Molecular Mechanisms of KDELC2 on Glioblastoma Tumorigenesis and Temozolomide Resistance
Abstract
1. Introduction
2. Materials and Methods
2.1. In Silico Study
2.2. Normal Brain Lysates, Human Glioma Cell Preparation, and Western Blot Analysis
2.3. RNA Isolation and Real-Time Reverse Quantitative Transcription-Polymerase Chain Reaction (qRT-PCR)
2.4. Stable Expression of shRNAs
2.5. MTT Assay
2.6. Wound Healing and Cell Invasion Assays
2.7. Gelatin Zymography
2.8. Flow Cytometry Analysis
2.9. Immunofluorescence (IF) Staining
2.10. Three-Dimensional (3D) Collagen Spheroid Assay
2.11. Tube Formation Assay
2.12. Orthotopic Xenograft Animal Model
2.13. Tissue Microarray Slide Preparation and Immunohistochemical (IHC) Staining
2.14. Assessment of IHC Scores of KDELC2 in Human Glioma Tissues
2.15. Statistical Analysis of KDELC2 Expression and Overall Survival Time of Human Glioma Tissues
3. Results
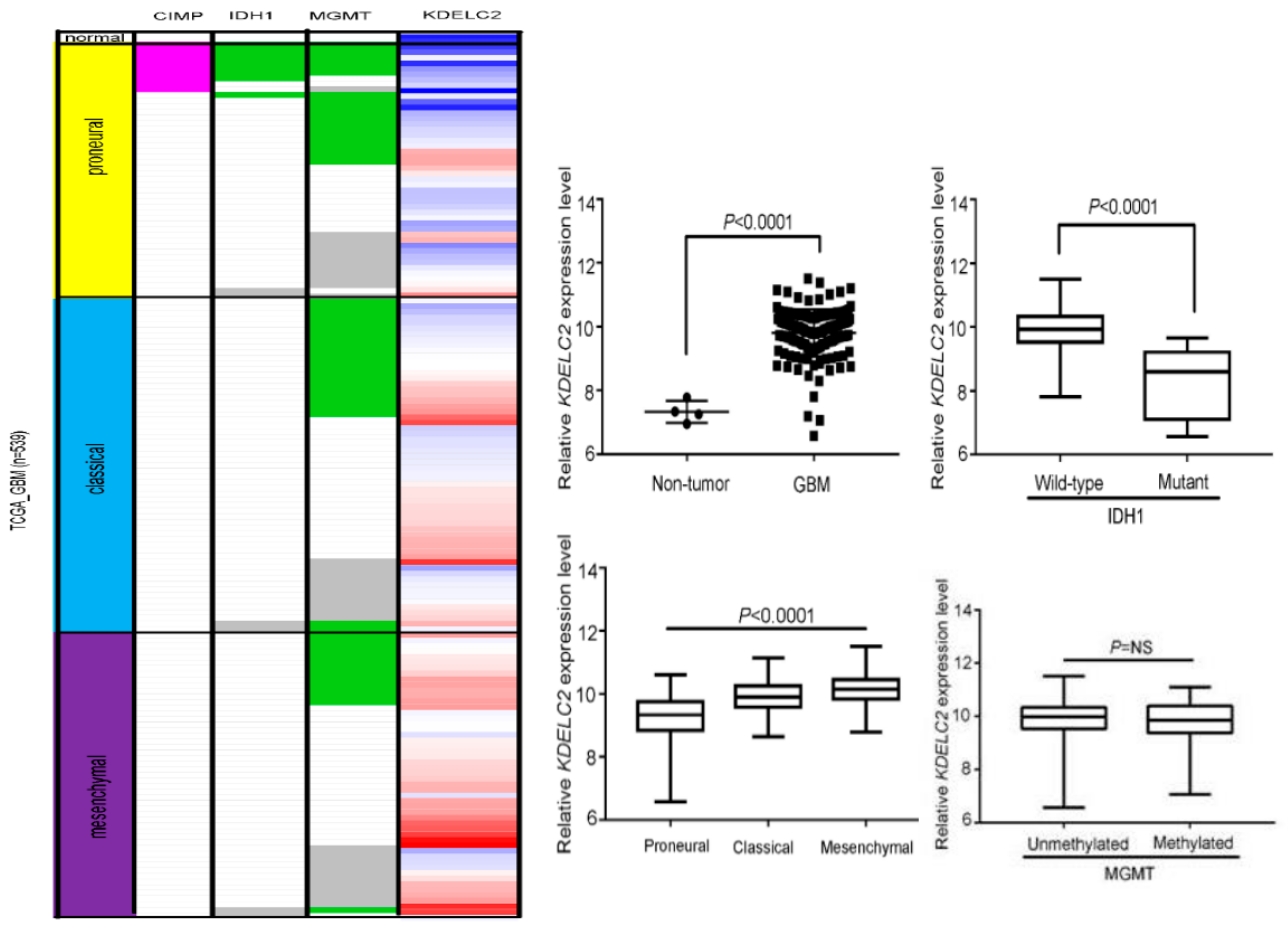
3.1. KDELC2 mRNA Expression Correlates with Non-GCIMP and IDH1 Wild-Type GBMs in the TCGA Database
3.2. KDELC2 mRNA Expression Correlated with WHO Classification, IDH1 Status, and 1p/19q Co-Deletion of Gliomas in the CGGA Database
3.3. KDELC2 Overexpression in GBM Cell Lines
3.4. KDELC2 Knockdown Suppressed Tumor Proliferation in GBMs
3.5. KDELC2 Knockdown Interrupted Cell Cycle of GBMs
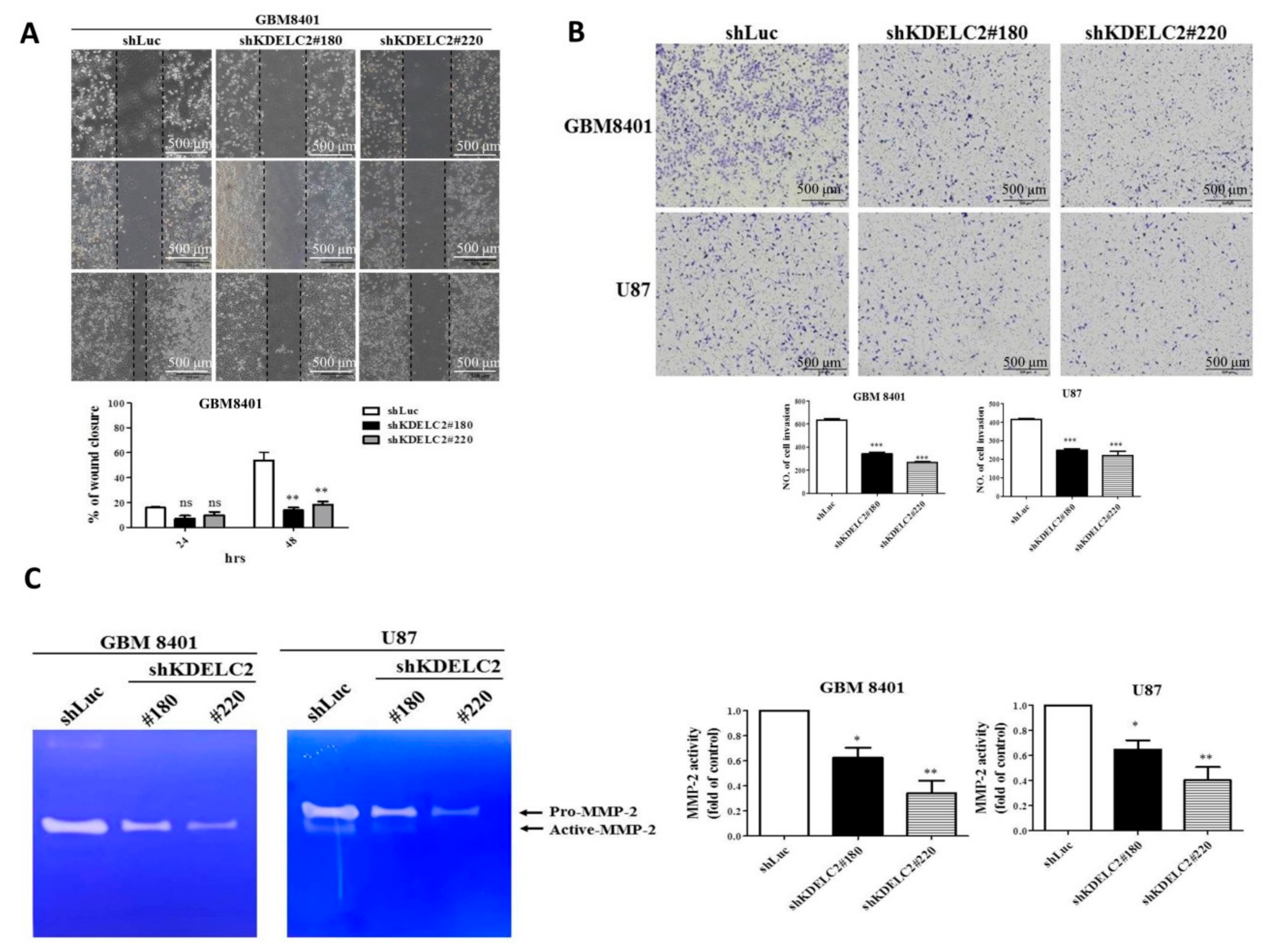
3.6. KDELC2 Knockdown Inhibited Tumor Migration and Invasion of GBMs by Downregulating Matrix Metalloproteinase-2 (MMP2) Expression
3.7. KDELC2 Knockdown Suppressed 3D Tumor Spheroid Formation and Stemness Factors’ Expression of GBMs
