Regulation of JAM2 Expression in the Lungs of Streptozotocin-Induced Diabetic Mice and Human Pluripotent Stem Cell-Derived Alveolar Organoids
Abstract
1. Introduction
2. Materials and Methods
2.1. hPVC Cultures and HG Treatment
2.2. Cell Proliferation Assay
2.3. hPVC Differentiation
2.4. Preparation of MSC-CM
2.5. Mice and CM Administration
2.6. Morphological Analysis of the Mouse Lung
2.7. Immunofluorescence Staining
2.8. Microarray and Data Analysis
2.9. Real-Time Quantitative PCR
2.10. Chromatin Precipitation (ChIP)
2.11. Western Blot Analysis
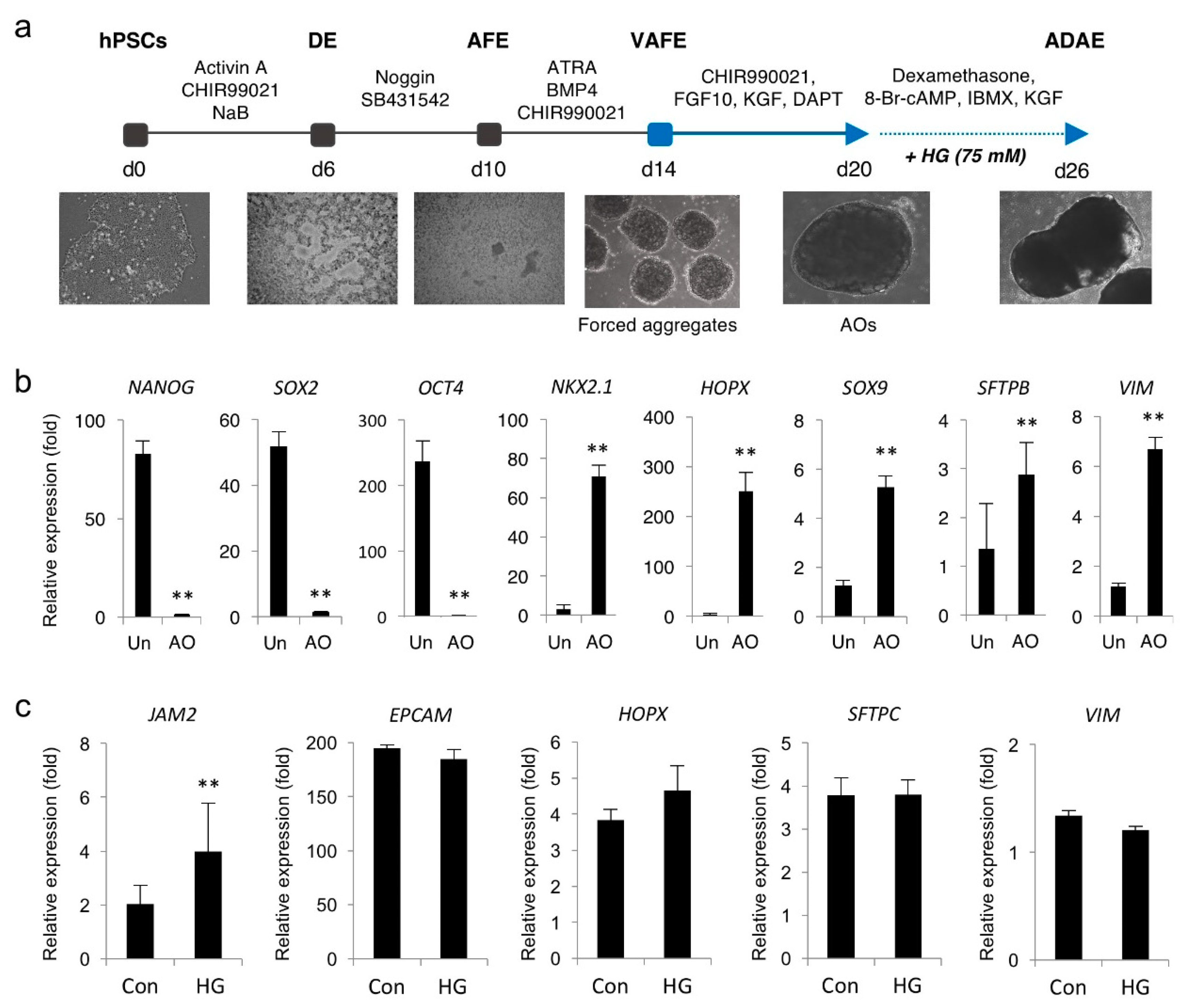
2.12. Generation of Multicellular AOs from hPSCs and HG Treatment
2.13. Statistical Analysis
3. Results
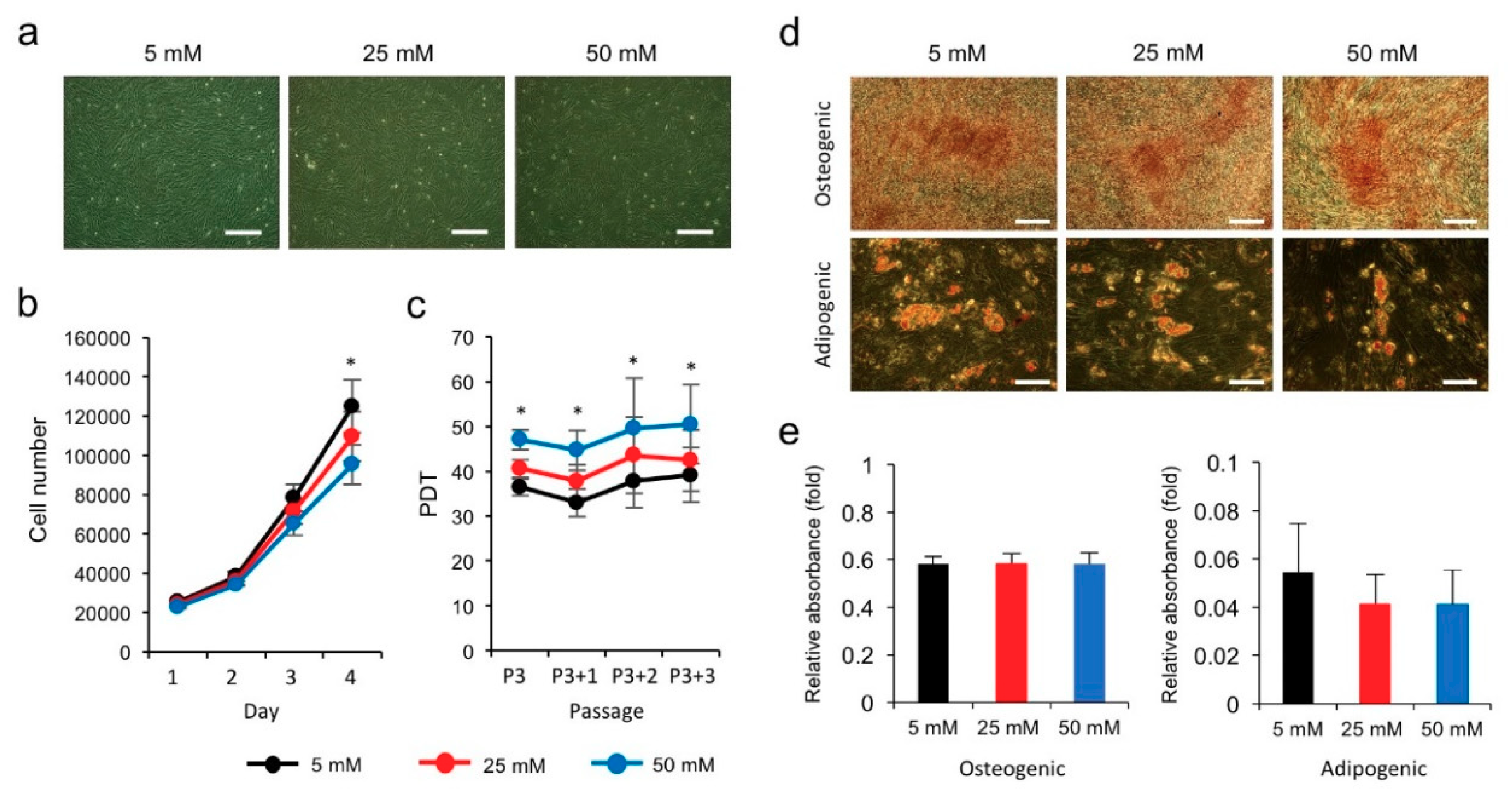
3.1. High Glucose Inhibits the Proliferation of hPVCs
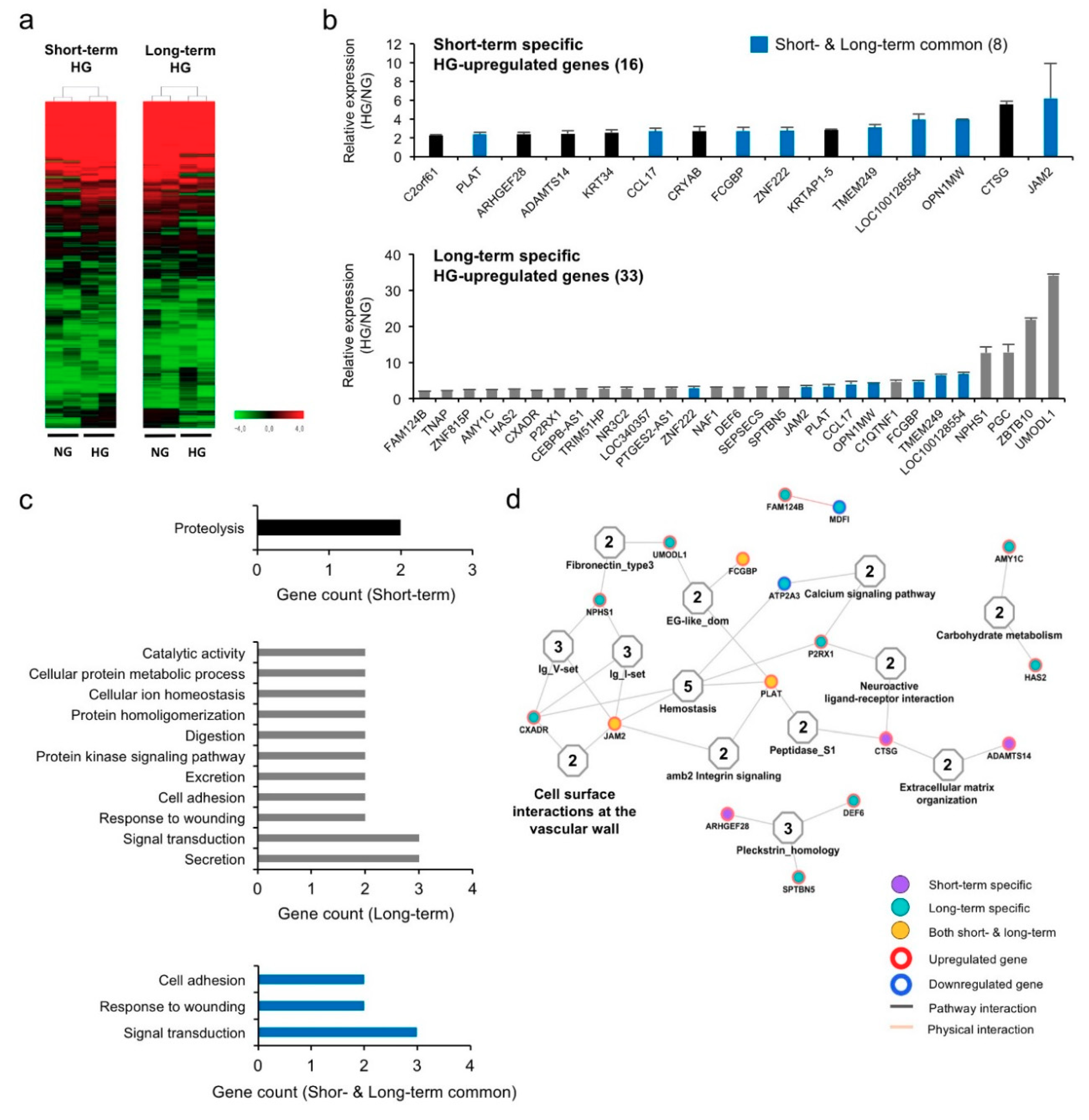
3.2. Hyperglycemia Alters Gene Expression Profiles in hPVCs
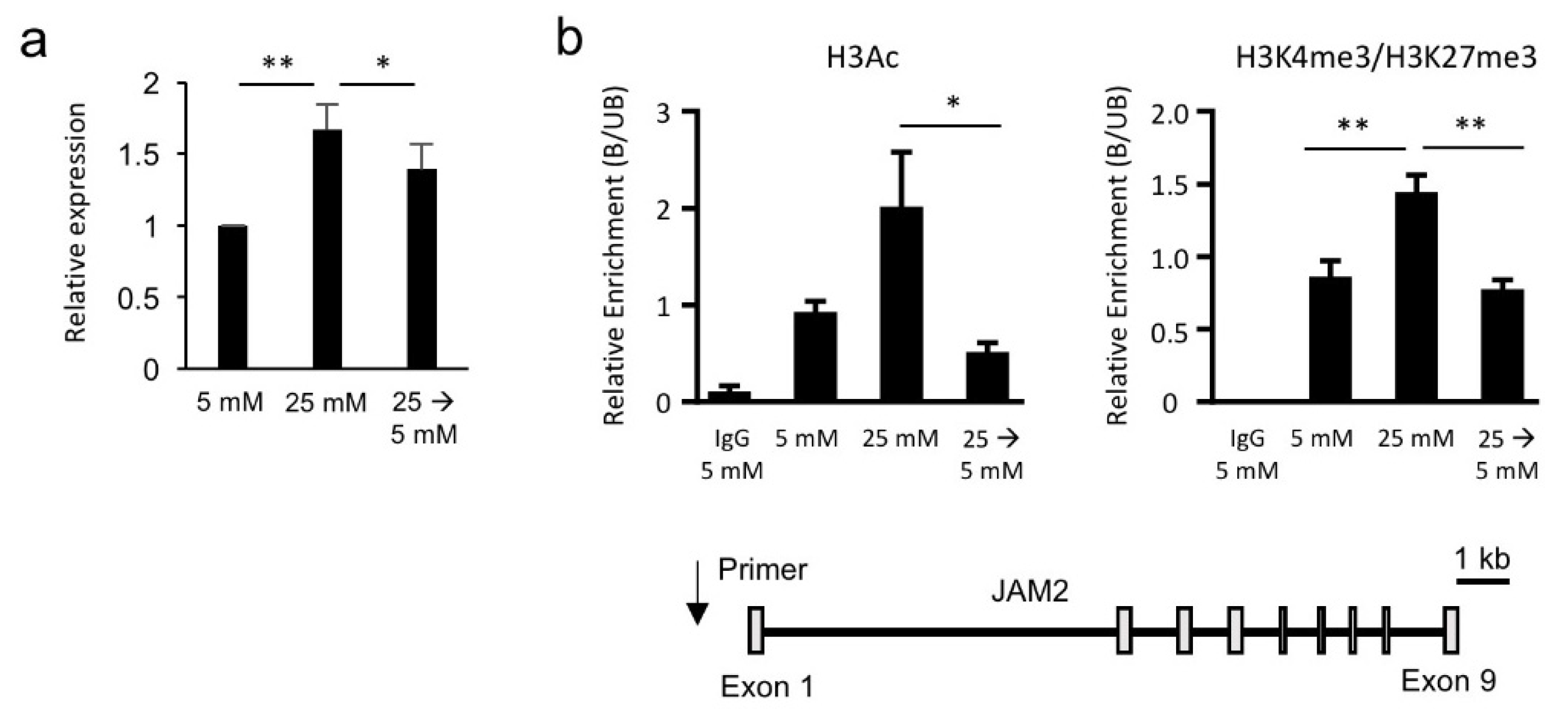
3.3. Hyperglycemia-Induced JAM2 Expression Is Transcriptionally and Epigenetically Reversible
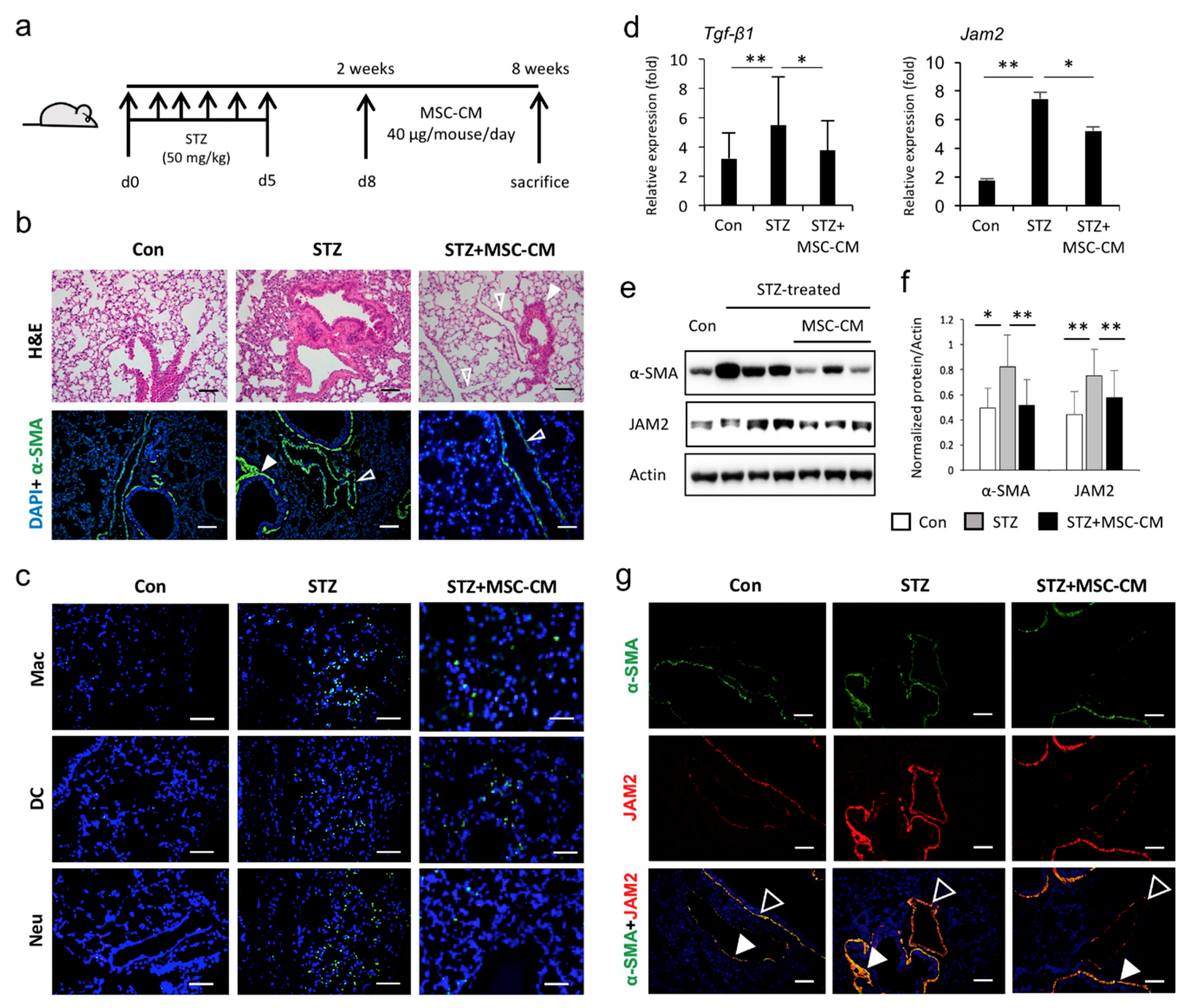
3.4. Enhanced JAM2 Expression and Histophysiological Alterations in Lung Tissue of Diabetic Mice Were Reduced via Paracrine Action of MSCs
3.5. Hyperglycemia Induces JAM2 Upregulation in hPSC-Derived AOs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Rask-Madsen, C.; King, G.L. Vascular complications of diabetes: Mechanisms of injury and protective factors. Cell Metab. 2013, 17, 20–33. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Lee, J.Y.; Ha, K.S.; Han, E.T.; Park, W.S.; Min, C.K.; Hong, S.H. Perivascular Cells and NADPH Oxidase Inhibition Partially Restore Hyperglycemia-Induced Alterations in Hematopoietic Stem Cell and Myeloid-Derived Suppressor Cell Populations in the Bone Marrow. Int. J. Stem Cells 2018, 12, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Oguntibeju, O.O. Type 2 diabetes mellitus, oxidative stress and inflammation: Examining the links. Int. J. Physiol. Pathophysiol. Pharmacol. 2019, 11, 45–63. [Google Scholar] [PubMed]
- Fintha, A.; Gasparics, A.; Rosivall, L.; Sebe, A. Therapeutic Targeting of Fibrotic Epithelial-Mesenchymal Transition-An Outstanding Challenge. Front. Pharmacol. 2019, 10, 388. [Google Scholar] [CrossRef]
- Talakatta, G.; Sarikhani, M.; Muhamed, J.; Dhanya, K.; Somashekar, B.S.; Mahesh, P.A.; Sundaresan, N.; Ravindra, P.V. Diabetes induces fibrotic changes in the lung through the activation of TGF-beta signaling pathways. Sci. Rep. 2018, 8, 11920. [Google Scholar] [CrossRef]
- Lei, S.; Liu, Y.; Liu, H.; Yu, H.; Wang, H.; Xia, Z. Effects of N-acetylcysteine on nicotinamide dinucleotide phosphate oxidase activation and antioxidant status in heart, lung, liver and kidney in streptozotocin-induced diabetic rats. Yonsei Med. J. 2012, 53, 294–303. [Google Scholar] [CrossRef]
- Oztay, F.; Sancar-Bas, S.; Gezginci-Oktayoglu, S.; Ercin, M.; Bolkent, S. Exendin-4 partly ameliorates-hyperglycemia-mediated tissue damage in lungs of streptozotocin-induced diabetic mice. Peptides 2018, 99, 99–107. [Google Scholar] [CrossRef]
- Pitocco, D.; Fuso, L.; Conte, E.G.; Zaccardi, F.; Condoluci, C.; Scavone, G.; Incalzi, R.A.; Ghirlanda, G. The diabetic lung--a new target organ? Rev. Diabet. Stud. 2012, 9, 23–35. [Google Scholar] [CrossRef]
