petunia hybrida
| Cyanidin-3-O-glucoside | 7.72± 0.8 µmolgFW 5.61± 0.39 | NR | [95] |
| Petunia (Petunia exserta) | Cyanidin-3-O-sophoroside Cyanidin-3-O-glucoside Peonidin-3-O-glucoside | NR | NR | [96] |
| Cyanidin-3-O-glucoside | NR | NR | [97] | |
| Petunia (Petunia hybrida) | Peonidin-3-O-(6-(6-coumaryl rhamnosyl)-glucoside)-5-O-glucoside | NR | NR | [98] |
| Red frangipani (Plumeria rubra) | Cyanidin-3-O-β-(2”-glucopyranosyl-O-β-galactopyranoside) | NR | NR | [73] |
| Pomegranate (Punica granatum) | Pelargonidin-3-O-glucoide Pelargonidin-3-O-diglucoside | NR | NR | [99] |
| Korean edible rose (Rosa hybrida cv. Noblered) | Cyanidin-3,5-di-O-glucoside | 375+_9.6 | NR | [100] |
Rosa rugosa
| Peonidin-3,5-di-O-glucoside Cyanidin-3,5-di-O-glucoside Cyanidin-3,5-di-O-glucoside | 165 ± 17.2 44.95 ± 0.38 14.01 ± 0.61 | NR | [101] |
| Nasturtium (Tropaeolum majus) | Delphinidin-3-O-dihexoside Pelargonidin-3-O-sophoroside | 245.5±167.3 880.3± 18.3 | 31.9± 21.7 114.5±17.22 | [62] |
| Nasturtium (Tropaeolum majus) | Pelargonidin-3-O-sophoroside | NR | NR | [102] |
| Marigold (Tagetes erecta) | Cyanidin-di-hexoside | NR | NR | |
| Aracress (Spilanthes oleracea) | Cyanidin-3-O-glucoside Delphinidina-3-O-glucuronide | NR | NR | |
| Tulip (Tulipa fosteriana ‘Albert heijn’) | Pelargonidin-3-O-acetylrutinoside Cyanidin-3-O-rutinoside | NR | NR | [103] |
| Blue periwinkle (Vinca. major L.) | Delphinidin-3-O-[6-O-(α-rhamnopyranosyl)-β-galactopyranoside]-7-O-(α-rhamnopyranoside) | NR | NR | [104] |
| Blue periwinkle (Vinca. minor L.) | Delphinidin-3-O-[2-O- (β-xylopyranosyl)-6-O-(α-rhamnopyranosyl)-β-galactopyranoside]-7-O-(α-rhamnopyranoside) | NR | NR | |
| Garden pansy (Viola wittrockiana) | Delphinidin-3-O-rhamnosyl-glucoside | NR | 0.57 ± 1.2 | [105] |
| Yunnan edible rose (An ning) | Cyanidin-3,5-O-diglucoside Cyanidin-3-O-glucoside | 353.56 ± 2.50 | NR | [106] |
| False shamrock (Oxalis triangularis) | Malvidin-3-O-(6-O-(4-O-malonyl-a-rhamnopyranosyl)-β-glucopyranoside)-5-O-β-glucopyranoside Malvidin-3-O-(6-O-(4-O-malonyl-a-rhamnopyranosyl)-β-glucopyranoside)-5-O-β-glucopyranoside | NR | NR | [107] |
| Malvidin-3-O-rutinoside-5-O-glucoside. | NR | NR | [108] | |
| Mavidin-3-O-(6-O-(4-O-malonyl-a-rhamnopyranosyl-β-glucopyranoside)-5-O-b-glucopyranoside | NR | [109] |
4.2. Fruits and Vegetables
| Source | Major Anthocyanin Reported | Conc. mg/100 g FW | Conc. mg/100 g DW | Conc. mg/100 mL | Ref. |
|---|---|---|---|---|---|
| Chokeberry (Aronia melanocarpa) | Cyanidin-3-O-galactoside | 900 | NR | [125] | |
| Cyanidin-3-O-galactoside | 36.7 | NR | NR | [126] | |
| Cyanidin-3-O-galactoside Cyanidin-3-O-arabinoside | NR | 8.63 | [127] | ||
| Cyanidin-3-O-galactoside | 51.3 | NR | NR | [128] | |
| Cyanidin-3-O-galactoside | NR | NR | NR | [129] | |
| Cyanidin-3-O-galactoside | 93.3 | NR | NR | [115] | |
| Cyanidin-3-O-galactoside | 906.9 | NR | NR | [130] | |
| Cyanidin-3-O-galactoside | 248.24 | NR | NR | [117] | |
| Cyanidin-3-O-galactoside | 403 | NR | NR | [131] | |
| Cyanidin-3-O-galactoside | NR | 8286.4 | NR | [132] | |
| Cyanidin-3-O-galactoside | NR | NR | 30.1 | [133] | |
| Cyanidin-3-O-galactoside | NR | 40 | NR | [134] | |
| Cyanidin-3-O-galactoside | NR | 627 | NR | [135] | |
| Cyanidin-3-O-galactoside | NR | 798.08 | NR | [126] | |
| Cranberries (Vaccinium macrocarpon) | Peonidin-3-O-galactoside | NR | 75.26 | [136] | |
| Peonidin-3-O-galactoside | NR | 255 | NR | [137] | |
| Cyanidin-3-O-arabinose | NR | 588 | NR | [138] | |
| Peonidin-3-O-glucoside | NR | NR | 20.40 | [139] | |
| Cyanidin-3-O-galactoside | 15.7 | NR | NR | [117] | |
| Bilberry (Vaccinium spp.) | Delphinidin-3-O-glucoside | 47.7 | NR | NR | [140] |
| Delphinidin-3-O-glucoside | NR | 1761 | NR | [141] | |
| Delphinidin-3-O-glucoside | NR | NR | 57.41 | [139] | |
| Cyanidin-3-O-glucoside | NR | NR | 25.68 | [127] | |
| Delphinidin-3-O-galactoside | 177.97 | NR | NR | [117] | |
| Blueberry (Vaccinium spp.) | Maldivin-3-O-galactoside | 101.88 | NR | NR | [16] |
| Maldivin-3-O-galactoside | 194 | NR | NR | [142] | |
| Maldivin-3-O-galactoside | 178 | NR | NR | [143] | |
| Cyanidin-3-O-glucoside | 282 | NR | NR | [144] | |
| Petunidin-3-O-glucoside | 77.5 | NR | NR | [118] | |
| Maldivin-3-O-galactoside, Delphinidin-3-O-galactoside | 259.2 | NR | NR | [145] | |
| Delphinidin-3-O-glucoside | NR | 143.90 ± 1.56 | [124] | ||
| Delphinidin-3-O-galactoside | 55.37 ± 26.2 | NR | NR | [117] | |
| Maldivin-3-O-hexoside | NR | 1218 | NR | [146] | |
| Maldivin-3-O-galactoside Maldivin-3-O-glucoside | 36.24 ± 0.6 | NR | NR | [147] | |
| Cyanidin glycosides | 424.2 | NR | NR | [17] | |
| Delphinidin-3-O-glucoside | NR | 1435 | NR | [1] | |
| Maldivin-3-O-galactoside | 32.8 ± 18.9 | NR | NR | [148] | |
| Delphinidin-3-O-glucoside | 32893 ± 2.47 | NR | NR | [149] | |
| Maldivin glycosides | 790.7 ± 19 | NR | [150] | ||
| Maldivin-3-O-galactoside | 286.4 ± 37.97 | NR | NR | [151] | |
| Delphinidin-3-O-glucoside | 0.172 | NR | NR | [152] | |
| Blackberry (Rubus spp.) | Cyanidin-3-O-glucoside | NR | NR | 17.28 ± 0.0088 | [119] |
| Cyanidin-3-O-glucoside | 148.9 ± 69 | NR | NR | [117] | |
| Cyanidin-3-O-glucoside | NR | 606 | NR | [153] | |
| Cyanidin-3-O-rutinoside | NR | NR | 3.735 | [154] | |
| Cyanidin-3-O-glucoside | NR | 710 ± 0.02 | NR | [155] | |
| Cyanidin-3-O-glucoside | 124.3 | NR | [114] | ||
| Delphinidin-3-O-glucoside | 647.0 ± 19.2 | NR | [118] | ||
| Cyanidin-3-O-glucoside | NR | 811.85 ± 2.76 | NR | [156] | |
| Blackcurrant (Ribes nigrum) | Cyanidin-3-O-glucoside | 6.599 | NR | NR | [157] |
| Cyanidin-3-O-glucoside | NR | NR | NR | [158] | |
| Delphinidin-3-O-rutinoside | 27.85 ± 16.0 | NR | NR | [117] | |
| Delphinidin-3-O-rutinoside | 2.653 ± 1.82 | NR | NR | [152] | |
| Cyanidin-3-O-rutinoside Delphinidin-3-O-rutinoside | NR | NR | 8.94 | [127] | |
| Delphinidin-3-O-rutinoside | NR | NR | 140.75 ± 1.77 | [134] | |
| Delphinidin-3-O-rutinoside | NR | NR | 10.163 | [154] | |
| Delphinidin-3-O-glucoside | 644 ± 113 | NR | [132] | ||
| Blackthorn (Prunus spinose) | Peonidin-3-O-rutinoside | NR | 0.034 ± 0.03 | NR | [159] |
| Cyanidin-3-O-rutinoside | NR | NR | NR | [160] | |
| Cyanidin-3-O-glucoside | 128.648 ± 116.07 | NR | NR | [161] | |
| Redcurrant (Ribes rubrum) | Cyanidin-3-O-glucoside | 1.697 | NR | NR | [157] |
| Cyanidin-3-O-xylosylrutinoside | 6.85 ± 2.8 | NR | NR | [117] | |
| Cyanidin-3-O-rutinoside | NR | NR | [158] | ||
| Cyanidin-3-O-xylosyl-rutinoside | 104 ± 1.6 | NR | NR | [122] | |
| Elderberry (Sambucus spp.) | Cyanidin-3-O-glucoside | NR | 0.3738 ± 0.147 | [131] | |
| Cyanidin-3-O-glucoside | NR | NR | NR | [162] | |
| Cyanidin-3-O-glucoside Cyanidin-3-O-sambubioside | NR | NR | NR | [163] | |
| Cyanidin-3-O-glucoside | 132.17 ± 131.9 | NR | NR | [117] | |
| Strawberry (Fragaria spp.) | Pelargonidin-3-O-glucoside | NR | 15.13 | [139] | |
| Pelargonidin-3-O-glucoside | 61.11 ± 0.13 | NR | NR | [164] | |
| Pelargonidin-3-O-glucoside | 52.22 ± 46.4 | NR | NR | [117] | |
| Pelargonidin-3-O-glucoside | 57.9 ± 3.4 | NR | NR | [165] | |
| Pelargonidin-3-O-glucoside | 736.98 ± 178.9 | NR | [166] | ||
| Pelargonidin-3-O-glucoside | 18.581 | NR | NR | [167] | |
| Pelargonidin-3-O-glucoside | 407.8 ± 16.8 | NR | NR | [118] | |
| Pelargonidin-3-O-glucoside | 21.31 ± 1.11 | NR | NR | [132] | |
| Pelargonidin-3-O-glucoside | NR | NR | 20.1 | [168] | |
| Pelargonidin-3-O-glucoside | NR | 107 | NR | [165] | |
| Pelargonidin-3-O-glucoside | 33.27 | NR | NR | [123] | |
| Mulberry (Morus spp). | Cyanidin-3-O-glucoside | NR | 669 ± 34 | NR | [130] |
| Cyanidin-3-O-glucoside | NR | NR | 49.2 ± 0.0099 | [119] | |
| Cyanidin-3-O-glucoside | 156.1 ± 42.3 | NR | NR | [117] | |
| Cyanidin-3-O-glucoside | 1.543 ± 0.06 | NR | NR | [152] | |
| Sour Cherry (Prunus cerasus L) | Cyanidin-3-O-glucosyl-rutinoside | 1269.2 ± 23.3 | NR | NR | [169] |
| Cyanidin-3-O-glucosyl-rutinoside | NR | NR | NR | [170] | |
| Cyanidin-3-O-glucosyl-rutinoside | NR | 372.84 ± 1.67 | NR | [171] | |
| Cyanidin-3-O-glucosyl-rutinoside | 39.02 | NR | NR | [172] | |
| Cyanidin-3-O-glucosyl-rutinoside | 59.75 ± 5.06 | NR | NR | [173] | |
| Cyanidin-3-O-glucosyl-rutinoside | NR | NR | NR | [174] | |
| Cyanidin-3-O-glucosyl-rutinoside | NR | NR | NR | [175] | |
| Cyanidin-3-O-glucosyl-rutinoside | NR | NR | 73.67 | [139] | |
| Raspberries (Rubus idaeus) | Cyanidin-3-O-rutinoside | NR | NR | NR | [176] |
| Cyanidin-3-O-rutinoside | NR | NR | NR | [158] | |
| Cyanidin-3-O-sophoroside | 30.56 ± 33.7 | NR | NR | [117] | |
| Cyanidin-3-O-sophoroside | NR | 233 | NR | [153] | |
| Pelargonidin-3-O-glucoside | 0.313 ± 0.01 | NR | NR | [152] | |
| Cyanidin-3-O-sophoroside | NR | 8.098 | [154] | ||
| Cyanidin-3-O-glucoside Petunidin-3-O-glucoside | 133.9 ± 8.4 | NR | NR | [118] | |
| Plums (Prunus spp.) | Peonidin-3-O-rutinoside | NR | 0.034 ± 0.03 | NR | [173] |
| Lingonberries (Vaccinium vitis-idaea) | Cyanidin-3-O-glucoside | NR | NR | 60.5 ± 0.054 | [129] |
| Cyanidin-3-O-galactoside | 34.86 ± 21.5 | NR | NR | [117] | |
| Rosehip (Rosa spp.) | Cyanidin-3-O-glucoside | NR | 0.0068 | NR | [159] |
| Cyanidin-3-O-glucoside | NR | 0.92 ± 2.6 | NR | [177] | |
| Pomegranate (Punica granatum) | Cyanidin-3,5-O-diglucoside | NR | NR | NR | [178] |
| Cyanidin-3,5-O-diglucoside | NR | NR | NR | [179] | |
| Delphinidin-3,5-O-diglucoside | NR | NR | NR | [180] | |
| Cyanidin-3,5-O-diglucoside | NR | NR | 1.471 ± 0.32 | [181] | |
| Delphinidin-3,5-O-diglucoside | NR | NR | NR | [182] | |
| Cyanidin-3,5-O-diglucoside | NR | NR | NR | [183] | |
| Pelargodin-3,5-O-diglucoside | 17.9 ± 7.9 | NR | NR | [184] | |
| Cyanidin-3-O-glucoside | 43.99 ± 4.67 | NR | NR | [185] | |
| Cyanidin-3-O-monoglucoside | NR | NR | NR | [186] | |
| Cyanidin-3-O-glucoside | NR | 85 ± 0.02 | NR | [187] | |
| Cyanidin-3,5-O-diglucoside | NR | NR | [188] | ||
| Cyanidin-3,5-O-diglucoside | NR | 75.78 ± 3.78 | NR | [189] | |
| Cyanidin-3-O-glucoside | NR | NR | 2.816 ± 0.56 | [190] | |
| Cyanidin-3-O-glucoside | NR | NR | NR | [191] | |
| Cyanidin-3-O-glucoside | NR | NR | NR | [192] | |
| Malvidin-3-O-glucoside | NR | 117 ± 4 | NR | [193] | |
| Figs (Ficus carica) | Cyanidin-3-O-rutinoside | 1.191 ±6.33 | NR | NR | [194] |
| Cyanidin-3-O-rutinoside | NR | NR | [195] | ||
| Cyanidin-3-O-rutinoside | 4.82 | NR | NR | [196] | |
| Gooseberry (Ribes uva-crispa) | Cyanidin-3-O-glucoside | 37.79 ± 38.1 | NR | NR | [117] |
| Delphinidin-3-O-rutinoside | 61.19 | NR | NR | [197] | |
| Cyanidin-3-O-glucoside | 0.957 ± 1.66 | NR | NR | [157] | |
| Cyanidin-3-O-glucoside | 37.79 | NR | NR | [117] | |
| Acai (Euterpe oleracea) | Cyanidin-3-O-rutinoside | NR | 106.7 ± 125.95 | NR | [198] |
| Cyanidin-3-O-glucoside | 57 ± 0.39 | NR | NR | [199] | |
| Cyanidin-3-O-glucoside | NR | NR | [200] | ||
| Acerola (Malpighia emarginata) | Cyanidin-3-O-rhamnoside, Pelargonidin-3-O-rhamnoside | 12 ± 0.03 | NR | NR | [201] |
| Maqui (Aristotelia chilensis) | Delphinidin-3-O-glucoside | 715 ± 0.12 | NR | NR | [202] |
| Delphinidin-3-O-glucoside | 4235 ± 0.08 | NR | [203] | ||
| Cyanidin-3-O-sambubioside-5-O-glucoside+ Cyanidin-diglucoside | 2610 | NR | NR | [204] | |
| Delphinidin-3-O-glucoside-5-O-glucoside | NR | 1278 | NR | [205] | |
| Delphinidin-3-O-glucoside | 789 ± 0.14 | NR | NR | [206] | |
| Blood orange (Citrus × sinensis) | Cyanidin-3-O-(6″-malonyl glucoside) | NR | 1.20 ± 0.02 | [207] | |
| Red apples | Cyanidin-3-O-galactoside | 73.94 ± 31.7 | NR | NR | [208] |
| Cyanidin-3-O-galactoside Cyanidin-3-O-glucoside | NR | NR | NR | [209] | |
| Cyanidin-3-O-galactoside | 21.32 | NR | NR | [210] | |
| Cyanidin-3-O-galactoside | NR | NR | NR | [211] | |
| Dogberry (Cornus mas) | Cyanidin-3-O-rutinozit chloride | NR | NR | 342 | [212] |
| Cyanidin-3-O-galactoside | 123.5 ± 19.7 | NR | NR | [213] | |
| Peonidin-3-O-glucoside | 103.37 ± 5.77 | NR | NR | [214] | |
| Cyanidin-3-O-galactoside | 104.66 | NR | NR | [215] | |
| Pelargonidin-3-O-glucoside | NR | 1403 | NR | [216] | |
| Pelargonidin-3-O-glucoside | NR | NR | 38 ± 0.052 | [131] |
| Source | Major Anthocyanin | Conc. mg/100 g FW | Conc. mg/100 g DW | Conc. mg/100 mL | Ref. |
|---|---|---|---|---|---|
| Native Andean Potatoes (Solanum tuberosum, stenotonum, phureja and chaucha) | Petunidin-3-coumaroylrutinoside-5-glucoside Pelargonidin-3-coumaroylrutinoside-5- glucoside | NR | NR | NR | [217] |
| Red onion (Allium cepa) | Cyanidin-3-(6”-malonylglucoside) | 3.012 ± 1.62 | NR | NR | [218] |
| Cyanidin-3-(6”-malonyl)-glucopyranoside | 29.99 ± 1.19 | NR | NR | [156] | |
| Peonidin-3-O-glucoside | NR | 0.19 | NR | [219] | |
| Red cabbage (Brassica oleracea var. capitata f. rubra) | Cyanidin-3,5-O-diglucoside | NR | 232 | NR | [220] |
| Cyanidin-3,5-O-diglucoside | NR | NR | NR | [221] | |
| Cyanidin-3,5-O-diglucoside | NR | 629 ± 0.25 | NR | [222] | |
| Cyanidin-3,5-O-diglucoside | NR | 588.44 ± 146.5 | NR | [223] | |
| Cy 3-(feruloyl)diglucoside-5-glucoside | 34.28 ± 1.60 | NR | NR | [224] | |
| Cyanidin-3,5-O-diglucoside | NR | 630 ± 0.09 | NR | [225] | |
| Cyanidin-3,5-O-diglucoside | NR | NR | NR | [226] | |
| Cyanidin-3,5-O-diglucoside | NR | NR | NR | [227] | |
| Cyanidin-3-(sinapoyl)-O-diglucoside-5-O-glucoside | NR | NR | NR | [228] | |
| Cyanidin-3,5-O-diglucoside | NR | NR | NR | [229] | |
| Cyanidin-3-coumaroyl-dihexoside-5-hexoside | 23.93 ± 1.02 | NR | NR | [230] | |
| Cyanidin-3-(sinapoyl)-O-diglucoside-5-O-glucoside | NR | NR | NR | [231] | |
| Cyanidin derivates | NR | 73.1 ± 203 | NR | [232] | |
| Purple corn (Zea mays indurata) | Cyanidin-3-O-glucoside | NR | NR | [233] | |
| Cyanidin-3-O-glucoside | NR | 38.035 ± 3.39 | NR | [234] | |
| Cyanidin-3-O-glucoside | NR | NR | [235] | ||
| Cyanidin-3-O-glucoside | NR | 83.45 ± 11.44 | NR | [236] | |
| Cyanidin-3-O-glucoside | NR | 350 | NR | [237] | |
| Cyanidin-3-(6”-malonylglucoside) | NR | 4000 ± 0.3 | NR | [238] | |
| Cyanidin-3-O-glucoside | NR | 140.69 ± 68.92 | NR | [239] | |
| Cyanidin-3-O-glucoside | NR | 3.081 | NR | [240] | |
| Cyanidin-3-O-glucoside | NR | NR | [241] | ||