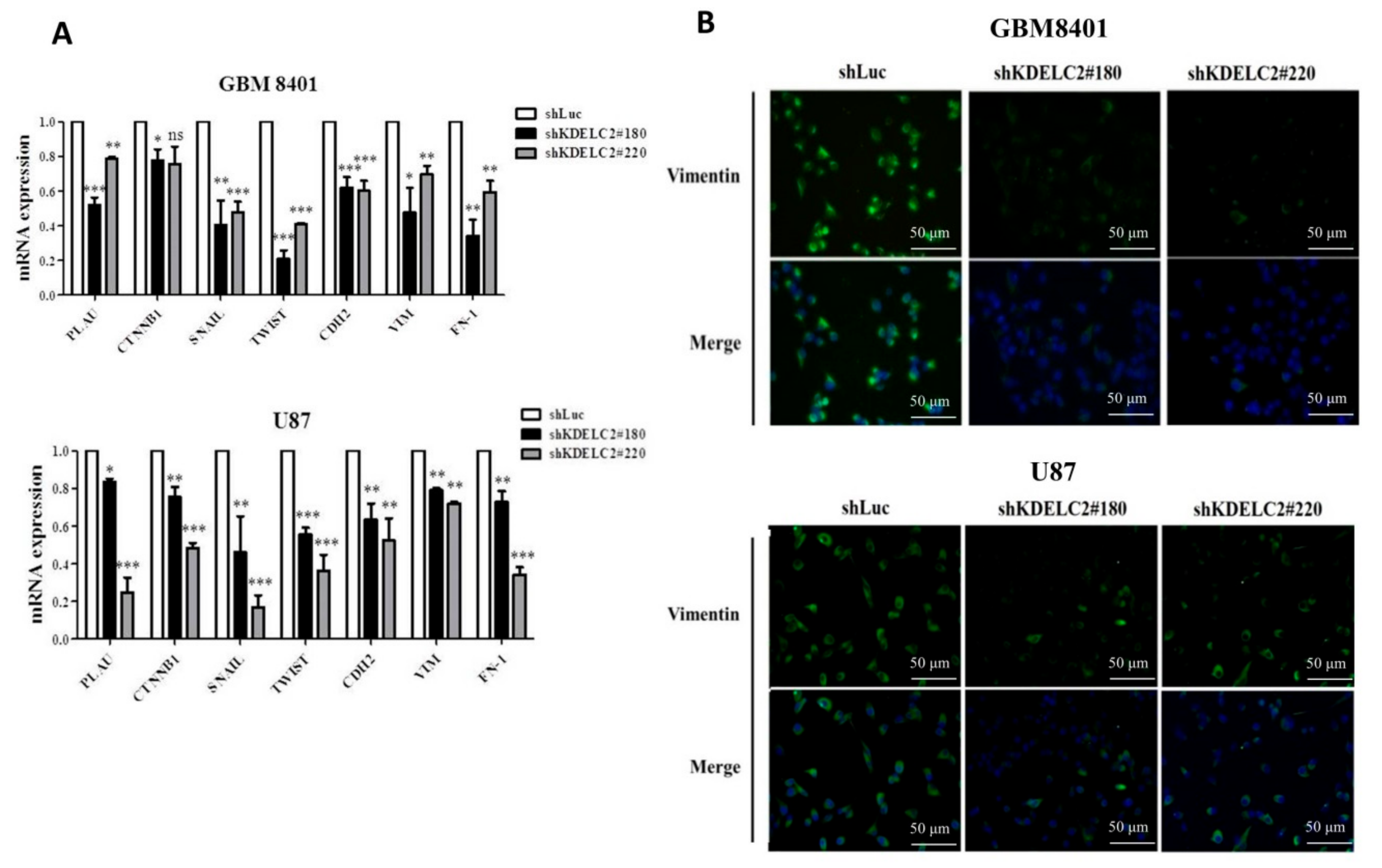
3.8. KDELC2 Knockdown Inhibited Epithelial–Mesenchymal Transition of GBMs
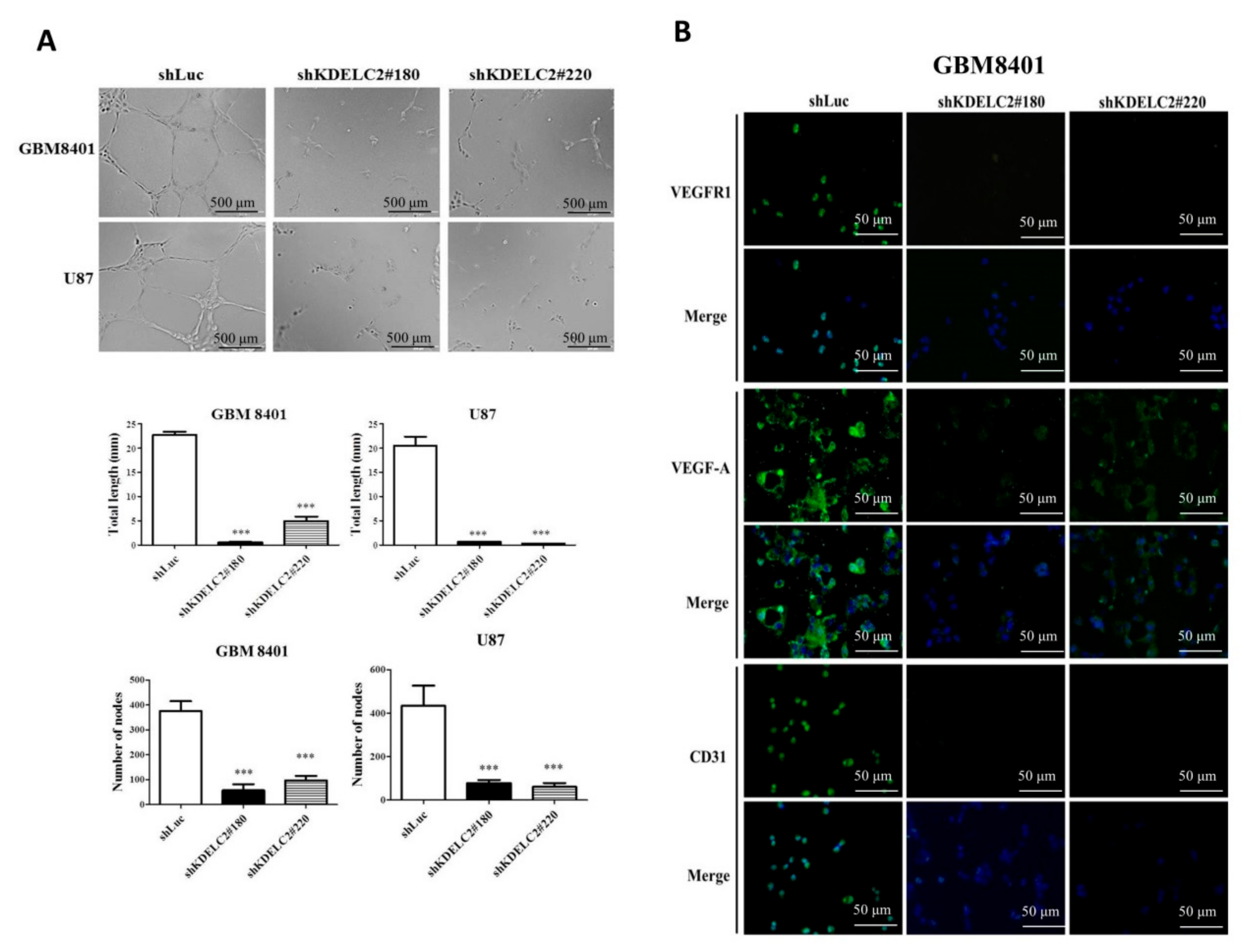
3.9. Downregulation of KDELC2 Suppressed GBM Angiogenesis
3.10. KDELC2 Knockdown Downregulated PI3k/mTOR/Akt, MAPK/ERK, and NF/kB Signaling Pathways by Suppression of Notch Receptor Expression
3.11. KDELC2 Knockdown Promoted TMZ Cytotoxic Effect by Decreasing MGMT Expression
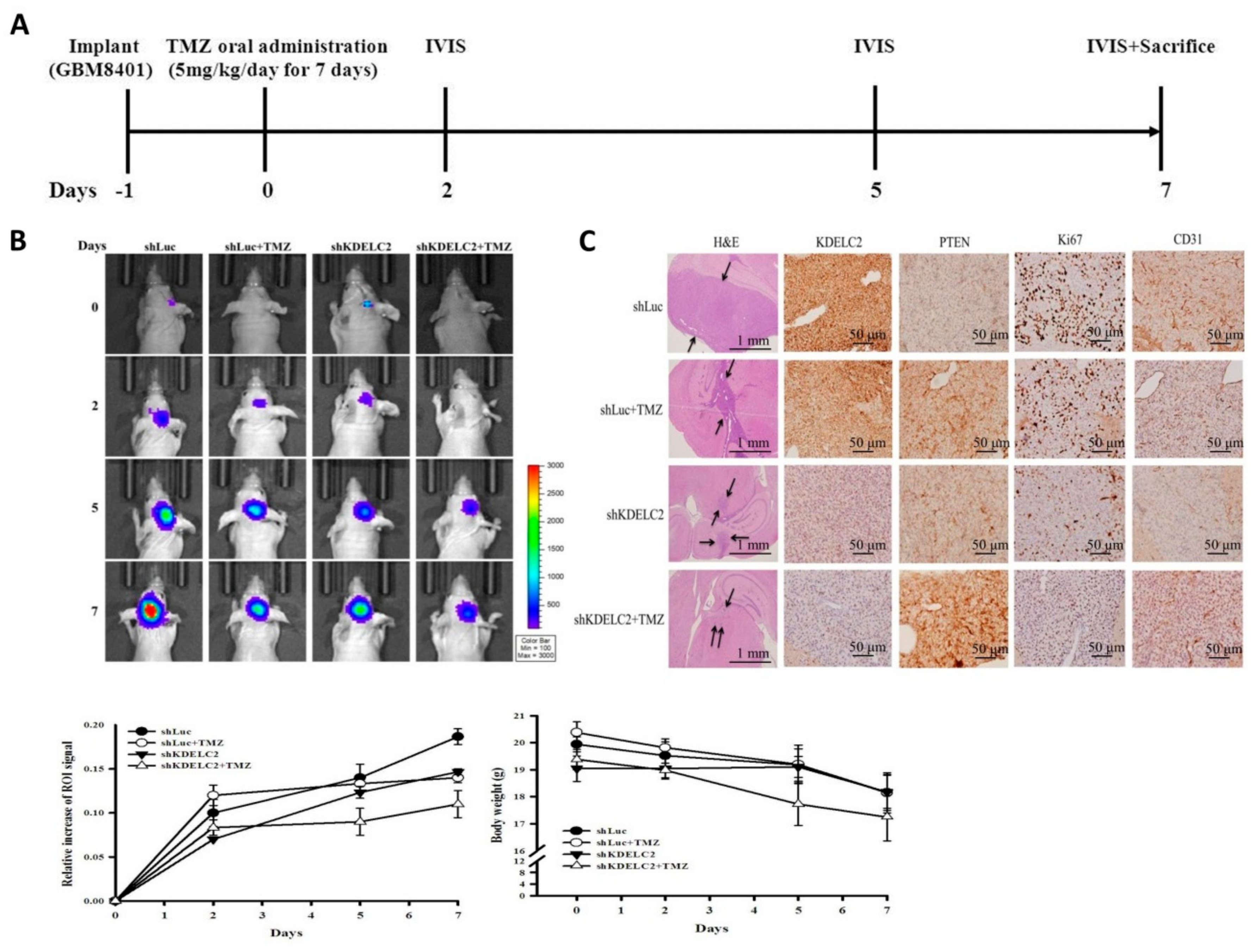
3.12. KDELC2 Knockdown Inhibited Tumor Proliferation and Angiogenesis in Orthotropic Human GBM Xenograft Mouse Models
3.13. Higher KDELC2 Expression Correlated with Advanced Tumor Grades and Poor Prognosis in Human Glioma Tissue Microarrays
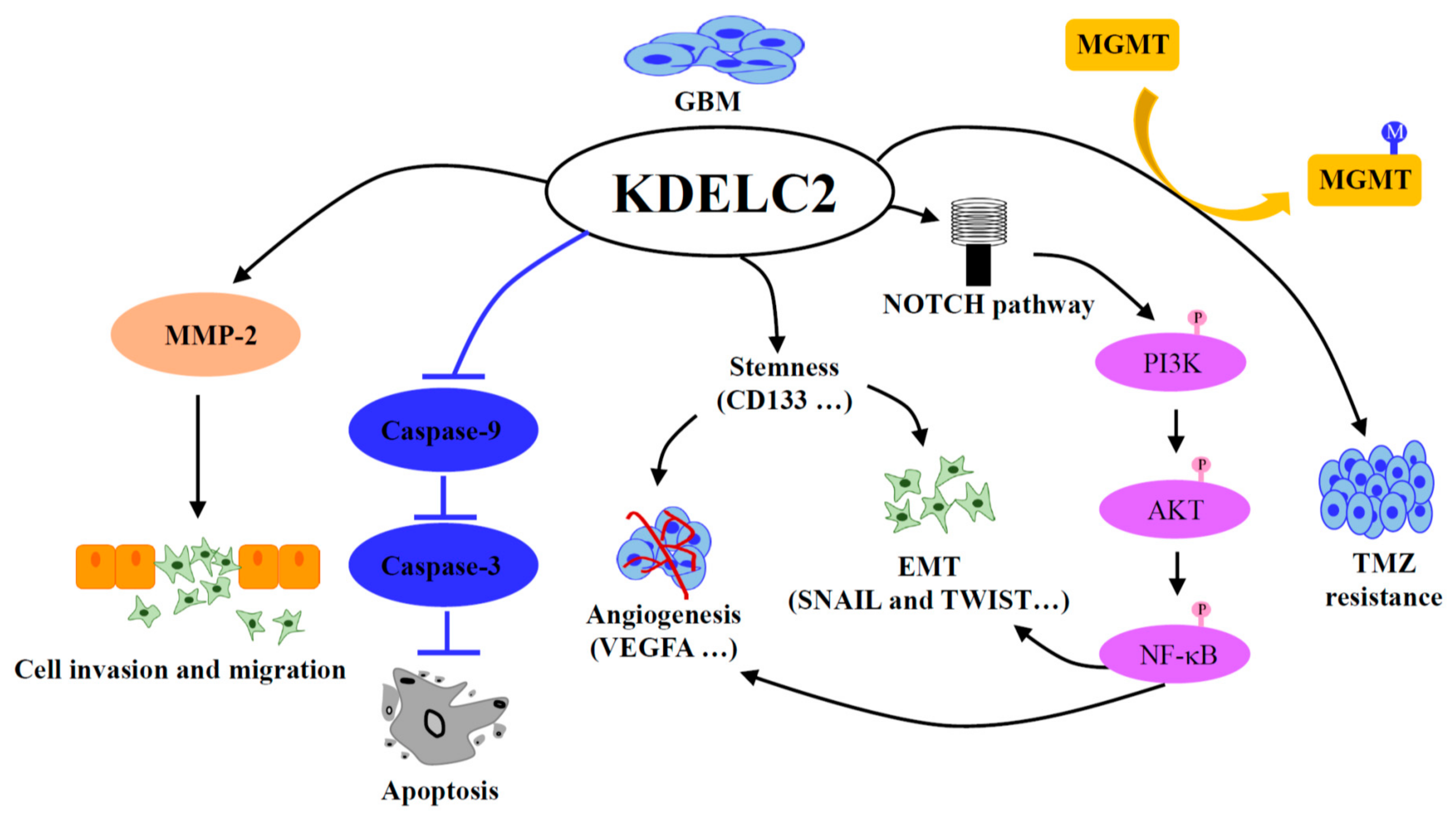
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- GBD 2016 Brain and Other CNS Cancer Collaborators. Global, regional, and national burden of brain and other CNS cancer, 1990-2016: A systematic analysis for the global burden of disease study 2016. Lancet Neurol. 2019, 18, 376–393. [Google Scholar] [CrossRef]
- Guruharsha, K.G.; Kankel, M.W.; Artavanis-Tsakonas, S. The Notch signalling system: Recent insights into the complexity of a conserved pathway. Nat. Rev. Genet. 2012, 13, 654–666. [Google Scholar] [CrossRef] [PubMed]
- Andersson, E.R.; Sandberg, R.; Lendahl, U. Notch signaling: Simplicity in design, versatility in function. Development 2011, 138, 3593–3612. [Google Scholar] [CrossRef] [PubMed]
- Faigle, R.; Song, H. Signaling mechanisms regulating adult neural stem cells and neurogenesis. Biochim. Biophys. Acta 2013, 1830, 2435–2448. [Google Scholar] [CrossRef]