- Ehrlich, S.F.; Quesenberry, C.P., Jr.; Van Den Eeden, S.K.; Shan, J.; Ferrara, A. Patients diagnosed with diabetes are at increased risk for asthma, chronic obstructive pulmonary disease, pulmonary fibrosis, and pneumonia but not lung cancer. Diabetes Care 2010, 33, 55–60. [Google Scholar] [CrossRef]
- Kolahian, S.; Leiss, V.; Nurnberg, B. Diabetic lung disease: Fact or fiction? Rev. Endocr. Metab. Disord. 2019, 20, 303–319. [Google Scholar] [CrossRef]
- Kim, J.; Kim, W.J.; Ha, K.S.; Han, E.T.; Park, W.S.; Yang, S.R.; Hong, S.H. Perivascular Stem Cells Suppress Inflammasome Activation during Inflammatory Responses in Macrophages. Int. J. Stem Cells 2019, 12, 419–429. [Google Scholar] [CrossRef] [PubMed]
- An, B.; Kim, E.; Song, H.; Ha, K.S.; Han, E.T.; Park, W.S.; Ahn, T.G.; Yang, S.R.; Na, S.; Hong, S.H. Gestational Diabetes Affects the Growth and Functions of Perivascular Stem Cells. Mol. Cells 2017, 40, 434–439. [Google Scholar] [CrossRef]
- Park, M.; Hong, S.H.; Park, S.H.; Kim, Y.S.; Yang, S.C.; Kim, H.R.; Noh, S.; Na, S.; Lee, H.K.; Lim, H.J.; et al. Perivascular Stem Cell-Derived Cyclophilin A Improves Uterine Environment with Asherman’s Syndrome via HIF1alpha-Dependent Angiogenesis. Mol. Ther. 2020, 28, 1818–1832. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.; An, B.; Kim, J.H.; Han, H.W.; Kim, J.H.; Heo, H.R.; Ha, K.S.; Han, E.T.; Park, W.S.; Hong, S.H. BMP4 and perivascular cells promote hematopoietic differentiation of human pluripotent stem cells in a differentiation stage-specific manner. Exp. Mol. Med. 2020, 52, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Hogmalm, A.; Bry, M.; Bry, K. Pulmonary IL-1beta expression in early life causes permanent changes in lung structure and function in adulthood. Am. J. Physiol. Lung Cell Mol. Physiol. 