Brassicasterol from Edible Aquacultural Hippocampus abdominalis Exerts an Anti-Cancer Effect by Dual-Targeting AKT and AR Signaling in Prostate Cancer
Abstract
:1. Introduction
2. Experimental Section
2.1. Preparation of SHL
2.2. Gas Chromatography-Mass Spectrometry (GC-MS)
2.3. Cell Culture
2.4. Cell Viability Assay
2.5. Western Blotting
2.6. RNA Extraction and qRT-PCR
2.7. Fluorescence-Activated Cell Sorting (FACS) Analysis
2.8. Wound-Healing Assay
2.9. 3D Tumor Organoids
2.10. siRNA Transfection
2.11. Crystal Violet Staining and Cell Growth Assay
2.12. Statistical Analyses
3. Results
3.1. Seahorse Lipid Extract (SHL) Inhibits Androgen Receptor (AR) Expression in Dihydrotestosterone (DHT)-Induced LNCaP Cells
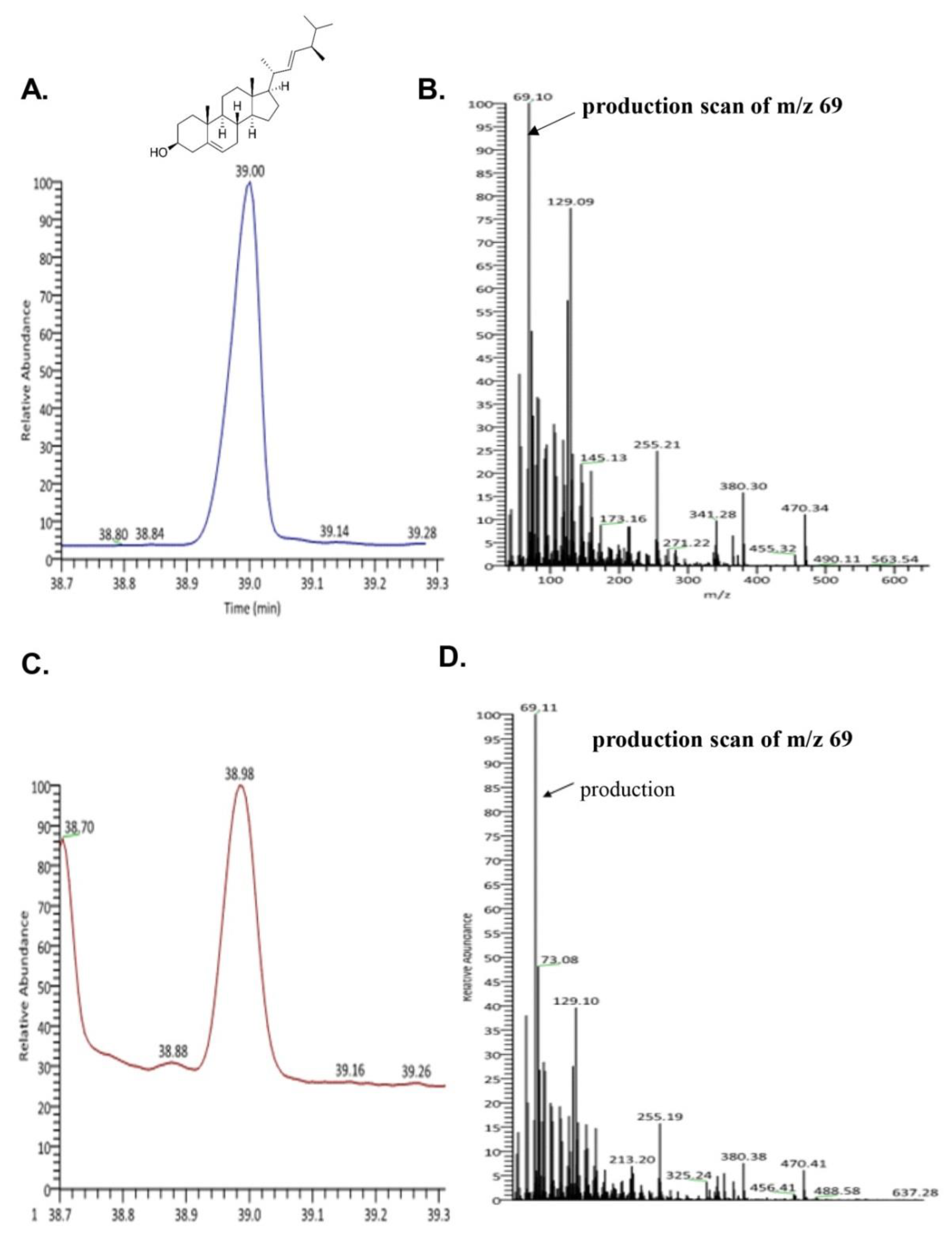
3.2. Identification of Brassicasterol from SHL Using GC-MS
3.3. Brassicasterol Inhibits AR and PSA Expression in LNCaP Cells
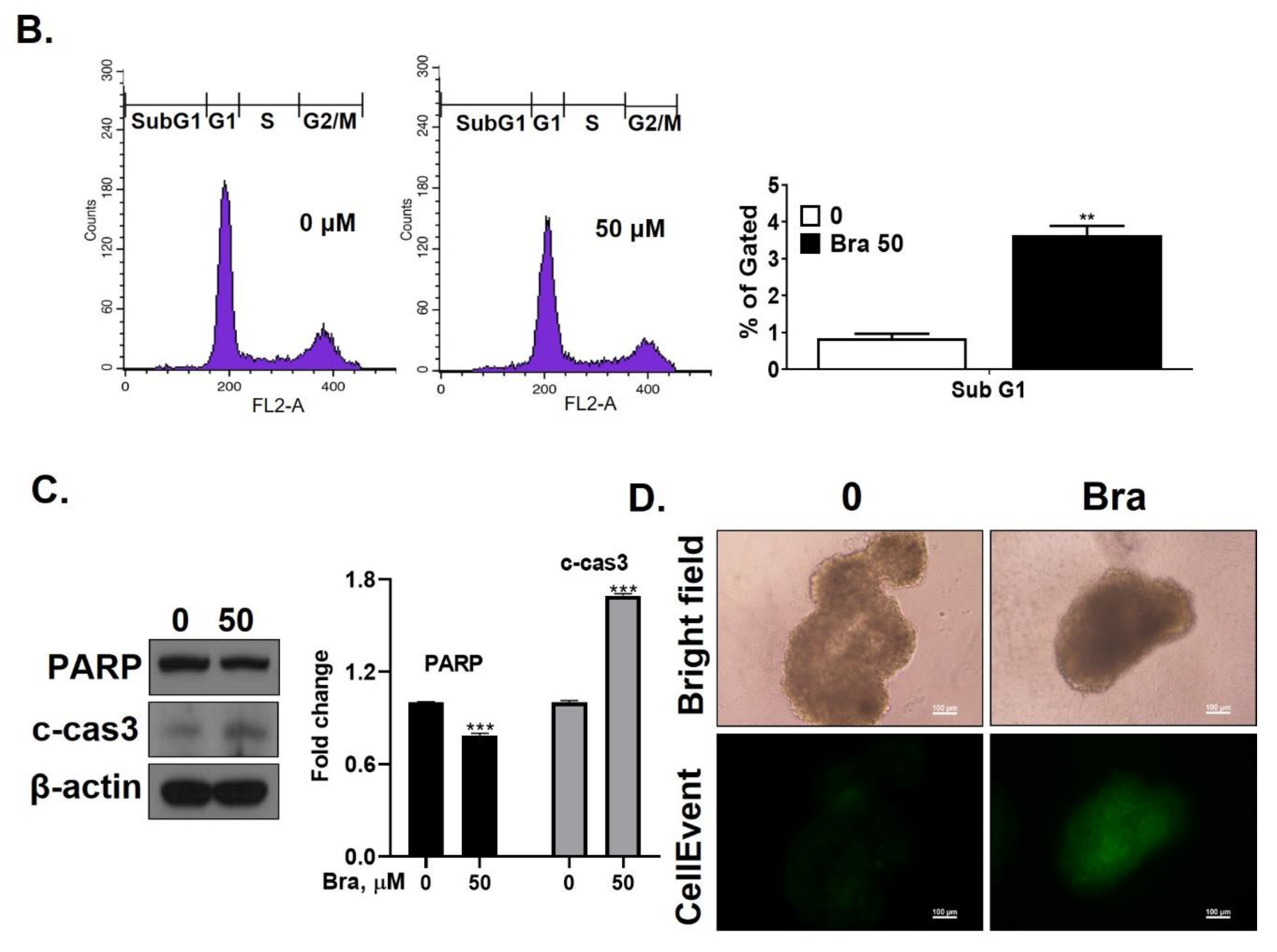
3.4. Brassicasterol Inhibits Cell Growth and Induces Sub-G1 Phase Arrest in LNCaP Cells
3.5. AR Mediates Brassicasterol-Induced Suppression of Cell Migration and EMT in LNCaP Cells
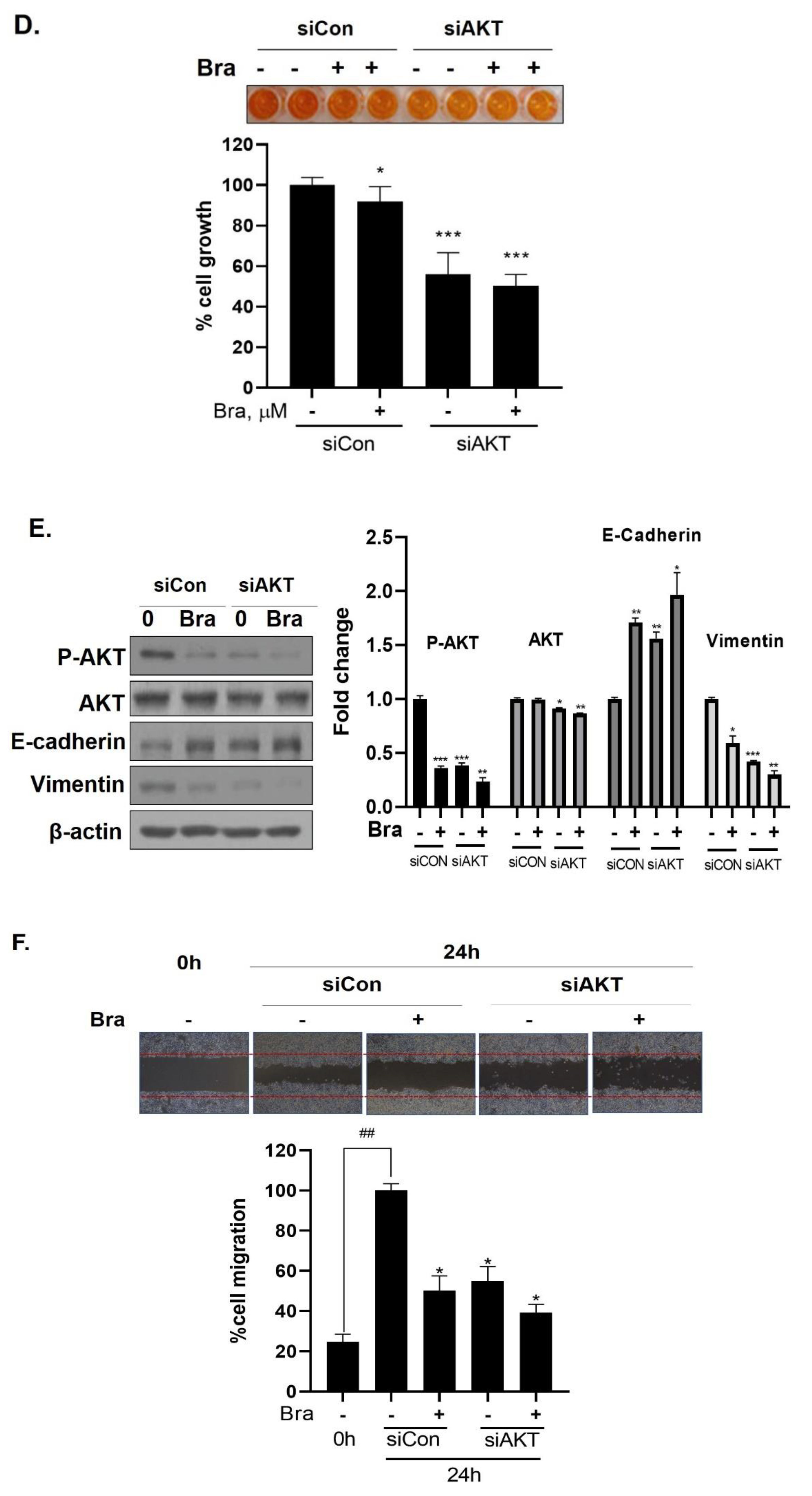
3.6. AKT Mediates Brassicasterol-Induced Suppression of the AR Signaling Pathway
3.7. Brassicasterol Exerts an Anti-Cancer Effect in AR-Independent Cancer as well as AR-Dependent Cells by Inhibiting AKT
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rawla, P. Epidemiology of Prostate Cancer. World J. Oncol. 2019, 10, 63–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayoclinic. Available online: http://www.Mayoclinic.org/diseases-conditions/prostate-cancer/symptoms-causes/syc-20353087 (accessed on 5 January 2020).
- Andriole, G.; Bruchovsky, N.; Chung, L.W.; Matsumoto, A.M.; Rittmaster, R.; Roehrborn, C.; Russell, D.; Tindall, D. Dihydrotestosterone and the prostate: The scientific rationale for 5alpha-reductase inhibitors in the treatment of benign prostatic hyperplasia. J. Urol. 2004, 172, 1399–1403. [Google Scholar] [CrossRef] [PubMed]