- Morrison, S.J.; Perez, S.E.; Qiao, Z.; Verdi, J.M.; Hicks, C.; Weinmaster, G.; Anderson, D.J. Transient Notch activation initiates an irreversible switch from neurogenesis to gliogenesis by neural crest stem cells. Cell 2000, 101, 499–510. [Google Scholar] [CrossRef]
- Jiang, X.; Xing, H.; Kim, T.M.; Jung, Y.; Huang, W.; Yang, H.W.; Song, S.; Park, P.J.; Carroll, R.S.; Johnson, M.D. Numb regulates glioma stem cell fate and growth by altering epidermal growth factor receptor and Skp1-Cullin-F-box ubiquitin ligase activity. Stem Cells 2012, 30, 1313–1326. [Google Scholar] [CrossRef]
- Li, L.; Liu, X.; Ma, X.; Deng, X.; Ji, T.; Hu, P.; Wan, R.; Qiu, H.; Cui, D.; Gao, L. Identification of key candidate genes and pathways in glioblastoma by integrated bioinformatical analysis. Exp. Ther. Med. 2019, 18, 3439–3449. [Google Scholar] [CrossRef]
- Moloney, D.J.; Shair, L.H.; Lu, F.M.; Xia, J.; Locke, R.; Matta, K.L.; Haltiwanger, R.S. Mammalian Notch1 is modified with two unusual forms of O-linked glycosylation found on epidermal growth factor-like modules. J. Biol. Chem. 2000, 275, 9604–9611. [Google Scholar] [CrossRef]
- Haines, N.; Irvine, K.D. Glycosylation regulates Notch signalling. Nat. Rev. Mol. Cell. Biol. 2003, 4, 786–797. [Google Scholar] [CrossRef]
- Matsuura, A.; Ito, M.; Sakaidani, Y.; Kondo, T.; Murakami, K.; Furukawa, K.; Nadano, D.; Matsuda, T.; Okajima, T. O-linked N-acetylglucosamine is present on the extracellular domain of notch receptors. J. Biol. Chem. 2008, 283, 35486–35495. [Google Scholar] [CrossRef]