2018, 314, L936–L945. [Google Scholar] [CrossRef]
- Heo, J.; Noh, B.J.; Lee, S.; Lee, H.Y.; Kim, Y.; Lim, J.; Ju, H.; Yu, H.Y.; Ryu, C.M.; Lee, P.C.; et al. Phosphorylation of TFCP2L1 by CDK1 is required for stem cell pluripotency and bladder carcinogenesis. EMBO Mol. Med. 2020, 12, e10880. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Gotoh, S.; Korogi, Y.; Seki, M.; Konishi, S.; Ikeo, S.; Sone, N.; Nagasaki, T.; Matsumoto, H.; Muro, S.; et al. Long-term expansion of alveolar stem cells derived from human iPS cells in organoids. Nat. Methods 2017, 14, 1097–1106. [Google Scholar] [CrossRef]
- Hong, S.H.; Rampalli, S.; Lee, J.B.; McNicol, J.; Collins, T.; Draper, J.S.; Bhatia, M. Cell fate potential of human pluripotent stem cells is encoded by histone modifications. Cell Stem Cell 2011, 9, 24–36. [Google Scholar] [CrossRef]
- Mangialardi, G.; Katare, R.; Oikawa, A.; Meloni, M.; Reni, C.; Emanueli, C.; Madeddu, P. Diabetes causes bone marrow endothelial barrier dysfunction by activation of the RhoA-Rho-associated kinase signaling pathway. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 555–564. [Google Scholar] [CrossRef]
- Chen, C.M.; Juan, S.H.; Pai, M.H.; Chou, H.C. Hyperglycemia induces epithelial-mesenchymal transition in the lungs of experimental diabetes mellitus. Acta Histochem. 2018, 120, 525–533. [Google Scholar] [CrossRef]