| Purple carrot (Daucus carota subsp. sativus) | Cyanidin-3-xylosyl(feruloylglucosyl)-galactoside | NR | 1986 ± 0.36 | NR | [242] |
| Cyanidin-3-O-glucoside | 290 | NR | NR | [243] | |
| Cyanidin-3-xylosyl(feruloylglucosyl)galactoside | 82.2 ± 0.14 | NR | NR | [244] | |
| Cyanidin-3-xylosyl(feruloylglucosyl)galactoside | NR | NR | [245] | ||
| Radicchio (Cichorium intybus) | Cyanidin-3,5-di-O-(600-O-malonyl)-glucoside | NR | NR | [246] | |
| Cyanidin-3-O-(600-malonyl)-glucoside | NR | NR | [247] | ||
| Cyanidin-3- O -(6”- O -malonyl)-glucoside | 54.9 | NR | NR | [248] | |
| Cyanidin-3-O-(6”-O-malonyl)-glucoside | NR | NR | [249] | ||
| Cyanidin-3-O-(6”-Omalonyl)- glucoside | 51.15 ± 23.5 | NR | NR | [250] | |
| Purple asparagus (Asparagus officinalis) | Cyanidin-3-O-rutinoside | 3.34 ± 5.28 | NR | NR | [251] |
| Purple kale (Brassica oleracea) | Cyanidin-3-(sinapoyl)diglucoside-5- glucoside | NR | NR | NR | [252] |
| Rhubarb (Rheum rhabarbarum) | Cyanidin-3-O-glucoside Cyanidin-3-O-rutinoside | NR | 341.1 ± 41.6 | NR | [8] |
| Red radish (Raphanus raphanistrum subsp. sativus) | Pelargonidin-3-O-(6-O-p-coumaroyl-2-O-feruloyl)-sophoroside-5-O-(6-O-malonyl)-glucoside | NR | NR | NR | [253] |
| Cyanidin-3-O-sophoroside-5-O-glucoside | NR | NR | NR | [254] | |
| Pelargonidin-3-diglucoside-5 (malonyl)-glucoside | NR | NR | NR | [255] | |
| Black beans (Phaseolus vulgaris) | Petunidin-3-O-glucoside | 206 | NR | NR | [256] |
| Cyanidin-3-O-glucoside | NR | NR | NR | [257] | |
| Black rice (Oryza sativa) | Cyanidin-3-O-glucoside | 123.9 ± 31 | NR | NR | [258] |
| Cyanidin-3-O-glucoside | NR | 140.4 ± 336 | NR | [259] | |
| Cyanidin-3-O-glucoside | NR | 41692 ± 0.36 | NR | [260] | |
| Delphinidin-3-O-galactoside | NR | 74 | NR | [261] | |
| Cyanidin-3-O-glucoside | NR | NR | 278 | [262] | |
| Cyanidin-3-O-glucoside | NR | NR | NR | [263] | |
| Kohlrabi (Brassica oleracea Gongylodes Group) | Cyanidin-3,5-O-diglucoside | NR | NR | NR | [221] |
| Cyanidin-3-(caffeoyl) p-coumaroyl (sinapoyl) diglucoside-5-glucoside | NR | 302 ± 0.21 | NR | [264] | |
| Cyanidin-3-(sinapoyl)-diglucoside-5-glucoside | NR | 2.3 | NR | [265] | |
| Cyanidin-3-(feruloyl) diglucoside-5-glucoside | NR | 30 ± 0.01 | NR | [266] | |
| Eggplant (Solanum melongena) | Delphinidin-3-glucoside-5-(coumaryl) dirhamnoside | NR | 110 | NR | [267] |
| Delphinidin-3-O-rutinoside | 4810 | NR | NR | [268] | |
| Malvidin 3-(p-coumaroyl)rhamnoside(glucoside)-5-glucoside | 202.6 ± 0.286 | NR | [269] | ||
| Malvidin-3-rutinoside-5-glucoside | NR | NR | NR | [270] | |
| Artichoke (Cynara cardunculus) | Cyanidin-3-(6′-malonylglucoside) | NR | 124 ± 0.04 | NR | [271] |
| Purple sweet potato (Ipomoea batatas) | Peo-3-caffeoyl-feruloylsoph-5-glucoside | NR | 83.8 ± 0.4 | NR | [272] |
| Cyanidin-3-caffeoyl-p-hydroxybenzoyl sophoroside- 5-glucoside | NR | NR | [273] | ||
| Peonidin 3-caffeoyl-p-hydroxybenzoyl-sophoroside-5-glucoside | NR | 714 ± 0.28 | NR | [274] | |
| Peonidin-3-(6”-caffeoyl-6”‘-p-hydroxybenzoylsoph)-5-glucoside | NR | 68.4 | NR | [264] | |
| Peonidin-3-caffeoyl-p-hydroxybenzoyl sophoroside-5-glucoside | NR | NR | [248] | ||
| Peonidin-3-(caffeoylferuloyl sophoroside)-5-glucoside | NR | 730.3 ± 99.1 | NR | [275] | |
| Peonidin-3-caffeoyl-feruloyl sophoroside-5-glucoside | NR | NR | [276] | ||
| Cyanidin-3-(6”- caffeoyl-feruloyl sophoroside)-5-glcucoside | NR | 455.08 | NR | [277] | |
| Peonidin-3-(6”caffeoyl-6”‘p-hydroxybenzoyl sophoroside)-5-glucoside | NR | NR | [101] |
5. Anthocyanins’ Potential for Health Benefits
6. Anthocyanins Involvement in Cancer Prevention
7. Anthocyanins as Potential Agents for Melanoma Prevention
| Cell Line | Anthocyanins Sources | Conc. | Biological Effect | Ref. |
|---|---|---|---|---|
| C1 41 | Black Raspberry | 0–100 μg/mL | ↓tumor progression | [347] |
| B16-F1 | Mulberry | 0–5 mg/mL | ↓cell proliferation ↓viability | [31] |
| B16 | Cyanidin-3-α-O-rhamnoside Pelargodin-3- α-O-rhamnoside | 0–20 μg/mL | ↓melanin content ✓skin-lightening ↓tyrosinase activity | [25] |
| MeOMEZlan-a mouse melanocytes | Red wine | 4–500 mg/L | ↓melanogenic activity ↓tyrosinase activity | [346] |
| TVM-A12 | Cyanidin-3-O-β-glucopyranoside | 5/10 µM | ↓cell proliferation induced ✓morphological differentiation | [319] |
| B16-F1 | Mulberry | 0–3 mg/mL | ↓cells proliferation | [31] |
| B16 | Liriope platyphylla | 0–500 μg/mL | ↓tyrosinase activity ↓melanin content | [348] |
| B16-F10 | Blueberry | 0–800 μg/mL | ✓antioxidant activity ↓cells proliferation ✓apoptosis ↑LDH activity | [19] |
| A375 | Hibiscus sabdariffa Linn. | 5–50 mg/mL | ↓ melanin synthesis ↓tyrosinase activity | [349] |
| B16-F10 | Strawberry | ↓cell proliferation | [55] | |
| B16-F10 | Blueberry and blackcurrant juices | 0–500 µg/mL | ↓cell proliferation | [21] |
| B16-F10 | Delphinidin | 10 μg/mL | ↓endothelial cells proliferation | [295] |
| B-16 | Fructus Sorbi acupariae | 5 mL/kg | ✓antitumor activity ✓antimetastatic activity ↑stromal progenitor cells | [350] |
| A375, A549 | Rubus fairholmianus | 10–40 µg/mL | ↓cell proliferation ↓viability ↑cytotoxicity ✓apoptosis | [351] |
| A375, B16-F10 | Chokeberry, red grape | 0–400 µg/mL | ↓cell proliferation ↑oxidative stress biomarkers ↓Δψ | [21] |
| B16-F10 | Blueberry | 0–800 μg/mL | ↓viability ↓cell proliferation ✓blocked cell cycle G0/G1 phase ✓apoptosis | [20] |
| A375 | Houttuynia cordata Thunb | 25–200 μg/mL | ↓viability ↓cell proliferation ✓apoptosis | [352] |
| B16-F10 | Elderberries | ↓cell proliferation ↑LDH activity | [20] | |
| B16-F10 | Dendrobium | 0–120 μg/mL | ↓cell viability ↑melanin inhibition ↑enzyme inhibition | [353] |
| WM35 | Origanum vulgare | 0–4 μg/mL | ↓cell viability | [22] |
| B16-F1 | Hibiscus sabdariffa calyx | 0–1 mg/mL | ↓cell growth ↓ migration ↓tube formation ↓MMP–2/–9 and VEGF ↓migration and angiogenesis | [354] |
8. Anthocyanins as Functional Ingredients in Cosmetics
9. Conclusions and Future Perspectives
Funding
Conflicts of Interest
References
- Zhou, Y.; Zheng, J.; Li, Y.; Xu, D.-P.; Li, S.; Chen, Y.-M.; Li, H.-B. Natural polyphenols for prevention and treatment of cancer. Nutrients 2016, 8, 515. [Google Scholar] [CrossRef]
- Lage, N.N.; Layosa, M.A.A.; Arbizu, S.; Chew, B.P.; Pedrosa, M.L.; Mertens-Talcott, S.; Talcott, S.; Noratto, G.D. Dark sweet cherry (prunus avium) phenolics enriched in anthocyanins exhibit enhanced activity against the most aggressive breast cancer subtypes without toxicity to normal breast cells. J. Funct. Foods 2020, 64, 12. [Google Scholar] [CrossRef]
- Koch, W. Dietary polyphenols-important non-nutrients in the prevention of chronic noncommunicable diseases. A systematic review. Nutrients 2019, 11, 1039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isah, T. Stress and defense responses in plant secondary metabolites production. Biol. Res. 2019, 52, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lila, M.A. Anthocyanins and human health: An in vitro investigative approach. J. Biomed. Biotechnol. 2004, 2004, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A.; Johnson, I.T.; Saltmarsh, M. Polyphenols: Antioxidants and beyond. Am. J. Clin. Nutr. 2005, 81, 215S–217S. [Google Scholar] [CrossRef]
- Wallace, T.C.; Giusti, M.M. Anthocyanins. Adv. Nutr. 2015, 6, 620–622. [Google Scholar] [CrossRef] [Green Version]
- Takeoka, G.R.; Dao, L.; Harden, L.; Pantoja, A.; Kuhl, J.C. Antioxidant activity, phenolic and anthocyanin contents of various rhubarb (rheum spp.) varieties. Food Sci. Technol. 2013, 48, 172–178. [Google Scholar]
- Bimpilas, A.; Panagopoulou, M.; Tsimogiannis, D.; Oreopoulou, V. Anthocyanin copigmentation and color of wine: The effect of naturally obtained hydroxycinnamic acids as cofactors. Food Chem. 2016, 197, 39–46. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef] [Green Version]
- Lin, B.W.; Gong, C.C.; Song, H.F.; Cui, Y.Y. Effects of anthocyanins on the prevention and treatment of cancer. Br. J. Pharmacol. 2017, 174, 1226–1243. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Feng, P.; He, W.; Du, X.; Chen, C.; Suo, L.; Liang, M.; Zhang, N.; Na, A.; Zhang, Y. The prevention and inhibition effect of anthocyanins on colorectal cancer. Curr. Pharm. Des. 2019, 25, 4919–4927. [Google Scholar] [CrossRef] [PubMed]
- Rager, E.L.; Bridgeford, E.P.; Ollila, D.W. Cutaneous melanoma: Update on prevention, screening, diagnosis, and treatment. Am. Fam. Physician 2005, 72, 269–276. [Google Scholar] [PubMed]
- Hurst, E.A.; Harbour, J.W.; Cornelius, L.A. Ocular melanoma: A review and the relationship to cutaneous melanoma. Arch. Dermatol. 2003, 139, 1067–1073. [Google Scholar] [CrossRef] [PubMed]
- Wiecker, T.S.; Luther, H.; Buettner, P.; Bauer, J.; Garbe, C. Moderate sun exposure and nevus counts in parents are associated with development of melanocytic nevi in childhood: A risk factor study in 1,812 kindergarten children. Cancer 2003, 97, 628–638. [Google Scholar] [CrossRef]
- Bunea, A.; Rugina, D.; Sconta, Z.; Pop, R.M.; Pintea, A.; Socaciu, C.; Tabaran, F.; Grootaert, C.; Struijs, K.; VanCamp, J. Anthocyanin determination in blueberry extracts from various cultivars and their antiproliferative and apoptotic properties in b16-f10 metastatic murine melanoma cells. Phytochemistry 2013, 95, 436–444. [Google Scholar] [CrossRef]
- Wang, E.; Liu, Y.; Xu, C.; Liu, J. Antiproliferative and proapoptotic activities of anthocyanin and anthocyanidin extracts from blueberry fruits on b16-f10 melanoma cells. Food Nutr. Res. 2017, 61, 1325308. [Google Scholar] [CrossRef] [Green Version]
- Diaconeasa, Z.; Ayvaz, H.; Rugina, D.; Leopold, L.; Stanila, A.; Socaciu, C.; Tabaran, F.; Luput, L.; Mada, D.C.; Pintea, A.; et al. Melanoma inhibition by anthocyanins is associated with the reduction of oxidative stress biomarkers and changes in mitochondrial membrane potential. Plant Foods Hum. Nutr. 2017, 72, 404–410. [Google Scholar] [CrossRef]
- Benedec, D.; Oniga, I.; Cuibus, F.; Sevastre, B.; Stiufiuc, G.; Duma, M.; Hanganu, D.; Iacovita, C.; Stiufiuc, R.; Lucaciu, C.M. Origanum vulgare mediated green synthesis of biocompatible gold nanoparticles simultaneously possessing plasmonic, antioxidant and antimicrobial properties. Int. J. Nanomed. 2018, 13, 1041–1058. [Google Scholar] [CrossRef] [Green Version]
- Rugină, D.; Hanganu, D.; Diaconeasa, Z.; Tăbăran, F.; Coman, C.; Leopold, L.; Bunea, A.; Pintea, A. Antiproliferative and apoptotic potential of cyanidin-based anthocyanins on melanoma cells. Int. J. Mol. Sci. 2017, 18, 0949. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.N.; Panchanathan, R.; Lee, W.S.; Kim, H.J.; Kim, D.H.; Choi, Y.H.; Kim, G.S.; Shin, S.C.; Hong, S.C. Anthocyanins from the fruit of vitis coignetiae pulliat inhibit tnf-augmented cancer proliferation, migration, and invasion in a549 cells. Asian Pac. J. Cancer Prev. Apjcp 2017, 18, 2919–2923. [Google Scholar] [PubMed]
- Hanamura, T.; Uchida, E.; Aoki, H. Skin-lightening effect of a polyphenol extract from acerola (Malpighia emarginata DC.) fruit on uv-induced pigmentation. Biosci. Biotechnol. Biochem. 2008, 72, 3211–3218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazza, G.; Francis, F.J. Anthocyanins in grapes and grape products. Crit. Rev. Food Sci. Nutr. 1995, 35, 341–371. [Google Scholar] [CrossRef]
- Felgines, C.; Talavera, S.; Texier, O.; Gil-Izquierdo, A.; Lamaison, J.L.; Remesy, C. Blackberry anthocyanins are mainly recovered from urine as methylated and glucuronidated conjugates in humans. J. Agric. Food Chem. 2005, 53, 7721–7727. [Google Scholar] [CrossRef] [PubMed]
- Andersen, O.M.; Markham, K.R. The Anthocyanins. In Flavonoids Chemistry, Biochemistry and Applications; Andersen, O.M., Markham, K.R., Eds.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2006; pp. 471–551. [Google Scholar]
- Hsu, Y.H.; Tagami, T.; Matsunaga, K.; Okuyama, M.; Suzuki, T.; Noda, N.; Suzuki, M.; Shimura, H. Functional characterization of udp-rhamnose-dependent rhamnosyltransferase involved in anthocyanin modification, a key enzyme determining blue coloration in lobelia erinus. Plant J. 2017, 89, 325–337. [Google Scholar] [CrossRef] [Green Version]
- Vicente Dragano, N.R.; Castro Marques, A.Y. Chapter 11—Native Fruits, Anthocyanins in Nutraceuticals, and the Insulin Receptor/Insulin Receptor Substrate-1/Akt/Forkhead Box Protein Pathway. In Molecular Nutrition and Diabetes; Mauricio, D., Ed.; Academic Press: San Diego, CA, USA, 2016; pp. 131–145. [Google Scholar]
- Wang, L.-S.S.; Gary, D. Anthocyanins and their role in cancer prevention. Cancer Lett. 2008, 269, 281–290. [Google Scholar] [CrossRef] [Green Version]
- Trouillas, P.; Sancho-García, J.C.; De Freitas, V.; Gierschner, J.; Otyepka, M.; Dangles, O. Stabilizing and modulating color by copigmentation: Insights from theory and experiment. Chem. Rev. 2016, 116, 4937–4982. [Google Scholar] [CrossRef] [Green Version]
- Boulton, R. The copigmentation of anthocyanins and its role in the color of red wine: A critical review. Am. J. Enol. Vitic. 2001, 52, 67–87. [Google Scholar]
- Huang, H.-P.; Shih, Y.-W.; Chang, Y.-C.; Hung, C.-N.; Wang, C.-J. Chemoinhibitory effect of mulberry anthocyanins on melanoma metastasis involved in the ras/pi3k pathway. J. Agric. Food Chem. 2008, 56, 9286–9293. [Google Scholar] [CrossRef]
- Tsuda, T. Dietary anthocyanin-rich plants: Biochemical basis and recent progress in health benefits studies. Mol. Nutr. Food Res. 2012, 56, 159–170. [Google Scholar] [CrossRef]
- Yeh, C.T.; Yen, G.C. Induction of apoptosis by the anthocyanidins through regulation of bcl-2 gene and activation of c-jun n-terminal kinase cascade in hepatoma cells. J. Agric. Food Chem. 2005, 53, 1740–1749. [Google Scholar] [CrossRef] [PubMed]
- Katsube, N.; Iwashita, K.; Tsushida, T.; Yamaki, K.; Kobori, M. Induction of apoptosis in cancer cells by bilberry (vaccinium myrtillus) and the anthocyanins. J. Agric. Food Chem 2003, 51, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Borges, G.; Roowi, S.; Rouanet, J.M.; Duthie, G.G.; Lean, M.E.; Crozier, A. The bioavailability of raspberry anthocyanins and ellagitannins in rats. Mol. Nutr. Food Res. 2007, 51, 714–725. [Google Scholar] [CrossRef] [PubMed]