- Takeuchi, H.; Schneider, M.; Williamson, D.B.; Ito, A.; Takeuchi, M.; Handford, P.A.; Haltiwanger, R.S. Two novel protein O-glucosyltransferases that modify sites distinct from POGLUT1 and affect Notch trafficking and signaling. Proc. Natl. Acad. Sci. USA 2018, 115, E8395–E8402. [Google Scholar] [CrossRef]
- Jafar-Nejad, H.; Leonardi, J.; Fernandez-Valdivia, R. Role of glycans and glycosyltransferases in the regulation of Notch signaling. Glycobiology 2010, 20, 931–949. [Google Scholar] [CrossRef] [PubMed]
- Okajima, T.; Irvine, K.D. Regulation of notch signaling by o-linked fucose. Cell 2002, 111, 893–904. [Google Scholar] [CrossRef]
- Shi, S.; Stanley, P. Protein O-fucosyltransferase 1 is an essential component of Notch signaling pathways. Proc. Natl. Acad. Sci. USA 2003, 100, 5234–5239. [Google Scholar] [CrossRef] [PubMed]
- Sasamura, T.; Sasaki, N.; Miyashita, F.; Nakao, S.; Ishikawa, H.O.; Ito, M.; Kitagawa, M.; Harigaya, K.; Spana, E.; Bilder, D.; et al. Neurotic, a novel maternal neurogenic gene, encodes an O-fucosyltransferase that is essential for Notch-Delta interactions. Development 2003, 130, 4785–4795. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Valdivia, R.; Takeuchi, H.; Samarghandi, A.; Lopez, M.; Leonardi, J.; Haltiwanger, R.S.; Jafar-Nejad, H. Regulation of mammalian Notch signaling and embryonic development by the protein O-glucosyltransferase Rumi. Development 2011, 138, 1925–1934. [Google Scholar] [CrossRef]
- Chang, Y.C.; Tsai, H.F.; Huang, S.P.; Chen, C.L.; Hsiao, M.; Tsai, W.C. Enrichment of aldolase C correlates with low non-mutated IDH1 expression and predicts a favorable prognosis in glioblastomas. Cancers 2019, 11, 1238. [Google Scholar] [CrossRef]
