Effect of Hypoxia Preconditioned Secretomes on Lymphangiogenic and Angiogenic Sprouting: An in Vitro Analysis
Abstract
:1. Introduction
2. Experimental Setup
2.1. Ethical Approval
2.2. Preparation of Blood Plasma/Serum and Hypoxia Preconditioned Plasma (HPP)/Serum (HPS) Samples
2.3. Preparation of Adipose-Derived Cell Suspension (ADCS)
2.4. Quantitative Analysis of VEGF-C, TSP-1, PF-4 Concentration in Blood-Derived Secretomes
2.5. Analysis of the Effect of Blood-Derived Secretomes on Microvessel Sprouting In Vitro
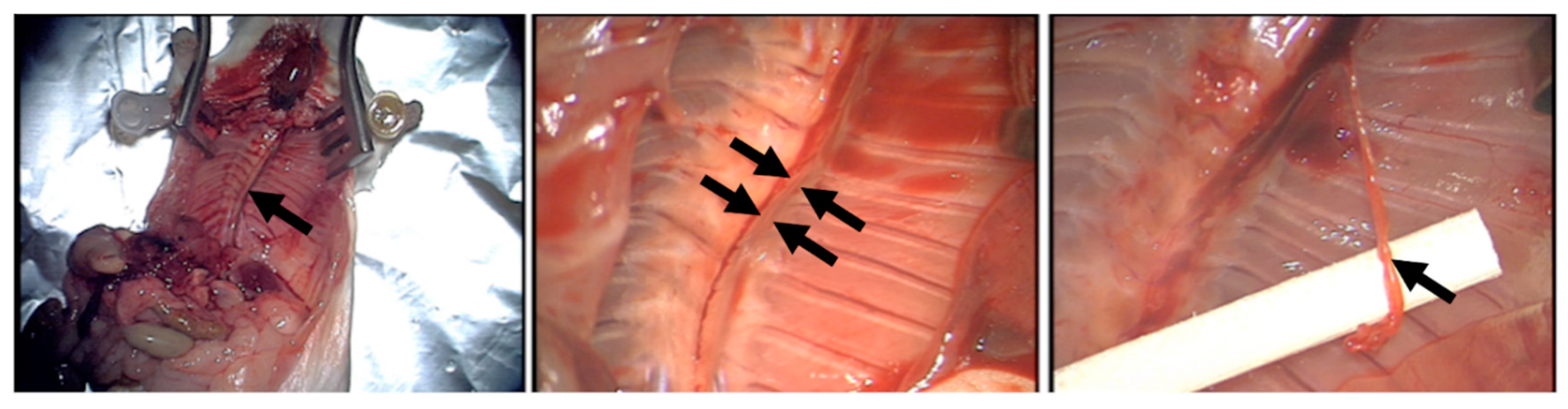
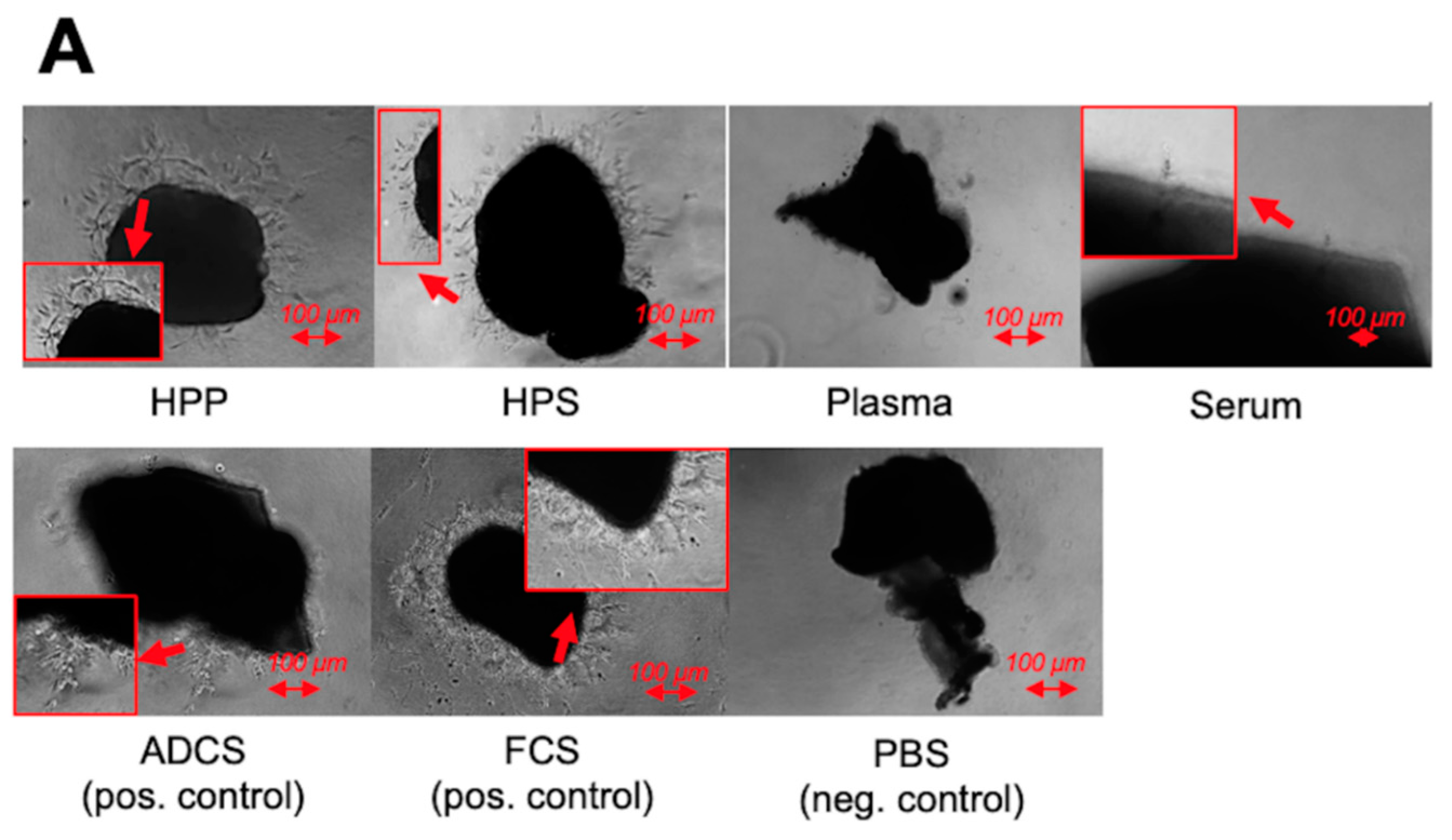
2.6. Analysis of the Effect of Blood-Derived Secretomes on Lymphatic Vessel Sprouting In Vitro
2.7. Immunohistochemical Staining of Lymphatic Sprouts
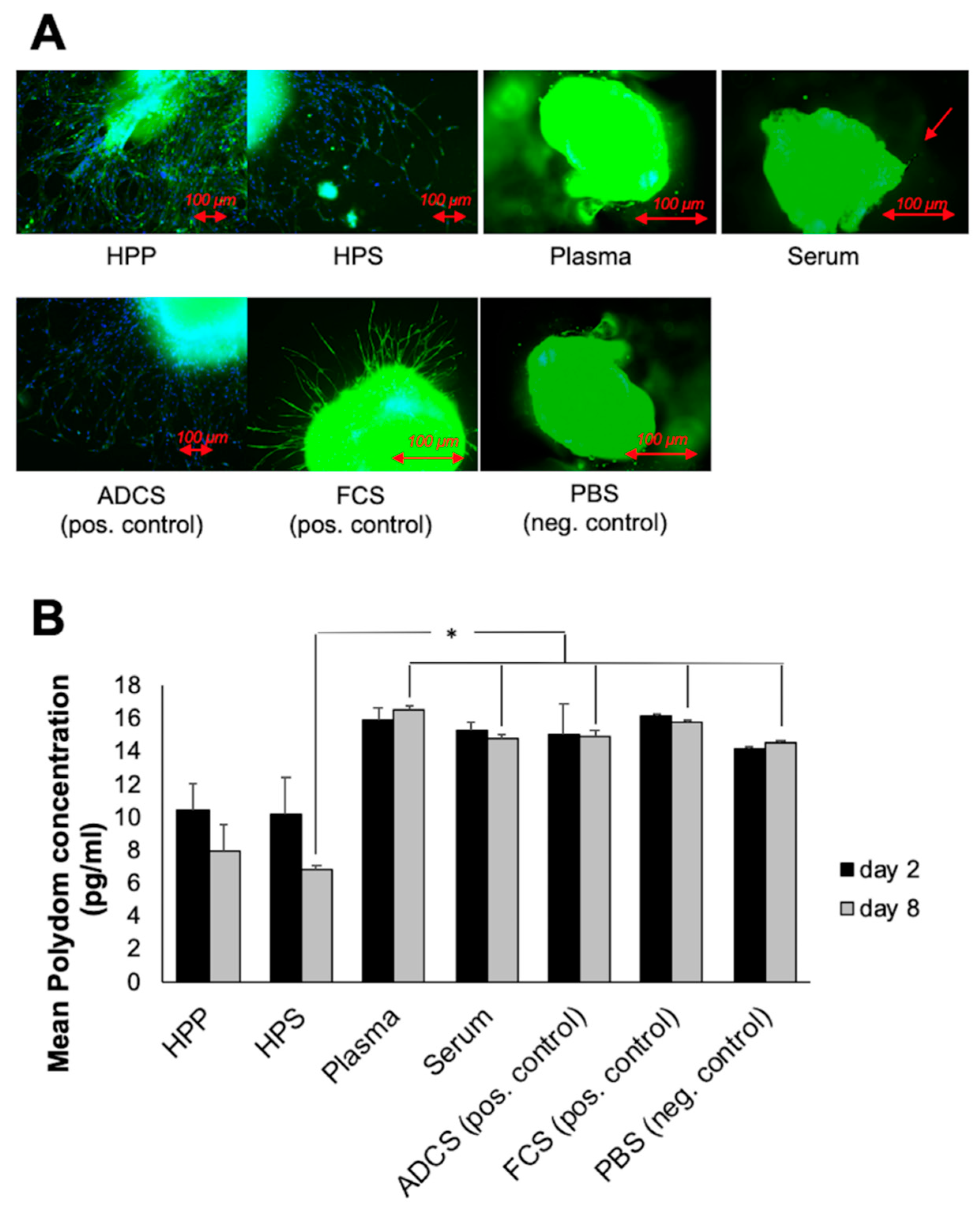
2.8. Quantitative Analysis of Polydom Concentration in Thoracic Duct Ring Assay
2.9. Statistical Analysis
3. Results
3.1. Quantitative Analysis of pro- (VEGF-C) and anti- (TSP-1, PF-4) Angiogenic/Lymphangiogenic Growth Factor Concentration in Hypoxia Preconditioned Blood-Derived Secretomes
3.2. Analysis of the Ability of Hypoxia Preconditioned Blood-Derived Secretomes to induce Angiogenic Sprouting In Vitro
3.3. Qualitative and Quantitative Validation of the Lymphatic Sprouting Assay
3.4. Analysis of the Ability of Hypoxia Preconditioned Blood-Derived Secretomes to induce Lymphatic Sprouting In Vitro
4. Discussion
5. Conclusion
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ADCS | adipose-derived cell suspension |
| ASC | adipose-derived stem cells |
| EWS | extracorporeal wound simulation |
| FCS | fetal calf serum |
| HPP | hypoxia preconditioned plasma |
| HPS | hypoxia preconditioned serum |
| LYVE-1 | lymphatic vessel endothelial hyaluronan receptor-1 |
| PBC | peripheral blood cells |
| PBS | phosphate buffered saline |
| PF-4 | platelet factor-4 |
| TSP-1 | thrombospondin-1 |
| VEGF | vascular endothelial growth factor |
| FGF | fibroblast growth factor |
| MMP | matrix metalloproteinase |
References