- Feitelson, M.A.; Arzumanyan, A.; Kulathinal, R.J.; Blain, S.W.; Holcombe, R.F.; Mahajna, J.; Marino, M.; Martinez-Chantar, M.L.; Nawroth, R.; Sanchez-Garcia, I.; et al. Sustained proliferation in cancer: Mechanisms and novel therapeutic targets. Semin. Cancer Biol. 2015, 35, S25–S54. [Google Scholar] [CrossRef] [PubMed]
- Heinlein, C.A.; Chang, C. Androgen receptor in prostate cancer. Endocr. Rev. 2004, 25, 276–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taplin, M.E.; Balk, S.P. Androgen receptor: A key molecule in the progression of prostate cancer to hormone independence. J. Cell. Biochem. 2004, 91, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Riegman, P.; Vlietstra, R.; Van Der Korput, J.; Romijn, J.; Trapman, J. Characterization of the Prostate-specific Antigen gene: A novel human kallikrein-like gene. Biochem. Biophys. Res. Commun. 1989, 159, 95–102. [Google Scholar] [CrossRef]
- Lorente, D.; De Bono, J.S. Molecular alterations and emerging targets in castration resistant prostate cancer. Eur. J. Cancer 2014, 50, 753–764. [Google Scholar] [CrossRef]
- Hobisch, A.; Culig, Z.; Radmayr, C.; Bartsch, G.; Klocker, H.; Hittmair, A. Distant Metastases from Prostatic Carcinoma Express Androgen Receptor Protein. Cancer Res. 1995, 55, 3068–3072. [Google Scholar]
- Gregory, C.W.; Johnson, R.T.; Mohler, J.L.; French, F.S.; Wilson, E.M. Androgen Receptor Stabilization in Recurrent Prostate Cancer Is Associated with Hypersensitivity to Low Androgen. Cancer Res. 2001, 61, 2892–2898. [Google Scholar]
- Visakorpi, T.; Hyytinen, E.; Koivisto, P.; Tanner, M.; Keinanen, R.; Palmberg, C.; Palotie, A.; Tammela, T.; Isola, J.; Kallioniemi, O.P. In vivo amplification of the androgen receptor gene and progression of human prostate cancer. Nat. Genet. 1995, 9, 401–406. [Google Scholar] [CrossRef]
- Scher, H.I.; Sawyers, C.L. Biology of progressive, castration-resistant prostate cancer: Directed therapies targeting the androgen-receptor signaling axis. J. Clin. Oncol. 2005, 23, 8253–8261. [Google Scholar] [CrossRef] [PubMed]