- Felgines, C.; Texier, O.; Besson, C.; Fraisse, D.; Lamaison, J.L.; Rémésy, C. Blackberry anthocyanins are slightly bioavailable in rats. J. Nutr. 2002, 132, 1249–1253. [Google Scholar] [CrossRef] [Green Version]
- Felgines, C.; Talavéra, S.; Gonthier, M.P.; Texier, O.; Scalbert, A.; Lamaison, J.L.; Rémésy, C. Strawberry anthocyanins are recovered in urine as glucuro- and sulfoconjugates in humans. J. Nutr. 2003, 133, 1296–1301. [Google Scholar] [CrossRef] [Green Version]
- Ichiyanagi, T.; Shida, Y.; Rahman, M.M.; Hatano, Y.; Konishi, T. Bioavailability and tissue distribution of anthocyanins in bilberry (vaccinium myrtillus l.) extract in rats. J. Agric. Food Chem. 2006, 54, 6578–6587. [Google Scholar] [CrossRef]
- Marczylo, T.H.; Cooke, D.; Brown, K.; Steward, W.P.; Gescher, A.J. Pharmacokinetics and metabolism of the putative cancer chemopreventive agent cyanidin-3-glucoside in mice. Cancer Chemother. Pharmacol. 2009, 64, 1261–1268. [Google Scholar] [CrossRef]
- Matsumoto, H.; Ichiyanagi, T.; Iida, H.; Ito, K.; Tsuda, T.; Hirayama, M.; Konishi, T. Ingested delphinidin-3-rutinoside is primarily excreted to urine as the intact form and to bile as the methylated form in rats. J. Agric. Food Chem. 2006, 54, 578–582. [Google Scholar] [CrossRef]
- Fang, J. Bioavailability of anthocyanins. Drug Metab. Rev. 2014, 46, 508–520. [Google Scholar] [CrossRef]
- Czank, C.; Cassidy, A.; Zhang, Q.; Morrison, D.J.; Preston, T.; Kroon, P.A.; Botting, N.P.; Kay, C.D. Human metabolism and elimination of the anthocyanin, cyanidin-3-glucoside: A (13)c-tracer study. Am. J. Clin. Nutr. 2013, 97, 995–1003. [Google Scholar] [CrossRef] [Green Version]
- Passamonti, S.; Vrhovsek, U.; Vanzo, A.; Mattivi, F. The stomach as a site for anthocyanins absorption from food. Febs Lett. 2003, 544, 210–213. [Google Scholar] [CrossRef] [Green Version]
- Passamonti, S.; Vrhovsek, U.; Vanzo, A.; Mattivi, F. Fast access of some grape pigments to the brain. J. Agric. Food Chem. 2005, 53, 7029–7034. [Google Scholar] [CrossRef] [PubMed]
- Vanzo, A.; Terdoslavich, M.; Brandoni, A.; Torres, A.M.; Vrhovsek, U.; Passamonti, S. Uptake of grape anthocyanins into the rat kidney and the involvement of bilitranslocase. Mol. Nutr. Food Res. 2008, 52, 1106–1116. [Google Scholar] [CrossRef]
- Tian, Q.; Giusti, M.M.; Stoner, G.D.; Schwartz, S.J. Urinary excretion of black raspberry (rubus occidentalis) anthocyanins and their metabolites. J. Agric. Food Chem. 2006, 54, 1467–1472. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Pittman, H.E., III; McKay, S.; Prior, R.L. Aglycones and sugar moieties alter anthocyanin absorption and metabolism after berry consumption in weanling pigs. J. Nutr. 2005, 135, 2417–2424. [Google Scholar] [CrossRef] [PubMed]
- de Ferrars, R.M.; Czank, C.; Zhang, Q.; Botting, N.P.; Kroon, P.A.; Cassidy, A.; Kay, C.D. The pharmacokinetics of anthocyanins and their metabolites in humans. Br. J. Pharmacol. 2014, 171, 3268–3282. [Google Scholar] [CrossRef] [Green Version]
- Keppler, K.; Humpf, H.U. Metabolism of anthocyanins and their phenolic degradation products by the intestinal microflora. Bioorganic Med. Chem. 2005, 13, 5195–5205. [Google Scholar] [CrossRef]
- Talavéra, S.; Felgines, C.; Texier, O.; Besson, C.; Gil-Izquierdo, A.; Lamaison, J.L.; Rémésy, C. Anthocyanin metabolism in rats and their distribution to digestive area, kidney, and brain. J. Aagric. Food Chem. 2005, 53, 3902–3908. [Google Scholar] [CrossRef]
- Forni, C.; Braglia, R.; Mulinacci, N.; Urbani, A.; Ronci, M.; Gismondi, A.; Tabolacci, C.; Provenzano, B.; Lentini, A.; Beninati, S. Antineoplastic activity of strawberry (fragaria × ananassa duch.) crude extracts on b16-f10 melanoma cells. Mol. Biosyst. 2014, 10, 1255–1263. [Google Scholar] [CrossRef]
- Konczak, I.; Zhang, W. Anthocyanins—More than nature’s colours. J. Biomed. Biotechnol. 2004, 2004, 239–240. [Google Scholar] [CrossRef] [Green Version]
- Hogan, S.; Chung, H.; Zhang, L.; Li, J.; Lee, Y.; Dai, Y.; Zhou, K. Antiproliferative and antioxidant properties of anthocyanin-rich extract from açai. Food Chem. 2010, 118, 208–214. [Google Scholar] [CrossRef]
- Jaakola, L. New insights into the regulation of anthocyanin biosynthesis in fruits. Trends Plant Sci. 2013, 18, 477–483. [Google Scholar] [CrossRef] [Green Version]
- Fang, J. Classification of fruits based on anthocyanin types and relevance to their health effects. Nutrition 2015, 31, 1301–1306. [Google Scholar] [CrossRef] [PubMed]
- Escribano-Bailón, M.T.; Santos-Buelga, C.; Rivas-Gonzalo, J.C. Anthocyanins in cereals. J. Chromatogr. A 2004, 1054, 129–141. [Google Scholar] [CrossRef]
- Kruger, M.J.; Davies, N.; Myburgh, K.H.; Lecour, S. Proanthocyanidins, anthocyanins and cardiovascular diseases. Food Res. Int. 2014, 59, 41–52. [Google Scholar] [CrossRef]
- Hertog, M.G.; Feskens, E.J.; Hollman, P.C.; Katan, M.B.; Kromhout, D. Dietary antioxidant flavonoids and risk of coronary heart disease: The zutphen elderly study. Lancet 1993, 342, 1007–1011. [Google Scholar] [CrossRef]
- Skibola, C.F.; Smith, M.T. Potential health impacts of excessive flavonoid intake. Free Radic Biol Med. 2000, 29, 375–383. [Google Scholar] [CrossRef]
- Markakis, P. Anthocyanins as Food Colors; Academic Press: Cambridge, MA, USA, 1982. [Google Scholar]
- Burton-Freeman, B.; Sandhu, A.; Edirisinghe, I. Chapter 35—Anthocyanins. In Nutraceuticals; Gupta, R.C., Ed.; Academic Press: Boston, MA, USA, 2016; pp. 489–500. [Google Scholar]
- Garzón, G.A.; Manns, D.C.; Riedl, K.; Schwartz, S.J.; Padilla-Zakour, O. Identification of phenolic compounds in petals of nasturtium flowers (tropaeolum majus) by high-performance liquid chromatography coupled to mass spectrometry and determination of oxygen radical absorbance capacity (orac). J. Agric. Food Chem. 2015, 63, 1803–1811. [Google Scholar] [CrossRef]
- Park, K.-I.; Hoshino, A.; Saito, N.; Tatsuzawa, F. Anthocyanins in the flowers of ipomoea tricolor cav. (convolvulaceae). Biochem. Syst. Ecol. 2014, 54, 15–18. [Google Scholar] [CrossRef]
- Phippen, W.B.; Simon, J.E. Anthocyanins in basil (ocimum basilicum l.). J. Agric. Food Chem. 1998, 46, 1734–1738. [Google Scholar] [CrossRef]
- Szymanowska, U.; Zlotek, U.; Karas, M.; Baraniak, B. Anti-inflammatory and antioxidative activity of anthocyanins from purple basil leaves induced by selected abiotic elicitors. Food Chem. 2015, 172, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Goupy, P.; Vian, M.A.; Chemat, F.; Caris-Veyrat, C. Identification and quantification of flavonols, anthocyanins and lutein diesters in tepals of crocus sativus by ultra performance liquid chromatography coupled to diode array and ion trap mass spectrometry detections. Ind. Crop. Prod. 2013, 44, 496–510. [Google Scholar] [CrossRef]
- Nørbæk, R.; Kondo, T. Further anthocyanins from flowers of crocus antalyensis (iridaceae). Phytochemistry 1999, 50, 325–328. [Google Scholar] [CrossRef]
- Kazuma, K.; Kogawa, K.; Noda, N.; Kato, N.; Suzuki, M. Identification of delphinidin 3-o-(6’’-o-malonyl)-beta-glucoside-3’-o-beta-glucoside, a postulated intermediate in the biosynthesis of ternatin c5 in the blue petals of clitoria ternatea (butterfly pea). Chem. Biodivers. 2004, 1, 1762–1770. [Google Scholar] [CrossRef] [PubMed]
- Nair, V.; Bang, W.Y.; Schreckinger, E.; Andarwulan, N.; Cisneros-Zevallos, L. Protective role of ternatin anthocyanins and quercetin glycosides from butterfly pea (clitoria ternatea leguminosae) blue flower petals against lipopolysaccharide (lps)-induced inflammation in macrophage cells. J. Agric. Food Chem. 2015, 63, 6355–6365. [Google Scholar] [CrossRef]
- Grajeda-Iglesias, C.; Salas, E.; Barouh, N.; Barea, B.; Figueroa-Espinoza, M.C. Lipophilization and ms characterization of the main anthocyanins purified from hibiscus flowers. Food Chem. 2017, 230, 189–194. [Google Scholar] [CrossRef]
- Hashimoto, M.; Suzuki, T.; Iwashina, T. New acylated anthocyanins and other flavonoids from the red flowers clematis cultivars. Sage J. 2011, 6, 1631–1636. [Google Scholar] [CrossRef] [Green Version]
- Jia, N.; Shu, Q.-Y.; Wang, L.-S.; Du, H.; Xu, Y.-J.; Liu, Z.-A. Analysis of petal anthocyanins to investigate coloration mechanism in herbaceous peony cultivars. Sci. Hortic. 2008, 117, 167–173. [Google Scholar] [CrossRef]
- Byamukama, R.; Namukobe, J.; Jordheim, M.; Andersen, Ø.M.; Kiremire, B.T. Anthocyanins from ornamental flowers of red frangipani, plumeria rubra. Sci. Hortic. 2011, 129, 840–843. [Google Scholar] [CrossRef]
- Terahara, N.; Oda, M.; Matsui, T.; Osajima, Y.; Saito, N.; Toki, K.; Honda, T. Five new anthocyanins, ternatins a3, b4, b3, b2, and d2, from clitoria ternatea flowers. J. Nat. Prod. 1996, 59, 139–144. [Google Scholar] [CrossRef]
- Li, J.-B.; Hashimoto, F.; Shimizu, K.; Sakata, Y. Anthocyanins from red flowers of camellia cultivar ‘dalicha’. Phytochemistry 2008, 69, 3166–3171. [Google Scholar] [CrossRef] [PubMed]
- Li, J.B.; Hashimoto, F.; Shimizu, K.; Sakata, Y. A new acylated anthocyanin from the red flowers of camellia hongkongensis and characterization of anthocyanins in the section camellia species. J. Integr. Plant Biol. 2009, 51, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Nørbæk, R.; Brandt, K.; Nielsen, J.K.; Ørgaard, M.; Jacobsen, N. Flower pigment composition of crocus species and cultivars used for a chemotaxonomic investigation. Biochem. Syst. Ecol. 2002, 30, 763–791. [Google Scholar] [CrossRef]
- Park, C.H.; Chae, S.C.; Park, S.Y.; Kim, J.K.; Kim, Y.J.; Chung, S.O.; Arasu, M.V.; Al-Dhabi, N.A.; Park, S.U. Anthocyanin and carotenoid contents in different cultivars of chrysanthemum (dendranthema grandiflorum ramat.) flower. Molecules 2015, 20, 11090–11102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.-M.; Li, C.-H.; Zhu, X.-R.; Deng, Y.-M.; Sun, W.; Wang, L.-S.; Chen, F.-D.; Zhang, Z. The identification of flavonoids and the expression of genes of anthocyanin biosynthesis in the chrysanthemum flowers. Biol. Plant. 2012, 56, 458–464. [Google Scholar] [CrossRef]
- Sakaguchi, K.; Kitajima, J.; Iwashina, T. Acylated delphinidin glycosides from violet and violet-blue flowers of clematis cultivars and their coloration. Nat. Prod. Commun 2013, 8, 1563–1566. [Google Scholar] [CrossRef] [Green Version]
- Nakayama, M.; Koshioka, M.; Yoshida, H.; Kan, Y.; Fukui, Y.; Koike, A.; Yamaguchi, M.-a. Cyclic malyl anthocyanins in dianthus caryophyllus. Phytochemistry 2000, 55, 937–939. [Google Scholar] [CrossRef]
- Okamura, M.; Nakayama, M.; Umemoto, N.; Cano, E.A.; Hase, Y.; Nishizaki, Y.; Sasaki, N.; Ozeki, Y. Crossbreeding of a metallic color carnation and diversification of the peculiar coloration by ion-beam irradiation. Euphytica 2013, 191, 45–56. [Google Scholar] [CrossRef] [Green Version]
- Fukui, Y.; Tanaka, Y.; Kusumi, T.; Iwashita, T.; Nomoto, K. A rationale for the shift in colour towards blue in transgenic carnation flowers expressing the flavonoid 3′,5′-hydroxylase gene. Phytochemistry 2003, 63, 15–23. [Google Scholar] [CrossRef]
- Bloor, S.J. A macrocyclic anthocyanin from red\mauve carnation flowers. Phytochemistry 1998, 49, 225–228. [Google Scholar] [CrossRef]
- Koike, A.; Barreira, J.C.; Barros, L.; Santos-Buelga, C.; Villavicencio, A.L.; Ferreira, I.C. Edible flowers of viola tricolor l. As a new functional food: Antioxidant activity, individual phenolics and effects of gamma and electron-beam irradiation. Food Chem 2015, 179, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Liang, L.; Meng, X.; Li, Y.; Gao, F.; Liu, X.; Wang, S.; Gao, X.; Wang, L. Biochemical and molecular characterization of a flavonoid 3-o-glycosyltransferase responsible for anthocyanins and flavonols biosynthesis in freesia hybrida. Front. Plant Sci. 2016, 7, 410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grajeda-Iglesias, C.; Figueroa-Espinoza, M.C.; Barouh, N.; Baréa, B.; Fernandes, A.; de Freitas, V.; Salas, E. Isolation and characterization of anthocyanins from hibiscus sabdariffa flowers. J. Nat. Prod. 2016, 79, 1709–1718. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Luo, G.; Yu, F.; Jia, Q.; Zheng, Y.; Bi, X.; Lei, J. Characterization of anthocyanins in the hybrid progenies derived from iris dichotoma and domestica by hplc-dad-esi/ms analysis. Phytochemistry 2018, 150, 60–74. [Google Scholar] [CrossRef]
- Yabuya, T.; Yamaguchi, M.-a.; Imayama, T.; Katoh, K.; Ino, I. Anthocyanin 5-o-glucosyltransferase in flowers of iris ensata. Plant Sci. 2002, 162, 779–784. [Google Scholar] [CrossRef]
- Imayama, T.; Yoshihara, N.; Fukuchi-Mizutani, M.; Tanaka, Y.; Ino, I.; Yabuya, T. Isolation and characterization of a cdna clone of udp-glucose: Anthocyanin 5-o-glucosyltransferase in iris hollandica. Plant Sci. 2004, 167, 1243–1248. [Google Scholar] [CrossRef]
- Saito, N.; Toki, K.; Kuwano, H.; Moriyama, H.; Shigihara, A.; Honda, T. Acylated cyanidin 3-rutinoside-5,3′-diglucoside from the purple-red flower of lobelia erinus. Phytochemistry 1995, 39, 423–426. [Google Scholar] [CrossRef]
- Markham, K.R.; Mitchell, K.A.; Boase, M.R. Malvidin-3-o-glucoside-5-o-(6-acetylglucoside) and its colour manifestation in ‘johnson’s blue’ and other ‘blue’ geraniums. Phytochemistry 1997, 45, 417–423. [Google Scholar] [CrossRef]
- Jia, N.; Shu, Q.; Wang, D.; Wang, L.; Liu, Z.; Ren, H.; Xu, Y.; Tian, D.; Tilt, K.M. Identification and characterization of anthocyanins by high-performance liquid chromatography–electrospray ionization–mass spectrometry in herbaceous peony species. J. Amer. Soc. Hort. Sci. 2008, 133, 418–426. [Google Scholar] [CrossRef]
- Du, H.; Wu, J.; Ji, K.-X.; Zeng, Q.-Y.; Bhuiya, M.-W.; Su, S.; Shu, Q.-Y.; Ren, H.-X.; Liu, Z.-A.; Wang, L.-S. Methylation mediated by an anthocyanin, o-methyltransferase, is involved in purple flower coloration in paeonia. J. Exp. Bot. 2015, 66, 6563–6577. [Google Scholar] [CrossRef] [Green Version]