- Bradfield, P.F.; Nourshargh, S.; Aurrand-Lions, M.; Imhof, B.A. JAM family and related proteins in leukocyte migration (Vestweber series). Arterioscler. Thromb. Vasc. Biol. 2007, 27, 2104–2112. [Google Scholar] [CrossRef] [PubMed]
- Kuznik, B.I.; Linkova, N.S.; Kolchina, N.V.; Kukanova, E.O.; Khavinson, V.K. The JAM Family of Molecules and Their Role in the Regulation of Physiological and Pathological Processes. Usp. Fiziol. Nauk 2016, 47, 76–97. [Google Scholar] [PubMed]
- Christen, S.; Coppieters, K.; Rose, K.; Holdener, M.; Bayer, M.; Pfeilschifter, J.M.; Hintermann, E.; von Herrath, M.G.; Aurrand-Lions, M.; Imhof, B.A.; et al. Blockade but not overexpression of the junctional adhesion molecule C influences virus-induced type 1 diabetes in mice. PLoS ONE 2013, 8, e54675. [Google Scholar] [CrossRef]
- Hintermann, E.; Bayer, M.; Conti, C.B.; Fuchs, S.; Fausther, M.; Leung, P.S.; Aurrand-Lions, M.; Taubert, R.; Pfeilschifter, J.M.; Friedrich-Rust, M.; et al. Junctional adhesion molecules JAM-B and JAM-C promote autoimmune-mediated liver fibrosis in mice. J. Autoimmun. 2018, 91, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A.; Ramalingam, T.R. Mechanisms of fibrosis: Therapeutic translation for fibrotic disease. Nat. Med. 2012, 18, 1028–1040. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, F.; Wang, D.; Li, L.; Si, H.; Wang, C.; Liu, J.; Chen, Y.; Cheng, J.; Lu, Y. Mesenchymal Stem Cells Attenuate Diabetic Lung Fibrosis via Adjusting Sirt3-Mediated Stress Responses in Rats. Oxid. Med. Cell Longev. 2020, 2020, 8076105. [Google Scholar] [CrossRef]