- Culig, Z.; Klocker, H.; Bartsch, G.; Steiner, H.; Hobisch, A. Androgen receptors in prostate cancer. J. Urol. 2003, 170, 1363–1369. [Google Scholar] [CrossRef]
- Linja, M.J.; Savinainen, K.J.; Saramäki, O.R.; Tammela, T.L.; Vessella, R.L.; Visakorpi, T. Amplification and Overexpression of Androgen Receptor Gene in Hormone-Refractory Prostate Cancer. Cancer Res. 2001, 61, 3550–3555. [Google Scholar] [PubMed]
- Dehm, S.M.; Tindall, D.J. Alternatively spliced androgen receptor variants. Endocr. Relat. Cancer 2011, 18, R183–R196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, B.S.; Schultz, N.; Hieronymus, H.; Gopalan, A.; Xiao, Y.; Carver, B.S.; Arora, V.K.; Kaushik, P.; Cerami, E.; Reva, B.; et al. Integrative genomic profiling of human prostate cancer. Cancer Cell 2010, 18, 11–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bitting, R.L.; Armstrong, A.J. Targeting the PI3K/Akt/mTOR pathway in castration-resistant prostate cancer. Endocr. Relat. Cancer 2013, 20, R83–R99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mulholland, D.J.; Tran, L.M.; Li, Y.; Cai, H.; Morim, A.; Wang, S.; Plaisier, S.; Garraway, I.P.; Huang, J.; Graeber, T.G.; et al. Cell autonomous role of PTEN in regulating castration-resistant prostate cancer growth. Cancer Cell 2011, 19, 792–804. [Google Scholar] [CrossRef] [Green Version]
- Carver, B.S.; Chapinski, C.; Wongvipat, J.; Hieronymus, H.; Chen, Y.; Chandarlapaty, S.; Arora, V.K.; Le, C.; Koutcher, J.; Scher, H.; et al. Reciprocal feedback regulation of PI3K and androgen receptor signaling in PTEN-deficient prostate cancer. Cancer Cell 2011, 19, 575–586. [Google Scholar] [CrossRef] [Green Version]
- Gong, F.; Zhang, Y.; Lin, J.; Li, C.; Zhou, C.; Hong, P.; Ryu, B.; Qian, Z.J. 1-(5-Bromo-2-hydroxy-4-methoxyphenyl)ethanone [SE1] Inhibits MMP-9 Expression by Regulating NF-kappaB and MAPKs Signaling Pathways in HT1080 Human Fibrosarcoma Cells. Evid. Based Complement. Altern. Med. 2018, 2018, 5639486. [Google Scholar] [CrossRef]