- Owlarn, S.; Klenner, F.; Schmidt, D.; Rabert, F.; Tomasso, A.; Reuter, H.; Mulaw, M.A.; Moritz, S.; Gentile, L.; Weidinger, G.; et al. Generic wound signals initiate regeneration in missing-tissue contexts. Nat. Commun. 2017, 8, 2282. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yin, H.; Lei, X.; Lau, J.N.Y.; Yuan, M.; Wang, X.; Zhang, F.; Zhou, F.; Qi, S.; Shu, B.; et al. A systematic review and meta-analysis of clinical effectiveness and safety of hydrogel dressings in the management of skin wounds. Front. Bioeng. Biotechnol. 2019, 7, 342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, G.; Ceilley, R. Chronic wound healing: A review of current management and treatments. Adv. Ther. 2017, 34, 599–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, C.; Wang, C.; Liu, H.; Li, Q.; Li, R.; Zhang, Y.; Liu, Y.; Shao, Y.; Wang, J. Selection of appropriate wound dressing for various wounds. Front. Bioeng. Biotechnol. 2020, 8, 182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tammela, T.; Alitalo, K. Lymphangiogenesis: Molecular mechanisms and future promise. Cell 2010, 140, 460–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alderfer, A.; Wei, A.; Hanjaya-Putra, D. Lymphatic tissue engineering and regeneration. J. Biol. Eng. 2018, 12, 32. [Google Scholar] [CrossRef]
- Skobe, M.; Detmar, M. Structure, function, and molecular control of the skin lymphatic system. J. Investig. Dermatol. Symp. Proc. 2000, 5, 14–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiig, H.; Swartz, M. Interstitial fluid and lymph formation and transport: Physiological regulation and roles in inflammation and cancer. Physiol. Rev. 2012, 92, 1005–1060. [Google Scholar] [CrossRef]
- Schaupper, M.; Jeltsch, M.; Rohringer, S.; Redl, H.; Holnthoner, W. Lymphatic vessels in regenerative medicine and tissue engineering. Tissue Eng. Part B Rev. 2016, 22, 395–407. [Google Scholar] [CrossRef]
- Dixon, J.B.; Raghunathan, S.; Swartzm, M.A. A tissue-engineered model of the intestinal lacteal for evaluating lipid transport by lymphatics. Biotechnol. Bioeng. 2009, 103, 1224–1235. [Google Scholar] [CrossRef] [Green Version]
- Alitalo, K. The lymphatic vasculature in disease. Nat. Med. 2011, 17, 1371–1380. [Google Scholar] [CrossRef] [PubMed]