- Chen, Y.H.; Hueng, D.Y.; Tsai, W.C. Proteolipid protein 2 overexpression indicates aggressive tumor behavior and adverse prognosis in human gliomas. Int. J. Mol. Sci. 2018, 19, 3353. [Google Scholar] [CrossRef]
- Bao, S.; Wu, Q.; McLendon, R.E.; Hao, Y.; Shi, Q.; Hjelmeland, A.B.; Dewhirst, M.W.; Bigner, D.D.; Rich, J.N. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 2006, 444, 756–760. [Google Scholar] [CrossRef]
- Liebelt, B.D.; Shingu, T.; Zhou, X.; Ren, J.; Shin, S.A.; Hu, J. Glioma stem cells: Signaling, microenvironment, and therapy. Stem Cells Int. 2016, 2016, 7849890. [Google Scholar] [CrossRef]
- Mei, X.; Chen, Y.S.; Chen, F.R.; Xi, S.Y.; Chen, Z.P. Glioblastoma stem cell differentiation into endothelial cells evidenced through live-cell imaging. Neuro Oncol. 2017, 19, 1109–1118. [Google Scholar] [CrossRef] [PubMed]
- Bazzoni, R.; Bentivegna, A. Role of Notch signaling pathway in glioblastoma pathogenesis. Cancers 2019, 11, 292. [Google Scholar] [CrossRef] [PubMed]
- Verhaak, R.G.; Hoadley, K.A.; Purdom, E.; Wang, V.; Qi, Y.; Wilkerson, M.D.; Miller, C.R.; Ding, L.; Golub, T.; Mesirov, J.P.; et al. Cancer Genome Atlas Research Network. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 2010, 17, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Guardia, G.D.A.; Correa, B.R.; Araujo, P.R.; Qiao, M.; Burns, S.; Penalva, L.O.F.; Galante, P.A.F. Proneural and mesenchymal glioma stem cells display major differences in splicing and lncRNA profiles. NPJ Genom. Med. 2020, 5, 2. [Google Scholar] [CrossRef]