- Guo, Y.; Yu, Y.; Hu, S.; Chen, Y.; Shen, Z. The therapeutic potential of mesenchymal stem cells for cardiovascular diseases. Cell Death Dis. 2020, 11, 349. [Google Scholar] [CrossRef]
- Zanoni, M.; Cortesi, M.; Zamagni, A.; Tesei, A. The Role of Mesenchymal Stem Cells in Radiation-Induced Lung Fibrosis. Int. J. Mol. Sci. 2019, 20, 3876. [Google Scholar] [CrossRef]
- Vizoso, F.J.; Eiro, N.; Cid, S.; Schneider, J.; Perez-Fernandez, R. Mesenchymal stem cell secretome: Toward cell-free therapeutic strategies in regenerative medicine. Int. J. Mol. Sci. 2017, 18, 1852. [Google Scholar] [CrossRef]
- Zang, L.; Hao, H.; Liu, J.; Li, Y.; Han, W.; Mu, Y. Mesenchymal stem cell therapy in type 2 diabetes mellitus. Diabetol. Metab. Syndr. 2017, 9, 36. [Google Scholar] [CrossRef]
- Kang, I.; Lee, B.C.; Choi, S.W.; Lee, J.Y.; Kim, J.J.; Kim, B.E.; Kim, D.H.; Lee, S.E.; Shin, N.; Seo, Y.; et al. Donor-dependent variation of human umbilical cord blood mesenchymal stem cells in response to hypoxic preconditioning and amelioration of limb ischemia. Exp. Mol. Med. 2018, 50, 35. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Song, X.; Yu, H.; Sun, J.; Zhao, Y. Therapeutic Potentials of Extracellular Vesicles for the Treatment of Diabetes and Diabetic Complications. Int. J. Mol. Sci. 2020, 21, 5163. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; Zhang, J.; Zhu, Y.; Shi, H.; Yin, S.; Sun, F.; Wang, Q.; Zhang, L.; Yan, Y.; Zhang, X.; et al. Exosomes derived from hucMSC attenuate renal fibrosis through CK1delta/beta-TRCP-mediated YAP degradation. Cell Death Dis. 2020, 11, 327. [Google Scholar] [CrossRef] [PubMed]