- Baluk, P.; Tammela, T.; Ator, E.; Lyubynska, N.; Achen, M.G.; Hicklin, D.J.; Jeltsch, M.; Petrova, T.V.; Pytowski, B.; Stacker, S.A.; et al. Pathogenesis of persistent lymphatic vessel hyperplasia in chronic airway inflammation. J. Clin. Invest. 2005, 115, 247–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kataru, R.P.; Jung, K.; Jang, C.; Yang, H.; Schwendener, R.A.; Baik, J.E.; Han, S.H.; Alitalo, K.; Koh, G.H. Critical role of CD11b+ macrophages and VEGF in inflamma- tory lymphangiogenesis, antigen clearance and inflammation resolution. Blood 2009, 113, 5650–5659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huggenberger, R.; Siddiqui, S.S.; Brander, D.; Ullmann, S.; Zimmermann, K.; Antsiferova, M.; Werner, S.; Alitalo, K.; Detmar, M. An important role of lymphatic vessel activation in limiting acute inflammation. Blood 2011, 117, 4667–4678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marino, D.; Luginbühl, J.; Scola, S.; Meuli, M.; Reichmann, E. Bioengineering dermo-epidermal skin grafts with blood and lymphatic capillaries. Sci. Transl. Med. 2014, 6, 221. [Google Scholar] [CrossRef] [Green Version]
- Hanjaya-Putra, D.; Shen, Y.I.; Wilson, A.; Fox-Talbot, K.; Khetan, S.; Burdick, J.A.; Steenbergen, C.; Gerecht, S. Integration and regression of implanted engineered human vascular networks during deep wound healing. Stem. Cells Transl. Med. 2013, 2, 297–306. [Google Scholar] [CrossRef]
- Tammela, T.; Enholm, B.; Alitalo, K.; Paavonen, K. The biology of vascular endothelial growth factors. Cardiovasc Res. 2005, 65, 550–563. [Google Scholar] [CrossRef]
- Cursiefen, C.; Maruyama, K.; Bock, F.; Saban, D.; Sadrai, Z.; Lawler, J.; Dana, R.; Masli, S. Thrombospondin 1 inhibits inflammatory lymphangiogenesis by CD36 ligation on monocytes. J. Exp. Med. 2011, 208, 1083–1092. [Google Scholar] [CrossRef]
- Wong, B.W. Lymphatic vessels in solid organ transplantation and immunobiology. Am. J. Transplant. 2020, 20, 1992–2000. [Google Scholar] [CrossRef]
- Karpanen, T.; Alitalo, K. Molecular biology and pathology of lymphangiogenesis. Annu. Rev. Pathol. 2007, 3, 367–397. [Google Scholar] [CrossRef]
- Schöllhorn, L.; Bock, F.; Cursiefen, C. Thrombospondin-1 as a regulator of corneal inflammation and lymphangiogenesis: Effects on dry eye disease and corneal graft immunology. J. Ocul. Pharmacol. Ther. 2015, 31, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.J.; Xie, F.M. Influence of angiogenesis inhibitors, endostatin and PF-4, on lymphangiogenesis. Lymphology 2005, 38, 1–8. [Google Scholar] [PubMed]