- Bocangel, D.B.; Finkelstein, S.; Schold, S.C.; Bhakat, K.K.; Mitra, S.; Kokkinakis, D.M. Multifaceted resistance of gliomas to temozolomide. Clin. Cancer Res. 2002, 8, 2725–2734. [Google Scholar]
- Hegi, M.E.; Diserens, A.C.; Gorlia, T.; Hamou, M.F.; de Tribolet, N.; Weller, M.; Kros, J.M.; Hainfellner, J.A.; Mason, W.; Mariani, L.; et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N. Engl. J. Med. 2005, 352, 997–1003. [Google Scholar] [CrossRef]







| Number of Cases | Average Intensity | Average Tumor (%) | Average Score | Difference or Correlation * | |
|---|---|---|---|---|---|
| Normal brain tissue | 5 | 0.67 | 6.67 | 6.67 | |
| Classification of gliomas | |||||
| Pilocytic astrocytoma | 1 | 0 | 0 | 0 | |
| Diffuse astrocytoma, IDH-mutant | 2 | 0 | 0 | 0 | Significant difference (p < 0.001 *) |
| Diffuse astrocytoma, IDH-WT | 12 | 1.17 | 24.58 | 43.33 | |
| Anaplastic astrocytoma, IDH-mutant | 3 | 0.67 | 3.33 | 3.33 | Significant difference (p = 0.012 *) |
| Anaplastic astrocytoma, IDH-WT | 6 | 1.14 | 23.57 | 50.71 | |
| Glioblastoma, IDH-mutant | 4 | 1.33 | 17.5 | 31.67 | Significant difference (p = 0.039 *) |
| Glioblastoma, IDH-WT | 33 | 1.69 | 42.14 | 85.14 | |
| Diffuse midline glioma, H3 K27M-mutant | 8 | 1.5 | 42.86 | 72.86 | |
| Oligodendroglioma, IDH-mutant | 1 | 0 | 0 | 0 | Not significant difference (p = 1) |
| Oligodendroglioma, IDH-WT | 2 | 0 | 0 | 0 | |
| Anaplastic oligodendroglioma, IDH-mutant | 2 | 0.25 | 13.75 | 13.75 | No significant difference (p = 0.258) |
| Anaplastic oligodendroglioma, IDH-WT | 2 | 1.5 | 32.5 | 57.5 | |
| WHO grades of gliomas | |||||