- Prinsi, B.; Negri, A.S.; Quattrocchio, F.M.; Koes, R.E.; Espen, L. Proteomics of red and white corolla limbs in petunia reveals a novel function of the anthocyanin regulator anthocyanin1 in determining flower longevity. J. Proteom. 2016, 131, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Ando, T.; Tatsuzawa, F.; Saito, N.; Takahashi, M.; Tsunashima, Y.; Numajir, H.; Watanabe, H.; Kokubun, H.; Hara, R.; Seki, H.; et al. Differences in the floral anthocyanin content of red petunias and petunia exserta. Phytochemistry 2000, 54, 495–501. [Google Scholar] [CrossRef]
- Griesbach, R.J.; Stehmann, J.R.; Meyer, F. Anthocyanins in the “red” flowers of petunia exserta. Phytochemistry 1999, 51, 525–528. [Google Scholar] [CrossRef]
- Saito, R.; Fukuta, N.; Ohmiya, A.; Itoh, Y.; Ozeki, Y.; Kuchitsu, K.; Nakayama, M. Regulation of anthocyanin biosynthesis involved in the formation of marginal picotee petals in petunia. Plant Sci. 2006, 170, 828–834. [Google Scholar] [CrossRef]
- Zhang, L.; Fu, Q.; Zhang, Y. Composition of anthocyanins in pomegranate flowers and their antioxidant activity. Food Chem. 2011, 127, 1444–1449. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, H.-J.; Choung, M.-G. Anthocyanin compositions and biological activities from the red petals of korean edible rose (rosa hybrida cv. Noblered). Food Chem. 2011, 129, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-L.; Luo, C.-L.; Zhou, Q.; Zhang, Z.-C. Isolation and identification of two major acylated anthocyanins from purple sweet potato (ipomoea batatas l. Cultivar eshu no. 8) by uplc-qtof-ms/ms and nmr. Food Sci. Technol. 2018, 53, 1932–1941. [Google Scholar] [CrossRef]
- Navarro-González, I.; González-Barrio, R.; García-Valverde, V.; Bautista-Ortín, A.B.; Periago, M.J. Nutritional composition and antioxidant capacity in edible flowers: Characterisation of phenolic compounds by hplc-dad-esi/ms(n). Int. J. Mol. Sci. 2015, 16, 805–822. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Y.; Ma, X.; Tang, D.; Shi, Y. Comparison of anthocyanin components, expression of anthocyanin biosynthetic structural genes, and tff3′h1 sequences between tulipa fosteriana ‘Albert heijn’ and its reddish sport. Sci. Hortic. 2014, 175, 16–26. [Google Scholar] [CrossRef]
- Tatsuzawa, F. Differences in the floral anthocyanin content of violet–blue flowers of vinca minor l. And v. Major l. (apocynaceae). Phytochem. Lett. 2015, 13, 365–369. [Google Scholar] [CrossRef]
- González-Barrio, R.; Periago, M.J.; Luna-Recio, C.; Garcia-Alonso, F.J.; Navarro-González, I. Chemical composition of the edible flowers, pansy (viola wittrockiana) and snapdragon (antirrhinum majus) as new sources of bioactive compounds. Food Chem. 2018, 252, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Ge, Q.; Ma, X. Composition and antioxidant activity of anthocyanins isolated from yunnan edible rose (an ning). Food Sci. Hum. Wellness 2013, 2, 68–74. [Google Scholar] [CrossRef] [Green Version]
- Fossen, T.; Rayyan, S.; Holmberg, M.H.; Nateland, H.S.; Andersen, O.M. Acylated anthocyanins from leaves of oxalis triangularis. Phytochemistry 2005, 66, 1133–1140. [Google Scholar] [CrossRef] [PubMed]
- Alexandra Pazmiño-Durán, E.; Mónica Giusti, M.; Wrolstad, R.E.; Glória, M.B.A. Anthocyanins from oxalis triangularis as potential food colorants. Food Chem. 2001, 75, 211–216. [Google Scholar] [CrossRef]
- Fossen, T.; Rayyan, S.; Holmberg, M.H.; Nimtz, M.; Andersen, O.M. Covalent anthocyanin-flavone dimer from leaves of oxalis triangularis. Phytochemistry 2007, 68, 652–662. [Google Scholar] [CrossRef]
- Harborne, A.J. Phytochemical Methods a Guide to Modern Techniques Plant Analysis; Springer Science & Business Media: Berlin, Germany, 1998. [Google Scholar]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Remesy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr 2005, 81, 230s–242s. [Google Scholar] [CrossRef] [Green Version]
- Tsao, R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef]
- Ariza, M.T.; Reboredo-Rodríguez, P.; Cervantes, L.; Soria, C.; Martínez-Ferri, E.; González-Barreiro, C.; Cancho-Grande, B.; Battino, M.; Simal-Gándara, J. Bioaccessibility and potential bioavailability of phenolic compounds from achenes as a new target for strawberry breeding programs. Food Chem. 2018, 248, 155–165. [Google Scholar] [CrossRef]
- Van de Velde, F.; Grace, M.H.; Esposito, D.; Pirovani, M.É.; Lila, M.A. Quantitative comparison of phytochemical profile, antioxidant, and anti-inflammatory properties of blackberry fruits adapted to argentina. J. Food Compos. Anal. 2016, 47, 82–91. [Google Scholar] [CrossRef]
- Hwang, S.J.; Yoon, W.B.; Lee, O.-H.; Cha, S.J.; Kim, J.D. Radical-scavenging-linked antioxidant activities of extracts from black chokeberry and blueberry cultivated in korea. Food Chem. 2014, 146, 71–77. [Google Scholar] [CrossRef]
- Thi, N.; Hwang, E.-S. Effects of black chokeberry extracts on metastasis and cell-cycle arrest in sk-hep1 human liver cancer cell line. Asian Pac. J. Trop. Biomed. 2018, 8, 285. [Google Scholar] [CrossRef]
- Veberic, R.; Slatnar, A.; Bizjak, J.; Stampar, F.; Mikulic-Petkovsek, M. Anthocyanin composition of different wild and cultivated berry species. Lwt Food Sci. Technol. 2015, 60, 509–517. [Google Scholar] [CrossRef]
- Marhuenda, J.; Alemán, M.D.; Gironés-Vilaplana, A.; Pérez, A.; Caravaca, G.; Figueroa, F.; Mulero, J.; Zafrilla, P. Phenolic composition, antioxidant activity, and in vitro availability of four different berries. J. Chem. 2016, 2016, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Sang, J.; Ma, Q.; Li, C.-q. Development and validation of green chromatography for the determination of anthocyanins in haskap berry, mulberry and blackberry. Anal. Methods 2017, 9, 2535–2545. [Google Scholar] [CrossRef]
- Tomas, M.; Toydemir, G.; Boyacioglu, D.; Hall, R.; Beekwilder, J.; Capanoglu, E. The effects of juice processing on black mulberry antioxidants. Food Chem. 2015, 186, 277–284. [Google Scholar] [CrossRef]
- Antolak, H.; Czyzowska, A.; Sakač, M.; Mišan, A.; Đuragić, O.; Kregiel, D. Phenolic compounds contained in little-known wild fruits as antiadhesive agents against the beverage-spoiling bacteria asaia spp. Molecules 2017, 22, 1256. [Google Scholar] [CrossRef]
- Mattila, P.H.; Hellström, J.; Karhu, S.; Pihlava, J.-M.; Veteläinen, M. High variability in flavonoid contents and composition between different north-european currant (ribes spp.) varieties. Food Chem. 2016, 204, 14–20. [Google Scholar] [CrossRef]
- Feng, R.; Ni, H.M.; Wang, S.Y.; Tourkova, I.L.; Shurin, M.R.; Harada, H.; Yin, X.M. Cyanidin-3-rutinoside, a natural polyphenol antioxidant, selectively kills leukemic cells by induction of oxidative stress. J. Biol. Chem. 2007, 282, 13468–13476. [Google Scholar] [CrossRef] [Green Version]
- Diaconeasa, Z.; Leopold, L.; Rugina, D.; Ayvaz, H.; Socaciu, C. Antiproliferative and antioxidant properties of anthocyanin rich extracts from blueberry and blackcurrant juice. Int. J. Mol. Sci. 2015, 16, 2352–2365. [Google Scholar] [CrossRef] [Green Version]
- Taheri, R.; Connolly, B.A.; Brand, M.H.; Bolling, B.W. Underutilized chokeberry (aronia melanocarpa, aronia arbutifolia, aronia prunifolia) accessions are rich sources of anthocyanins, flavonoids, hydroxycinnamic acids, and proanthocyanidins. J. Agric. Food Chem. 2013, 61, 8581–8588. [Google Scholar] [CrossRef]
- Wojdyło, A.; Oszmiański, J.; Teleszko, M.; Sokół-Łętowska, A. Composition and quantification of major polyphenolic compounds, antioxidant activity and colour properties of quince and mixed quince jams. Int. J. Food Sci. Nutr. 2013, 64, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Šavikin, K.; Zdunić, G.; Janković, T.; Gođevac, D.; Stanojković, T.; Pljevljakušić, D. Berry fruit teas: Phenolic composition and cytotoxic activity. Food Res. Int. 2014, 62, 677–683. [Google Scholar] [CrossRef]
- Gardana, C.; Ciappellano, S.; Marinoni, L.; Fachechi, C.; Simonetti, P. Bilberry adulteration: Identification and chemical profiling of anthocyanins by different analytical methods. J. Agric. Food Chem. 2014, 62, 10998–11004. [Google Scholar] [CrossRef] [PubMed]
- Worsztynowicz, P.; Napierała, M.; Białas, W.; Grajek, W.; Olkowicz, M. Pancreatic α-amylase and lipase inhibitory activity of polyphenolic compounds present in the extract of black chokeberry (aronia melanocarpa l.). Process. Biochem. 2014, 49, 1457–1463. [Google Scholar] [CrossRef]
- Rugină, D.; Diaconeasa, Z.; Coman, C.; Bunea, A.; Socaciu, C.; Pintea, A. Chokeberry anthocyanin extract as pancreatic β-cell protectors in two models of induced oxidative stress. Oxidative Med. Cell. Longev. 2015, 2015, 10. [Google Scholar] [CrossRef] [Green Version]
- Vlachojannis, C.; Zimmermann, B.F.; Chrubasik-Hausmann, S. Quantification of anthocyanins in elderberry and chokeberry dietary supplements. Phytother. Res. Ptr 2015, 29, 561–565. [Google Scholar] [CrossRef]
- Oszmiański, J.; Lachowicz, S. Effect of the production of dried fruits and juice from chokeberry (aronia melanocarpa l.) on the content and antioxidative activity of bioactive compounds. Molecules 2016, 21, 1098. [Google Scholar] [CrossRef]
- Tomić, M.; Ignjatović, Đ.; Tovilović-Kovačević, G.; Krstić-Milošević, D.; Ranković, S.; Popović, T.; Glibetić, M. Reduction of anxiety-like and depression-like behaviors in rats after one month of drinking aronia melanocarpa berry juice. Food Funct. 2016, 7, 3111–3120. [Google Scholar] [CrossRef]
- Ćujić, N.; Šavikin, K.; Janković, T.; Pljevljakušić, D.; Zdunić, G.; Ibrić, S. Optimization of polyphenols extraction from dried chokeberry using maceration as traditional technique. Food Chem. 2016, 194, 135–142. [Google Scholar] [CrossRef]
- Ćujić, N.; Savikin, K.; Miloradovic, Z.; Ivanov, M.; Vajic, U.-J.; Karanovic, D.; Grujic-Milanovic, J.; Jovovic, D.; Mihailovic-Stanojevic, N. Characterization of dried chokeberry fruit extract and its chronic effects on blood pressure and oxidative stress in spontaneously hypertensive rats. J. Funct. Foods 2018, 44, 330–339. [Google Scholar] [CrossRef]
- Lee, J. Proanthocyanidin a2 purification and quantification of american cranberry (vaccinium macrocarpon ait.) products. J. Funct. Foods 2013, 5, 144–153. [Google Scholar] [CrossRef]
- Hummer, K.; Durst, R.; Zee, F.; Atnip, A.; Giusti, M.M. Phytochemicals in fruits of hawaiian wild cranberry relatives. J. Sci. Food Agric. 2014, 94, 1530–1536. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Zhao, S.; Yagiz, Y.; Gu, L. Static, kinetic, and isotherm adsorption performances of macroporous adsorbent resins for recovery and enrichment of bioactive procyanidins from cranberry pomace. J. Food Sci. 2018, 83, 1249–1257. [Google Scholar] [CrossRef] [PubMed]
- Díaz-García, M.C.; Obón, J.M.; Castellar, M.R.; Collado, J.; Alacid, M. Quantification by uhplc of total individual polyphenols in fruit juices. Food Chem. 2013, 138, 938–949. [Google Scholar] [CrossRef]
- Aaby, K.; Grimmer, S.; Holtung, L. Extraction of phenolic compounds from bilberry (vaccinium myrtillus l.) press residue: Effects on phenolic composition and cell proliferation. Lwt Food Sci. Technol. 2013, 54, 257–264. [Google Scholar] [CrossRef]
- Primetta, A.K.; Jaakola, L.; Ayaz, F.A.; Inceer, H.; Riihinen, K.R. Anthocyanin fingerprinting for authenticity studies of bilberry (vaccinium myrtillus l.). Food Control. 2013, 30, 662–667. [Google Scholar] [CrossRef]
- Buran, T.J.; Sandhu, A.K.; Li, Z.; Rock, C.R.; Yang, W.W.; Gu, L. Adsorption/desorption characteristics and separation of anthocyanins and polyphenols from blueberries using macroporous adsorbent resins. J. Food Eng. 2014, 128, 167–173. [Google Scholar] [CrossRef]
- Correa-Betanzo, J.; Allen-Vercoe, E.; McDonald, J.; Schroeter, K.; Corredig, M.; Paliyath, G. Stability and biological activity of wild blueberry (vaccinium angustifolium) polyphenols during simulated in vitro gastrointestinal digestion. Food Chem. 2014, 165, 522–531. [Google Scholar] [CrossRef]
- Paes, J.; Dotta, R.; Barbero, G.F.; Martínez, J. Extraction of phenolic compounds and anthocyanins from blueberry (vaccinium myrtillus l.) residues using supercritical co2 and pressurized liquids. J. Supercrit. Fluids 2014, 95, 8–16. [Google Scholar] [CrossRef]
- Zhang, S.-L.; Deng, P.; Xu, Y.-C.; Lü, S.-W.; Wang, J.-J. Quantification and analysis of anthocyanin and flavonoids compositions, and antioxidant activities in onions with three different colors. J. Integr. Agric. 2016, 15, 2175–2181. [Google Scholar] [CrossRef] [Green Version]
- Cardenosa, V.; Girones-Vilaplana, A.; Muriel, J.L.; Moreno, D.A.; Moreno-Rojas, J.M. Influence of genotype, cultivation system and irrigation regime on antioxidant capacity and selected phenolics of blueberries (vaccinium corymbosum l.). Food Chem 2016, 202, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Figueira, M.E.; Oliveira, M.; Direito, R.; Rocha, J.; Alves, P.; Serra, A.T.; Duarte, C.; Bronze, R.; Fernandes, A.; Brites, D.; et al. Protective effects of a blueberry extract in acute inflammation and collagen-induced arthritis in the rat. Biomed. Pharm. 2016, 83, 1191–1202. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Li, B.; Ma, Y.; Sun, X.; Lin, Y.; Meng, X. Polyphenols, anthocyanins, and flavonoids contents and the antioxidant capacity of various cultivars of highbush and half-high blueberries. J. Food Compos. Anal. 2017, 62, 84–93. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, Q.; Chen, X.Y.; Li, X.; Wang, Y.; Zhang, J.L. Comparison and screening of bioactive phenolic compounds in different blueberry cultivars: Evaluation of anti-oxidation and alpha-glucosidase inhibition effect. Food Res. Int 2017, 100, 312–324. [Google Scholar] [CrossRef] [PubMed]
- Zorenc, Z.; Veberic, R.; Stampar, F.; Koron, D.; Mikulic-Petkovsek, M. Thermal stability of primary and secondary metabolites in highbush blueberry (vaccinium corymbosum l.) purees. Lwt Food Sci. Technol. 2017, 76, 79–86. [Google Scholar] [CrossRef]
- Oh, H.D.; Yu, D.J.; Chung, S.W.; Chea, S.; Lee, H.J. Abscisic acid stimulates anthocyanin accumulation in ‘jersey’ highbush blueberry fruits during ripening. Food Chem. 2018, 244, 403–407. [Google Scholar] [CrossRef]