- Kim, Y.T.; Kim, S.K.; Jeon, Y.J.; Park, S.J. Seahorse-derived peptide suppresses invasive migration of HT1080 fibrosarcoma cells by competing with intracellular alpha-enolase for plasminogen binding and inhibiting uPA-mediated activation of plasminogen. BMB Rep. 2014, 47, 691–696. [Google Scholar] [CrossRef]
- Sun, D.; Wu, S.; Jing, C.; Zhang, N.; Liang, D.; Xu, A. Identification, synthesis and characterization of a novel antimicrobial peptide HKPLP derived from Hippocampus kuda Bleeker. J. Antibiot. (Tokyo) 2012, 65, 117–121. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Q.; He, L.; Qian, Z.J.; Zhou, C.; Hong, P.; Wang, Z.; Wang, Y.; Sun, S.; Li, C. Significantly Accelerated Osteoblast Cell Growth on TiO2/SrHA Composite Mediated by Phenolic Compounds (BHM) from Hippocamp us kuda Bleeler. ACS Appl. Mater. Interfaces 2018, 10, 30214–30226. [Google Scholar] [CrossRef] [PubMed]
- Ryu, B.; Qian, Z.J.; Kim, S.K. Purification of a peptide from seahorse, that inhibits TPA-induced MMP, iNOS and COX-2 expression through MAPK and NF-kappaB activation, and induces human osteoblastic and chondrocytic differentiation. Chem. Biol. Interact. 2010, 184, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Kim, S.Y.; Fernando, I.P.S.; Sanjeewa, K.K.A.; Wang, L.; Lee, S.H.; Ko, S.C.; Kang, M.C.; Jayawardena, T.U.; Jeon, Y.J. Free radical scavenging activity of the peptide from the Alcalase hydrolysate of the edible aquacultural seahorse (Hippocampus abdominalis). J. Food Biochem. 2019, 43, e12833. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.; Ahn, C.B.; Yoon, N.Y.; Nam, K.H.; Kim, Y.K.; Je, J.Y. Protective effect of enzymatic hydrolysates from seahorse (Hippocampus abdominalis) against H2O2-mediated human umbilical vein endothelial cell injury. Biomed. Pharmacother. 2018, 108, 103–110. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, C.; Zhou, C.; Hong, P.; Zhang, Y.; Sun, S.; Qian, Z.J. 2′-Hydroxy-5′-methoxyacetophenone attenuates the inflammatory response in LPS-induced BV-2 and RAW264.7 cells via NF-kappaB signaling pathway. J. Neuroimmunol. 2019, 330, 143–151. [Google Scholar] [CrossRef]