- Hadjipanayi, E.; Bauer, A.T.; Moog, P.; Salgin, B.; Kükrek, H.; Fersch, B.; Hopfner, U.; Meissner, T.; Schlüter, A.; Ninkovic, M.; et al. Cell-free carrier system for localised delivery of peripheral blood cell-derived engineered factor signaling: Towards development of a one-step device for autologous angiogenic therapy. J. Control. Release 2013, 169, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Hadjipanayi, E.; Schilling, A.F. Hypoxia- based strategies for angiogenic induction. Organog. Landes Biosci. 2013, 9, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hadjipanayi, E.; Schilling, A.F. Regeneration through autologous hypoxia preconditioned plasma. Organogenesis 2014, 10, 164–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hadjipanayi, E.; Bekeran, S.; Moog, P. Extracorporeal wound simulation as a foundation for tissue repair and regeneration therapies. Int. J. Transpl. Plast. Surg. 2018, 2, 1–10. [Google Scholar]
- Hadjipanayi, E.; Moog, P.; Bekeran, S.; Kirchhoff, K.; Berezhnoi, A.; Aguirre, J.; Bauer, A.T.; Kükrek, H.; Schmauss, D.; Hopfner, U.; et al. In vitro characterization of hypoxia preconditioned serum (HPS)-fibrin hydrogels: Basis for an injectable biomimetic tissue regeneration therapy. J. Funct. Biomater. 2019, 13, 22. [Google Scholar] [CrossRef] [Green Version]
- Moog, P.; Kirchhoff, K.; Bekeran, S.; Bauer, A.T.; Isenburg, S.; Dornseifer, U.; Machens, H.G.; Schilling, A.F.; Hadjipanayi, E. Comparative evaluation of the angiogenic potential of hypoxia preconditioned blood-derived secretomes and platelet-rich plasma: An in vitro analysis. Biomedicines 2020, 8, 16. [Google Scholar] [CrossRef] [Green Version]
- Hadjipanayi, E.; Kuhn, P.H.; Moog, P.; Bauer, A.T.; Kuekrek, H.; Mirzoyan, L.; Hummel, A.; Kirchhoff, K.; Salgin, B.; Isenburg, S.; et al. The fibrin matrix regulates angiogenic responses within the hemostatic microenvironment through biochemical control. PLoS ONE 2015, 10, 1–20. [Google Scholar] [CrossRef]
- Moog, P.; Jensch, M.; Hughes, J.; Salgin, B.; Dornseifer, U.; Machens, H.G.; Schilling, A.F.; Hadjipanayi, E. Use of oral anticoagulation and diabetes do not inhibit the angiogenic potential of hypoxia preconditioned blood-derived secretomes. Biomedicines 2020, 8, 283. [Google Scholar] [CrossRef]