- Chen, L.; Xin, X.; Yuan, Q.; Su, D.; Liu, W. Phytochemical properties and antioxidant capacities of various colored berries: Phytochemical properties and antioxidant capacities of colored berries. J. Sci. Food Agric. 2014, 94, 180–188. [Google Scholar] [CrossRef]
- Blando, F.; Gerardi, C.; Renna, M.; Castellano, S.; Serio, F. Characterisation of bioactive compounds in berries from plants grown under innovative photovoltaic greenhouses. J. Berry Res. 2018, 8, 55–69. [Google Scholar] [CrossRef]
- Ljevar, A.; Ćurko, N.; Tomašević, M.; Radošević, K.; Srček, V.G.; Ganić, K.K. Phenolic composition, antioxidant capacity and in vitro cytotoxicity assessment of fruit wines. Food Technol. Biotechnol. 2016, 54, 145–155. [Google Scholar] [CrossRef]
- Ivanovic, J.; Tadic, V.; Dimitrijevic, S.; Stamenic, M.; Petrovic, S.; Zizovic, I. Antioxidant properties of the anthocyanin-containing ultrasonic extract from blackberry cultivar “čačanska bestrna”. Ind. Crop. Prod. 2014, 53, 274–281. [Google Scholar] [CrossRef]
- Dos Santos, S.S.; Rodrigues, L.M.; Da Costa, S.C.; De Cassia Bergamasco, R.; Madrona, G.S. Microencapsulation of bioactive compounds from blackberry pomace (rubus fruticosus) by spray drying technique. Int. J. Food Eng. 2017, 13. [Google Scholar] [CrossRef]
- Laczko-Zold, E.; Komlosi, A.; Ulkei, T.; Fogarasi, E.; Croitoru, M.; Fulop, I.; Domokos, E.; Stefanescu, R.; Varga, E. Extractability of polyphenols from black currant, red currant and gooseberry and their antioxidant activity. Acta Biol Hung. 2018, 69, 156–169. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Su, S.; Wang, L.; Wu, J.; Tang, Z.; Xu, Y.; Shu, Q.; Wang, L. Antioxidant capacities and anthocyanin characteristics of the black–red wild berries obtained in northeast china. Food Chem. 2016, 204, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Guimaraes, R.; Barros, L.; Duenas, M.; Carvalho, A.M.; Queiroz, M.J.; Santos-Buelga, C.; Ferreira, I.C. Characterisation of phenolic compounds in wild fruits from northeastern portugal. Food Chem 2013, 141, 3721–3730. [Google Scholar] [CrossRef] [Green Version]
- Velickovic, J.; Kostic, D.; Stojanovic, G.; Mitic, S.; Mitic, M.; Randjelovic, S.; Djordjevic, A. Phenolic composition, antioxidant and antimicrobial activity of the extracts from prunus spinosa l. Fruit. Hem. Ind. 2014, 68, 297–303. [Google Scholar] [CrossRef] [Green Version]
- Mikulic-Petkovsek, M.; Stampar, F.; Veberic, R.; Sircelj, H. Wild prunus fruit species as a rich source of bioactive compounds: Wild prunus-rich source of bioactive compound. J. Food Sci. 2016, 81, C1928–C1937. [Google Scholar] [CrossRef]
- Duymuş, H.G.; Göger, F.; Başer, K.H.C. In vitro antioxidant properties and anthocyanin compositions of elderberry extracts. Food Chem. 2014, 155, 112–119. [Google Scholar] [CrossRef]
- Szalóki-Dorkó, L.; Csizmadia, G.; Abrankó, L.; Stéger-Máté, M. Examination of anthocyanin content of some elderberry cultivars grown in hungary. Acta Hortic. 2015, 79–86. [Google Scholar] [CrossRef]
- Giampieri, F.; Alvarez-Suarez, J.M.; Mazzoni, L.; Forbes-Hernandez, T.Y.; Gasparrini, M.; Gonzàlez-Paramàs, A.M.; Santos-Buelga, C.; Quiles, J.L.; Bompadre, S.; Mezzetti, B.; et al. An anthocyanin-rich strawberry extract protects against oxidative stress damage and improves mitochondrial functionality in human dermal fibroblasts exposed to an oxidizing agent. Food Funct. 2014, 5, 1939. [Google Scholar] [CrossRef]
- Misran, A.; Padmanabhan, P.; Sullivan, J.A.; Khanizadeh, S.; Paliyath, G. Composition of phenolics and volatiles in strawberry cultivars and influence of preharvest hexanal treatment on their profiles. Can. J. Plant Sci. 2014, 95, 115–126. [Google Scholar] [CrossRef]
- Fernandez-Lara, R.; Gordillo, B.; Rodriguez-Pulido, F.J.; Lourdes Gonzalez-Miret, M.; Del Villar-Martinez, A.A.; Davila-Ortiz, G.; Heredia, F.J. Assessment of the differences in the phenolic composition and color characteristics of new strawberry (fragaria x ananassa duch.) cultivars by hplc-ms and imaging tristimulus colorimetry. Food Res. Int 2015, 76, 645–653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bursac Kovacevic, D.; Putnik, P.; Dragovic-Uzelac, V.; Vahcic, N.; Babojelic, M.S.; Levaj, B. Influences of organically and conventionally grown strawberry cultivars on anthocyanins content and color in purees and low-sugar jams. Food Chem 2015, 181, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Arend, G.D.; Adorno, W.T.; Rezzadori, K.; Di Luccio, M.; Chaves, V.C.; Reginatto, F.H.; Petrus, J.C.C. Concentration of phenolic compounds from strawberry ( fragaria x ananassa duch ) juice by nanofiltration membrane. J. Food Eng. 2017, 201, 36–41. [Google Scholar] [CrossRef]
- Kołodziejczyk, K.; Sójka, M.; Abadias, M.; Viñas, I.; Guyot, S.; Baron, A. Polyphenol composition, antioxidant capacity, and antimicrobial activity of the extracts obtained from industrial sour cherry pomace. Ind. Crop. Prod. 2013, 51, 279–288. [Google Scholar] [CrossRef]
- Yılmaz, F.M.; Karaaslan, M.; Vardin, H. Optimization of extraction parameters on the isolation of phenolic compounds from sour cherry (prunus cerasus l.) pomace. J. Food Sci. Technol. 2015, 52, 2851–2859. [Google Scholar] [CrossRef] [Green Version]
- Zorić, Z.; Pedisić, S.; Kovačević, D.B.; Ježek, D.; Dragović-Uzelac, V. Impact of packaging material and storage conditions on polyphenol stability, colour and sensory characteristics of freeze-dried sour cherry (prunus cerasus var. Marasca). J. Food Sci. Technol. 2016, 53, 1247–1258. [Google Scholar] [CrossRef] [Green Version]
- Karaaslan, M.; Yılmaz, F.M.; Karaaslan, A.; Vardin, H. Synthesis and accumulation of anthocyanins in sour cherries during ripening in accordance with antioxidant capacity development and chalcone synthase expression. Eur. Food Res. Technol. 2016, 242, 189–198. [Google Scholar] [CrossRef]
- Cao, J.; Jiang, Q.; Lin, J.; Li, X.; Sun, C.; Chen, K. Physicochemical characterisation of four cherry species (prunus spp.) grown in china. Food Chem. 2015, 173, 855–863. [Google Scholar] [CrossRef]
- Picariello, G.; De Vito, V.; Ferranti, P.; Paolucci, M.; Volpe, M.G. Species- and cultivar-dependent traits of prunus avium and prunus cerasus polyphenols. J. Food Compos. Anal. 2016, 45, 50–57. [Google Scholar] [CrossRef]
- Picariello, G.; Ferranti, P.; De Cunzo, F.; Sacco, E.; Volpe, M.G. Polyphenol patterns to trace sweet (prunus avium) and tart (prunus cerasus) varieties in cherry jam. J. Food Sci. Technol. 2017, 54, 2316–2323. [Google Scholar] [CrossRef]
- Kula, M.; Majdan, M.; Głód, D.; Krauze-Baranowska, M. Phenolic composition of fruits from different cultivars of red and black raspberries grown in poland. J. Food Compos. Anal. 2016, 52, 74–82. [Google Scholar] [CrossRef]
- Cunja, V.; Mikulic-Petkovsek, M.; Zupan, A.; Stampar, F.; Schmitzer, V. Frost decreases content of sugars, ascorbic acid and some quercetin glycosides but stimulates selected carotenes in rosa canina hips. J. Plant Physiol 2015, 178, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Türkyılmaz, M. Anthocyanin and organic acid profiles of pomegranate (punica granatum l.) juices from registered varieties in turkey. Int. J. Food Sci. Technol. 2013, 48, 2086–2095. [Google Scholar]
- Sentandreu, E.; Cerdán-Calero, M.; Sendra, J.M. Phenolic profile characterization of pomegranate (punica granatum) juice by high-performance liquid chromatography with diode array detection coupled to an electrospray ion trap mass analyzer. J. Food Compos. Anal. 2013, 30, 32–40. [Google Scholar] [CrossRef]
- Gomez-Caravaca, A.M.; Verardo, V.; Toselli, M.; Segura-Carretero, A.; Fernandez-Gutierrez, A.; Caboni, M.F. Determination of the major phenolic compounds in pomegranate juices by hplc-dad-esi-ms. J. Agric. Food Chem. 2013, 61, 5328–5337. [Google Scholar] [CrossRef] [PubMed]
- Fazaeli, M.; Yousefi, S.; Emam-Djomeh, Z. Investigation on the effects of microwave and conventional heating methods on the phytochemicals of pomegranate (punica granatum l.) and black mulberry juices. Food Res. Int. 2013, 50, 568–573. [Google Scholar] [CrossRef]
- Calani, L.; Beghe, D.; Mena, P.; Del Rio, D.; Bruni, R.; Fabbri, A.; Dall’asta, C.; Galaverna, G. Ultra-hplc-ms(n) (poly)phenolic profiling and chemometric analysis of juices from ancient punica granatum l. Cultivars: A nontargeted approach. J. Agric. Food Chem. 2013, 61, 5600–5609. [Google Scholar] [CrossRef] [PubMed]
- Turkyilmaz, M.; Ozkan, M. Effects of condensed tannins on anthocyanins and colour of authentic pomegranate (punica granatum l.) juices. Food Chem 2014, 164, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Sengul, H.; Surek, E.; Nilufer-Erdil, D. Investigating the effects of food matrix and food components on bioaccessibility of pomegranate (punica granatum) phenolics and anthocyanins using an in-vitro gastrointestinal digestion model. Food Res. Int. 2014, 62, 1069–1079. [Google Scholar] [CrossRef] [Green Version]
- Radunic, M.; Jukic Spika, M.; Goreta Ban, S.; Gadze, J.; Diaz-Perez, J.C.; MacLean, D. Physical and chemical properties of pomegranate fruit accessions from croatia. Food Chem 2015, 177, 53–60. [Google Scholar] [CrossRef]
- Lantzouraki, D.Z.; Sinanoglou, V.J.; Tsiaka, T.; Proestos, C.; Zoumpoulakis, P. Total phenolic content, antioxidant capacity and phytochemical profiling of grape and pomegranate wines. Rsc Adv. 2015, 5, 101683–101692. [Google Scholar] [CrossRef]
- Gonzalez-Trujano, M.E.; Pellicer, F.; Mena, P.; Moreno, D.A.; Garcia-Viguera, C. Antinociceptive and anti-inflammatory activities of a pomegranate (punica granatum l.) extract rich in ellagitannins. Int. J. Food Sci. Nutr. 2015, 66, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Ben-Simhon, Z.; Judeinstein, S.; Trainin, T.; Harel-Beja, R.; Bar-Ya’akov, I.; Borochov-Neori, H.; Holland, D. A “white” anthocyanin-less pomegranate (punica granatum l.) caused by an insertion in the coding region of the leucoanthocyanidin dioxygenase (ldox; ans) gene. PLoS ONE 2015, 10, e0142777. [Google Scholar] [CrossRef] [PubMed]
- De Arajuno Santiago, M.C.P.; Nogueira, R.I.; Paim, D.R.S.F.; Gouvêa, A.C.M.S.; de Oliveira Godoy, R.L.; Peixoto, F.M.; Pacheco, S.; Freitas, S.P. Effects of encapsulating agents on anthocyanin retention in pomegranate powder obtained by the spray drying process. LWT 2016, 73, 551–556. [Google Scholar] [CrossRef]
- Fanali, C.; Belluomo, M.G.; Cirilli, M.; Cristofori, V.; Zecchini, M.; Cacciola, F.; Russo, M.; Muleo, R.; Dugo, L. Antioxidant activity evaluation and hplc-photodiode array/ms polyphenols analysis of pomegranate juice from selected italian cultivars: A comparative study. Electrophoresis 2016, 37, 1947–1955. [Google Scholar] [CrossRef] [PubMed]
- Ambigaipalan, P.; de Camargo, A.C.; Shahidi, F. Identification of phenolic antioxidants and bioactives of pomegranate seeds following juice extraction using hplc-dad-esi-ms(n). Food Chem 2017, 221, 1883–1894. [Google Scholar] [CrossRef]
- Brighenti, V.; Groothuis, S.F.; Prencipe, F.P.; Amir, R.; Benvenuti, S.; Pellati, F. Metabolite fingerprinting of punica granatum l. (pomegranate) polyphenols by means of high-performance liquid chromatography with diode array and electrospray ionization-mass spectrometry detection. J. Chromatogr. A 2017, 1480, 20–31. [Google Scholar] [CrossRef]
- Perez-Ramirez, I.F.; Reynoso-Camacho, R.; Saura-Calixto, F.; Perez-Jimenez, J. Comprehensive characterization of extractable and nonextractable phenolic compounds by high-performance liquid chromatography-electrospray ionization-quadrupole time-of-flight of a grape/pomegranate pomace dietary supplement. J. Agric. Food Chem. 2018, 66, 661–673. [Google Scholar] [CrossRef] [Green Version]
- Trad, M.; Le Bourvellec, C.; Gaaliche, B.; Ginies, C.; Renard, C.M.G.C.; Mars, M. Caprification modifies polyphenols but not cell wall concentrations in ripe figs. Sci. Hortic. 2013, 160, 115–122. [Google Scholar] [CrossRef]
- Ammar, S.; del Mar Contreras, M.; Belguith-Hadrich, O.; Segura-Carretero, A.; Bouaziz, M. Assessment of the distribution of phenolic compounds and contribution to the antioxidant activity in tunisian fig leaves, fruits, skins and pulps using mass spectrometry-based analysis. Food Funct. 2015, 6, 3663–3677. [Google Scholar] [CrossRef]
- Pereira, C.; López-Corrales, M.; Serradilla, M.J.; del Carmen Villalobos, M.; Ruiz-Moyano, S.; Martín, A. Influence of ripening stage on bioactive compounds and antioxidant activity in nine fig (ficus carica l.) varieties grown in extremadura, spain. J. Food Compos. Anal. 2017, 64, 203–212. [Google Scholar] [CrossRef]
- Bochi, V.C.; Godoy, H.T.; Giusti, M.M. Anthocyanin and other phenolic compounds in ceylon gooseberry (dovyalis hebecarpa) fruits. Food Chem. 2015, 176, 234–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carvalho, A.V.; Ferreira Ferreira da Silveira, T.; Mattietto, R.A.; de Padilha Oliveira, M.D.; Godoy, H.T. Chemical composition and antioxidant capacity of acai (euterpe oleracea) genotypes and commercial pulps. J. Sci. Food Agric. 2017, 97, 1467–1474. [Google Scholar] [CrossRef] [PubMed]
- Garzon, G.A.; Narvaez-Cuenca, C.E.; Vincken, J.P.; Gruppen, H. Polyphenolic composition and antioxidant activity of acai (euterpe oleracea mart.) from colombia. Food Chem. 2017, 217, 364–372. [Google Scholar] [CrossRef]
- Peris, C.S.; Caiado, R.; Lima-Filho, A.; Rodrigues, E.; Eid, F.; Batista Gonçalves, M.; de Queiroz Alves, B.; Guilherme Palma Urushima, J.; Ragazzi, R.; Maia, M. Analysis anthocyanins extracted from the acai fruit (euterpe oleracea): A potential novel vital dye for chromovitrectomy. J. Ophthalmol. 2018, 2018, 9. [Google Scholar] [CrossRef] [Green Version]
- Alvarez-Suarez, J.M.; Giampieri, F.; Gasparrini, M.; Mazzoni, L.; Santos-Buelga, C.; Gonzalez-Paramas, A.M.; Forbes-Hernandez, T.Y.; Afrin, S.; Paez-Watson, T.; Quiles, J.L.; et al. The protective effect of acerola (malpighia emarginata) against oxidative damage in human dermal fibroblasts through the improvement of antioxidant enzyme activity and mitochondrial functionality. Food Funct. 2017, 8, 3250–3258. [Google Scholar] [CrossRef]