| WHO grade I | 1 | 0 | 0 | 0 | Positive correlation (p < 0.001 #) |
| WHO grade II | 17 | 0.83 | 16.67 | 29.17 | Positive correlation (p < 0.001 #) |
| WHO grade III | 13 | 0.88 | 18.44 | 33.44 | |
| WHO grade IV | 45 | 1.6 | 39.64 | 76.18 |
| Variable | Total | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | ||
| Sex | |||||
| Male | 47 | 1 | |||
| Female | 29 | 1.56 (1.48–1.73) | 0.064 | 1.61 (1.51–1.84) | 0.116 |
| Age | |||||
| <50 | 36 | 1 | |||
| ≥50 | 40 | 2.64 (1.70–8.90) | 0.017 * | 1.99 (1.54–3.83) | 0.100 |
| IDH1 R132H | |||||
| Negative | 63 | 1 | |||
| Positive | 13 | 0.25 (0.11–0.62) | 0.009 * | 0.27 (0.10–0.65) | 0.109 |
| ATRX | |||||
| Preserve | 39 | 1 | |||
| Loss of expression | 37 | 0.83 (0.69–0.81) | 0.276 | 0.70 (0.59–0.76) | 0.116 |
| H3K27M | |||||
| Negative | 67 | 1 | |||
| Positive | 9 | 1.26 (0.79–1.53) | 0.485 | 1.23 (0.77–1.49) | 0.212 |
| MGMT | |||||
| Preserved | 43 | 1 | |||
| Loss of expression | 33 | 1.10 (1.05–1.22) | 0.678 | 1.08 (1.04–1.15) | <0.001 * |
| EGFR | |||||
| Negative | 69 | 1 | |||
| Positive | 7 | 1.55 (0.94–1.90) | 0.228 | 1.52 (1.10–1.86) | 0.357 |
| EGFRvIII | |||||
| Negative | 58 | 1 | |||
| Positive | 18 | 2.28 (2.17–2.52) | <0.001 * | 2.49 (2.29–2.92) | 0.004 * |
| P53 | |||||
| Negative | 36 | 1 | |||
| Overexpression | 40 | 1.19 (1.10–1.35) | 0.482 | 1.11 (1.05–1.25) | 0.034 * |
| Neurofilament | |||||
| Negative | 59 | 1 | |||
| Positive | 17 | 0.59 (0.18–0.85) | 0.126 | 0.62 (0.18–0.87) | 0.253 |
| NF1 | |||||
| Negative | 42 | 1 | |||
| Positive | 34 | 1.58 (1.42–1.89) | 0.063 | 1.51 (1.37–1.85) | 0.001 * |
| AxL | |||||
| Negative | 29 | 1 | |||