- Xu, D.H.; Wang, L.H.; Mei, X.T.; Li, B.J.; Lv, J.L.; Xu, S.B. Protective effects of seahorse extracts in a rat castration and testosterone-induced benign prostatic hyperplasia model and mouse oligospermatism model. Environ. Toxicol. Pharmacol. 2014, 37, 679–688. [Google Scholar] [CrossRef]
- Himaya, S.W.; Ryu, B.; Qian, Z.J.; Kim, S.K. Paeonol from Hippocampus kuda Bleeler suppressed the neuro-inflammatory responses in vitro via NF-kappaB and MAPK signaling pathways. Toxicol. In Vitro 2012, 26, 878–887. [Google Scholar] [CrossRef]
- Himaya, S.W.; Ryu, B.; Qian, Z.J.; Li, Y.; Kim, S.K. 1-(5-bromo-2-hydroxy-4-methoxyphenyl)ethanone [SE1] suppresses pro-inflammatory responses by blocking NF-kappaB and MAPK signaling pathways in activated microglia. Eur. J. Pharmacol. 2011, 670, 608–616. [Google Scholar] [CrossRef]
- Schwartz, H.; Ollilainen, V.; Piironen, V.; Lampi, A.-M. Tocopherol, tocotrienol and plant sterol contents of vegetable oils and industrial fats. J. Food Compos. Anal. 2008, 21, 152–161. [Google Scholar] [CrossRef]
- Schroder, M.; Fricke, C.; Pilegaard, K.; Poulsen, M.; Wester, I.; Lutjohann, D.; Mortensen, A. Effect of rapeseed oil-derived plant sterol and stanol esters on atherosclerosis parameters in cholesterol-challenged heterozygous Watanabe heritable hyperlipidaemic rabbits. Br. J. Nutr. 2009, 102, 1740–1751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horoszewicz, J.S.; Leong, S.S.; Kawinski, E.; Karr, J.P.; Rosenthal, H.; Chu, T.A.; Mirand, E.; Murphy, G.P. LNCaP Model of Human Prostatic Carcinoma. Cancer Res. 1983, 43, 1809–1818. [Google Scholar] [PubMed]
- Chen, M.; Chen, J.-Z.; Ge, Y.-Q.; Shi, S.-L.; Zhang, G.-J.; Cheng, R.-B. Research progress on chemical constituents and pharmacological activities of Hippocampus. Chin. Tradit. Herb. Drugs 2017, 48, 4089–4099. [Google Scholar] [CrossRef]