- Lucas-Gonzalez, R.; Navarro-Coves, S.; Pérez-Álvarez, J.A.; Fernández-López, J.; Muñoz, L.A.; Viuda-Martos, M. Assessment of polyphenolic profile stability and changes in the antioxidant potential of maqui berry (aristotelia chilensis (molina) stuntz) during in vitro gastrointestinal digestion. Ind. Crop. Prod. 2016, 94, 774–782. [Google Scholar] [CrossRef]
- Genskowsky, E.; Puente, L.A.; Perez-Alvarez, J.A.; Fernandez-Lopez, J.; Munoz, L.A.; Viuda-Martos, M. Determination of polyphenolic profile, antioxidant activity and antibacterial properties of maqui [aristotelia chilensis (molina) stuntz] a chilean blackberry. J. Sci. Food Agric. 2016, 96, 4235–4242. [Google Scholar] [CrossRef]
- Brauch, J.E.; Buchweitz, M.; Schweiggert, R.M.; Carle, R. Detailed analyses of fresh and dried maqui (aristotelia chilensis (mol.) stuntz) berries and juice. Food Chem. 2016, 190, 308–316. [Google Scholar] [CrossRef]
- Brauch, J.E.; Reuter, L.; Conrad, J.; Vogel, H.; Schweiggert, R.M.; Carle, R. Characterization of anthocyanins in novel chilean maqui berry clones by hplc–dad–esi/msn and nmr-spectroscopy. J. Food Compos. Anal. 2017, 58, 16–22. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Lucas-Gonzalez, R.; Ballester-Costa, C.; Perez-Alvarez, J.A.; Munoz, L.A.; Fernandez-Lopez, J. Evaluation of protective effect of different dietary fibers on polyphenolic profile stability of maqui berry (aristotelia chilensis (molina) stuntz) during in vitro gastrointestinal digestion. Food Funct. 2018, 9, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Sommella, E.; Pagano, F.; Pepe, G.; Ostacolo, C.; Manfra, M.; Chieppa, M.; Di Sanzo, R.; Carabetta, S.; Campiglia, P.; Russo, M. Flavonoid composition of tarocco (citrus sinensis l. Osbeck) clone “lempso” and fast antioxidant activity screening by dpph-uhplc-pda-it-tof. Phytochem Anal. 2017, 28, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Zupan, A.; Mikulic-Petkovsek, M.; Cunja, V.; Stampar, F.; Veberic, R. Comparison of phenolic composition of healthy apple tissues and tissues affected by bitter pit. J. Agric. Food Chem. 2013, 61, 12066–12071. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wei, Z.; Ma, F. The effects of fruit bagging on levels of phenolic compounds and expression by anthocyanin biosynthetic and regulatory genes in red-fleshed apples. Process. Biochem. 2015, 50, 1774–1782. [Google Scholar] [CrossRef]
- Jakobek, L.; Barron, A.R. Ancient apple varieties from croatia as a source of bioactive polyphenolic compounds. J. Food Compos. Anal. 2016, 45, 9–15. [Google Scholar] [CrossRef]
- Rehman, R.N.U.; You, Y.; Yang, C.; Khan, A.R.; Li, P.; Ma, F. Characterization of phenolic compounds and active anthocyanin degradation in crabapple (malus orientalis) flowers. Hortic. Environ. Biotechnol. 2017, 58, 324–333. [Google Scholar] [CrossRef]
- Sengul, M.; Eser, Z.; Ercıslı, S. Chemical properties and antioxidant capacity of cornelian cherry genotypes grown in coruh valley of turkey. Acta Sci. Pol. Hortorum Cultus 2014, 13, 73–82. [Google Scholar]
- Sozanski, T.; Kucharska, A.Z.; Szumny, A.; Magdalan, J.; Bielska, K.; Merwid-Lad, A.; Wozniak, A.; Dzimira, S.; Piorecki, N.; Trocha, M. The protective effect of the cornus mas fruits (cornelian cherry) on hypertriglyceridemia and atherosclerosis through pparalpha activation in hypercholesterolemic rabbits. Phytomedicine 2014, 21, 1774–1784. [Google Scholar] [CrossRef]
- Drkenda, P.; Spahić, A.; Begić-Akagić, A.; Gaši, F.; Vranac, A.; Hudina, M.; Blanke, M. Pomological characteristics of some autochthonous genotypes of cornelian cherry (cornus mas l.) in bosnia and herzegovina. Erwerbs Obstbau 2014, 56, 59–66. [Google Scholar] [CrossRef]
- Kucharska, A.Z.; Szumny, A.; Sokół-Łętowska, A.; Piórecki, N.; Klymenko, S.V. Iridoids and anthocyanins in cornelian cherry (cornus mas l.) cultivars. J. Food Compos. Anal. 2015, 40, 95–102. [Google Scholar] [CrossRef]
- Milenkovic Andjelkovic, A.; Andjelkovic, M.; Radovanovic, A.; Radovanović, B.; Nikolic, V. Phenol composition, dpph radical scavenging antimicrobial activity cornelian cherry (cornus mas) fruit leaf extracts. Hem. Ind. 2014, 69, 46. [Google Scholar] [CrossRef] [Green Version]
- Giusti, M.M.; Polit, M.F.; Ayvaz, H.; Tay, D.; Manrique, I. Characterization and quantitation of anthocyanins and other phenolics in native andean potatoes. J. Agric. Food Chem. 2014, 62, 4408–4416. [Google Scholar] [CrossRef] [PubMed]
- Tedesco, I.; Carbone, V.; Spagnuolo, C.; Minasi, P.; Russo, G.L. Identification and quantification of flavonoids from two southern italian cultivars of allium cepa l., tropea (red onion) and montoro (copper onion), and their capacity to protect human erythrocytes from oxidative stress. J. Agric. Food Chem. 2015, 63, 5229–5238. [Google Scholar] [CrossRef] [PubMed]
- Fredotović, Ž.; Šprung, M.; Soldo, B.; Ljubenkov, I.; Budić-Leto, I.; Bilušić, T.; Čikeš-Čulić, V.; Puizina, J. Chemical composition and biological activity of allium cepa l. And allium × cornutum (clementi ex visiani 1842) methanolic extracts. Molecules 2017, 22, 448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiczkowski, W.; Szawara-Nowak, D.; Topolska, J. Red cabbage anthocyanins: Profile, isolation, identification, and antioxidant activity. Food Res. Int. 2013, 51, 303–309. [Google Scholar] [CrossRef]
- Sun, J.; Xiao, Z.; Lin, L.-z.; Lester, G.E.; Wang, Q.; Harnly, J.M.; Chen, P. Profiling polyphenols in five Brassica species microgreens by UHPLC-PDA-ESI/HRMSn. J. Agric. Food Chem. 2013, 61, 10960–10970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiczkowski, W.; Topolska, J.; Honke, J. Anthocyanins profile and antioxidant capacity of red cabbages are influenced by genotype and vegetation period. J. Funct. Foods 2014, 7, 201–211. [Google Scholar] [CrossRef]
- Park, S.; Arasu, M.V.; Jiang, N.; Choi, S.-H.; Lim, Y.P.; Park, J.-T.; Al-Dhabi, N.A.; Kim, S.-J. Metabolite profiling of phenolics, anthocyanins and flavonols in cabbage (brassica oleracea var. Capitata). Ind. Crop. Prod. 2014, 60, 8–14. [Google Scholar] [CrossRef]
- Podsedek, A.; Redzynia, M.; Klewicka, E.; Koziolkiewicz, M. Matrix effects on the stability and antioxidant activity of red cabbage anthocyanins under simulated gastrointestinal digestion. Biomed Res. Int. 2014, 2014, 365738. [Google Scholar] [CrossRef]
- Wiczkowski, W.; Szawara-Nowak, D.; Topolska, J. Changes in the content and composition of anthocyanins in red cabbage and its antioxidant capacity during fermentation, storage and stewing. Food Chem. 2015, 167, 115–123. [Google Scholar] [CrossRef]
- Ahmadiani, N.; Robbins, R.J.; Collins, T.M.; Giusti, M.M. Molar absorptivity (ε) and spectral characteristics of cyanidin-based anthocyanins from red cabbage. Food Chem. 2016, 197, 900–906. [Google Scholar] [CrossRef] [PubMed]
- Socquet-Juglard, D.; Bennett, A.A.; Manns, D.C.; Mansfield, A.K.; Robbins, R.J.; Collins, T.M.; Griffiths, P.D. Effects of growth temperature and postharvest cooling on anthocyanin profiles in juvenile and mature Brassica Oleracea. J. Agric. Food Chem. 2016, 64, 1484–1493. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, Z.; Liu, X. Characterization of acylated anthocyanins in red cabbage via comprehensive two-dimensional high performance liquid chromatography and hplc-ms. J. Food Process. Preserv. 2017, 41, e13129. [Google Scholar] [CrossRef]
- Wiczkowski, W.; Szawara-Nowak, D.; Romaszko, J. The impact of red cabbage fermentation on bioavailability of anthocyanins and antioxidant capacity of human plasma. Food Chem. 2016, 190, 730–740. [Google Scholar] [CrossRef]
- Murador, D.C.; Mercadante, A.Z.; de Rosso, V.V. Cooking techniques improve the levels of bioactive compounds and antioxidant activity in kale and red cabbage. Food Chem. 2016, 196, 1101–1107. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Z.; Zhang, H.; Liu, Y.; Zhang, S.; Meng, Q.; Liu, W. Isolation of high purity anthocyanin monomers from red cabbage with recycling preparative liquid chromatography and their photostability. Molecules 2018, 23, 991. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Oh, I.-N.; Kim, J.; Jung, D.; Cuong, N.P.; Kim, Y.; Lee, J.; Kwon, O.; Park, S.U.; Lim, Y.; et al. Phenolic compound profiles and their seasonal variations in new red-phenotype head-forming chinese cabbages. LWT 2018, 90, 433–439. [Google Scholar] [CrossRef]
- West, M.E.; Mauer, L.J. Color and chemical stability of a variety of anthocyanins and ascorbic acid in solution and powder forms. J. Agric. Food Chem. 2013, 61, 4169–4179. [Google Scholar] [CrossRef]
- Harakotr, B.; Suriharn, B.; Tangwongchai, R.; Scott, M.P.; Lertrat, K. Anthocyanins and antioxidant activity in coloured waxy corn at different maturation stages. J. Funct. Foods 2014, 9, 109–118. [Google Scholar] [CrossRef] [Green Version]
- Huang, B.; Wang, Z.; Park, J.H.; Ryu, O.H.; Choi, M.K.; Lee, J.Y.; Kang, Y.H.; Lim, S.S. Anti-diabetic effect of purple corn extract on c57bl/ksj db/db mice. Nutr Res. Pr. 2015, 9, 22–29. [Google Scholar] [CrossRef]
- Lago, C.; Landoni, M.; Cassani, E.; Cantaluppi, E.; Doria, E.; Nielsen, E.; Giorgi, A.; Pilu, R. Study and characterization of an ancient european flint white maize rich in anthocyanins: Millo corvo from galicia. PLoS ONE 2015, 10, e0126521. [Google Scholar]
- Lao, F.; Giusti, M.M. Quantification of purple corn (zea mays l.) anthocyanins using spectrophotometric and hplc approaches: Method comparison and correlation. Food Anal. Methods 2016, 9, 1367–1380. [Google Scholar] [CrossRef]
- Deineka, V.I.; Sidorov, A.N.; Deineka, L.A. Determination of purple corn husk anthocyanins. J. Anal. Chem. 2016, 71, 1145–1150. [Google Scholar] [CrossRef]
- Paucar-Menacho, L.M.; Martínez-Villaluenga, C.; Dueñas, M.; Frias, J.; Peñas, E. Optimization of germination time and temperature to maximize the content of bioactive compounds and the antioxidant activity of purple corn (zea mays l.) by response surface methodology. Lwt Food Sci. Technol. 2017, 76, 236–244. [Google Scholar] [CrossRef]
- Paulsmeyer, M.; Chatham, L.; Becker, T.; West, M.; West, L.; Juvik, J. Survey of anthocyanin composition and concentration in diverse maize germplasms. J. Agric. Food Chem. 2017, 65, 4341–4350. [Google Scholar] [CrossRef]
- Chen, L.; Yang, M.; Mou, H.; Kong, Q. Ultrasound-assisted extraction and characterization of anthocyanins from purple corn bran. J. Food Process. Preserv. 2018, 42, e13377. [Google Scholar] [CrossRef] [Green Version]
- Gras, C.C.; Carle, R.; Schweiggert, R.M. Determination of anthocyanins from black carrots by uhplc-pda after ultrasound-assisted extraction. J. Food Compos. Anal. 2015, 44, 170–177. [Google Scholar] [CrossRef]
- Abdel-Moemin, A.R. Analysis of phenolic acids and anthocyanins of pasta-like product enriched with date kernels (phoenix dactylifera l.) and purple carrots (daucus carota l. Sp. Sativus var. Atrorubens). J. Food Meas. Charact. 2016, 10, 507–519. [Google Scholar] [CrossRef]
- Esatbeyoglu, T.; Rodriguez-Werner, M.; Schlosser, A.; Liehr, M.; Ipharraguerre, I.; Winterhalter, P.; Rimbach, G. Fractionation of plant bioactives from black carrots (daucus carota subspecies sativus varietas atrorubens alef.) by adsorptive membrane chromatography and analysis of their potential anti-diabetic activity. J. Agric. Food Chem. 2016, 64, 5901–5908. [Google Scholar] [CrossRef]
- Gras, C.C.; Bogner, H.; Carle, R.; Schweiggert, R.M. Effect of genuine non-anthocyanin phenolics and chlorogenic acid on color and stability of black carrot (daucus carota ssp. Sativus var. Atrorubens alef.) anthocyanins. Food Res. Int. 2016, 85, 291–300. [Google Scholar] [CrossRef]
- Carazzone, C.; Mascherpa, D.; Gazzani, G.; Papetti, A. Identification of phenolic constituents in red chicory salads (cichorium intybus) by high-performance liquid chromatography with diode array detection and electrospray ionisation tandem mass spectrometry. Food Chem. 2013, 138, 1062–1071. [Google Scholar] [CrossRef] [PubMed]
- Ferioli, F.; Manco, M.A.; D’Antuono, L.F. Variation of sesquiterpene lactones and phenolics in chicory and endive germplasm. J. Food Compos. Anal. 2015, 39, 77–86. [Google Scholar] [CrossRef]
- Cefola, M.; Carbone, V.; Minasi, P.; Pace, B. Phenolic profiles and postharvest quality changes of fresh-cut radicchio ( cichorium intybus l.): Nutrient value in fresh vs. Stored leaves. J. Food Compos. Anal. 2016, 51, 76–84. [Google Scholar] [CrossRef]
- Papetti, A.; Maietta, M.; Corana, F.; Marrubini, G.; Gazzani, G. Polyphenolic profile of green/red spotted italian cichorium intybus salads by rp-hplc-pda-esi-msn. J. Food Compos. Anal. 2017, 63, 189–197. [Google Scholar] [CrossRef]
- Tardugno, R.; Pozzebon, M.; Beggio, M.; Del Turco, P.; Pojana, G. Polyphenolic profile of cichorium intybus l. Endemic varieties from the veneto region of italy. Food Chem. 2018, 266, 175–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slatnar, A.; Mikulic-Petkovsek, M.; Stampar, F.; Veberic, R.; Horvat, J.; Jakse, M.; Šircelj, H. Game tones: Sugars, organic acids, phenolics in green purple asparagus (asparagus officinalis l.) cultivars. Turk. J. Agric. For. 2018, 42, 55–66. [Google Scholar] [CrossRef]
- Jeon, J.; Kim, J.K.; Kim, H.; Kim, Y.J.; Park, Y.J.; Kim, S.J.; Kim, C.; Park, S.U. Transcriptome analysis and metabolic profiling of green and red kale (brassica oleracea var. Acephala) seedlings. Food Chem. 2018, 241, 7–13. [Google Scholar] [CrossRef]
- Papetti, A.; Milanese, C.; Zanchi, C.; Gazzani, G. Hplc–dad–esi/msn characterization of environmentally friendly polyphenolic extract from raphanus sativus l. Var. “Cherry belle” skin and stability of its red components. Food Res. Int. 2014, 65, 238–246. [Google Scholar] [CrossRef]
- Baenas, N.; Ferreres, F.; García-Viguera, C.; Moreno, D.A. Radish sprouts—Characterization and elicitation of novel varieties rich in anthocyanins. Food Res. Int. 2015, 69, 305–312. [Google Scholar] [CrossRef]
- Park, C.H.; Baskar, T.B.; Park, S.Y.; Kim, S.J.; Valan Arasu, M.; Al-Dhabi, N.A.; Kim, J.K.; Park, S.U. Metabolic profiling and antioxidant assay of metabolites from three radish cultivars (raphanus sativus). Molecules 2016, 21, 157. [Google Scholar] [CrossRef] [Green Version]