- Sabry, D.; Mohamed, A.; Monir, M.; Ibrahim, H.A. The Effect of Mesenchymal Stem Cells Derived Microvesicles on the Treatment of Experimental CCL4 Induced Liver Fibrosis in Rats. Int. J. Stem Cells 2019, 12, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Worthington, E.N.; Hagood, J.S. Therapeutic Use of Extracellular Vesicles for Acute and Chronic Lung Disease. Int. J. Mol. Sci. 2020, 21, 2318. [Google Scholar] [CrossRef]
- Siller, R.; Greenhough, S.; Park, I.H.; Sullivan, G.J. Modelling human disease with pluripotent stem cells. Curr. Gene Ther. 2013, 13, 99–110. [Google Scholar] [CrossRef]
- Ho, B.X.; Pek, N.M.Q.; Soh, B.S. Disease Modeling Using 3D Organoids Derived from Human Induced Pluripotent Stem Cells. Int. J. Mol. Sci. 2018, 19, 936. [Google Scholar] [CrossRef]
- Leibel, S.L.; Winquist, A.; Tseu, I.; Wang, J.; Luo, D.; Shojaie, S.; Nathan, N.; Snyder, E.; Post, M. Reversal of Surfactant Protein B Deficiency in Patient Specific Human Induced Pluripotent Stem Cell Derived Lung Organoids by Gene Therapy. Sci. Rep. 2019, 9, 13450. [Google Scholar] [CrossRef]
- Strikoudis, A.; Cieslak, A.; Loffredo, L.; Chen, Y.W.; Patel, N.; Saqi, A.; Lederer, D.J.; Snoeck, H.W. Modeling of Fibrotic Lung Disease Using 3D Organoids Derived from Human Pluripotent Stem Cells. Cell Rep. 2019, 27, 3709–3723 e3705. [Google Scholar] [CrossRef]
- Korogi, Y.; Gotoh, S.; Ikeo, S.; Yamamoto, Y.; Sone, N.; Tamai, K.; Konishi, S.; Nagasaki, T.; Matsumoto, H.; Ito, I.; et al. In Vitro Disease Modeling of Hermansky-Pudlak Syndrome Type 2 Using Human Induced Pluripotent Stem Cell-Derived Alveolar Organoids. Stem Cell Rep. 2019, 12, 431–440. [Google Scholar] [CrossRef]