- Saijo, H.; Suzuki, K.; Yoshimoto, H.; Imamura, Y.; Yamashita, S.; Tanaka, K. Paracrine effects of adipose-derived stem cells promote lymphangiogenesis in irradiated lymphatic endothelial cells. Plast. Reconstr. Surg. 2019, 143, 1189–1200. [Google Scholar] [CrossRef] [PubMed]
- Mazini, L.; Rochette, L.; Admou, B.; Amal, S.; Malka, G. Hopes and limits of Adipose-Derived Stem Cells (ADSCs) and Mesenchymal Stem Cells (MSCs) in wound healing. Int. J. Mol. Sci. 2020, 21, 1306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bachmann, S.; Jennewein, M.; Bubel, M.; Guthörl, S.; Pohlemann, T.; Oberringer, M. Interacting adipose-derived stem cells and microvascular endothelial cells provide a beneficial milieu for soft tissue healing. Mol. Biol. Rep. 2019, 47, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Komaki, M.; Numata, Y.; Morioka, C.; Honda, I.; Tooi, M.; Yokoyama, N.; Ayame, H.; Iwasaki, K.; Taki, A.; Oshima, N. Exosomes of human placenta-derived mesenchymal stem cells stimulate angiogenesis. Stem. Cell Res. Ther. 2017, 8, 219. [Google Scholar] [CrossRef] [PubMed]
- Winnier, G.E.; Valnzuela, N.; Alt, C.; Alt, E.U. Isolation of adipose tissue derived regenerative cells from human subcutaneous tissue with or without the use of an enzymatic reagent. PLoS ONE 2018, 14, 1–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baker, M.; Robinson, S.D.; Lechertier, T.; Barber, P.R.; Tavora, B.; D’Amico, G.; Jones, D.T.; Vojnovic, B.; Hodivala-Dilke, K. Use of the mouse aortic ring assay to study angiogenesis. Nat. Protoc. 2011, 7, 89–104. [Google Scholar] [CrossRef]
- Bruyère, F.; Melen-La, L.; Berndt, S.; Peulen, O.; Foidart, J.M.; Noël, A. The lymphatic ring assay: A 3D-culture model of lymphangiogenesis. Protoc. Exch. (Nat. Publ. Group) 2016, 1–18. [Google Scholar] [CrossRef]
- Morooka, N.; Futaki, S.; Sato-Nishiuchi, R.; Nishino, M.; Totani, Y.; Shimono, C.; Nakano, I.; Nakajima, H.; Mochizuki, N.; Sekiguchi, K. Polydom is an extracellular matrix protein involved in lymphatic vessel remodeling. Circ. Res. 2017, 120, 1276–1288. [Google Scholar] [CrossRef]
- Karpanen, T.; Padberg, Y.; van de Pavert, S.A.; Dierkes, C.; Morooka, N.; Peterson-Maduro, J.; van de Hoek, G.; Adrian, M.; Mochizuki, N.; Sekiguchi, K.; et al. An evolutionarily conserved role for polydom/svep1 during lymphatic vessel formation. Circ. Res. 2017, 120, 1263–1275. [Google Scholar] [CrossRef]