| Positive | 47 | 2.11 (1.58–4.58) | 0.008 * | 1.95 (1.46–4.46) | <0.001 * |
| p-AxL | |||||
| Negative | 22 | 1 | |||
| Positive | 54 | 1.36 (1.01–3.09) | 0.354 | 1.29 (0.98–2.90) | 0.013 * |
| NUR77 | |||||
| Negative | 27 | 1 | |||
| Positive | 49 | 1.89 (1.45–3.70) | 0.024 * | 2.13 (1.59–4.99) | 0.006 * |
| H3Lys27 | |||||
| Preserved | 67 | 1 | |||
| Loss of expression | 9 | 3.25 (1.33–9.98) | 0.039 * | 6.08 (1.54–14.36) | 0.001 * |
| PDGFRA | |||||
| Negative | 5 | 1 | |||
| Positive | 71 | 0.71 (0.23–1.27) | 0.528 | 1.43 (1.25–5.25) | 0.721 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, Y.-L.; Chang, H.-H.; Chen, Y.-C.; Chang, Y.-C.; Chen, Y.; Tsai, W.-C. Molecular Mechanisms of KDELC2 on Glioblastoma Tumorigenesis and Temozolomide Resistance. Biomedicines 2020, 8, 339. https://doi.org/10.3390/biomedicines8090339
Tsai Y-L, Chang H-H, Chen Y-C, Chang Y-C, Chen Y, Tsai W-C. Molecular Mechanisms of KDELC2 on Glioblastoma Tumorigenesis and Temozolomide Resistance. Biomedicines. 2020; 8(9):339. https://doi.org/10.3390/biomedicines8090339
Chicago/Turabian StyleTsai, Yu-Ling, Hsin-Han Chang, Ying-Chuan Chen, Yu-Chan Chang, Ying Chen, and Wen-Chiuan Tsai. 2020. "Molecular Mechanisms of KDELC2 on Glioblastoma Tumorigenesis and Temozolomide Resistance" Biomedicines 8, no. 9: 339. https://doi.org/10.3390/biomedicines8090339
APA StyleTsai, Y.-L., Chang, H.-H., Chen, Y.-C., Chang, Y.-C., Chen, Y., & Tsai, W.-C. (2020). Molecular Mechanisms of KDELC2 on Glioblastoma Tumorigenesis and Temozolomide Resistance. Biomedicines, 8(9), 339. https://doi.org/10.3390/biomedicines8090339