- Dye, B.R.; Hill, D.R.; Ferguson, M.A.; Tsai, Y.H.; Nagy, M.S.; Dyal, R.; Wells, J.M.; Mayhew, C.N.; Nattiv, R.; Klein, O.D.; et al. In vitro generation of human pluripotent stem cell derived lung organoids. eLife 2015, 4. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rasaei, R.; Kim, E.; Kim, J.-Y.; Na, S.; Kim, J.-H.; Heo, J.; Shin, D.-M.; Choi, S.S.; Hong, S.-H. Regulation of JAM2 Expression in the Lungs of Streptozotocin-Induced Diabetic Mice and Human Pluripotent Stem Cell-Derived Alveolar Organoids. Biomedicines 2020, 8, 346. https://doi.org/10.3390/biomedicines8090346
Rasaei R, Kim E, Kim J-Y, Na S, Kim J-H, Heo J, Shin D-M, Choi SS, Hong S-H. Regulation of JAM2 Expression in the Lungs of Streptozotocin-Induced Diabetic Mice and Human Pluripotent Stem Cell-Derived Alveolar Organoids. Biomedicines. 2020; 8(9):346. https://doi.org/10.3390/biomedicines8090346
Chicago/Turabian StyleRasaei, Roya, Eunbi Kim, Ji-Young Kim, Sunghun Na, Jung-Hyun Kim, Jinbeom Heo, Dong-Myung Shin, Sun Shim Choi, and Seok-Ho Hong. 2020. "Regulation of JAM2 Expression in the Lungs of Streptozotocin-Induced Diabetic Mice and Human Pluripotent Stem Cell-Derived Alveolar Organoids" Biomedicines 8, no. 9: 346. https://doi.org/10.3390/biomedicines8090346
APA StyleRasaei, R., Kim, E., Kim, J.-Y., Na, S., Kim, J.-H., Heo, J., Shin, D.-M., Choi, S. S., & Hong, S.-H. (2020). Regulation of JAM2 Expression in the Lungs of Streptozotocin-Induced Diabetic Mice and Human Pluripotent Stem Cell-Derived Alveolar Organoids. Biomedicines, 8(9), 346. https://doi.org/10.3390/biomedicines8090346