- Chen, L.; Shen, X.; Chen, G.; Cao, X.; Yang, J. Effect of Three-spot Seahorse Petroleum Ether Extract on Lipopolysaccharide Induced Macrophage RAW264.7 Inflammatory Cytokine Nitric Oxide and Composition Analysis. J. Oleo Sci. 2015, 64, 933–942. [Google Scholar] [CrossRef] [PubMed]
- Yazawa, Y.; Ikarashi, N.; Hoshino, M.; Kikkawa, H.; Sakuma, F.; Sugiyama, K. Inhibitory effect of ergosterol on bladder carcinogenesis is due to androgen signaling inhibition by brassicasterol, a metabolite of ergosterol. J. Nat. Med. 2020, 74, 680–688. [Google Scholar] [CrossRef]
- Wenzel, C.; Riefke, B.; Grundemann, S.; Krebs, A.; Christian, S.; Prinz, F.; Osterland, M.; Golfier, S.; Rase, S.; Ansari, N.; et al. 3D high-content screening for the identification of compounds that target cells in dormant tumor spheroid regions. Exp. Cell Res. 2014, 323, 131–143. [Google Scholar] [CrossRef] [Green Version]
- Vlietstra, R.J.; Van Alewijk, D.C.; Hermans, K.G.; Van Steenbrugge, G.J.; Trapman, J. Frequent Inactivation of PTEN in Prostate Cancer Cell Lines and Xenografts. Cancer Res. 1998, 58, 2720–2723. [Google Scholar]
- Nesterov, A.; Lu, X.; Johnson, M.; Miller, G.J.; Ivashchenko, Y.; Kraft, A.S. Elevated AKT activity protects the prostate cancer cell line LNCaP from TRAIL-induced apoptosis. J. Biol. Chem. 2001, 276, 10767–10774. [Google Scholar] [CrossRef] [Green Version]
- Maehama, T.; Dixon, J.E. The Tumor Suppressor, PTEN/MMAC1, Dephosphorylates the Lipid Second Messenger, Phosphatidylinositol 3,4,5-Trisphosphate. J. Biol. Chem. 1998, 273, 13375–13378. [Google Scholar] [CrossRef] [Green Version]
- Fang, J.; Ding, M.; Yang, L.; Liu, L.Z.; Jiang, B.H. PI3K/PTEN/AKT signaling regulates prostate tumor angiogenesis. Cell. Signal. 2007, 19, 2487–2497. [Google Scholar] [CrossRef] [Green Version]