- Liu, Y.; Cox, S.R.; Morita, T.; Kourembanas, S. Hypoxia regulates vascular endothelial growth factor gene expression in endothelial cells. Identification of a 5′ enhancer. Circ. Res. 1995, 77, 638–643. [Google Scholar] [CrossRef]
- Sumi, M.; Sata, M.; Toya, N.; Yanaga, K.; Ohki, T.; Nagai, R. Transplantation of adipose stromal cells, but not mature adipocytes, augments ischemia-induced angiogenesis. Life Sci. 2007, 80, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Hawighorst, T. Angiogenesis, lymphangiogenesis, and tumor progression. Zent. Gynakol 2002, 124, 497–505. [Google Scholar]
- Nicosia, R.F.; Ottinetti, A. Growth of microvessels in serum- free matrix culture of rat aorta. A quantitative assay of angiogenesis in vitro. Lab Invest. 1990, 63, 115–122. [Google Scholar] [PubMed]
- Klein-Soyer, C.; Duhamel-Clerin, E.; Ravanat, C.; Orvain, C.; Lanza, F.; Cazenave, J.P. PF4 inhibits thrombin- stimulated MMP-1 and MMP-3 metalloproteinase expression in human vascular endothelial cells. C R Acad. Sci. 1997, 320, 857–868. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moog, P.; Schams, R.; Schneidinger, A.; Schilling, A.F.; Machens, H.-G.; Hadjipanayi, E.; Dornseifer, U. Effect of Hypoxia Preconditioned Secretomes on Lymphangiogenic and Angiogenic Sprouting: An in Vitro Analysis. Biomedicines 2020, 8, 365. https://doi.org/10.3390/biomedicines8090365
Moog P, Schams R, Schneidinger A, Schilling AF, Machens H-G, Hadjipanayi E, Dornseifer U. Effect of Hypoxia Preconditioned Secretomes on Lymphangiogenic and Angiogenic Sprouting: An in Vitro Analysis. Biomedicines. 2020; 8(9):365. https://doi.org/10.3390/biomedicines8090365
Chicago/Turabian StyleMoog, Philipp, Rahmin Schams, Alexander Schneidinger, Arndt F. Schilling, Hans-Günther Machens, Ektoras Hadjipanayi, and Ulf Dornseifer. 2020. "Effect of Hypoxia Preconditioned Secretomes on Lymphangiogenic and Angiogenic Sprouting: An in Vitro Analysis" Biomedicines 8, no. 9: 365. https://doi.org/10.3390/biomedicines8090365
APA StyleMoog, P., Schams, R., Schneidinger, A., Schilling, A. F., Machens, H. -G., Hadjipanayi, E., & Dornseifer, U. (2020). Effect of Hypoxia Preconditioned Secretomes on Lymphangiogenic and Angiogenic Sprouting: An in Vitro Analysis. Biomedicines, 8(9), 365. https://doi.org/10.3390/biomedicines8090365