- Mojica, L.; Meyer, A.; Berhow, M.A.; de Mejía, E.G. Bean cultivars (phaseolus vulgaris l.) have similar high antioxidant capacity, in vitro inhibition of α-amylase and α-glucosidase while diverse phenolic composition and concentration. Food Res. Int. 2015, 69, 38–48. [Google Scholar] [CrossRef]
- Jhan, J.K.; Chung, Y.C.; Chen, G.H.; Chang, C.H.; Lu, Y.C.; Hsu, C.K. Anthocyanin contents in the seed coat of black soya bean and their anti-human tyrosinase activity and antioxidative activity. Int. J. Cosmet. Sci. 2016, 38, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Caro, G.; Watanabe, S.; Crozier, A.; Fujimura, T.; Yokota, T.; Ashihara, H. Phytochemical profile of a japanese black–purple rice. Food Chem. 2013, 141, 2821–2827. [Google Scholar] [CrossRef] [PubMed]
- Bordiga, M.; Gomez-Alonso, S.; Locatelli, M.; Travaglia, F.; Coïsson, J.D.; Hermosin-Gutierrez, I.; Arlorio, M. Phenolics characterization and antioxidant activity of six different pigmented oryza sativa l. Cultivars grown in piedmont (italy). Food Res. Int. 2014, 65, 282–290. [Google Scholar] [CrossRef]
- Hao, J.; Zhu, H.; Zhang, Z.; Yang, S.; Li, H. Identification of anthocyanins in black rice (oryza sativa l.) by uplc/q-tof-ms and their in vitro and in vivo antioxidant activities. J. Cereal Sci. 2015, 64, 92–99. [Google Scholar] [CrossRef]
- Asem, I.D.; Imotomba, R.K.; Mazumder, P.B.; Laishram, J.M. Anthocyanin content in the black scented rice (chakhao): Its impact on human health and plant defense. Symbiosis 2015, 66, 47–54. [Google Scholar] [CrossRef]
- Das, A.B.; Goud, V.V.; Das, C. Extraction of phenolic compounds and anthocyanin from black and purple rice bran ( oryza sativa l.) using ultrasound: A comparative analysis and phytochemical profiling. Ind. Crop. Prod. 2017, 95, 332–341. [Google Scholar] [CrossRef]
- Zhu, Y.; Sun, H.; He, S.; Lou, Q.; Yu, M.; Tang, M.; Tu, L. Metabolism and prebiotics activity of anthocyanins from black rice (oryza sativa l.) in vitro. PLoS ONE 2018, 13, e0195754. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Hu, Z.; Zhu, M.; Zhu, Z.; Wang, Z.; Tian, S.; Chen, G. Anthocyanin accumulation and molecular analysis of correlated genes in purple kohlrabi (Brassica oleracea var. gongylodes L.). J. Agric. Food Chem. 2015, 63, 4160–4169. [Google Scholar] [CrossRef]
- Rahim, M.A.; Robin, A.H.K.; Natarajan, S.; Jung, H.-J.; Lee, J.; Kim, H.; Kim, H.-T.; Park, J.-I.; Nou, I.-S. Identification and characterization of anthocyanin biosynthesis-related genes in kohlrabi. Appl. Biochem. Biotechnol. 2018, 184, 1120–1141. [Google Scholar] [CrossRef]
- Park, C.H.; Yeo, H.J.; Kim, N.S.; Eun, P.Y.; Kim, S.-J.; Arasu, M.V.; Al-Dhabi, N.A.; Park, S.-Y.; Kim, J.K.; Park, S.U. Metabolic profiling of pale green and purple kohlrabi (Brassica oleracea var. gongylodes L.). Appl. Biol. Chem. 2017, 60, 249–257. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, Z.; Chu, G.; Huang, C.; Tian, S.; Zhao, Z.; Chen, G. Anthocyanin accumulation and molecular analysis of anthocyanin biosynthesis-associated genes in eggplant (Solanum melongena L.). J. Agric. Food Chem. 2014, 62, 2906–2912. [Google Scholar] [CrossRef] [PubMed]
- García-Salas, P.; Gómez-Caravaca, A.M.; Morales-Soto, A.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Identification and quantification of phenolic compounds in diverse cultivars of eggplant grown in different seasons by high-performance liquid chromatography coupled to diode array detector and electrospray-quadrupole-time of flight-mass spectrometry. Food Res. Int. 2014, 57, 114–122. [Google Scholar] [CrossRef]
- Zhang, Y.; Chu, G.; Hu, Z.; Gao, Q.; Cui, B.; Tian, S.; Wang, B.; Chen, G. Genetically engineered anthocyanin pathway for high health-promoting pigment production in eggplant. Mol. Breed. 2016, 36, 54. [Google Scholar] [CrossRef]
- Ferarsa, S.; Zhang, W.; Moulai-Mostefa, N.; Ding, L.; Jaffrin, M.Y.; Grimi, N. Recovery of anthocyanins and other phenolic compounds from purple eggplant peels and pulps using ultrasonic-assisted extraction. Food Bioprod. Process. 2018, 109, 19–28. [Google Scholar] [CrossRef]
- Pandino, G.; Meneghini, M.; Tavazza, R.; Lombardo, S.; Mauromicale, G. Phytochemicals accumulation and antioxidant activity in callus and suspension cultures of Cynara scolymus L. Plant Cell Tissue Organ Cult. 2017, 128, 223–230. [Google Scholar] [CrossRef]
- Lee, M.J.; Park, J.S.; Choi, D.S.; Jung, M.Y. Characterization and quantitation of anthocyanins in purple-fleshed sweet potatoes cultivated in korea by hplc-dad and hplc-esi-qtof-ms/ms. J. Agric. Food Chem. 2013, 61, 3148–3158. [Google Scholar] [CrossRef]
- Xu, J.; Su, X.; Lim, S.; Griffin, J.; Carey, E.; Katz, B.; Tomich, J.; Smith, J.S.; Wang, W. Characterisation and stability of anthocyanins in purple-fleshed sweet potato p40. Food Chem. 2015, 186, 90–96. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.J.; Park, W.S.; Bae, J.-Y.; Kang, S.Y.; Yang, M.H.; Lee, S.; Lee, H.-S.; Kwak, S.-S.; Ahn, M.-J. Variations in the carotenoid and anthocyanin contents of korean cultural varieties and home-processed sweet potatoes. J. Food Compos. Anal. 2015, 41, 188–193. [Google Scholar] [CrossRef]
- Gras, C.C.; Nemetz, N.; Carle, R.; Schweiggert, R.M. Anthocyanins from purple sweet potato ( ipomoea batatas (l.) lam.) and their color modulation by the addition of phenolic acids and food-grade phenolic plant extracts. Food Chem. 2017, 235, 265–274. [Google Scholar] [CrossRef]
- Ge, J.; Hu, Y.; Wang, H.; Huang, Y.; Zhang, P.; Liao, Z.; Chen, M. Profiling of anthocyanins in transgenic purple-fleshed sweet potatoes by hplc-ms/ms: Profiling of anthocyanins in transgenic purple-fleshed sweet potatoes. J. Sci. Food Agric. 2017, 97, 4995–5003. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xu, Y.; Cao, Y.; Fang, K.; Xia, W.; Jiang, Q. Combined effect of microwave and steam cooking on phytochemical compounds and antioxidant activity of purple sweet potatoes. Food Sci. Technol. Res. 2017, 23, 193–201. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.J.; Wang, J.M.; Lin, W.L.; Chu, C.Y.; Chou, F.P.; Tseng, T.H. Protective effect of hibiscus anthocyanins against tert-butyl hydroperoxide-induced hepatic toxicity in rats. Food Chem Toxicol 2000, 38, 411–416. [Google Scholar] [CrossRef]
- Smith, M.A.L.; Marley, K.A.; Seigler, D.; Singletary, K.W.; Meline, B. Bioactive properties of wild blueberry fruits. J. Food Sci. 2000, 65, 352–356. [Google Scholar] [CrossRef]
- Rugină, D.; Sconţa, Z.; Leopold, L.; Pintea, A.; Bunea, A.; Socaciu, C. Antioxidant activities of chokeberry extracts and the cytotoxic action of their anthocyanin fraction on hela human cervical tumor cells. J. Med. Food 2012, 15, 700–706. [Google Scholar] [CrossRef] [Green Version]
- Tsuda, T.; Horio, F.; Uchida, K.; Aoki, H.; Osawa, T. Dietary cyanidin 3-o-beta-d-glucoside-rich purple corn color prevents obesity and ameliorates hyperglycemia in mice. J. Nutr. 2003, 133, 2125–2130. [Google Scholar] [CrossRef]
- Boyer, J.; Brown, D.; Liu, R.H. Uptake of quercetin and quercetin 3-glucoside from whole onion and apple peel extracts by caco-2 cell monolayers. J. Agric. Food Chem 2004, 52, 7172–7179. [Google Scholar] [CrossRef]
- Riboli, E.; Norat, T. Epidemiologic evidence of the protective effect of fruit and vegetables on cancer risk. Am. J. Clin. Nutr. 2003, 78, 559s–569s. [Google Scholar] [CrossRef] [Green Version]
- Kwon, J.Y.; Lee, K.W.; Hur, H.J.; Lee, H.J. Peonidin inhibits phorbol-ester-induced cox-2 expression and transformation in jb6 p+ cells by blocking phosphorylation of erk-1 and -2. Ann. N. Y. Acad. Sci. 2007, 1095, 513–520. [Google Scholar] [CrossRef]
- Lazze, M.C.; Pizzala, R.; Savio, M.; Stivala, L.A.; Prosperi, E.; Bianchi, L. Anthocyanins protect against DNA damage induced by tert-butyl-hydroperoxide in rat smooth muscle and hepatoma cells. Mutat Res. 2003, 535, 103–115. [Google Scholar] [CrossRef]
- Ramirez-Tortosa, C.; Andersen, O.M.; Cabrita, L.; Gardner, P.T.; Morrice, P.C.; Wood, S.G.; Duthie, S.J.; Collins, A.R.; Duthie, G.G. Anthocyanin-rich extract decreases indices of lipid peroxidation and DNA damage in vitamin e-depleted rats. Free Radic. Biol. Med. 2001, 31, 1033–1037. [Google Scholar] [CrossRef]
- Rossi, A.; Serraino, I.; Dugo, P.; Di Paola, R.; Mondello, L.; Genovese, T.; Morabito, D.; Dugo, G.; Sautebin, L.; Caputi, A.P.; et al. Protective effects of anthocyanins from blackberry in a rat model of acute lung inflammation. Free Radic. Res. 2003, 37, 891–900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, J.-M.; Chia, L.-S.; Goh, N.-K.; Chia, T.-F.; Brouillard, R. Analysis and biological activities of anthocyanins. Phytochemistry 2003, 64, 923–933. [Google Scholar] [CrossRef]
- He, J.; Giusti, M.M. Anthocyanins: Natural colorants with health-promoting properties. Annu. Rev. Food Sci. Technol. 2010, 1, 163–187. [Google Scholar] [CrossRef] [PubMed]
- Allen, R.G.; Tresini, M. Oxidative stress and gene regulation. Free Radic. Biol. Med. 2000, 28, 463–499. [Google Scholar] [CrossRef]
- Bors, W.; Heller, W.; Michel, C.; Saran, M. Flavonoids as antioxidants: Determination of radical-scavenging efficiencies. Methods Enzymol. 1990, 186, 343–355. [Google Scholar]
- Wang, H.; Cao, G.; Prior, R.L. Oxygen radical absorbing capacity of anthocyanins. J. Agric. Food Chem. 1997, 45, 304–309. [Google Scholar] [CrossRef]
- Renis, M.; Calandra, L.; Scifo, C.; Tomasello, B.; Cardile, V.; Vanella, L.; Bei, R.; La Fauci, L.; Galvano, F. Response of cell cycle/stress-related protein expression and DNA damage upon treatment of caco2 cells with anthocyanins. Br. J. Nutr. 2008, 100, 27–35. [Google Scholar] [CrossRef] [Green Version]
- Parry, J.; Su, L.; Moore, J.; Cheng, Z.; Luther, M.; Rao, J.N.; Wang, J.Y.; Yu, L.L. Chemical compositions, antioxidant capacities, and antiproliferative activities of selected fruit seed flours. J. Agric. Food Chem. 2006, 54, 3773–3778. [Google Scholar] [CrossRef]
- Keravis, T.; Favot, L.; Abusnina, A.A.; Anton, A.; Justiniano, H.; Soleti, R.; Alabed Alibrahim, E.; Simard, G.; Andriantsitohaina, R.; Lugnier, C. Delphinidin inhibits tumor growth by acting on vegf signalling in endothelial cells. PLoS ONE 2015, 10, e0145291. [Google Scholar] [CrossRef] [Green Version]
- Meyers, K.J.; Watkins, C.B.; Pritts, M.P.; Liu, R.H. Antioxidant and antiproliferative activities of strawberries. J. Agric. Food Chem. 2003, 51, 6887–6892. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Hu, Y.; Jiang, X.; Chen, T.; Ma, Y.; Wu, S.; Sun, J.; Jiao, R.; Li, X.; Deng, L.; et al. Cyanidin-3-o-glucoside inhibits the uvb-induced ros/cox-2 pathway in hacat cells. J. Photochem. Photobiol. B Biol. 2017, 177, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Mladěnka, P.; Říha, M.; Martin, J.; Gorová, B.; Matějíček, A.; Spilková, J. Fruit extracts of 10 varieties of elderberry (sambucus nigra l.) interact differently with iron and copper. Phytochem. Lett. 2016, 18, 232–238. [Google Scholar] [CrossRef]
- Singh, R.P.; Dhanalakshmi, S.; Agarwal, R. Phytochemicals as cell cycle modulators--a less toxic approach in halting human cancers. Cell Cycle 2002, 1, 156–161. [Google Scholar] [CrossRef] [Green Version]
- Afaq, F.; Adhami, V.M.; Mukhtar, H. Photochemoprevention of ultraviolet b signaling and photocarcinogenesis. Mutat. Res. 2005, 571, 153–173. [Google Scholar] [CrossRef]
- Olsson, M.E.; Gustavsson, K.E.; Andersson, S.; Nilsson, A.; Duan, R.D. Inhibition of cancer cell proliferation in vitro by fruit and berry extracts and correlations with antioxidant levels. J. Agric. Food Chem. 2004, 52, 7264–7271. [Google Scholar] [CrossRef]
- Stoner, G.D.; Sardo, C.; Apseloff, G.; Mullet, D.; Wargo, W.; Pound, V.; Singh, A.; Sanders, J.; Aziz, R.; Casto, B.; et al. Pharmacokinetics of anthocyanins and ellagic acid in healthy volunteers fed freeze-dried black raspberries daily for 7 days. J. Clin. Pharmacol. 2005, 45, 1153–1164. [Google Scholar] [CrossRef]
- Galvano, F.; La Fauci, L.; Lazzarino, G.; Fogliano, V.; Ritieni, A.; Ciappellano, S.; Battistini, N.C.; Tavazzi, B.; Galvano, G. Cyanidins: Metabolism and biological properties. J. Nutr. Biochem. 2004, 15, 2–11. [Google Scholar] [CrossRef]
- Hakimuddin, F.; Paliyath, G.; Meckling, K. Selective cytotoxicity of a red grape wine flavonoid fraction against mcf-7 cells. Breast Cancer Res. Treat. 2004, 85, 65–79. [Google Scholar] [CrossRef]
- Chen, P.-N.; Chu, S.-C.; Chiou, H.-L.; Kuo, W.-H.; Chiang, C.-L.; Hsieh, Y.-S. Mulberry anthocyanins, cyanidin 3-rutinoside and cyanidin 3-glucoside, exhibited an inhibitory effect on the migration and invasion of a human lung cancer cell line. Cancer Lett. 2006, 235, 248–259. [Google Scholar] [CrossRef]
- Ding, M.; Feng, R.; Wang, S.Y.; Bowman, L.; Lu, Y.; Qian, Y.; Castranova, V.; Jiang, B.-H.; Shi, X. Cyanidin-3-glucoside, a natural product derived from blackberry, exhibits chemopreventive and chemotherapeutic activity. J. Biol. Chem. 2006, 281, 17359–17368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roy, M.; Sen, S.; Chakraborti, A.S. Action of pelargonidin on hyperglycemia and oxidative damage in diabetic rats: Implication for glycation-induced hemoglobin modification. Life Sci. 2008, 82, 1102–1110. [Google Scholar] [CrossRef] [PubMed]
- Seeram, N.P.; Adams, L.S.; Zhang, Y.; Lee, R.; Sand, D.; Scheuller, H.S.; Heber, D. Blackberry, black raspberry, blueberry, cranberry, red raspberry, and strawberry extracts inhibit growth and stimulate apoptosis of human cancer cells in vitro. J. Agric. Food Chem. 2006, 54, 9329–9339. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Xu, J.; Kim, J.; Chen, T.-Y.; Su, X.; Standard, J.; Carey, E.; Griffin, J.; Herndon, B.; Katz, B.; et al. Role of anthocyanin-enriched purple-fleshed sweet potato p40 in colorectal cancer prevention. Mol. Nutr. Food Res. 2013, 57, 1908–1917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, D.Y.; Lee, W.S.; Kim, S.H.; Kim, M.J.; Yun, J.W.; Lu, J.N.; Lee, S.J.; Tsoy, I.; Kim, H.J.; Ryu, C.H.; et al. Anti-invasive activity of anthocyanins isolated from Vitis coignetiae in human hepatocarcinoma cells. J. Med. Food 2009, 12, 967–972. [Google Scholar] [CrossRef] [PubMed]