- Xin, L.; Teitell, M.A.; Lawson, D.A.; Kwon, A.; Mellinghoff, I.K.; Witte, O.N. Progression of prostate cancer by synergy of AKT with genotropic and nongenotropic actions of the androgen receptor. Proc. Natl. Acad. Sci. USA 2006, 103, 7789–7794. [Google Scholar] [CrossRef] [Green Version]
- Ha, S.; Ruoff, R.; Kahoud, N.; Franke, T.F.; Logan, S.K. Androgen receptor levels are upregulated by Akt in prostate cancer. Endocr. Relat. Cancer 2011, 18, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Mikhailova, M.; Wang, Y.; Bedolla, R.; Lu, X.-H.; Kreisberg, J.I.; Ghosh, P.M. AKT Regulates Androgen Receptor-dependent Growth and PSA Expression in Prostate Cancer; Springer: New York, NY, USA, 2008; Volume 617. [Google Scholar]
- Thomas, C.; Lamoureux, F.; Crafter, C.; Davies, B.R.; Beraldi, E.; Fazli, L.; Kim, S.; Thaper, D.; Gleave, M.E.; Zoubeidi, A. Synergistic targeting of PI3K/AKT pathway and androgen receptor axis significantly delays castration-resistant prostate cancer progression in vivo. Mol. Cancer Ther. 2013, 12, 2342–2355. [Google Scholar] [CrossRef] [Green Version]
- Tai, S.; Sun, Y.; Squires, J.M.; Zhang, H.; Oh, W.K.; Liang, C.Z.; Huang, J. PC3 is a cell line characteristic of prostatic small cell carcinoma. Prostate 2011, 71, 1668–1679. [Google Scholar] [CrossRef] [Green Version]
- Shukla, S.; Maclennan, G.T.; Hartman, D.J.; Fu, P.; Resnick, M.I.; Gupta, S. Activation of PI3K-Akt signaling pathway promotes prostate cancer cell invasion. Int. J. Cancer 2007, 121, 1424–1432. [Google Scholar] [CrossRef] [PubMed]
- Thiery, J.P. Epithelial-mesenchymal transitions in tumour progression. Nat. Rev. Cancer 2002, 2, 442–454. [Google Scholar] [CrossRef]
- Grünert, S.; Jechlinger, M.; Beug, H.; Grünert, S. Diverse cellular and molecular mechanisms contribute to epithelial plasticity and metastasis. Nat. Rev. Mol. Cell Biol. 2003, 4, 657–665. [Google Scholar] [CrossRef]
- Mittal, V. Epithelial Mesenchymal Transition in Tumor Metastasis. Annu. Rev. Pathol. Mech. Dis. 2018, 13, 395–412. [Google Scholar] [CrossRef]
- Lin, C.Y.; Jan, Y.J.; Kuo, L.K.; Wang, B.J.; Huo, C.; Jiang, S.S.; Chen, S.C.; Kuo, Y.Y.; Chang, C.R.; Chuu, C.P. Elevation of androgen receptor promotes prostate cancer metastasis by induction of epithelial-mesenchymal transition and reduction of KAT5. Cancer Sci. 2018, 109, 3564–3574. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Yang, Z.; Lu, N. A new role for the PI3K/Akt signaling pathway in the epithelial-mesenchymal transition. Cell Adhes. Migr. 2015, 9, 317–324. [Google Scholar] [CrossRef] [Green Version]
- Dong, J.; Zhai, B.; Sun, W.; Hu, F.; Cheng, H.; Xu, J. Activation of phosphatidylinositol 3-kinase/AKT/snail signaling pathway contributes to epithelial-mesenchymal transition-induced multi-drug resistance to sorafenib in hepatocellular carcinoma cells. PLoS ONE 2017, 12, e0185088. [Google Scholar] [CrossRef] [PubMed] [Green Version]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Ryu, S.; Lee, Y.-K.; Lee, H.-J. Brassicasterol from Edible Aquacultural Hippocampus abdominalis Exerts an Anti-Cancer Effect by Dual-Targeting AKT and AR Signaling in Prostate Cancer. Biomedicines 2020, 8, 370. https://doi.org/10.3390/biomedicines8090370
Xu Y, Ryu S, Lee Y-K, Lee H-J. Brassicasterol from Edible Aquacultural Hippocampus abdominalis Exerts an Anti-Cancer Effect by Dual-Targeting AKT and AR Signaling in Prostate Cancer. Biomedicines. 2020; 8(9):370. https://doi.org/10.3390/biomedicines8090370
Chicago/Turabian StyleXu, Yinzhu, Sooin Ryu, You-Kyung Lee, and Hyo-Jeong Lee. 2020. "Brassicasterol from Edible Aquacultural Hippocampus abdominalis Exerts an Anti-Cancer Effect by Dual-Targeting AKT and AR Signaling in Prostate Cancer" Biomedicines 8, no. 9: 370. https://doi.org/10.3390/biomedicines8090370
APA StyleXu, Y., Ryu, S., Lee, Y.-K., & Lee, H.-J. (2020). Brassicasterol from Edible Aquacultural Hippocampus abdominalis Exerts an Anti-Cancer Effect by Dual-Targeting AKT and AR Signaling in Prostate Cancer. Biomedicines, 8(9), 370. https://doi.org/10.3390/biomedicines8090370