- Ali, Z.; Yousaf, N.; Larkin, J. Melanoma epidemiology, biology and prognosis. Ejc Suppl. 2013, 11, 81–91. [Google Scholar] [CrossRef] [Green Version]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in globocan 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef]
- Leonardi, G.C.; Falzone, L.; Salemi, R.; Zanghi, A.; Spandidos, D.A.; McCubrey, J.A.; Candido, S.; Libra, M. Cutaneous melanoma: From pathogenesis to therapy (review). Int. J. Oncol. 2018, 52, 1071–1080. [Google Scholar] [CrossRef] [Green Version]
- Rastrelli, M.; Tropea, S.; Rossi, C.R.; Alaibac, M. Melanoma: Epidemiology, risk factors, pathogenesis, diagnosis and classification. In vivo (Athensgreece) 2014, 28, 1005–1011. [Google Scholar]
- Ross, M.I.; Gershenwald, J.E. Evidence-based treatment of early-stage melanoma. J. Surg. Oncol. 2011, 104, 341–353. [Google Scholar] [CrossRef]
- Villanueva, J.; Herlyn, M. Melanoma and the tumor microenvironment. Curr. Oncol. Rep. 2008, 10, 439–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laikova, K.V.; Oberemok, V.V.; Krasnodubets, A.M.; Gal’chinsky, N.V.; Useinov, R.Z.; Novikov, I.A.; Temirova, Z.Z.; Gorlov, M.V.; Shved, N.A.; Kumeiko, V.V.; et al. Advances in the understanding of skin cancer: Ultraviolet radiation, mutations, and antisense oligonucleotides as anticancer drugs. Molecules 2019, 24, 1516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swope, V.B.; Abdel-Malek, Z.A. MC1R: Front and center in the bright side of dark eumelanin and DNA repair. Int. J. Mol. Sci. 2018, 19, 2667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serafino, A.; Sinibaldi-Vallebona, P.; Lazzarino, G.; Tavazzi, B.; Rasi, G.; Pierimarchi, P.; Andreola, F.; Moroni, G.; Galvano, G.; Galvano, F.; et al. Differentiation of human melanoma cells induced by cyanidin-3-O-β-glucopyranoside. FASEB J. 2004, 18, 1940–1942. [Google Scholar] [CrossRef]
- Videira, I.F.; Moura, D.F.; Magina, S. Mechanisms regulating melanogenesis. An. Bras. Bermatologia 2013, 88, 76–83. [Google Scholar] [CrossRef] [Green Version]
- Park, K.-C.; Huh, S.Y.; Choi, H.R.; Kim, D.-S. Biology of melanogenesis and the search for hypopigmenting agents. Dermatol. Sin. 2010, 28, 53–58. [Google Scholar] [CrossRef] [Green Version]
- Kanitakis, J. Anatomy, histology and immunohistochemistry of normal human skin. Eur. J. Dermatol. Ejd 2002, 12, 390–399. [Google Scholar]
- Farage, M.A.; Miller, K.W.; Berardesca, E.; Maibach, H.I. Clinical implications of aging skin: Cutaneous disorders in the elderly. Am. J. Clin. Dermatol. 2009, 10, 73–86. [Google Scholar] [CrossRef]
- Pennello, G.; Devesa, S.; Gail, M. Association of surface ultraviolet b radiation levels with melanoma and nonmelanoma skin cancer in United States blacks. Cancer Epidemiol. Biomark. Prev. 2000, 9, 291–297. [Google Scholar]
- Leonel, E.; Rojo, D.E.R.; Graf, B.; Diana; Chung, M.; Ribnicky, D.; Fridlender, B.; Raskin, I. Role of Anthocyanins in Skin Aging and UV Induced Skin Damage. In Anthocyanins in Health and Disease; Wallace, T.C., Giusti, M.M., Eds.; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Bauer, J.; Garbe, C. Acquired melanocytic nevi as risk factor for melanoma development. A comprehensive review of epidemiological data. Pigment Cell Res. 2003, 16, 297–306. [Google Scholar] [CrossRef]
- Hawkes, J.E.; Truong, A.; Meyer, L.J. Genetic predisposition to melanoma. Semin. Oncol. 2016, 43, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.E.; Torrisi, E.; Bevelacqua, Y.; Perrotta, R.; Libra, M.; McCubrey, J.A.; Spandidos, D.A.; Stivala, F.; Malaponte, G. Melanoma: Molecular pathogenesis and emerging target therapies (review). Int. J. Oncol. 2009, 34, 1481–1489. [Google Scholar] [PubMed] [Green Version]
- Hirst, N.G.; Gordon, L.G.; Scuffham, P.A.; Green, A.C. Lifetime cost-effectiveness of skin cancer prevention through promotion of daily sunscreen use. Value Health 2012, 15, 261–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ives, N.J.; Stowe, R.L.; Lorigan, P.; Wheatley, K. Chemotherapy compared with biochemotherapy for the treatment of metastatic melanoma: A meta-analysis of 18 trials involving 2621 patients. J. Clin. Oncol. 2007, 25, 5426–5434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bishayee, A. Cancer prevention and treatment with resveratrol: From rodent studies to clinical trials. Cancer Prev. Res. 2009, 2, 409–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aquilato, A.; Lopez, V.; Doonan, B.; Hsieh, T.-C.; Pinto, J.T.; Wu, E.; Wu, J.M. Chapter 102—Braf Mutation in Melanoma and Dietary Polyphenols as Adjunctive Treatment Strategy. In Polyphenols in Human Health Disease; Academic Press: San Diego, CA, USA, 2014; pp. 1353–1365. [Google Scholar]
- Shishodia, S.; Aggarwal, B.B. Resveratrol: A polyphenol for all seasons. In Resveratrol in Health Disease; Aggarwal, B.B., Shishodia, S., Eds.; CRC Press: Boca Raton, FL, USA, 2006; pp. 1–16. [Google Scholar]
- Imtiaz, A.; Siddiqui, R.S.T.; Chamcheu, J.C.; Mukhtar, H. Bioactive Food Components For Melanoma: An Overview, Skin Cancer Overview; Xi, Y., Ed.; IntechOpen: London, UK, 2011. [Google Scholar]
- Chinembiri, T.N.; du Plessis, L.H.; Gerber, M.; Hamman, J.H.; du Plessis, J. Review of natural compounds for potential skin cancer treatment. Molecules 2014, 19, 11679–11721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giampieri, F.; Alvarez-Suarez, J.M.; Tulipani, S.; Gonzàles-Paramàs, A.M.; Santos-Buelga, C.; Bompadre, S.; Quiles, J.L.; Mezzetti, B.; Battino, M. Photoprotective potential of strawberry (fragaria × ananassa) extract against uv-a irradiation damage on human fibroblasts. J. Agric. Food Chem. 2012, 60, 2322–2327. [Google Scholar] [CrossRef]
- Saewan, N.; Jimtaisong, A. Photoprotection of natural flavonoids. J. Appl. Pharm. Sci. 2013, 3, 129–141. [Google Scholar]
- Syed, D.N.; Afaq, F.; Maddodi, N.; Johnson, J.J.; Sarfaraz, S.; Ahmad, A.; Setaluri, V.; Mukhtar, H. Inhibition of human melanoma cell growth by the dietary flavonoid fisetin is associated with disruption of wnt/[beta]-catenin signaling and decreased mitf levels. J. Invest. Dermatol. 2011, 131, 1291–1299. [Google Scholar] [CrossRef] [Green Version]
- Murapa, P.; Dai, J.; Chung, M.; Mumper, R.J.; D’Orazio, J. Anthocyanin-rich fractions of blackberry extracts reduce uv-induced free radicals and oxidative damage in keratinocytes. Phytother. Res. 2012, 26, 106–112. [Google Scholar] [CrossRef]
- Bae, J.Y.; Lim, S.S.; Kim, S.J.; Choi, J.S.; Park, J.; Ju, S.M.; Han, S.J.; Kang, I.J.; Kang, Y.H. Bog blueberry anthocyanins alleviate photoaging in ultraviolet-b irradiation-induced human dermal fibroblasts. Mol. Nutr. Food Res. 2009, 53, 726–738. [Google Scholar] [CrossRef] [PubMed]
- Afaq, F.; Katiyar, S.K. Polyphenols: Skin photoprotection and inhibition of photocarcinogenesis. Mini Rev. Med. Chem. 2011, 11, 1200–1215. [Google Scholar] [PubMed] [Green Version]
- Harborne, J.B. Spectral methods of characterizing anthocyanins. Biochem. J. 1958, 70, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.F.; Lien, C.Y.; Lai, Y.C.; Huang, C.L.; Liao, W.C. Influence of purple sweet potato extracts on the uv absorption properties of a cosmetic cream. J. Cosmet. Sci. 2010, 61, 333–341. [Google Scholar]
- Tsoyi, K.; Park, H.B.; Kim, Y.M.; Chung, J.I.; Shin, S.C.; Shim, H.J.; Lee, W.S.; Seo, H.G.; Lee, J.H.; Chang, K.C.; et al. Protective effect of anthocyanins from black soybean seed coats on uvb-induced apoptotic cell death in vitro and in vivo. J. Agric. Food Chem. 2008, 56, 10600–10605. [Google Scholar] [CrossRef]
- Miko Enomoto, T.; Johnson, T.; Peterson, N.; Homer, L.; Walts, D.; Johnson, N. Combination glutathione and anthocyanins as an alternative for skin care during external-beam radiation. Am. J. Surg. 2005, 189, 627–630. [Google Scholar] [CrossRef]
- Gómez-Cordovés, C.; Bartolomé, B.; Vieira, W.; Virador, V.M. Effects of wine phenolics and sorghum tannins on tyrosinase activity and growth of melanoma cells. J. Agric. Food Chem. 2001, 49, 1620–1624. [Google Scholar] [CrossRef]
- Lu, H.; Li, J.; Zhang, D.; Stoner, G.D.; Huang, C. Molecular mechanisms involved in chemoprevention of black raspberry extracts: From transcription factors to their target genes. Nutr. Cancer 2006, 54, 69–78. [Google Scholar] [CrossRef]
- Choung, M.G.; Hwang, Y.S.; Kim, G.P.; Ahn, K.G.; Shim, H.S.; Hong, S.B.; Choi, J.H.; Yu, C.Y.; Chung, I.M.; Kim, S.H.; et al. Antimelanogenic effect and whitening of anthocyanin rich fraction from seeds of liriope platyphylla. Korean J. Med. Crop. Sci. 2013, 21, 361–371. [Google Scholar] [CrossRef]
- Hwang, J.-M.; Kuo, H.-C.; Lin, C.-T.; Kao, E.-S. Inhibitory effect of liposome-encapsulated anthocyanin on melanogenesis in human melanocytes. Pharm. Biol. 2013, 51, 941–947. [Google Scholar] [CrossRef]
- Razina, T.G.; Zueva, E.P.; Ulrich, A.V.; Rybalkina, O.Y.; Chaikovskii, A.V.; Isaikina, N.V.; Kalinkina, G.I.; Zhdanov, V.V.; Zyuz’kov, G.N. Antitumor effects of sorbus aucuparia l. Extract highly saturated with anthocyans and their mechanisms. Bull. Exp. Biol. Med. 2016, 162, 93–97. [Google Scholar] [CrossRef] [PubMed]
- George, B.P.A.; Abrahamse, H.; Hemmaragala, N.M. Caspase dependent apoptotic inhibition of melanoma and lung cancer cells by tropical rubus extracts. Biomed. Pharmacother. 2016, 80, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Yanarojana, M.; Nararatwanchai, T.; Thairat, S.; Tancharoen, S. Antiproliferative activity and induction of apoptosis in human melanoma cells by houttuynia cordata thunb extract. Anticancer Res. 2017, 37, 6619–6628. [Google Scholar] [PubMed]
- Kanlayavattanakul, M.; Lourith, N.; Chaikul, P. Biological activity and phytochemical profiles of dendrobium: A new source for specialty cosmetic materials. Ind. Crop. Prod. 2018, 120, 61–70. [Google Scholar] [CrossRef]
- Su, C.-C.; Wang, C.-J.; Huang, K.-H.; Lee, Y.-J.; Chan, W.-M.; Chang, Y.-C. Anthocyanins from hibiscus sabdariffa calyx attenuate in vitro and in vivo melanoma cancer metastasis. J. Funct. Foods 2018, 48, 614–631. [Google Scholar] [CrossRef]
- Forni, C.; Frattarelli, A.; Lentini, A.; Beninati, S.; Lucioli, S.; Caboni, E. Assessment of the antiproliferative activity on murine melanoma cells of extracts from elicited cell suspensions of strawberry, strawberry tree, blackberry and red raspberry. Plant Biosyst. 2016, 150, 1233–1239. [Google Scholar] [CrossRef]
- Afaq, F.; Syed, D.N.; Malik, A.; Hadi, N.; Sarfaraz, S.; Kweon, M.-H.; Khan, N.; Zaid, M.A.; Mukhtar, H. Delphinidin, an anthocyanidin in pigmented fruits and vegetables, protects human hacat keratinocytes and mouse skin against uvb-mediated oxidative stress and apoptosis. J. Investig. Dermatol. 2007, 127, 222–232. [Google Scholar] [CrossRef] [Green Version]
- Zillich, O.V.; Schweiggert-Weisz, U.; Eisner, P.; Kerscher, M. Polyphenols as active ingredients for cosmetic products. Int. J. Cosmet. Sci. 2015, 37, 455–464. [Google Scholar] [CrossRef]
- Draelos, Z.D. Hair, sun, regulation, and beauty. J. Cosmet. Dermatol. 2014, 13, 1–2. [Google Scholar] [CrossRef] [Green Version]
- Pillai, S.; Cornell, M.; Oresajo, C. Epidermal Barrier Part 1: Skin Physiology Pertinent to Cosmetic Dermatology; L’Oréal Research: Clark, NJ, USA, 2018. [Google Scholar]
- Darlenski, R.; Kazandjieva, J.; Hristakieva, E.; Fluhr, J.W. Atopic dermatitis as a systemic disease. Clin. Dermatol. 2014, 32, 409–413. [Google Scholar] [CrossRef]
- Brown, S.J.; McLean, W.H. One remarkable molecule: Filaggrin. J. Investig. Dermatol. 2012, 132, 751–762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moser, K.; Kriwet, K.; Naik, A.; Kalia, Y.N.; Guy, R.H. Passive skin penetration enhancement and its quantification in vitro. Eur. J. Pharm. Biopharm. 2001, 52, 103–112. [Google Scholar] [CrossRef]
- Elias, P.M. Skin barrier function. Curr. Allergy Asthma Rep. 2008, 8, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Trommer, H.; Neubert, R.H.H. Overcoming the stratum corneum: The modulation of skin penetration. Ski. Pharmacol. Physiol. 2006, 19, 106–121. [Google Scholar] [CrossRef]
- Westfall, A. Evaluation of the Efficacy of Anthocyanins as Biologically Active Ingredients in Lipstick Formulations. Master’s Thesis, The Ohio State University, Columbus, OH, USA, 2015. [Google Scholar]
- Lai, J.; Xin, C.; Zhao, Y.; Feng, B.; He, C.; Dong, Y.; Fang, Y.; Wei, S. Study of active ingredients in black soybean sprouts and their safety in cosmetic use. Molecules 2012, 17, 11669–11679. [Google Scholar] [CrossRef]
- Plundrich, N.; Grace, M.H.; Raskin, I.; Ann Lila, M. Bioactive polyphenols from muscadine grape and blackcurrant stably concentrated onto protein-rich matrices for topical applications. Int. J. Cosmet. Sci. 2013, 35, 394–401. [Google Scholar] [CrossRef]

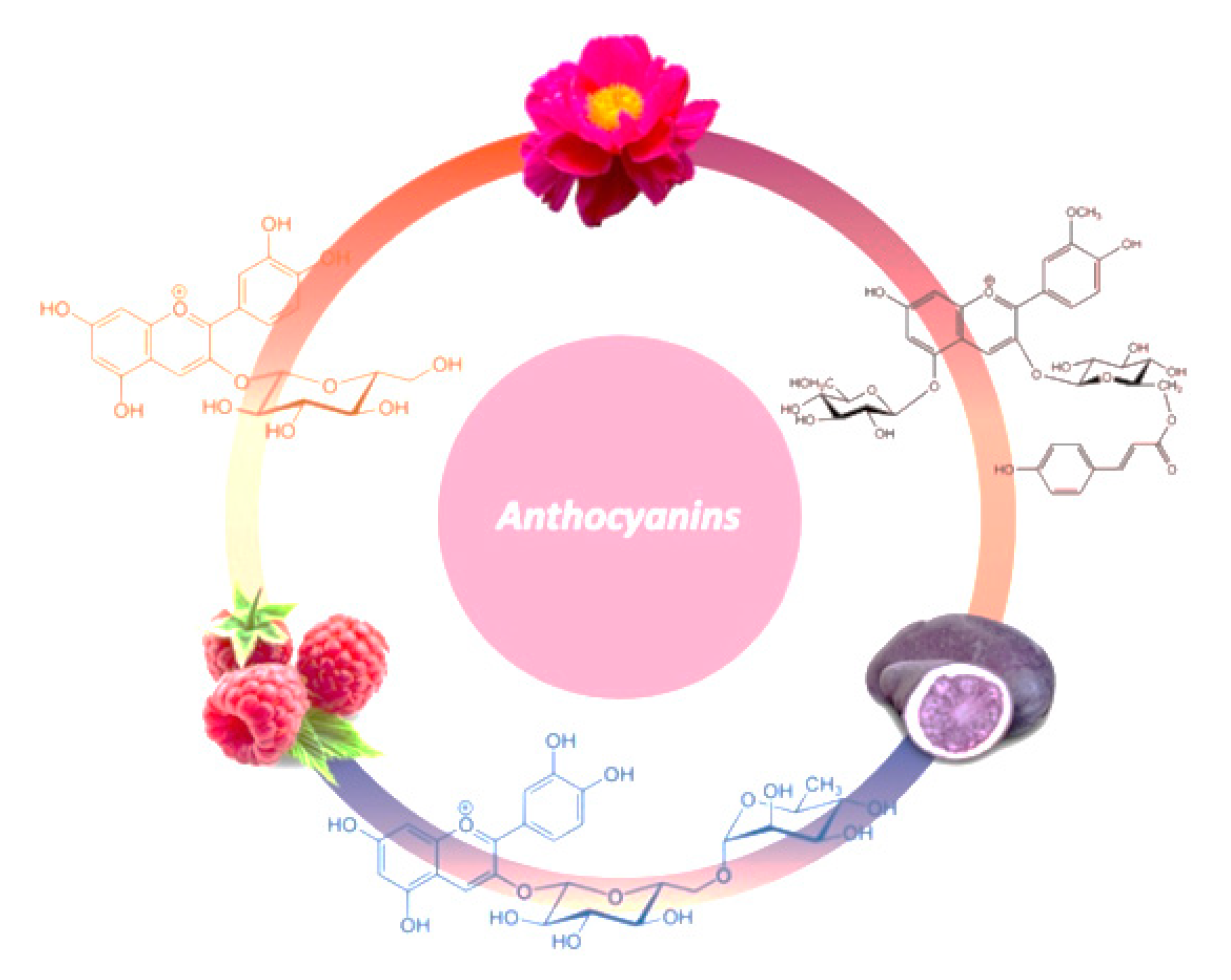

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diaconeasa, Z.; Știrbu, I.; Xiao, J.; Leopold, N.; Ayvaz, Z.; Danciu, C.; Ayvaz, H.; Stǎnilǎ, A.; Nistor, M.; Socaciu, C. Anthocyanins, Vibrant Color Pigments, and Their Role in Skin Cancer Prevention. Biomedicines 2020, 8, 336. https://doi.org/10.3390/biomedicines8090336
Diaconeasa Z, Știrbu I, Xiao J, Leopold N, Ayvaz Z, Danciu C, Ayvaz H, Stǎnilǎ A, Nistor M, Socaciu C. Anthocyanins, Vibrant Color Pigments, and Their Role in Skin Cancer Prevention. Biomedicines. 2020; 8(9):336. https://doi.org/10.3390/biomedicines8090336
Chicago/Turabian StyleDiaconeasa, Zorița, Ioana Știrbu, Jianbo Xiao, Nicolae Leopold, Zayde Ayvaz, Corina Danciu, Huseyin Ayvaz, Andreea Stǎnilǎ, Mǎdǎlina Nistor, and Carmen Socaciu. 2020. "Anthocyanins, Vibrant Color Pigments, and Their Role in Skin Cancer Prevention" Biomedicines 8, no. 9: 336. https://doi.org/10.3390/biomedicines8090336
APA StyleDiaconeasa, Z., Știrbu, I., Xiao, J., Leopold, N., Ayvaz, Z., Danciu, C., Ayvaz, H., Stǎnilǎ, A., Nistor, M., & Socaciu, C. (2020). Anthocyanins, Vibrant Color Pigments, and Their Role in Skin Cancer Prevention. Biomedicines, 8(9), 336. https://doi.org/10.3390/biomedicines8090336














